Abstract
The evolution of longevity requires a low risk of mortality from extrinsic factors, relative to intrinsic factors, so that individuals that differentially invest in physiological self-maintenance and minimize their annual reproductive costs will maximize lifetime fitness through a prolonged reproductive lifespan. The trade-off between reproductive effort and self-maintenance, as measured by immune function, has been well documented in short-lived birds, but is difficult to demonstrate in long-lived birds. To assess self-maintenance in a long-lived seabird, we measured serum protein levels, including immunoglobulin G (IgG = IgY), in 30 breeding pairs of common terns (Sterna hirundo) and their first-hatched (A) chicks. Most parents were of known age from banding as hatchlings; our sample was selected to contrast young breeders (6–9 years) with very old birds (17–23 years). Body-mass of the parents declined by 5% during the chick-rearing period, while serum protein levels were stable. Serum IgG levels were higher in parents of offspring with faster growth rates, while IgG levels were lower in parents whose broods were reduced by starvation. A-chicks in broods of two had higher IgG levels than singleton chicks. Albumin levels were not related to reproductive performance. Thus, despite adequate statistical power, we could find no evidence for a trade-off between reproduction and self-maintenance in common terns, even in old age. The results are consistent with life-history predictions for long-lived vertebrates, in which selection favors sustained self-maintenance across the reproductive lifespan. The positive relationships between IgG levels and reproductive performance indicate that IgG can be used as an index of parental “quality.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds are remarkable for their exceptional longevity in the face of high metabolic turnover (Holmes and Ottinger 2003). Although some avian taxa show actuarial (Ricklefs 2000), reproductive (Møller and de Lope 1999), and immunological senescence (Saino et al. 2003; Cichon et al. 2003), rates of senescence vary widely and appear to be associated with other aspects of life history (Sanz and Moreno 2000). Birds with high risks of mortality from extrinsic factors (e.g. weather, food shortage, or predation) are expected to have rapid development, high reproductive investment, and low annual survival rates (Williams 1966; Stearns 1992). These taxa display trade-offs between reproductive effort and self-maintenance, and consequently appear to show physiological deterioration with age (Gustafsson and Pärt 1990). For these short-lived taxa, costs of reproduction are manifested in reduced annual survival (Nur 1984a) and future reproductive performance (Roskaft 1985) of the parents, as well as offspring morphology and recruitment (Nur 1984b). In addition, experimentally increased reproductive effort results in depressed antibody responses in parents (Deerenberg et al. 1997; Nordling et al. 1998), indicating reduced self-maintenance.
In contrast, taxa with low risks from extrinsic hazards typically have prolonged growth and developmental periods, delayed sexual maturation, low annual fecundity, and high survival rates and longevity (Stearns 1992). Seabirds breeding on predator-free islands typify this suite of traits (Hamer et al. 2002). Slow offspring growth rates of seabirds are thought to reflect the parents’ strategy of provisioning their offspring secondarily to maintaining their own body condition, thereby minimizing their costs of reproduction and lengthening their reproductive lifespans (Ricklefs 1992; Sæther et al. 1993).
In long-lived species, individual differences in physiological performance or “quality,” may hinder the detection of costs of reproduction and senescence. In at least some species, individuals that are proficient foragers are more likely to survive and consistently produce offspring, yielding positive covariance between reproduction and survival (Cam et al. 2002). These individuals predominate in the oldest cohorts, so that reproductive performance may remain high or increase with age in cross-sectional (cohort) comparisons, even if it declines with age within individuals (Nisbet 2001). Longitudinal (repeated measures) analysis of reproductive performance of individuals provides the best evidence for identifying high-quality individuals (Cam and Monnat 2000). These individuals show a positive relationship between reproductive output and self-maintenance as both are potentially driven by the superior foraging efficiency of high-quality birds. Currently, research is being undertaken to identify physiological biomarkers of individual quality (Moreno 2003).
The common tern (Sterna hirundo) population that one of us (ICTN) studied for >30 years allows the study of the relationship between reproductive success and self-maintenance across the lifespan of this long-lived seabird. This species shows little or no evidence for age-related declines in survival (Nisbet and Cam 2002), reproductive performance (Nisbet et al. 2002a), endocrine (Nisbet et al. 1999), or immune function (Apanius and Nisbet 2003). Most of the parents selected for this study were of known age (6–23 years), allowing us to test whether any of the relationships examined varied with parental age across the entire lifespan of the species. In this paper, we ask whether high-quality parents, as measured by offspring number and growth rate, also show enhanced self-maintenance, as measured by one component of immune function (serum immunoglobulin G [IgG] levels). In long-lived seabirds, we expect positive covariation in parental performance and immune-mediated self-maintenance, especially if the immunological mechanism entails memory of previous antigenic exposures.
IgG (=IgY) is the predominant circulating antibody molecule in birds and is produced by bursally derived (B-) lymphocytes, which are responsible for life-long immunological memory of humoral responses (Warr 1995). Serum levels reflect systemic production directed against dietary and environmental antigens that breach mucosal surfaces and low levels are associated with recurrent opportunistic bacterial infections in humans (Root and Ryan 1985) and domestic animals (Kanecko 1997). Metabolic studies in laboratory animals and humans show that serum IgG levels are maintained around a homeostatic set-point (Waldmann et al. 1970). In poultry species, these homeostatic levels show heritable variation (Rees and Nordskog 1981) that responds to artificial selection (Sarker et al. 1999). Selection for elevated IgG levels increased experimentally induced antibody responses (Sarker et al. 2000). Hence, baseline IgG levels are an evolutionarily labile trait. Under natural conditions, circulating IgG levels will reflect a genotype-by-environment interaction as baseline levels are shifted by exposure to environmental antigens (Lemke et al. 2004). Higher IgG levels can be regarded as a greater allocation of host resources to this immune mechanism either proximately, as a phenotypic response to the antigenic environment, or ultimately, as a genetic response to selection. Among practical considerations, repeated blood sampling to measure IgG does not influence the immunological phenotype and the values obtained are comparable among species (ms, in prep.).
In both parent terns and their offspring, we compared serum levels of IgG with those of albumin which is the most abundant serum protein and provides nutritional and transport functions (Kaneko 1997). Circulating levels of albumin are controlled homeostatically, independently of IgG, and are sensitive to protein malnutrition in growing chickens (Leveille and Sauberlich 1961). Thus, albumin levels can provide additional information about body condition, but are not predicted to be key components of the hypothesized relationship between immune self-maintenance and longevity.
Materials and methods
Study area and population
This study was conducted at Bird Island, Marion, MA (41°40′N, 70°43′W), in May–June 1999 as described in Nisbet et al. (2002a). We sampled 59 parents on 30 nests; 1 parent was not captured. For this study, we used samples restricted to a narrow range of dates to minimize the effects of laying date (Arnold et al. 2004). In 1999, egg-laying occurred between May 7 and June 4 (Nisbet et al. 2002a). Clutches of our study birds were initiated between May 7 and 16 and the first egg of each clutch hatched between May 29 and June 7. Parents were sampled between May 30 and June 6, between 5 days earlier and 4 days later than the hatching of their first eggs. This sampling design yielded a strong positive correlation between hatch date and sampling date. Hence, it was not possible to distinguish statistically between effects of hatch date, season, and stage in the reproductive cycle within our 8-day sampling frame.
The exact ages of 41 parents were known (6–23 years) from banding as chicks. The sampling design attempted to balance the number of nests with a young parent (6–9 years, N=14) to those with an old parent (17–23 years, N=17); to the extent possible, these two groups were matched by hatch date and sampling date (Nisbet et al. 2002a). Mates of these known-age birds were either unbanded (unknown age) or were also known-age, among the latter cases we were able to test for age-based assortative mating. The birds aged 6–9 years were younger than the median age and those aged 17–23 years comprised the oldest 7% of the local population (Nisbet 2002). This increased the power of the study to detect age-related variation in reproductive (Nisbet et al. 2002a) and immunological variables (Apanius and Nisbet 2003). All study pairs nested earlier than others of the same age in the same colony (Nisbet et al. 2002a), so that both old and (especially) young birds were expected to be of above-average “quality” for their ages (Arnold et al. 2004).
A subset of 13 parents was recaptured between June 23 and 27 when their oldest chicks were close to fledging (days 21–26). There were no significant differences between singly and repeatedly sampled birds in any characteristic measured at the time of initial sampling: body-mass (ANOVA F 1,53=0.16, P=0.69), total protein (F 1,56=0.12, P=0.72), albumin (F 1,56=1.29, P=0.26), or IgG (F 1,56=1.24, P=0.27). Hence, we considered parents resampled at the time of fledging to be a random sample of the study population and used them in a repeated-measures analysis of variables between hatching and fledging timepoints.
Field procedures
Parents were captured using walk-in traps placed over their nest. At each capture (1) body-mass was measured with a Pesola spring balance (±1 g); (2) head length (back of skull to tip of bill) was measured with digital calipers (±0.2 mm); (3) blood (100—700 μl) was collected by jugular venipuncture. Birds in the nonoverlapping parts of the distribution for this species were sexed by head length (Nisbet 2002) resulting in 16 known-sex females and 16 males.
All study nests were checked daily, usually between 0900 and 1100, and were enclosed within low fences (minimum plot area 2 m2) to facilitate capture of cursorial chicks. All chicks were banded at hatching (day 0) and were weighed (±0.1 g) on each day until day 4 and on alternate days thereafter (±0.5 g) until fledging. Body-mass data collected on the same days as blood sampling were used in repeated-measures analyses. All body-mass measurements (n=14–21 per individual) were fitted to logistic growth curves. The logistic growth constant (k) and asymptotic body-mass (A) were uncorrelated (r=0.12, df=29, P=0.52) and are used as independent summary variables of offspring growth.
In the study nests, fledging success was 70% (45 fledglings/64 hatchlings) and productivity (1.50 fledglings/breeding pair) was significantly higher than the mean (0.95±0.11, N=40) for a sample of nests selected as representative of the colony (Nisbet et al. 2002a; Mann–Whitney test, P<0.001). This confirms our expectation that these earlybreeding birds were of above-average quality.
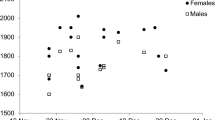
Survival outcomes of chicks in the study broods are summarized in Fig. 1. All pairs raised either one (N=15) or two chicks (N=15) to fledging. In 17 broods, one or two chicks died or disappeared (“brood reduction”). As in earlier studies of common terns (Nisbet 2002), most of the chicks that died or disappeared were the third-hatched (“C-chicks”) or second-hatched (“B-chicks”) within the brood; most had lost body-mass and appeared to have died from starvation, reflecting sibling competition that favors the oldest chick (“A-chick”) in each brood (Bollinger 1994). We classified each brood according to whether brood reduction occurred or not. For each focal A-chick at each sampling date, we scored the presence/absence of siblings, their number (1 or 2) if present and their summed body-masses. Fledging brood-size was as likely to be determined by brood reduction as not (17/30 vs. 13/30, respectively; Fisher exact test, P=0.45). Therefore, we treated these two variables as independent measures of parental performance.
Classification of first-hatched common tern (Sterna hirundo) chicks with regard to the presence (filled circle) or absence (open circle) of siblings, brood reduction (BR), number of fledglings (NF), and sample size (N=number of nests). Bars represent first- (a), second- (b), or third-hatched (c) chicks; truncated bars indicate death of a chick. BR indicates whether sibling mortality occurred or not. NA = not applicable
Blood samples (about 50 μl) were collected by jugular venipuncture from A-chicks at approximately 4-day intervals: day 4 (range 3–5, N=29), day 8 (7–9, N=27), day 12 (11–14, N=30), day 16 (15–17, N=30), day 20 (19–22, N=30), and day 24 (24–26, N=24). In addition, a single blood sample (about 20 μl) was taken from 19 chicks on days 0–2 to estimate the time-course of catabolism of maternally derived IgG (Apanius 1998). The volume of blood removed was 2.7–2.9% of the estimated total blood volume (10% of body-mass; Bond and Gilbert 1958; Fudge 2000) per week during the first 2 weeks of life. At day 4, we compared chicks that were previously sampled (N=19) with those that were not (N=11) and found no significant differences in body-mass (ANOVA: F 1,28=0.44, P=0.51), albumin (F 1,27=0.43, P=0.52), or IgG (F 1,27=0.62, P=0.44). Thus, there was no evidence that sampling of young chicks perturbed their growth or hematological variables. Weekly removal of 10% of the blood volume from adult American kestrels (Falco sparverius) did not perturb their hematological values (Rehder et al. 1982).
Hematological assays
Field-collected blood samples were placed in polypropylene microcentrifuge tubes, allowed to clot at ambient temperature for 2 h, and then placed in a chilled (4–10°C) container. Within 6 h, tubes were centrifuged (10,000 rpm for 10 min) and the serum was collected. Serum was frozen at −20°C for a maximum of 30 days, transported on dry ice and stored at −80°C.
Total serum protein was measured with Bradford reagent (Protein Assay, Biorad, Richmond, CA). Serum albumin was measured with bromcresol green reagent (B6671, Sigma, St. Louis, MO) following Doumas et al. (1971). In both cases, standard curves were constructed using bovine serum albumin (BP1600, Fisher, Pittsburg, PA). Common tern serum IgG was identified based on the molecular weight of the native protein and of subunits following reductive dissociation in two-dimensional electrophoresis following Apanius et al. (1983). Potential interference from lipoproteins was avoided by using an anionic detergent buffer (0.33% sodium dodecylsulfate in 0.5 M Tris–HCl pH 6.8 and 10% glycerol). IgG levels (g l−1) were measured by electrophoretic separation in 7.5% polyacrylamide gels followed by quantitative Coomassie G-250 staining protocol (Neuhoff et al. 1988; Apanius and Nisbet 2003). We constructed standard curves of 2, 4, 6, 8, 10 g l−1 of purified chicken IgG (I4881, Sigma) in every gel (all r 2>0.95).
Statistical methods
Repeated measurements of A-chick body-masses, albumin, and IgG levels on days 4, 8, 12, 16, 20, and 24 were analyzed using linear mixed models (PROC MIXED, v. 9.1, SAS Institute Inc., Cary, NC). The uniformly spaced time-points (age-classes) were treated as an ordinal categorical variable. An auto-regressive AR(1) covariance structure, in which the autocorrelation between measurements decreased exponentially with time, provided the best fit as determined by the Akaike information theoretic criterion (Littell et al. 2000). The intra-class correlation (autocorrelation) coefficient (ρ) was simultaneously estimated and represents the correlation between consecutive measurements from the same individual. Monotonic growth of each variable was indicated by a significant trend (1 df linear contrast) across age-classes. Significant differences between adjacent age-classes were identified using planned pairwise contrasts.
To test the effect of sibling competition on A-chick body-mass, albumin, and IgG, we categorized A-chicks by the presence or absence of siblings at the time of measurement and used age-class as a covariate. To test whether the number of siblings (or their summed body-masses) was related to body-mass, albumin, or IgG, we categorized broods as having one or two siblings present (or summed their body-masses) at the time of measurement and used age-class as a covariate. The significance of the age-class interaction was tested to determine if the effects of siblings were uniform across age-classes. Degrees of freedom reflect number of nests and age-classes that siblings were present or absent.
To test for the effect of brood reduction on A-chick body-mass, albumin, and IgG growth, we categorized nests by brood reduction and used age-class as a covariate. The significance of the age-class interaction was tested to determine if the effect of brood reduction was uniform across age-classes. Degrees of freedom for this test are based on the 24 nests for which brood reduction was possible; 6 nests with single hatchlings were excluded. To test for effects of fledging brood-size on A-chick body-mass, we categorized all broods by the number of offspring that fledged and used age-class as a covariate. The significance of the age-class interaction was tested to determine if the effect of fledging brood-size was uniform across age-classes.
All A-chick body-mass measurements were used to fit logistic growth curves (PROC NLIN) for estimation of the logistic growth constant (k) and the asymptotic body-mass (A). The repeated-measures analysis using age-classes was complementary to parameterization of growth by fitting to a specified function. Whereas the logistic equation assumes a canonical relationship between age and each variable, the changes in body-mass, albumin, and IgG levels across age-classes were not constrained to a particular functional form in the mixed models. This allowed detection of effects at specific age-classes as interactions.
For parents, assortative mating based on (rank-transformed) age, body-mass, albumin of IgG was tested by correlation analysis (PROC CORR). Correlation analysis was also used to test relationships between (rank-transformed) age, body-mass, albumin, and IgG levels at hatching and fledging. General linear models (PROC GLM) were used to test the relationships between parental (rank-transformed) age, body-mass, albumin, and IgG levels at hatching with reproductive and offspring growth variables. For parents sampled twice, general linear mixed models were employed to test for trends in parental body-mass, albumin, and IgG levels between hatching and fledging. The residuals of linear models were examined and satisfied the assumptions of normality and homogeneity of variances.
Statistical power (1-β) for the comparison of paired means was calculated using the means, standard deviations, intra-class correlation coefficients, and sample sizes of the hematological parameters at the time of hatching and fledging (PROC POWER). Power is reported as the minimum detectable difference (MDD) at β=0.20 and at α=0.05 or as the minimum significant r (MSR) at α=0.05.
Results
Offspring: body-mass
Body-mass of A-chicks increased with age, peaking at day 20 (Table 1; Fig. 2a), then appeared to decline on day 24 (P=0.055). The mean logistic growth constant k was 0.279±0.007 (SE) and the mean asymptotic mass A was 116.1±1.9 g, or 13.0±2.1 g (10.1%) less than the mean adult body-mass (F 1,84=37.35, P<0.0001).
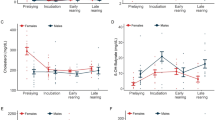
Characteristics of common tern A-chicks as a function of offspring age-class (0–24 days old) and the presence (filled circle) or absence (open circle) of siblings at the time of measurement for a body-mass, b albumin levels, and c IgG levels (mean ± 1 SEM). Adult values for a body-mass, b albumin, and c IgG are indicated by horizontal lines [mean (straight line) ± 1 SEM (dashed line)]. Numbers refer to the sample size for nests with siblings absent (top) or present (bottom) in a and c; numbers refer to the sample sizes of all nests in b
Of the 30 study nests, the number of nests with a single A-chick was 6 (20%) at hatching and 15 (50%) at fledging. Compared to singletons, A-chicks with a sibling at the time of measurement had lower body-masses on days 12 (P<0.0001), 16 (P<0.0001), and 20 (P=0.0072), but not at other age-classes, as shown by the significant age-class × body-mass interaction (Fig. 2a; Table 1). Similar results were obtained when the six nests with a single A-chick were excluded from the analysis, except that the interaction term was only marginally significant (F 5,104=2.05, P=0.078). For broods in which the younger sibling survived to fledging, A-chick body-mass was lighter at days 12 (P=0.050), 16 (P=0.12), and 20 (P=0.049), but not at other ages, compared to singletons (significant age-class × fledgling-number interaction, Table 1). At fledging, body-masses of A-chicks with a sibling were 9.9±2.8 g (8.6%) lighter than those without one (F 1,28=9.79, P=0.0041) but there was no difference in the logistic growth constant (F 1,28=0.09, P=0.77, MDD=0.028). In broods that fledged two chicks, body-mass of the A-chick was consistently 6.05±2.58 g (5.1%) heavier than the B-chick (F 1,28=5.47, P=0.027), with no evidence that this difference varied among age-classes (interaction F 5,128=1.09, P=0.37). There was no evidence that the number of siblings (1 vs. 2) or their summed body-masses at any age-class was related to the body-mass of the A-chick (all P>0.12). In summary, the presence of siblings decreased the body-mass of the A-chick starting on day 12 and through fledging.
Comparing across age-classes, neither A-chick body-mass nor the age-class interaction was related to brood reduction (Table 1). Logistic growth constants were 0.025±0.013 (9.0%) lower in A-chicks from broods with brood reduction compared to those without, although the statistical significance was marginal (F 1,28=3.64, P=0.067). The asymptotic mass of A-chicks did not differ between broods that were reduced or not (F 1,28=0.19, P=0.66, MDD=7.9 g). Thus, brood reduction was apparently associated with decreased growth rates of the A-chicks, but not with their fledging body-masses.
Offspring: hematology
Albumin levels in A-chicks were low and not significantly different between days 0 and 2 (F 1,24=0.79, P=0.38), consistently increased between days 4 and 16 (Table 1; Fig. 2b) and then remained stable afterward. Albumin levels were indistinguishable from adult levels on day 16 and thereafter (all P>0.50). Albumin levels were not correlated to body-mass at any age-class (all P>0.20). Albumin levels were not related to the presence of siblings, brood reduction, fledging brood-size (Table 1), or growth of A-chicks, as indexed by logistic growth constant and asymptotic mass (all P>0.19).
Catabolism of maternally derived IgG was evident from the declining levels observed in A-chicks between days 0 and 2 (Fig. 1c; F 1,24=20.92, P=0.0001). Subsequently, IgG levels were low and marginally increased between days 4 and 16 (P=0.058), then significantly increased between days 16 and 24 (P<0.0001). IgG levels on day 20 were indistinguishable from those in adults (F 1,85=1.91, P=0.17).
Controlling for differences among age-classes, IgG levels were 0.96±0.26 g l−1 greater in A-chicks with siblings present at the time of measurement compared to those without (Table 1; Fig. 2c). This difference was evident on day 4 (P=0.017) and was consistent across all age-classes, as indicated by the nonsignificant age-class × sib-present interaction (Table 1). The difference remained significant when the single-hatchling nests were omitted from the analysis (F 1,10=9.29, P=0.012). Post-hoc analysis indicated that IgG levels were not significantly related to the presence of siblings on day 16 (P=0.46) and day 24 (P=0.91). IgG levels were marginally related to the age-class × fledgling-number interaction (Table 1), with greater levels in A-chicks from broods of two on day 20 but not on other days. IgG levels were not related to brood reduction (Table 1). After controlling for age-class, IgG levels were not related to logistic growth constant or asymptotic body-mass (all P>0.52). IgG levels were not correlated to body-mass or albumin levels at any age-class (all P>0.33). In summary IgG, but not albumin, levels in A-chicks were higher when siblings were present.
Parents: age
Although assortative mating by age was expected (Bridge and Nisbet 2004), there was only marginal evidence for it in this study (r s=0.583, df=10 pairs, P=0.060, MSR=0.602). At a modest risk of pseudoreplication, the ages of parents were treated as statistically independent.
Parents: body-mass
Mean body-mass at the time of hatching did not differ between sexes (F 1,29=1.94, P=0.18, MDD=5.39). Body-mass was lower in older birds (mean slope=−0.22±0.12 g year−1, but this trend was only marginally significant (Table 2). Body-masses of parents declined by 5% between hatching and fledging of their offspring (Table 3). There was no evidence for assortative mating by body-mass (r=−0.046, df=26, P=0.82, MSR=0.388). Hence, body-masses of parents were treated as statistically independent.
Parents: hematology
At the time of hatching, parents did not differ significantly in albumin or IgG levels as a function of sex, age, or the interaction (all P>0.20). There was no evidence for assortative mating by albumin or IgG (all P>0.16). Hence, hematological measurements of parents were pooled across sexes and treated as statistically independent (Table 2).
In parents, albumin levels were positively correlated with IgG levels at both hatching and fledging (Table 2). Between hatching and fledging, there were no significant trends in albumin of IgG levels (Table 3). Parental body-mass was not related to hematological variables at either hatching or fledging (Table 2) nor was the decline in body-mass between hatching and fledging related to serum protein levels (all P>0.14).
Parents: reproductive performance
Parental age was not related to brood reduction or offspring growth, as assessed by logistic growth constants or asymptotic body-masses of A-chicks (Table 4). Parents that fledged two offspring were older by 3.39±1.72 years than those that fledged one, but the significance was borderline (Table 4).
Body-mass of parents at the time of hatching was not related to brood reduction, number of fledglings, nor offspring growth rate (Table 4). The rate of body-mass decline was greater for parents with two fledglings compared to one, but with questionable significance (F 1,10=3.38, P=0.096). The rate of body-mass decline was not related to the A-chick growth rate (F 1,10=0.10, P=0.76).
Parental IgG levels were positively correlated to logistic growth constants of their A-chicks (Table 4; Fig. 3) and were lower by 1.54±0.50 g l−1 (34%) in parents with brood reduction (Table 4). Albumin levels were not related to either reproductive or growth variables (Tables 3 and 4).
Parent–offspring correlations
Using measurements of A-chicks at fledging (day 20), body-mass, and albumin levels were not correlated between parents and offspring (Table 5). IgG levels in offspring were negatively correlated with those in their mothers and with the mean levels of both parents (mid-parent; Table 5).
Discussion
Although serum albumin and IgG in common tern parents were highly correlated, only IgG levels were associated with measures of reproductive performance. IgG levels were positively related to offspring growth rates and negatively related to brood reduction. To our knowledge, this is the first report of a positive association between reproductive performance and immune function. Previous observational and experimental studies in short-lived species have shown negative relationships between reproductive output and immune function, interpreted as trade-offs between reproductive effort and self-maintenance (Sheldon and Verhulst 1996).
This discrepancy is consistent with life-history theory. Previous studies used avian models that are short-lived, have high annual fecundity and show demographic senescence. The common tern has a long reproductive lifespan (3–25 years) and little evidence of physiological or demographic senescence (Nisbet et al. 1999, 2002a; Nisbet and Cam 2002). This life history is expected to reflect selection for sustained self-maintenance throughout the reproductive lifespan (Apanius and Nisbet 2003) rather than allocation of resources to maximize reproductive effort, as proposed in short-lived taxa (Sheldon and Verhulst 1996). In long-lived seabirds, the trade-off between reproduction and self-maintenance within individuals may be overridden by variation in phenotypic quality among individuals (Nisbet 2001). Among long-lived seabirds, consistent differences in reproductive performance among individuals have been reported in retrospective demographic analyses (Coulson and Thomas 1985; Mills 1989), but the physiological bases for differences in phenotypic “quality” are elusive. In accordance with a suggestion by Moreno (2003), we propose that self-maintenance through immunological mechanisms may be a key factor indexing individual quality. Moreover, we believe that the positive associations between reproductive success and immune function that we observed here may be common manifestations of the foraging efficiency of individual parents. Any change (whether genotypic or phenotypic) that increases the nutritional benefits per unit of foraging effort will increase the resources that are available for both reproduction and self-maintenance, both of which contribute to fitness in long-lived vertebrates.
The decrease in body-mass that we recorded in common tern parents between hatching and fledging of their chicks is similar to that reported by Wendeln and Becker (1996) in common terns between the last week of incubation and the first week of chick rearing. Although Wendeln and Becker attributed this difference to the stress of chick rearing, they gave no specific evidence for this conclusion and aerodynamic, for example, wing-loading, considerations may also be a plausible, and not mutually exclusive, explanation. Our study showed no relationship between the decrease in body-mass and measures of reproductive effort, including brood reduction and chick growth rates. More importantly, there was no hematological evidence that this decrease of body-mass in common terns was associated with a change in physiological condition. This contrasts with decreases in γ-globulin (γG) levels across the reproductive cycle in short-lived birds (γG levels are a less precise measure of serum IgG; Kaneko 1997). In great tits (Parus major), body-mass, packed cell volume, total plasma protein, albumin, and γG were lower in parents mid-way through the chick-rearing period compared to the pre-breeding period (Hõrak et al. 1998). For example, γG levels were 25 and 33% lower in females and males, respectively (Hõrak et al. 1998). In barn swallows (Hirundo rustica), γG levels peaked at egg-laying and declined during incubation in females, but did not change across the reproductive cycle in males (Saino et al. 2001). For males, γG levels were lower in those that bred later in the season; late-breeding males are believed to be of lower parental quality (Saino et al. 2001a). Higher γG levels were associated with greater overwinter survival in breeding barn swallows (Saino et al. 1997) and fledgling house martins (Delichon urbica; Christe et al. 2001). For short-lived species, γG as well as other hematological indicators point to a reduction in self-maintenance as a function of reproductive effort, which may negatively affect survival. Although our sample sizes (and statistical power) were limited, we could find no evidence of decreased self-maintenance across the reproductive cycle in common terns. Our preliminary finding warrants additional field studies.
Age-related declines in reproductive output (Møller and de Lope 1999), chick quality (Saino et al. 2003), and chick immune function (Cichon et al. 2003; Saino et al. 2002) have been observed in short-lived passerine birds. We found no evidence of age-related decline in reproductive success (Nisbet et al. 2002a) or IgG levels (Apanius and Nisbet 2003) among the oldest common terns, which represent the oldest 7% of the breeding population at this site (Nisbet 2002). Previous work has shown that early nesting common terns are more successful (Arnold et al. 2004; Tims et al. 2004) and are also older (Nisbet et al. 2002a; Tims et al. 2004). In this study, we found that chick growth and survival did not differ significantly between old and date-matched young parents or, if they did differ, older parents were more successful despite having lower body-masses. Our data suggest that common terns that breed early, regardless of age, are high-quality individuals that can maintain high reproductive effort and self-maintenance, rather than showing within-individual trade-offs between these two functions. Since our sample over-represented high-quality individuals, it remains to be seen whether our conclusions apply to all individuals in the population. Nonetheless, since “high quality” is operationally defined here as “above average reproductive success,” we believe that the physiological and life-history associations that we observed are driven by natural selection.
Previous work with this population has demonstrated that early laying (or hatching) dates predict above-average reproductive performance of the parents and can serve as proxies for parental quality (Arnold et al. 2004). We now have candidate physiological markers for investigating the mechanisms responsible for this phenological effect. Our study design precluded analysis of seasonal variation of the reproductive and physiological variables. A direct comparison of phenological versus physiological predictors of reproductive performance would require either a multi-year study of the same individual parents or experimental manipulation.
In common tern A-chicks, IgG levels did not show a trade-off with morphological growth. Again, this contrasts with the results obtained from studies of short-lived birds, in which reduced body growth is associated with reduced expression of immune function. In barn swallows, first-hatched chicks grew faster than later-hatched siblings but had lower γG levels at day 12 (Saino et al. 2001b). In sand martins (Riparia riparia), nestling γG levels were negatively related to the number and size of the offspring in the nest (Szep and Møller 1999). These results were interpreted as showing that morphological growth and immunological development were competing for limiting nutrients in rapidly growing nestlings.
The uncoupling of morphological growth from the ontogeny of IgG levels in common tern chicks is even more remarkable in the context of their rapid growth and sibling competition. We found that the presence of a sibling reduced the body-mass of A-chicks by 8–10%. Repeated-measures analysis showed that the negative effect of sibling competition was most apparent as chicks approached their peak body-mass, that is, between days 12 and 20. This overlapped with the ages when IgG levels were increasing at the greatest rate, for example, between days 16 and 24. Yet, IgG levels were higher in A-chicks with competing siblings, despite lower body-mass, while albumin levels did not differ. Two explanations are plausible. First, high-quality parents capable of provisioning two chicks might also provide a higher quality diet that allows greater expansion of the antibody repertoire and immunoglobulin production; however, this explanation is inconsistent with the observed negative parent–offspring correlations. Second, two-chick broods and sibling competition may be associated with higher circulating levels of androgens in the offspring (Eising and Groothuis 2003), which in turn may promote IgG production. This possibility is supported by the observation that black-headed gull chicks (Larus ridibundus) implanted with testosterone showed enhanced antibody responses (Ros et al. 1997). The widely held notion that testosterone is invariably immunosuppresive has been challenged recently (Hasselquist et al. 1999). Since one- and two-chick broods were interspersed and chicks intermingled in the dense breeding colony with considerable fecal contamination, it is unlikely that differing antigenic pressure was responsible for the difference in IgG levels.
The negative correlation between IgG levels in offspring and parents is puzzling. It may be due to differing antigenic loads in the diet. In common terns, parents feed mainly on small fish and crustaceans during the breeding season, whereas they feed their chicks mainly on larger fish (Nisbet 2002); exposures of parents and chicks to contaminants are also dissimilar (Nisbet et al. 2002b). Dietary factors may play a role in the expression of IgG levels. Lipopolysaccharides (LPS) from the gram-negative bacteria in the vertebrate gut are believed to stimulate B-lymphocyte proliferation and IgG production in chickens (Kuhlmann-Rabens et al. 1987).
In a cross-fostering experiment with house martins, γG levels were not related to brood of origin but to the rearing environment, which varied in intensity of ectoparasite infestation (Christe et al. 2000). However, experimentally increasing the number of nest ectoparasites (cimicid bugs) caused a reduction in nestling body-mass and increased total plasma protein, but no change in γG levels (de Lope et al. 1998), suggesting that ectoparasitism was not the causal mechanism. In our study, there was no evidence to suggest that elevated IgG levels indicated ectoparasitic or infectious disease based on (1) physical examination; (2) absence of a relationship between IgG and body-mass growth; (3) absence of hematazoan parasites (unpub. obs.); and (4) no evidence of elevated acute phase proteins, for example, transferrin, α-2-macroglobulin, haptoglobin, based on qualitative review of serum protein profiles (unpub. obs.). Since the study nests were interspersed in a dense breeding colony and ambulatory chicks intermingled with conspecifics after day 5, we find it reasonable to assume that antigenic stimulation was relatively homogenous between offspring. Switching eggs or hatchlings could potentially resolve the factors related to the negative correlation between parent and offspring IgG levels.
This negative correlation may be related to the activity of maternally transferred IgG. Mothers with higher levels of IgG would be expected to transfer a greater quantity, and potential quality, of IgG to the offspring (Kowalczyk et al. 1985). Maternally transferred antibodies were detectable in nestling blue and gold macaws (Ara ararauna) up to 42 days after hatching, indicating that they persist beyond the initial decline phase (Lung et al. 1996). Maternally derived antibody may bind to and block recognition of antigens by the offspring’s B-cells and thereby suppress the development of those specific antibodies (Solomon 1971); the net effect could reduce offspring IgG levels. Alternatively, maternally derived IgG could shape the developing antibody repertoire and lead to a phenotype with high-affinity antibodies (Lemke et al. 2004), but with lower overall IgG levels for the same level of protection. Phage display of the chicken antibody repertoire may be profitably adapted for addressing these speculations in the future (O’Brien and Aitken 2002).
A pertinent question is whether increased IgG levels signify greater investment in protective immunoglobulin production or a transient response to infection? Typically, IgG levels show long-term changes in response to chronic antigen loading typically associated with unhygienic diet and living conditions, which are especially notable in developing human populations (Rowe et al. 1968). IgG levels typically do not show short-term changes to acute opportunistic infections or single experimental immunizations since they represent the integration of innumerable antigen-specific responses (Benner et al. 1982). As noted above, ectoparasite manipulation induced pathological weight loss and increased total plasma proteins, but did not change IgG levels (de Lope et al. 1998). Injection of sheep red blood cells did not alter γG levels in barn swallows (Saino and Møller 1996) or IgG levels in prairie voles (Microtus ochrogaster) or laboratory mice (Mus musculus, De Vries et al. 1997). On the other hand, stress-induced increase in corticosterone was associated with reduced IgG levels (De Vries et al., 1997; Barnard et al. 1994) and prolonged glucocorticosteroid treatment severely decreased serum IgG levels in mice (Sabbele et al. 1983). Elevated corticosterone was found in seabird nestlings and parents facing nutrient limitation (Kitaysky et al. 1999, 2001) and is consistent with our hypothesis that IgG levels are physiologically linked to foraging efficiency of the parents. Under the hypothesis that parents with higher IgG levels were responding to chronic antigen dosing from persistent infections, it would be predicted that body-masses and albumin levels would be depressed and acute phase proteins would be elevated, but this was not observed. In fact, albumin and IgG levels were positively correlated at hatching and fledging, suggesting that parents with high IgG levels were on a superior nutritional plane. The role of corticosterone in mediating the relationship between IgG levels and reproductive performance warrants future investigation.
The central premise of the reproductive effort vs. self-maintenance trade-off is that limited nutritional resources are allocated into these mutually exclusive categories. In short-lived species, this assumption is supported by the observed decline in IgG levels with the reproductive cycle and negative relationship between IgG levels and offspring growth conditions. Yet the daily turnover of IgG in chickens is approximately 58 mg day−1 (Leslie and Clem 1970), while the maintenance protein intake is roughly 4 g day−1 (Klasing 1998, p. 142) so that IgG synthesis requires only about 1% of the daily protein intake. In mammals, the IgG turnover rate scales allometrically with body-mass with an exponent of −0.20 (Waldmann et al. 1970), indicating that smaller animals will have a more rapid turnover. At a given body-mass, IgG turnover rate is positively related to resting metabolic rate as well as serum concentration (Sabiston and Ste Rose 1976; Waldmann et al. 1970). The sum of these effects potentially increases the rate of IgG production in small birds to, at most, 5% in total protein intake. Either the cellular basis of immunoglobulin production is extremely costly, also unlikely for an adult, or some other explanation is required for understanding the regulation of IgG levels in a life-history context.
Serum IgG is the collective response to a lifetime of antigenic exposure (Lemke et al. 2004). Its value is expected to increase with reproductive lifespan as the antibody repertoire it contains is tuned to the individual’s antigenic environment. A small but consistent investment in IgG production and attendant memory may be more economical for controlling opportunistic environmental pathogens than intermittent up- and down-regulation of phagocytes and acute phase proteins (Lee and Klasing 2004). This also reduces the intra-individual variation in health status and may act to avoid vulnerable states when an individual expresses interleukin-1 induced sickness behavior (Lee and Klasing 2004). More importantly IgG increases the efficiency of bacterial clearance leading to a reduced reliance on free-radical mediated mechanisms used by granulocytes for bacterial destruction (Dröge 2002). To the extent that this form of free-radical damage is related to longevity, increased investment in and homeostasis of IgG levels would be favored in long-lived vertebrates.
In conclusion, circulating serum IgG levels in common terns were positively associated with parental quality, as indicated by chick growth rates and the occurrence of brood reduction. Thus, IgG-dependent self-maintenance positively covaried with reproductive performance in this long-lived species, as would be expected from a life-history-based theory of aging. The development of IgG levels in A-chicks did not appear to compete with morphological growth, as IgG levels were higher in chicks with younger siblings (i.e. in chicks raised by high-performing parents). There was little evidence for a decline in parental body as a function of parental effort. Our study is unique in using physiological and immunological indicators to assess reproductive performance and phenotypic quality of long-lived individuals.
References
Apanius V, Temple SA, Bale M (1983) Serum proteins of wild turkey vultures (Cathartes aura). Comp Biochem Physiol 76B:907–913
Apanius V (1998) Ontogeny of immune function. In: Starck JM, Ricklefs RE (eds) Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University Press, Oxford, pp 203–222
Apanius V, Nisbet ICT (2003) Serum immunoglobulin G levels in very old common terns Sterna hirundo. Exp Gerontol 38:761–764
Arnold JM, Hatch JJ, Nisbet ICT (2004) Seasonal declines in reproductive success of the common tern: timing or parental quality? J Avian Biol 35:33–45
Barnard C, Behnke J, Sewell J (1994) Social behaviour and susceptibility to infection in house mice (Mus musculus): effects of group size, aggressive behaviour and status-related hormonal responses prior to infection on resistance to Babesia microti. Parasitology 108:487–496
Benner R, van Oudenaren A, Björklund M, Ivars F, Holmberg D (1982) ‘Background’ immunoglobulin production: measurement, biological significance and regulation. Immunol Today 3:243–249
Bollinger PB (1994) Relative effects of hatching order, egg-size variation, and parental quality on chick survival in common terns. Auk 111:263–273
Bond C, Gilbert P (1958) Comparative study of blood volume in representative aquatic and non-aquatic birds. Am J Physiol 194:519–521
Bridge E, Nisbet ICT (2004) Wing molt and assortative mating in common terns: a test of the molt-signaling hypothesis. Condor 106:336–343
Cam E, Link WA, Cooch EG, Monnat JY, Danchin E (2002) Individual covariation in life-history traits: seeing the trees despite the forest. Am Nat 159:96–105
Cam E, Monnat JY (2000) Stratification based on reproductive state reveals contrasting patterns of age-related variation in demographic parameters in the kittiwake. Oikos 90:560–574
Cichon M, Sendecka J, Gustafsson L (2003) Age-related decline in humoral immune function in collared flycatchers. J Evol Biol 16:1205–1210
Christe P, Møller AP, Saino N, de Lope F (2000) Genetic and environmental components of phenotypic variation in immune response and body size of a colonial bird (Delichon urbica) (the house martin). Heredity 85:75–83
Christe P, de Lope F, Gonzalez G, Saino N, Møller AP (2001) The influence of environmental conditions on immune responses, morphology and recapture probability of nestling house martins (Delichon urbica). Oecologia 126:333–338
Coulson JC, Thomas CS (1985) Differences in the breeding performance of individual kittiwake gulls (Rissa tridactyla). In: Sibly RM, Smith RH (eds) Behavioural ecology. Blackwell Scientific Publications, Oxford, pp 489–503
Deerenberg C, Apanius V, Daan S, Bos N (1997) Reproductive effort decreases antibody responsiveness. Proc R Soc Lond B 264:1021–1029
de Lope F, Møller AP, de la Cruz C (1998) Parasitism, immune response and reproductive success in the house martin Delichon urbica. Oecologia 114:188–193
DeVries AC, et al (1997) Stress affects corticosteroid and immunoglobulin concentrations in male house mice (Mus musculus) and prairie voles (Microtus ochrogaster). Comp Biochem Physiol A 118:655–663
Doumas B, Watson W, Biggs H (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 1:87–96
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Eising CM, Groothuis TGG (2003) Yolk androgens and begging behaviour in black-headed gull chicks: an experimental field study. Anim Behav 66:1027–1034
Fudge AM (2000) Avian blood sampling and artifact considerations. In: Fudge AM (ed) Laboratory medicine: avian and exotic pets. WB Saunders, Philadelphia, pp 1–8
Gustafsson L, Pärt T (1990) Acceleration of senescence in the collared flycatcher Ficedula albicollis by reproductive costs. Nature 347:279–281
Hamer KC, Schreiber E, Burger J (2002) Breeding biology, life histories and life history-environment interactions in seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, FL, pp 217–261
Hasselquist D, Marsh JA, Sherman PW, Wingfield JC (1999) Is avian humoral immunocompetence suppressed by testosterone? Behav Ecol Sociobiol 45:167–175
Holmes DJ, Ottinger MA (2003) Birds as long-lived animal models for the study of aging. Exp Gerontol 38:1365–1375
Hõrak P, Jenni ES, Ots I, Tegelmann L (1998) Health and reproduction: the sex-specific clinical profile of great tits (Parus major) in relation to breeding. Can J Zool 76:2235–2244
Kaneko J (1997) Serum proteins and the dysproteinemias. In: Kaneko J, Harvey J, Bruss M (eds) Clinical biochemistry of domestic animals, 5th edn. Academic Press, New York, pp 117–138
Kitaysky AS, Piatt JF, Wingfield JC (1999) The adrenocortical stress-response of black-legged kittiwakes chicks in relation to dietary restrictions. J Comp Physiol B 169:303–310
Kitaysky AS, Wingfield JC, Piatt JF (2001) Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav Ecol 12:619–625
Klasing K (1998) Comparative avian nutrition. CAB International, New York
Kowalczyk K, Daiss J, Halpern J, Roth TF (1985) Quantitation of maternal-fetal IgG transport in the chicken. Immunology 54:755–762
Kuhlmann-Rabens I, Wanke R, Storandt F, Altmann B, Losch U, Merkenschlager M (1987) Attempts to localize the defect in dysgammaglobulinemia of UM-B19 chickens by studying the effect of immunomodulating substances on immunoglobulin and antibody production. Vet Immunol Immunopathol 14:123–143
Lee K, Klasing K (2004) A role for immunology in invasion biology. Trends Ecol Evol 19:523–529
Lemke H, Coutinho A, Lange H (2004) Lamarckian inheritance of somatically acquired maternal IgG phenotypes. Trends Immunol 25:180–186
Leslie G, Clem L (1970) Chicken immunoglobulins: biological half-lives and normal adult serum concentrations of IgM and IgY. Proc Soc Exp Biol Med 134:195–198
Leveille G, Sauberlich H (1961) Influence of dietary protein level on serum protein components and cholesterol in the growing chick. J Nutr 74:500–504
Littell RC, Pendergast J, Natarajan R (2000) Modelling covariance structure in the analysis of repeated measures data. Stat Med 2000:1793–1819
Lung N, Thompson J, Kollias G, Olsen J, Zdziarski J, Klein P (1996) Maternal immunoglobulin G antibody transfer and development of immunoglobulin G antibody responses in blue and gold Macaw (Ara ararauna) chicks. Am J Vet Res 57:1162–1167
Mills JA (1989) Red-billed gull. In: Newton I (ed) Lifetime reproductive success. Academic Press, London, pp 387–404
Møller AP, de Lope F (1999) Senescence in a short-lived migratory bird: age-dependent morphology, migration, reproduction and parasitism. J Anim Ecol 68:163–171
Moreno J (2003) Lifetime reproductive success in seabirds: interindividual differences and implications for conservation. Sci Mar 67(Suppl. 2):7–12
Nisbet ICT (2001) Detecting and measuring senescence in wild birds: experience with long-lived seabirds. Exp Gerontol 36:833–843
Nisbet ICT (2002) Common tern (Sterna hirundo). In: Poole A, Gill F (eds) The birds of North America, No. 618. The Birds of North America Inc., Philadelphia
Nisbet ICT, Cam E (2002) Test for age-specificity in survival of the common tern. J Appl Stat 29:65–83
Nisbet ICT, Apanius V, Friar MS (2002a) Breeding performance of very old common terns. J Field Ornithol 73:117–124
Nisbet ICT, Finch CE, Thompson N, Russek-Cohen E, Proudman JA, Ottinger MA (1999) Endocrine patterns during aging in the common tern (Sterna hirundo). Gen Comp Endocrinol 114:279–286
Nisbet ICT, Montoya JP, Burger J, Hatch JJ (2002b) Use of stable isotopes to investigate individual differences in diets and mercury exposures among common terns Sterna hirundo in breeding and wintering grounds. Mar Ecol Prog Ser 242:267–274
Nordling D, Andersson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond B 265:1291–1298
Neuhoff V, Arold N, Taube D, Ehrhardt W (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9:255–262
Nur N (1984a) The consequences of brood size for breeding blue tits I Adult survival, weight change and the cost of reproduction. J Anim Ecol 53:479–496
Nur N (1984b) The consequences of brood size for breeding blue tits II Nestling weight, offspring survival and optimal brood size. J Anim Ecol 53:497–517
O’Brien P, Aitken R (2002) Antibody phage display: methods and protocols. Humana Press, Totowa
Rees MJ, Nordskog AW (1981) Genetic control of serum immunoglobulin G levels in the chicken. J Immunogenet 8:425–431
Rehder NB, Bird DM, Lague PC (1982) Variations in blood packed cell volume of captive American kestrels. Comp Biochem Physiol 72A:105–109
Ricklefs RE (1992) The roles of parent and chick in determining feeding rates in Leach’s storm-petrel. Anim Behav 43:895–906
Ricklefs RE (2000) Intrinsic aging-related mortality in birds. J Avian Biol 31:103–111
Ros AFH, Groothuis TGG, Apanius V (1997) The relationship between gonadal steroids, immunocompetence, body mass, and behavior in young black-headed gulls (Larus ridibundus). Am Nat 150:201–219
Roskaft E (1985) The effect of enlarged brood size on the future reproductive potential of the rook. J Anim Ecol 54:255–260
Rowe D, McGregor I, Smith SJ (1968) Plasma immunoglobulin concentrations in a West African (Gambian) community and in a group of healthy British adults. Clin Exp Immunol 3:63–79
Sabbele N, van Oudenaren A, Benner R (1983) The effect of corticosteroids upon the number and organ distribution of ‘background’ immunoglobulin-secreting cells in mice. Cell Immunol 77:308–317
Sabiston B, Ste Rose J (1976) Effect of cold exposure on the metabolism of immunoglobulins in rabbits. J Immunol 116:106–111
Sæther BE, Andersen R, Pedersen HC (1993) Regulation of parental effort in a long-lived seabird—an experimental manipulation of the cost of reproduction in the Antarctic petrel, Thalassoica antarctica. Behav Ecol Sociobiol 33:147–150
Saino N, Bolzern AM, Møller AP (1997) Immunocompetence, ornamentation, and viability of male barn swallows (Hirundo rustica). Proc Nat Acad Sci U S A 94:549–552
Saino N, Martinelli R, Møller AP (2001a) Immunoglobulin plasma concentration in relation to egg laying and mate ornamentation of female barn swallows (Hirundo rustica). J Evol Biol 14:95–109
Saino N, Incagli M, Martinelli R, Ambrosini R, Møller AP (2001b) Immunity, growth and begging behaviour of nestling barn swallows Hirundo rustica in relation to hatching order. J Avian Biol 32:263–270
Saino N, Ambrosini R, Martinelli R, Møller AP (2002) Mate fidelity, senescence in breeding performance and reproductive trade-offs in the barn swallow. J Anim Ecol 71:309–319
Saino N, Ferrari RP, Romano M, Rubolini D, Møller AP (2003) Humoral immune response in relation to senescence, sex and sexual ornamentation in the barn swallow (Hirundo rustica). J Evol Biol 16:1127–1134
Sanz JJ, Moreno J (2000) Delayed senescence in a southern population of the pied flycatcher (Ficedula hypoleuca). Ecoscience 7:25–31
Sarker N, Tsudzuki M, Nishibori M, Yamamoto Y (1999) Direct and correlated response to divergent selection for serum immunoglobulin M and G levels in birds. Poult Sci 78:1–7
Sarker N, Tsudzuki M, Nishibori M, Yasue H, Yamamoto Y (2000) Cell-mediated and humoral immunity and phagocytic ability in chicken lines divergently selected for serum immunoglobulin M and G levels. Poult Sci 79:1705–1709
Sheldon BC, Verhulst S (1996) Ecological immunology, costly parasite defenses and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Solomon JB (1970) Unification of foetal and neonatal immunology. Nature 227:895–897
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Szep T, Møller AP (1999) Cost of parasitism and host immune defence in the sand martin (Riparia riparia): a role for parent-offspring conflict? Oecologia 119:9–15
Tims J, Nisbet ICT, Friar MS, Mostello C, Hatch JJ (2004) Characteristics and performance of common terns in old and newly-established colonies. Waterbirds 27:321–332
Waldmann T, Blaese R, Strober W (1970) Physiological factors controlling immunoglobulin metabolism. In: Rothschild M, Waldmann T (eds) Plasma protein metabolism: regulation of synthesis, distribution, and degradation. Academic Press, New York, pp 269–286
Warr GW, Magor KE, Higgins DA (1995) IgY: clues to the origins of modern antibodies. Immunol Today 16:392–398
Wendeln H, Becker PH (1996) Body mass change in breeding common terns Sterna hirundo. Bird Study 43:85–95
Williams GC (1966) Adaptation and natural selection. Princeton University Press, Princeton
Acknowledgments
We thank Suzanne Conlon, Margaret Friar, Tim Meehan, and Jeremy Hatch for help in the field in 1999, many other colleagues and assistants for banding chicks since 1976, and the Town of Marion for permission to work at Bird Island. Laboratory analyses were assisted by P. Scipio, Y. Sanchez, D. Rodriguez, and Y. Tran and supported by NIH-MBRS (GM61347). This research was approved by institutional, state, and federal agencies for compliance with animal welfare and migratory bird regulations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carol Vleck
Rights and permissions
About this article
Cite this article
Apanius, V., Nisbet, I.C. Serum immunoglobulin G levels are positively related to reproductive performance in a long-lived seabird, the common tern (Sterna hirundo). Oecologia 147, 12–23 (2006). https://doi.org/10.1007/s00442-005-0238-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0238-6