Abstract
Several infaunal bivalve taxa show patterns of decreased biomass in areas with higher densities of adjacent reef-associated predators (the snapper, Pagrus auratus and rock lobster, Jasus edwardsii). A caging experiment was used to test the hypothesis that patterns observed were caused by predation, using plots seeded with a known initial density of the bivalve Dosinia subrosea to estimate survivorship. The caging experiment was replicated at several sites inside and outside two highly protected marine reserves: predators are significantly more abundant inside these reserves. Survivorship in fully caged, partially caged and open plots were then compared at sites having either low (non reserve) or high (reserve) predator density. The highest rates of survivorship of the bivalve were found in caged plots inside reserves and in all treatments outside reserves. However, inside reserves, open and partially caged treatments exhibited low survivorship. It was possible to specifically attribute much of this mortality to predation by large rock lobsters, due to distinctive marks on the valves of dead D. subrosea. This suggests that predation by large rock lobster could indeed account for the distributional patterns previously documented for certain bivalve populations. Our results illustrate that protection afforded by marine reserves is necessary to investigate how depletion through fishing pressure can change the role of upper-level predators and trophic processes between habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotic interactions across habitat types have been observed to create distinct ‘halo’ patterns, often indicating foraging by fauna from a shelter to a food habitat (Ogden et al. 1973). Most investigations of these interactions have focused on meso grazing herbivores (Suchanek 1978) or secondary consumers (Fairweather 1988) rather than upper-level predators. ‘Haloes’ of reduced infaunal abundance have been observed in soft-sediment communities adjacent to rocky reefs and have been attributed to predation by reef-associated fauna (Davis et al. 1982; Posey and Ambrose 1994; Barros et al. 2001). However, these studies have been done with only limited replication over small spatial scales (Davis et al. 1982; Posey and Ambrose 1994) and have shown a lack of significant effects in caging studies designed to investigate predation (Posey and Ambrose 1994). Highly protected marine reserves have been suggested to provide a framework for large-scale experiments in which trophic interactions, involving more natural populations of predators can be observed (Dayton et al. 2000; Bohnsack 2003). Investigations of marine reserves have generally documented the recovery of previously exploited upper-level predators (Kelly et al. 2000; Willis et al. 2003). However, few have identified the trophic implications of increased populations and size distributions of these large, often relatively sedentary predators (Shears and Babcock 2002; Graham et al. 2003) and no previous studies have considered the importance of these trophic interactions across habitats.
Recent investigations in northeastern New Zealand have revealed patterns in soft-sediment communities near reefs that correlate well with the measured densities of two upper-level predators, the snapper, Pagrus auratus, and the rock lobster, Jasus edwardsii (Langlois et al. 2005). In that study, large-scale differences in abundances of predators found inside versus outside three comparable marine reserves were used to investigate the influence of these reef-associated predators on adjacent soft-sediment fauna. Snapper are generalist predators that take primarily invertebrate prey from both soft-sediment habitats and rocky reefs (Paul 1976; Babcock et al. 1999) and rock lobster, although commonly assumed to spend most of their time on rocky reefs, have been observed to forage over adjacent sandy areas (Kelly et al. 1999). MacDiarmid et al. (1991) found nocturnal foraging by J. edwardsii to be spatially limited, suggesting that any control these rock lobster may have on prey populations would be restricted to areas near day-time shelters. Langlois et al. (2005) found sites with consistently higher densities of snapper and lobster to have ‘haloes’ of lower biomass of several bivalve species adjacent to the reef, in particular Dosinia subrosea, the largest and third most abundant species of the infaunal assemblage. This pattern was consistent across the three locations that were separated by hundreds of kilometres. In addition, the comparison of sites with different densities of predators could not be correlated in any systematic way with other measured environmental variables, such as sediment characteristics, densities of bioturbating infauna or predatory infauna. These observations, however, although quantitative and replicated, do not provide conclusive evidence that differences in bivalve densities are due to predation by snapper and rock lobster, as correlation does not provide a basis for causal inference. Experimental caging manipulations are needed to establish that predators are causing the observed effects.
Caging manipulations are useful in that they can reduce or remove concerns relating to confounding environmental effects at larger scales. However, such experiments are successful only if there are significant levels of predation in the ‘uncaged’ treatment (Posey and Ambrose 1994; Connell 1997). We used the large-scale differences in density of upper-level predators inside versus outside two marine reserves as part of a large-scale caging manipulation, with replication at three spatial scales. Snapper and rock lobster are both heavily fished in northeastern New Zealand and they have been found to occur at significantly higher densities inside no-take marine reserves. Greater-than-legal-sized snapper (>270 mm fork length) and rock lobster (>100 mm carapace length (CL)) have been observed to be 14 times and 3.7 times more abundant, respectively, inside no-take reserves than outside (Babcock et al. 1999). However, the role of predators in structuring soft-sediment systems can be complex (Thrush 1999) and confounded by environmental heterogeneity at larger scales (Legendre et al. 1997). Here, by replicating the caging manipulation both inside and outside reserves, the relative sizes of any effects could be compared between sites of high (reserve) and low (non reserve) predator densities.
The model proposed was that the differences in the distribution of large bivalves observed inside versus outside marine reserves are due to greater levels of predation inside the reserves. The central hypothesis was that there would be significantly lower survivorship of the bivalve, D. subrosea, in uncaged areas (exposed to predation) than in caged areas where predators were excluded. It was also predicted that survivorship of bivalves would be lower in areas open to predators at sites having higher densities of predators (inside reserves) than in open areas with lower densities of predators (outside reserves).
Laboratory feeding trials of juvenile J. edwardsii on the mussel Perna canaliculus (James and Tong 1998) have described an innate feeding technique where the mandibles leave a distinctive damage pattern on the posterior margin of bivalve shells. Similarly, aquaria trials indicated that the method used by larger rock lobsters to open and consume D. subrosea also leaves distinctive marks on their shells (T. J. Langlois pers. obs.). Furthermore, shells of D. subrosea (∼50 mm) preyed on by large lobster (>120 mm CL) exhibit marks distinct from those preyed on by smaller lobster (< 90 mm CL) or other predators. By identifying these distinctive marks on shells of D. subrosea in the field, we also tested the hypothesis that predation of D. subrosea is primarily due to large rock lobster. Feeding on Dosinia species by snapper has been reported (Godfriaux 1970) but has not been widely observed.
Materials and methods
Study sites and sampling methods
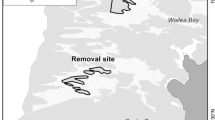
Two separate no-take marine reserve locations in northeastern New Zealand were studied between January and March of 2003. The Cape Rodney to Okakari Point (Leigh) Marine Reserve (36°16′S, 174°48′E) was gazetted in 1975 and the Tawharanui Marine Park (36°22′S, 174°50′E) was declared a no-take area in 1981 (Fig. 1). Six sites were designated at each location (three inside and three outside each marine reserve) at 12 m depth on sand flats adjacent to the extensive subtidal rocky reefs. They were chosen for similar wave exposure and reef/soft-sediment interfaces. To ensure interspersion, non reserve sites were selected on either side of each reserve at distances of more than 500 m apart (see Fig. 1). Within each site, 12 circular plots (each 0.5 m2) were established (5–7 m from the reef edge and marked with two steel stakes) within which all D. subrosea were removed before the plots were reseeded with ten live D. subrosea. These densities were comparable to densities observed in wild populations outside reserves (Langlois et al. 2005). No plots were more than 25 m away from each other. Dosinia subrosea were of similar size (~50 mm) and obtained from local populations. Exclusion cages were constructed over four of these plots, using steel mesh with a cross-mesh measurement of 75 mm, and anchored by steel stakes. Cages were circular, extended 150 mm into the sediment, and protruded 200 mm above. Lids to the cages were constructed out of the same material and hinged to allow easy access. Over four other plots, cage walls were constructed around half the circumference of the plots but lids were not added, creating partial exposure. The four remaining plots were left open. These three treatments (cage, partial cage and open plots) were haphazardly interspersed across each site. These bivalves are not thought to be highly mobile (Powell 1979) and a pilot study did not find any measurable migration of D. subrosea out of the plots (T.J. Langlois unpub. data). All plots were excavated after 2 weeks to estimate the survivorship of D. subrosea. Shells of dead D. subrosea were collected from an area of ~250 m2 around the plots and along the adjacent reef edge.
Environmental variables
Densities of snapper at the sites were estimated from data collected in April and May of 2002. Relative densities at these locations were shown to be fairly stable between years (Willis et al. 2003). Baited underwater video (BUV) (Willis and Babcock 2000) (n=4) was used at each site to estimate snapper density. Densities of lobster at each site were estimated by underwater visual census (UVC) of 25 m2 quadrats (n=10) during February and March 2003, and the sizes of lobsters were obtained using a visual method as described by MacDiarmid (1991). Feeding rates in captive J. edwardsii from January to March have been observed to be approximately 50% of the peak rates found in November and December (Kelly et al. 1999). Other species known to be predators of soft-sediment fauna (i.e. octopus and rays) were also identified during rock lobster census dives. Pilot studies suggested that caging materials presented a limited area of resistance to water movement within the experimental plots and therefore environmental variables, including grain size and organic content were not expected to change over short time periods. Any confounding effects of the caging were thus considered as likely to be detected by the comparison of survivorship in the partial cages versus that in the open plots.
Statistical analyses
The overall experimental design consisted of four factors: Location (random with two levels: Leigh and Tawharanui), Status (fixed with two levels: inside versus outside a marine reserve), Site (random with three levels, nested within Location × Status) and Treatment (fixed with three levels: full cage, partial cage and no cage). The density of snapper and rock lobster (greater than legal size) were analysed with a generalized linear model under the assumption of Poisson errors with over-dispersion. The survivorship of D. subrosea observed in the plots was analysed using a binomial generalized linear mixed model. Models were fit using the GLIMMIX routine (Littell et al. 1996) in the SAS statistical software package. The tests associated with each of the variance components for all random effects in the model and random interaction terms (i.e. Lo, Si(Lo×St), Lo×St, Lo×Tr, Lo×St×Tr, and Tr×Si(Lo×St)) had P-values >0.4. As a consequence, the model was refitted with the fixed effects and their interaction only. Effect sizes were calculated from maximum likelihood estimates from the SAS GLIMMIX procedure (see Willis and Millar 2001).
Shells of dead D. subrosea collected from each site were classified into one of three categories: ‘cracked’ (opened by a large lobster, see the short video of a large lobster feeding on D. subrosea provided as Electronic Supplementary Material), ‘nibbled’ (opened by either a small lobster or another predator) or ‘ambiguous’ (shell fragments or unmarked valves). The proportion of shells in each category recovered from non reserve and reserve sites were compared using a chi-squared test.
Results
Within reserves, where there are high densities of large snapper and rock lobster, levels of survivorship were found to be consistently lower for treatments open to predation (Fig. 2). At these reserve sites, large rock lobster (>legal size) were found to be consistently and significantly more abundant (Fig. 2, χ2 1=26.1, P<0.0001) with no significant interaction of Location and Status. The estimated effect was a 4.6-fold higher average density in greater-than-legal-size rock lobster at reserve sites compared to non reserve sites (with 95% confidence bounds of 3.2–7.1). Large snapper (>legal size) were also significantly more abundant at the reserve sites for both locations (Fig. 2, χ2 1=186.9, P<0.0001) with no significant interaction between Location and Status. The estimated effect was a 16-fold higher average density in snapper larger than legal size at reserve sites compared to non reserve sites (with 95% confidence bounds of 4.2–41.4). No octopuses or rays were observed during the censuses of rock lobster.
a Average (+1SE) survivorship of Dosina subrosea (n=12) within each treatment, reserve status and location. b Average (+1SE) density of legal-size snapper (Pagrus auratus) and rock lobster (Jasus edwardsii) at reserve and non reserve sites of each location. All sites and replicates were pooled. No greater-than-legal-sized rock lobster were observed at the non reserve sites within Leigh
Analysis of the survivorship of D. subrosea found significant interaction of Status and Treatment (Table 1, F 2, 137=10.65, P<0.001), indicating that the effects of cages on survivorship of D. subrosea should be analysed separately inside and outside reserves. Inside reserves, the survivorship of D. subrosea was significantly and consistently lower in uncaged plots than in caged plots (P<0.0001, Fig. 2a). The estimated probability of survivorship within reserves was 0.39 (with 95% confidence bounds of 0.34–0.44) in open plots compared to 0.93 (with 95% confidence bounds of 0.84–0.98) in caged treatments. In contrast, there were no effects of either cages or predators on survivorship of D. subrosea outside reserves (Fig. 2a). The likelihood of survivorship in plots open to predation outside reserves was estimated to have a probability of 0.94 (with 95% confidence bounds of 0.87–0.99) compared to 0.39 inside reserves.
No caging artefacts were detected, as there were no significant differences in survivorship between open plots and partial cages, either inside (P>0.31) or outside marine reserves (P>0.27, Fig. 2a). In addition, the lack of any significant differences among any of the treatments outside of marine reserves indicated that (a) there was a conspicuous lack of predation occurring outside reserves and (b) there were no detectable caging artefacts in the absence of predation. The loss of bivalves observed in fully caged plots was attributed to mortality from handling, as intact but empty valves were recovered from these plots.
Of the transplanted shells that did not survive or were missing from the plots at the end of the experiment, 49% were accounted for by the dead shells recovered around the plots and along the adjacent reef edge. Amongst these recovered shells, a significantly greater proportion from reserve sites (64.8%) than from non reserve sites (8.5%) were cracked distinctly (Fig. 3, χ2 1=124.5, P<0.0001), indicating large lobster had preyed on them. A significantly greater proportion of shells at non reserve sites (23.4%) compared to reserve sites (5.2%) were marked by ‘nibbles’ (Fig. 3, χ2 1=25, P<0.001), suggesting either a small lobster or another predator had opened them.
Discussion
There was no evidence of significant predation from the cage experiment after 2 weeks at sites where upper-level predators were exploited. However, within the reserves, where the densities of large rock lobster and large snapper were higher, predation resulted in lower survivorship of bivalves. This difference in the level of survivorship between the reserve and non reserve sites suggests that the significantly higher densities of rock lobster and snapper within the reserves can have a large influence on soft-sediment bivalve populations.
The majority of dead shells recovered from reserve sites exhibited distinctive markings attributable to predation by large rock lobster. Observations of rock lobster and snapper in aquaria suggest that rock lobsters readily prey on D. subrosea, while snapper do not (T. J. Langlois pers. obs.). These bivalves are not thought to be highly mobile (Powell 1979) and so predation by rock lobster or benthivorous fishes that then deposited any shell fragments outside the experimental sites may explain the 51% of shells not recovered. In their natural environment, rock lobster have been observed to forage over adjacent soft-sediments and return to the reef edge with bivalves (MacDiarmid 1991) and also to cover large distances (~km's) along and off the reef edge. This behaviour could have resulted in a wide dispersal of shell fragments.
This study does not provide unequivocal evidence to show that rock lobster predation is the only explanation for patterns previously described in soft sediment communities by Langlois et al. (2005). Snapper and other benthivorous fauna such as rays (Hines et al. 1997) and octopus (Luckens 1991) are also likely to prey on these organisms. However, this study provides strong evidence that predation by rock lobster can be an important factor in the survivorship of adult bivalve populations.
Langlois et al. (2005) and this study illustrate the possible trophic interactions between reef-associated predators and large bivalves in adjacent soft-sediment assemblages. The actions of these predators may result in further indirect effects. Shears and Babcock (2002) describe a trophic cascade of effects on rocky reef habitats in these marine reserves, where snapper affect densities of urchins that in turn affect distributions of kelp forests. However, the large-scale study by Langlois et al. (2005) found no evidence of further community effects beyond these direct effects of predators on bivalves in soft-sediment habitats at these locations. Regular disturbance of soft-sediment communities by the feeding activity of decapod crustaceans have been shown to indirectly impact infaunal assemblages (Bonsdorff and Pearson 1997) and feeding disturbance by rays has been found to indirectly regulate community structure (Thrush et al. 1991), but such effects may be lost at the large-scale of sampling employed by Langlois et al. (2005) as the inherent spatial variability in soft-sediment assemblages can mask subtler small-scale patterns (Legendre et al. 1997).
Strong evidence exists suggesting that epibenthic predators, such as rock lobster, can use various cues to locate infaunal prey, including chemical signals carried in the exhalent water from bivalve siphons (Vedel 1986; Zimmer et al. 1999). There is also an increasing amount of evidence suggesting relatively sessile prey such as bivalves (Nakaoka 2000) and urchins (Dill et al. 2003) can detect the presence of certain predators. Nakaoka (2000) illustrated how the presence of a specific predator resulted in avoidance behaviour by an infaunal bivalve, including reduced water flow through the siphons. This mechanism of predator avoidance could explain the persistence of small populations of D. subrosea in the presence of high densities of rock lobster (Langlois et al. 2005). The high predation rates detected by this study were likely to be the result of density-dependent feeding behaviour (Eggleston et al. 1992; Hines et al. 1997 and epibenthic predators responding to various cues (Zimmer et al. 1999). These cues may have been very strong because the densities of bivalves used were far higher than any previously observed in areas where high densities of rock lobster or snapper occur (Langlois et al. 2005).
The historical fishing pressure for rock lobster (Kelly et al. 2000) suggests that, before the exploitation of this upper-level predator, coastal populations of D. subrosea were likely to have been similar to those now found in marine reserves. Centuries of fishing pressure off the western coast of Europe has lead to a reduction of predation on benthic ophiuroids and the extension of their beds (Aronson 1989). Similarly, this study suggests the extraction of rock lobster in New Zealand has lead to populations of bivalves flourishing in the functional absence of this important predator.
Caging has long been used as a means of understanding predation processes in marine systems, but it can be difficult or impossible to eliminate the possibility that any effects detected might be due to caging artefacts (Connell 1997). Kennelly (1991) devised a means of assessing caging artefacts by examining effects of full and partial cages in the absence of predators. He achieved this by setting up caging experiments inside and outside larger-scaled exclusions. By using “cages inside cages”, his experiment provided a means of assessing artefacts of smaller cages that were independent of their role to exclude predators. In the present study, we replicated caging treatments inside and outside of marine reserves. This allowed the assessment of effects of predators in areas of different predator densities. More particularly, no predation was detected in areas where densities of predators were low (outside reserves), due to exploitation. The lack of any significant effects of cages in these predator-free areas provided a solid basis for inferring that caging artefacts, if any, did not confound interpretations of results (Kennelly 1991; Connell 1997). It also demonstrated how exploitation of higher-level predators can dramatically affect trophic interactions.
Previous experimental caging studies of predation in soft-sediment communities frequently have not detected direct effects on prey (Reise 1977; e.g. Bell and Coull 1978; Raffaelli et al. 1989; Hall et al. 1990). Features of soft-sediment communities used to explain the lack of direct negative effects by predators include: the absence of dramatic resource monopolization and the generalist nature of many predators (Peterson 1979), multiple trophic levels (Commito and Ambrose 1985) and the mobility of both predators and prey (Thrush 1986; Frid 1989; Hall et al. 1990). These studies assumed that predation plays a strong role in these systems, an assumption that we are increasingly realising may not be correct, due to the functional extinction of large predators by fishing pressure (Aronson 1989; Dayton et al. 1998). Our study found direct evidence of how fished areas, in comparison with reserves, act as a ‘sliding baseline’ when investigating the trophic implications of exploitation in marine communities (Pauly et al. 1998; Dayton et al. 2000; Langlois and Ballantine 2005).
Our study has benefited from the ability to contrast sites with different densities of predators, ensuring that rates of predation are high enough to be functionally important, and also ensuring that effects detected are not due to caging artefacts. These results indicate that reef-associated predators in this system, and in particular rock lobster, are capable of controlling a dominant species of macrofauna in adjacent soft sediments. Our study estimates that where lobsters are fished this role is reduced, and the estimated probability of survivorship by D. subrosea increases from 0.39 to 0.94. This study and Langlois et al. (2005) demonstrate that the comparison of highly protected and exploited areas is necessary to appreciate how predators, that are normally heavily fished, are capable of affecting populations of their prey and how trophic processes can structure adjacent habitats.
References
Aronson RB (1989) Brittlestar beds: low-predation anachronisms in the British Isles. Ecology 70:856–865
Babcock RC, Kelly S, Shears NT, Walker JW, Willis TJ (1999) Changes in community structure in temperate marine reserves. Mar Ecol Prog Ser 189:125–134
Barros F, Underwood AJ, Lindegarth M (2001) The influence of rocky reefs on structure of benthic macrofauna in nearby soft-sediments. Estuar Coast Shelf Sci 52:191–199
Bell SS, Coull BC (1978) Field evidence that shrimp predation regulates meiofauna. Oecologia 35:141–148
Bohnsack JA (2003) Shifting baselines, marine reserves, and Leopold’s biotic ethic. Gulf Caribb Res 14:1–7
Bonsdorff E, Pearson TH (1997) The relative impact of physical disturbance and predation by Crangon crangon on population density in Capitella capitata: an experimental study. Ophelia 46:1–10
Commito JA, Ambrose WG (1985) Multiple trophic levels in soft-bottom communities. Mar Ecol Prog Ser 26:289–293
Connell SD (1997) Exclusion of predatory fish on a coral reef: the anticipation, pre-emption and evaluation of some caging artefacts. J Exp Mar Biol Ecol 213:181–198
Davis N, VanBlaricom GR, Dayton PK (1982) Man-made structures on marine sediments: effects on adjacent benthic communities. Mar Biol 70:295–304
Dayton PK, Tegner MJ, Edwards PB, Riser KL (1998) Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol Appl 8:309–322
Dayton PK, Sala E, Tegner MJ, Thrush SF (2000) Marine reserves: parks, baselines, and fishery enhancement. Bull Mar Sci 66:617–634
Dill LM, Heithaus MR, Walters CJ (2003) Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 84:1151–1157
Eggleston DB, Lipcius RN, Hines AH (1992) Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Mar Ecol Prog Ser 85:55–68
Fairweather PG (1988) Predation creates haloes of bare space among prey on rocky seashores in New South Wales. J Exp Mar Biol Ecol 114:261–273
Frid CLJ (1989) The role of recolonization processes in benthic communities, with special reference to the interpretation of predator-induced effects. J Exp Mar Biol Ecol 126:163–171
Godfriaux BL (1970) Food of predatory demersal fish in hauraki gulf: part 1 food and feeding habits of snapper. N Z J Mar Freshw Res 3:518–544
Graham NAJ, Evans RD, Russ GR (2003) The effects of marine reserve protection on the trophic relationships of reef fishes on the Great Barrier Reef. Environ Conserv 30:200–208
Hall SJ, Raffaelli D, Turrell WR (1990) Predator-caging experiments in marine systems: a re-examination of their value. Am Nat 136:657–672
Hines AH et al. (1997) Nonlinear foraging response of a large marine predator to benthic prey: eagle ray pits and bivalves in a New Zealand sandflat. J Exp Mar Biol Ecol 216:191–210
James PJ, Tong LJ (1998) Feeding technique, critical size and size preference of Jasus edwardsii fed cultured and wild mussels. Mar Freshw Res 49:151–156
Kelly S, MacDiarmid AB, Babcock RC (1999) Characteristics of spiny lobster, Jasus edwardsii, aggregations in exposed reef and sandy areas. Mar Freshw Res 50:409–416
Kelly S, Scott D, MacDiarmid AB, Babcock RC (2000) Spiny lobster, Jasus edwardsii, recovery in New Zealand marine reserves. Biol Conserv 92:359–369
Kennelly SJ (1991) Caging experiments to examine the effects of fishes on understorey species in a sublittoral kelp community. J Exp Mar Biol Ecol 147:207–230
Langlois TJ, Anderson MJ, Babcock RC (2005) Reef associated predators influence adjacent soft-sediment communities. Ecology 86:1508–1519
Langlois TJ, Ballantine WJ (2005) Marine ecological research in New Zealand: developing predictive models using no-take marine reserves. Conserv Biol (in press)
Legendre P et al. (1997) Spatial structure of bivalves in a sandflat: scale and generating processes. J Exp Mar Biol Ecol 216:99–128
Littell R, Milliken G, Stroup W, Wolfinger R (1996) SAS system for mixed models. SAS, Cary
Luckens PA (1991) Distribution growth rate and death from octopod and gastropod predation of Tawera bollonsi (Bivalvia Veneridae) at the Auckland Islands. N Z J Mar Freshw Res 25:255–268
MacDiarmid AB (1991) Seasonal changes in depth distribution, sex ratio and size frequency of spiny lobster (Jasus edwardsii) on a coastal reef in northern New Zealand. Mar Ecol Prog Ser 70:129–141
MacDiarmid AB, Hickey B, Maller RA (1991) Daily movement patterns of the spiny lobster Jasus edwardsii (Hutton) on a shallow reef in northern New Zealand. J Exp Mar Biol Ecol 147:185–205
Nakaoka M (2000) Nonlethal effects of predators on prey populations: predator-mediated change in bivalve growth. Ecology 81:1031–1045
Ogden JC, Brown RA, Salesky N (1973) Grazing by echinoid diadema Antillarum philippi: formation of halos around West Indian patch reefs. Science 182:715–717
Paul LJ (1976) A study on age, growth, and population structure of the snapper, Chrysophrys auratus (Foster), in the Hauraki Gulf, New Zealand. In: Fisheries Research Bulletin No 13. New Zealand Ministry of Agriculture and Fisheries, Wellington, New Zealand
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F (1998) Fishing down marine food webs. Science 279:860–863
Peterson CH (1979) Importance of predation and competition in organizing the inter-tidal epifaunal communities of Barnegat Inlet, New Jersey. Oecologia 39:1–24
Posey MH, Ambrose WG (1994) Effects of proximity to an offshore hard-bottom reef on infaunal abundances. Mar Biol 118:745–753
Powell AWB (1979) New Zealand mollusca. William Collins Publishers Ltd, Auckland
Raffaelli D, Conacher A, McLachlan H, Emes C (1989) The role of epibenthic crustacean predators in an estuarine food web. Estuar Coast Shelf Sci 28:149–160
Reise K (1977) Predator exclusion experiments in an intertidal mud flat. Helgol Wiss Meeresunters 30:263–271
Shears NT, Babcock RC (2002) Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132:131–142
Suchanek TH (1978) The ecology of Mytilus edulis in exposed rocky inter tidal communities. J Exp Mar Biol Ecol 31:105–120
Thrush SF (1986) Community structure on the floor of a sea-lough: are large epibenthic predators important? J Exp Mar Biol Ecol 104:171–183
Thrush SF (1999) Complex role of predators in structuring soft-sediment macrobenthic communities: implications of changes in spatial scale for experimental studies. Aust J Ecol 24:344–354
Thrush SF, Pridmore RD, Hewitt JE, Cummings VJ (1991) Impact of ray feeding disturbances on sandflat macrobenthos: do communities dominated by polychaetes or shellfish respond differently? Mar Ecol Prog Ser 69:245–252
Vedel JP (1986) Morphology and physiology of a hair plate sensory organ located on the antenna of the rock lobster Palinurus vulgaris. J Neurobiol 17:65–76
Willis TJ, Babcock RC (2000) A baited underwater video system for the determination of relative density of carnivorous reef fish. Mar Freshw Res 51:755–763
Willis TJ, Millar RB (2001) Modified hooks reduce incidental mortality of snapper (Pagrus auratus:Sparidae) in the New Zealand commercial longline fishery. ICES J Mar Sci 58:830–841
Willis TJ, Millar RB, Babcock RC (2003) Protection of exploited fish in temperate regions: high density and biomass of snapper Pagrus auratus (Sparidae) in northern New Zealand marine reserves. J Appl Ecol 40:214–227
Zimmer RK, Commins JE, Browne KA (1999) Regulatory effects of environmental chemical signals on search behavior and foraging success. Ecology 80:1432–1446
Acknowledgements
This research was supported by a scholarship to T. J. Langlois from the Education Committee, States of Jersey, Channel Islands, UK and funds from the University of Auckland. We thank Bill Ballantine and Geordie Murman for ideas and suggestions. We also thank Taylor Heyl, Russell Millar, Simon Thrush, and one anonymous reviewer for their comments on a previous version of the manuscript. The supplementary video was filmed by Michelle Brock.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl
An erratum to this article can be found at http://dx.doi.org/10.1007/s00442-005-0237-7
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Langlois, T.J., Anderson, M.J., Babcock, R.C. et al. Marine reserves demonstrate trophic interactions across habitats. Oecologia 147, 134–140 (2006). https://doi.org/10.1007/s00442-005-0148-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0148-7