Abstract
About half of the Amazon rainforest is subject to seasonal droughts of 3 months or more. Despite this drought, several studies have shown that these forests, under a strongly seasonal climate, do not exhibit significant water stress during the dry season. In addition to deep soil water uptake, another contributing explanation for the absence of plant water stress during drought is the process of hydraulic redistribution; the nocturnal transfer of water by roots from moist to dry regions of the soil profile. Here, we present data on patterns of soil moisture and sap flow in roots of three dimorphic-rooted species in the Tapajós Forest, Amazônia, which demonstrate both upward (hydraulic lift) and downward hydraulic redistribution. We measured sap flow in lateral and tap roots of our three study species over a 2-year period using the heat ratio method, a sap-flow technique that allows bi-directional measurement of water flow. On certain nights during the dry season, reverse or acropetal flow (i.e.,in the direction of the soil) in the lateral roots and positive or basipetal sap flow (toward the plant) in the tap roots of Coussarea racemosa (caferana), Manilkara huberi (maçaranduba) and Protium robustum (breu) were observed, a pattern consistent with upward hydraulic redistribution (hydraulic lift). With the onset of heavy rains, this pattern reversed, with continuous night-time acropetal sap flow in the tap root and basipetal sap flow in lateral roots, indicating water movement from wet top soil to dry deeper soils (downward hydraulic redistribution). Both patterns were present in trees within a rainfall exclusion plot (Seca Floresta) and to a more limited extent in the control plot. Although hydraulic redistribution has traditionally been associated with arid or strongly seasonal environments, our findings now suggest that it is important in ameliorating water stress and improving rain infiltration in Amazonian rainforests. This has broad implications for understanding and modeling ecosystem process and forest function in this important biome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Approximately half of the evergreen forests of the Amazon Basin are subjected to dry seasons of at least 3 months duration with <0.5 mm d−1 of rainfall (Nepstad et al. 1994). During the severe drought episode associated with the 1997/1998 El Niño Southern Oscillation (ENSO), approximately one-fourth of the region’s forests depleted >70% of the plant-available soil moisture in the upper 10 m of soil (Nepstad et al. 2004). Interestingly, however, several studies have found that Amazon forests maintain high leaf area indices, high predawn leaf water potentials, and high productivity despite seasonal drought (Nepstad et al. 1994, 2004; Saleska et al. 2003; Asner et al. 2004; Rocha et al. 2004). Trees can grow substantially during the rainless season; in fact, this period is the only time of the year when eddy flux data shows that the Tapajós Forest, Central Amazônia becomes a sink for atmospheric carbon dioxide (Saleska et al. 2003).
Deep water uptake is one mechanism that has been suggested to explain the absence of seasonal water stress in Amazonian ecosystems. During the prolonged dry season, three-fourths of soil water uptake is supplied from below 2 m depth (Nepstad et al. 1994; Hoddnett et al. 1996; Jipp et al. 1998). However, since the majority of fine roots are present near the soil surface in these forests, we might expect that under conditions of high evaporative demand, deep soil water uptake will still be limited by the low densities of fine roots at depth (Nepstad et al. 1994; Jipp et al. 1998; Jackson et al. 2000; Ryel et al. 2002). An additional or alternative explanation for the absence of plant water stress during drought is via hydraulic redistribution of water that can move passively through the roots upward (hydraulic lift) or downward, whenever a gradient in soil water potential exists among soil layers which is stronger than the overall gradient between soil and atmosphere (e.g., especially during the night when evaporative demand is low, Burgess et al. 1998; Caldwell et al. 1998).
It has been suggested that hydraulic redistribution can buffer plants against seasonal or even chronic water deficits and allow trees to use soil water more effectively by generating continuous uptake from wet soil layers and facilitating root growth and survival in dry soil layers (Dawson 1993). Its occurrence may actually contradict expected plant and ecosystem responses to drought, because at least in the short-term, plants may ameliorate their own levels of water stress by essentially “extending” their rhizosphere, which will ultimately affect the rates of water transfer to the atmosphere. Therefore, documenting hydraulic redistribution is important for understanding and modeling ecosystem processes, water budgets (Dawson 1996; Emerman and Dawson 1996; Horton and Hart 1998; Jackson et al. 2000) and even regional-scale climate models (Feddes et al. 2001; Lee et al. 2005, in review).
Hydraulic redistribution (HR) has been well documented in arid and semiarid ecosystems, croplands, temperate and coniferous forests in North America, Australia and Africa (Richards and Caldwell 1987; Dawson 1993; Burgess et al. 1998; Caldwell et al. 1998; Brooks et al. 2002; Ludwig et al. 2003, 2004; Ryel et al. 2003; Espeleta et al. 2004; Meinzer et al. 2004). However, until now, HR had not been documented for wet-tropical ecosystems, probably because of the assumption that the high total annual precipitation of these ecosystems (>2,000 mm on average) precludes the development of water potential gradients in the soil; a requisite condition for HR. But even though total annual precipitation is high in some Amazonian (and other) rainforests, the absence of rain during several months in winter time may be enough to permit the upper soil layers to dry significantly. Tropical systems that exhibit these conditions are therefore just as likely as any other system to exhibit HR as an important contribution to plant and ecosystem water relations.
Recently, Rocha et al. (2004) observed nocturnal increases in shallow soil water content during the dry season in the Tapajós forest, central Amazonia and on the basis of this, they speculated that Amazonian rainforest trees may exhibit HR. In this same forest we experimentally tested Rocha et al.’s (2004) supposition that dimorphic-rooted species in the Amazon can redistribute water not only upwards during the dry season but also downwards during the dry-to-wet season transition.
Water use behaviors associated with drought such as HR are particularly important, given that El Niño events have increased drought severity in Amazonian regions, and predictions suggest the climate of this region will become drier as a consequence of global warming and land use change (Trenberth and Hoar 1997; Hulme and Vilmer 1998). Beginning in 1999, a throughfall reduction experiment was established in the Floresta Nacional do Tapajós (state of Pará, Brazil, described in Nepstad et al. 2002) with the aim of understanding integrated responses of moist tropical forests to severe droughts. This experiment was an ideal system to test the actual role of HR on plant and ecosystem water fluxes during extremely dry years in tropical ecosystems. HR can be especially important under drier conditions because it can contribute to drought avoidance, postponing the effects of surface soil desiccation and allowing tree and forest to function for longer periods as long as deep soil moisture is still present (Dawson 1993, 1996). As deep soil moisture is depleted, hydraulic redistribution should reverse the direction in response to rain events, transferring water from the soil surface to deeper soil layers; a process that could facilitate deep root growth (see Burgess et al. 2000b). In order to understand the response of these ecosystems and the plants that inhabit them to current and predicted changes in rainfall patterns, we need a better understanding of drought-related functional traits and strategies displayed by the key functional plant types. Therefore, the objective of this study was to determine direction, timing and magnitude of soil water redistribution by roots of three common Amazonian tree species and assess the role of such phenomenon in the broader scheme of plant water uptake in natural and drought conditions in an Amazonian forest.
2 Materials and methods
2.1 Study site
The study was carried out between June 2001 and August 2003 at the Floresta Nacional do Tapajós, located in east-central Amazônia (State of Pará, Brazil, 2.8968°S, 54.9519°), about 67 km south of the city of Santarém. The forest is characterized as broad leaf evergreen located on a broad, flat plateau. Mean annual rainfall is around 2,000 mm with a very distinct 3–5 month dry period (August–December). Less than 15% of total annual precipitation falls during this period, indicating strong seasonality in rainfall (Fig. 1). This forest can also experience severe drought during El Niño events, when annual rainfall falls to 800 mm. The soil type is Oxisol (Haplustox), dominated by kaolinite clay minerals and is free of a hardpan or iron oxide concretions in the upper 12 m. The water table is more than 100-m deep (Nepstad et al. 2002).
Sap flow measurements were made within the control and treatment plots of a throughfall exclusion experiment (“Seca Floresta”). This experimental “rainout” was initiated in 1999 to simulate the effects of extreme droughts (usually associated with El Niño years) on plant and forest functioning. The experiment consists of two 1-ha forest plots (control and treatment), both fully trenched (1.0–1.7 m depth) along the perimeters to isolate from the surrounding forest. Sternberg et al. (2002) have shown, using isotope labeling experiments, that no lateral movement of water occurred between inside and outside the plot. In addition, we selected trees that were located in the central part of each plot, at least 30 m from the trench. Rainfall was partially excluded from the treatment plot by plastic panels and wooden gutters, installed in the understory of the treatment plot, which excluded on average 75% of throughfall and 50% of total rainfall during the wet season period.
2.2 Study species
We chose three species that represent three different “functional types”: Coussarea racemosa A. Rich (Rubiaceae), representing an understory type; Protium robustum (Burseraceae), a mid-canopy species and Manilkara huberi (Ducke) Chevalier (Sapotaceae), representing a canopy species. We excavated the coarse root systems around the base of two individuals (one individual at each plot) and down to a depth of 1 m to investigate their rooting pattern. We observed that all individuals had a dimorphic root system with 2–12 laterals roots extending horizontally and a single descending tap root. We found that the diameter of C. racemosa tap roots was markedly reduced at 1 m depth, suggesting that the individuals of this species are not very deep rooted. However, the diameter of M. huberi and P. robustum tap roots remained similar to that of the stem at 1 m, suggesting that the roots of these species can extend very deeply in the soil. We monitored water flow in all tap roots and in two to four lateral roots.
Coussarea racemosa is the most common tree species in the Tapajós forest (I. Tohver, unpublished data). It is a shade tolerant, understory tree and can grow up to 15 m. M. huberi is another dominant species in the Tapajós forest and has great economic importance as a timber tree. It is an emergent tree that can reach up to 45 m. However, the individuals we chose in both plots had not yet reached the canopy: both were approximately 20 m tall. P. robustum is a mid-canopy tree, 20–25 m tall. We elected to compare trees of the same size class to control for this variable, so that all the individuals of the canopy and mid-canopy class were about 20 m in height. In addition, excavation of much larger specimens was not possible because of the disturbance it would have created for ongoing research in the experimental plot.
2.3 Environmental variables
Rainfall was measured daily at the site using an automated tipping-bucket rain gauge (RainWise Inc. Bar Harbor, Maine) installed at the top of a wooden tower, above the canopy. Soil water data were collected once a month from May 1999 to September 2003, using Time Domain Reflectrometry (TDR) probes installed to a depth of 11 m in both treatment and control plots. Each 30-cm long TDR sensor was inserted into undisturbed soil at the end of a 1.5-m auger hole drilled horizontally into the wall of 11 m-deep shaft and back-filled with soil. This minimized the effects the shaft might have on soil moisture measurements. Six shafts were dug (three per plot) and had two horizontal sensors placed in opposite walls, at 0.5, 1, 2, 3-m, and so on, at 1-m intervals to 11-m depth. Two vertically inserted sensors were also added above the array of horizontally installed sensors to capture surface dynamics. In addition, 50-cm TDR sensors were installed vertically at 144 grid points in each plot; VWC measurements were made using a cable tester (Tektronix 1502C) and the calibration equation was developed using a similar Belterra clay formation from eastern Amazônia (Jipp et al. 1998).
2.4 Sap-flow probe installation and measurements
We used the heat ratio method (Burgess et al. 1998, 2001a, b) to make continuous measurements of sap flow in roots and stems of our study species. The HRM is described in detail in Burgess et al. (2001a, b), but its principle is basically to measure the increase in temperature following a heat pulse at two symmetrical points, above and below a heater inserted 6 mm into the active sapwood. We decided to use this technique because it allows bi-directional measurements of sap flow and also measures very slow flow rates.
One heater and a pair of copper–constantan thermocouples were inserted radially into the xylem tissue of the major lateral roots (two to four per individual) and tap roots of all specimens. Each thermocouple had two junctions to measure sap velocity at two depths in the xylem tissue. A metal guide (with three holes carefully drilled on a parallel line, spaced out 6 mm apart) was used to help drilling holes and minimize probe misalignment during insertion. All probes were connected to a AM416 multiplexer (Campbell Scientific Inc. Logan, Utah, USA) by 8 m-long cable. The heater was set up to send a pulse every 30 min and temperature ratios were recorded continuously with a data logger (CR10x, Campbell Scientific Corp., Logan, UT, USA) that was connected to the multiplexer by four shielded conductor cables.
We calculated the heat pulse velocity (V h; cm h−1) following Burgess et al. (1998) as:
where k is the thermal diffusivity of the fresh wood, X is the distance between the heater and the thermocouples (fixed value of 0.6 cm in our study), and v1 and v2 are the differences between the initial temperature at the two thermocouples (downstream and upstream the flow in relation to the heater, respectively) and the temperature measured after a heat pulse. k has a fixed value at the beginning equal to 2.5×10−3 cm2 s−1 but it was corrected after we determined the thermal properties of wood. All corrections for wound and misalignment of the probes were made according to Burgess et al. (2001).
At the end of the study, we cut all the roots to stop sap flow. This procedure was suggested by Burgess et al. (2001) as the most accurate way to determine the reference velocity (zero) flow value. Once this zero flow value was determined, we were able to distinguish between reverse flow (from the root to the soil) and uptake (from the soil to the trunk). We opt here to present heat pulse velocities instead of volumetric flow rates because in the latter case, quantities would simply scale with the size of each individual root monitored. Not all roots could be monitored; and further, summing “all lateral roots” or all tap roots is difficult where roots with intermediate orientations are present.
3 Results
3.1 Precipitation
Seasonal and interannual variation in rainfall were high (Fig. 1). Rainfall was 1,660 mm in the first year (2001) and 2,140 mm in the second year (2002). A low-rainfall period always occurred between August and December (dry season). Total precipitation for the dry season of 2001 was 126 mm (7% of total annual rainfall) and 306 mm (14% of total annual precipitation) for 2002 (Fig. 1).
3.2 Soil moisture
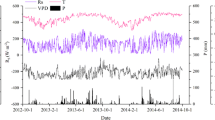
The effect of partial throughfall exclusion on soil moisture varied by depth (Fig. 2). Near the surface (0.5 m, Fig. 2a), seasonal peaks of soil moisture were reduced by the treatment, while dry season minima were similar to the treatment plot. At depth (10 m), soil moisture recharge was virtually eliminated, and VWC declined throughout the study period (Fig. 2c). When recharge of deep soil water was apparent, it lagged 2–4 months behind surface soil recharge. VWC at 4 m depth showed an intermediate pattern (Fig. 2b). In shallow soil of the control plot (50 cm), we observed a seasonal pattern of water depletion and recharge following seasonality in rainfall (Fig. 2a). At this plot, full recharge of deep soil moisture (10 m) usually lagged 2–4 months from surface soil recharge, suggesting that rainwater moved slowly in this soil by infiltration (Fig. 2c). In sum, partial exclusion of throughfall (~60%) decreased the soil moisture substantially and prevented full recharge of all soil compartments. Minor recharges were observed sporadically but were insufficient to reach the pretreatment levels of water content. Deep soil (10 m) dried out substantially, reaching a minimum of 0.32 m3 m−3 at the end of the exclusion period in July 2003, suggesting that plants depleted deep soil water sources during drought (Fig. 2c).
Long-term dynamics of volumetric water content (VWC) at three different depths in the control (gray line with diamonds) and treatment plots (black line with filled squares) of the “Seca Floresta” experiment at Floresta Nacional do Tapajós. Points represent averages (n=6±SE). The line at January 2000 represents the start of throughfall rain exclusion. Arrows represent simultaneous periods of recharge between shallow (0.5 m) and deep soil (10 m), suggesting downward hydraulic redistribution. Shaded bars represent periods of natural seasonal droughts
On a number of occasions during 2002 and 2003, we observed small increases in soil moisture at 10 m in the treatment plot coinciding with recharge of superficial soil (arrows in Fig. 2a, c). No peaks of recharge were observed at 4 m in the treatment plot during these periods (Fig. 2b).
3.3 Root sap flow
3.3.1 Trees in the control plot
During the night, we observed reverse sap flow in lateral roots (i.e., acropetal flow from the roots toward the soil) and positive sap flow (basipetal, towards the stem) in the tap root of M. huberi (Fig. 3) and C. racemosa (data not shown but the pattern is similar to M. huberi), in the control plot during several days in the dry season, which is a pattern consistent with upward hydraulic redistribution (hydraulic lift). With the onset of heavy, wet season rainfall, we observed basipetal nocturnal sap-flow rates (water uptake) in the lateral roots and continuous nighttime reversals in sap flow in the tap root of M. huberi in the control plot, indicating water movement from wet top soil into lateral roots and then towards deeper soils via the tap root (Fig. 3). This pattern was observed in this tree during 10 days in the transitional period between wet and dry seasons (December 2002), after a rainfall event of 42 mm. We also observed patterns that indicated both upward and downward HR by P. robustum in the control plot. Acropetal sap flow in lateral roots before rain and basipetal sap flow in the same roots after rain during the dry–wet season transition (Fig. 4) indicate HR, but we lack corresponding data for tap roots (due to sensor failure). P. robustum was also very responsive to rainfall, as sap velocity increased sharply after rewetting of soil by rain, indicating water uptake (Fig. 4).
Sap velocity (V h) in the tap root and lateral root of Manilkara huberi during a representative period of the dry–wet season transition (23 December to 15 January 2002) in the control plot of the “Seca Floresta” experiment in Floresta Nacional do Tapajós, Pará, Brazil. Before the onset of rains, night-time reversals in sap flow in the lateral roots and positive sap flow in the tap root show plants conducting upward hydraulic redistribution (shaded bars—nocturnal periods). With the onset of heavy, wet season rainfall, continuous nighttime reversals in sap flow were observed in the tap root, indicating water movement from wet top soil where lateral roots grew into deeper soils (downward hydraulic redistribution)
An example of sap velocity (V h) measured in the lateral root of P. robustum during the dry–wet season transition (January–Feburary 2003). A major rainfall event occurred on day 8 (dashed line), rewetting the upper soil; here, we observed a marked increase in nocturnal (positive) sap flow in the lateral root, suggesting downward HR. Shaded bars represent nighttime
3.3.2 Trees in the dry plot
Nocturnal acropetal flow in lateral roots and basipetal flow in tap roots (hydraulic lift) were recorded in all the species studied in the treatment plot (Figs. 5, 6, 7), where drought was more extreme than in natural conditions.
Sap velocity in the tap root and lateral root of P. robustum before and after removal of panels (dry–wet season transition) in the treatment plot at the Seca Floresta experiment. Hydraulic lift before rain and after a heavy rain event (36 mm), and reverse flow were observed in the tap root and positive sap-flow rates in the lateral root, suggesting downward HR. Shaded bars represent nighttime
Averages of nocturnal (between 8:30 p.m. and 5:30 a.m.) sap-flow velocity in the lateral root and tap root of P. robustum before and after (dashed line) the removal of panels in the treatment plot at Seca Floresta experiment. Hydraulic lift was observed before the rains, and with the onset of heavy, wet season rainfall, continuous night-time reversals in sap-flow were observed in the tap root and positive sap flow in lateral roots, indicating water movement from wet top soil to deeper soils (downward hydraulic redistribution)
Sap velocity (V h) in the tap root and lateral root of Manilkara huberi during a representative period of the exclusion period (manipulated drought during 10–22 August 2001) at the treatment plot of the “Seca Floresta” experiment in Floresta Nacional do Tapajós, Pará, Brazil. Night-time reverses in sap flow in the lateral roots and positive sap flow in the tap root—pattern consistent with upward hydraulic redistribution. Shaded bars represent nighttime
Downward hydraulic redistribution was also recorded in P. robustum in the dry plot when the plastic panels were removed and rainfall was permitted to reach the soil (Figs. 5 and 6). The reversals in sap flow in the tap root of this tree were of greater magnitude than those in the trees in the control plot and lasted for up to 16 days, probably as a result of the very steep water potential gradient between wet shallow soils and dry deep soils. Day-time acropetal flow in the tap root also suggests that deep soil was quite dry: such a pattern shows that the tap root was competing for water that would otherwise have moved towards the leaves via the stem (Fig. 5). Reverse sap flow in the tap root of P. robustum (August 2003) coincided with the period of increases in moisture content at surface and deep soil (Fig. 2a, c, last arrow—August 2003) in the treatment plot.
We never observed downward hydraulic redistribution in C. racemosa, and we were unable to document root sap flow dynamics in the roots of M. huberi at the dry plot during dry–wet season transition (due to sensor failure). During the dry season, sap flow traces in M. huberi indicated hydraulic lift (Fig. 7). Wet season downward redistribution in M. huberi would likely have been greater under the dry plot compared to that observed in the control plot.
4 Discussion
This study provides the first clear evidence that HR exists in tropical rainforest trees. One of the earliest descriptions of HR were presented in desert shrubs by Richards and Caldwell (1987) and since then it has been observed in a range of different plant life forms and vegetation types including Mediterranean-type scrublands (Burgess et al. 1998, 2000a, b), temperate forests and croplands (Brooks et al. 2002; Dawson 1993, 1996; Wan et al. 2000) and savannas (Ludwig et al. 2003, 2004, Sholz et al. 2002). A review of studies showing HR was last published by Caldwell et al. (1998); since this publication, the studies cited above have added examples showing that HR is far more widespread than first expected. Our findings now extend observations of HR to include tropical rainforest trees that inhabit an ecosystem type that covers 20% of terrestrial land surfaces (Whitmore 1998).
Our conclusions are based on two main lines of evidence: first, simultaneous peaks of recharge in water content between shallow and deep soil layers were observed several times in the treatment plot following the break of wet season. For example, in July 2003 at the treatment plot, the entire soil profile down to 10 m had dried out substantially after 3 years of throughfall exclusion. During this month, the plastic panels were removed and 82 mm of rainfall partially recharged shallow soil. At the same period, we observed an increase in water content at all depths from 6 m to 11 m (data presented only for 10 m), with no changes in water content in layers between 1 m and 4 m (data presented only for 4 m). If rainwater had moved by infiltration, we would have expected a gradual increase in moisture content displayed as a wetting front moving from upper to lower soil layers following rain. Though our soil moisture data were collected only once a month and might not have captured all of the fine-scale dynamics of water redistribution, we believe that the concomitant increase in soil moisture at different soil depths was the result of HR by plant roots and very unlikely to be the result of capillary rise from groundwater because at our site the water table occurs below 100 m depth (Nepstad et al. 2002).
There are three requirements for downward HR to occur: (1) soil with low hydraulic conductivity such that inverted vertical water potential gradients can be established following rain, (2) roots must be present in shallow and deep soil layers and connected by hydraulically intact pathways, and (3) leaf water potentials must be greater than water potentials in the dry soil layers. In the last case, when demand for water by leaves is a stronger sink for water than dry soil, water will not be exchanged between soil but instead all soil layers will supply water to the leaves. The soils at this forest have very high clay content and low hydraulic conductivity, which fulfill the first criteria. Measurements made in a similar Haplustox (Belterra Clay formation) soil in eastern Amazônia (Jipp et al. 1998; Nepstad et al. 2002) showed that recharge by water infiltration is estimated to take 3–4 months to reach a depth of 10 m (Jipp et al. 1998). Sternberg et al. (2002) suggested an even slower percolation rate of about 0.25 m/month following a deuterium pulse applied around trees in the “Seca Floresta” experimental plot. Deep soil layers at our site were very dry after 3 years of rainfall exclusion, and when surface soils became wet following rain, an inverted vertical gradient in water potential gradient was likely.
In terms of the second criteria, fine roots were more abundant in shallow soil but were also found down to 11 m during excavation of the shafts (Nepstad et al. 2002). Depletion of water at different soil depths following drought indicates that plant roots can absorb water throughout the 11 m profile but were probably most actively at the zone below 5 m at the end of drought. As suggested by Wan et al. (2002), rainfall exclusion can influence plants to develop more roots at depth. Sap flow reversals in the tap roots of P. robustum in the treatment plot in this same period (July 2003) supports the idea that downward HR could have been responsible for increase in water content at depth following rain as shown in other systems (Burgess et al. 2001c; Ryel et al. 2003). At certain times of the year we expected (based on soil water contents) increases in water content at depth due to downward HR but did not observe it. It is possible that during these times of the year, greater daytime transpiration may have prevented any net HR at our sampling frequency. Also, fine root activity, tunover and cavitation likely varies seasonally and must be considered when predicting HR from soil water contents alone.
A second line of evidence for HR comes from sap flow data measured in tree roots in both plots. Some of the observed differences of HR patterns among individual trees may be related to the drought treatment. In the control plot, sap flow traces indicative of downward HR in M. huberi and P. robustum (Figs. 3, 4) were documented only during the dry–wet season transitional period (December and January). In the treatment plot, downward HR in P. robustum was only recorded in July (Fig. 5), as a consequence of the removal of plastic panels, which allowed rain to rewet shallow soil. It appears that downward HR requires both a rainless period to dry the soil profile to depth followed by rainfall that rewets the surface soil, which are slow to recharge to depth. The magnitude and number of occasions that downward HR was observed were also greater in P. robustum in the dry plot when compared to trees in the control plot owing presumably to a steeper water potential gradient between shallow and deep soil in the dry plot. Reversals in sap flow in the tap root of this tree during daytime is further evidence that deep soil was very dry.
5 Ecological significance
Recent studies have challenged the idea that water supply is always abundant and reliable in tropical rainforests (Nepstad et al.1994, 2004; Trenberth and Hoar 1997; Dias et al. 2002). In fact, most of the Amazonian forests and other tropical rainforests around the world have been experiencing periods of severe and prolonged droughts (Curran et al. 1999; Goldammer 1999) usually associated with major climate events like El Niño years. At present, we know very little about the effects of water deficits on forest growth, nor the physiological mechanisms that tropical rainforest plants use to either avoid or tolerate water stress. Deep rooting (Nepstad et al. 1994) and stomata control (Cunningham 2004) are two well-known mechanisms that allow plants to cope with periods of high atmospheric demand and low water availability. As a further drought tolerance behavior, HR may also be important in tropical forests at a range of different scales. At the plant level, HR may help plants avoid drought stress by creating a readily available pool of water in superficial soil where most of a tree’s fine roots are located (Caldwell et al. 1998). Downward redistribution may also play a role in facilitating deep root growth since the propagation of the wetting front through the roots themselves means that roots will never outgrow their water supply (Burgess et al. 2001c).
At the stand level, HR can influence the amount of dry season evapotranspiration (Jackson et al. 2000; Ryel et al. 2002). Several studies have reported that Amazonian rainforests maintain transpiration and carbon sequestration during seasonal droughts by tapping water from deep water sources (Nepstad et al. 1994; Hodnett et al. 1995; Saleska et al. 2003; Goulden et al. 2004). However, fine root density in these forests is very low at depth, which might limit deep soil water uptake during periods of high atmospheric demand (Jackson et al. 2000). The superficial water pool originated by nocturnal HR should be accounted as another water source for transpiration during dry periods. Obviously, calculating the magnitude of these impacts at the ecosystem-level will require the collection of more extensive data than those currently at hand. We hope broader surveys of species differences will follow, including the influence of the canopy position. We expect fully emergent species or specimens to perhaps exhibit decreased HR compared to that measured in this study (due to greater evaporative demand, see point (3) above), but these trees may also have deeper roots, which may increase HR. The interplay between such dynamics requires further study.
Our data suggest that at broader scales knowledge of the occurrence of HR can enhance efforts to model ecosystem responses to water deficits. Such a model has been applied to the regional climate simulations of Amazonia and shows that the inclusion of this important plant behavior (HR) has a marked influence on both the ecosystem and climate of that region (Lee et al.2005, in review). Broadening our understanding of the role of species and improving our ability to calculate the magnitude of this phenomenon will no doubt contribute further to this process, particularly as land-use and the ecosystems impacts this change.
References
Asner GP, Nepstad DC, Cardinot G, Ray D (2004) Drought stress and carbon uptake in an Amazon forest measured with spaceborne imaging spectroscopy. Proc Nat Acad Sci USA 101:6039–6044
Brooks JR, Meinzer FC et al (2002) Hydraulic redistribution of soil water during summer drought in two contrasting Pacific Northwest coniferous forests. Tree Physiol 22(15–16):1107–1117
Burgess SSO, Adams MA, Turner NC, Ong CK (1998) The redistribution of soil water by tree root systems. Oecologia 115:306–311
Burgess SSO, Adams MA, Bleby T (2000a) Measurement of sap flow in roots of woody plants: a commentary. Tree Physiol 20:909–913
Burgess SSO, Pate JS, Adams MA, Dawson TE (2000b) Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes. Ann Bot 85:215–224
Burgess SSO, Adams MA, Turner NC, Beverly CR, Ong CK, Khan AAH, Bleby TM (2001a) An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21:589–598
Burgess SSO, Adams MA, Turner NC, Beverly CR, Ong CK, Khan AAH, Bleby TM (2001b) Correction: an improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21:1157
Burgess SSO, Adams MA, Turner NC, White DA, Ong CK (2001c) Tree roots: conduits for deep recharge of soil water. Oecologia 126:158–165
Caldwell MM, Dawson TE, Richards JH (1998) Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113:151–161
Cunningham SC (2004) Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Tress Struct Funct 18 (4):399–407
Curran LM, Caniago I, Paoli GD, Astianti D, Kusneti M, Leighton M, Nirarita CE, Haeruman H (1999) Impact of El Niño and logging on canopy tree recruitment in Borneo. Science 286:2184–2188
Dawson TE (1993) Hydraulic lift and water use in plants: implications for performance, water balance and plant–plant interactions. Oecologia 95:565–574
Dawson TE (1996) Determining water use by trees and forests from isotopic, energy balance, and transpiration analyses: the role of tree size and hydraulic lift. Tree Physiol 16:263–272
Dias MAFS, Rutledge S, Kabat P, Dias PLS, Nobre C, Fisch G, Dolman AJ, Zipser E, Garstang M, Manzi AO, Fuentes JD, Rocha HR, Marengo J, Plana-Fattori A, Sa LDA, Alvala RCS, Andreae MO, Artaxo P, Gielow R, Gatti L (2002) Cloud and rain processes in a biosphere-atmosphere interaction context in the Amazon Region. J Geophys Res Atmospheres 107(D20)
Emerman SH, Dawson TE (1996) Hydraulic lift and its influence on the water content of the rhizosphere: an example from sugar maple, Acer saccharum. Oecologia 108:273–278
Espeleta JF, West JB et al (2004) Species-specific patterns of hydraulic lift in co-occurring adult trees and grasses in a sandhill community. Oecologia 138(3):341–349
Feddes RAH, Hoff M, Bruen P, Dawson TE, de Rosnay P, Dirmeyer, Jackson RB, Kabat P, Kleidon A, Lilly A, Pitman AJ (2001) Modeling root water uptake in hydrological and climate models. Bull Am Meteorol Soc 82(12):2797–2810
Ford CR, McGuire MA, Mitchell RJ, Teskey RO (2004) Assessing variation in the radial profile of sap flux density in Pinus species and its effect on daily water use. Tree Physiol 24:241–249
Goldammer JG (1999) Forests on fire. Science 284 (5421):1782–1783
Goulden ML, Miller SD, Rocha HR, Menton MC, Freitas HC, Silva Figueira AM, Sousa CAD (2004) Diel and seasonal patterns of tropical forest CO2 exchange. Ecol Appl 14(4):S42—S54
Hodnett MG, Oyama MD, Tomasella J, Marques Filho AO (1996) Comparisons of long-term soil water storage behaviour under pasture and forest in three areas of Amazônia. In: Gash JHC, Nobre CA, Roberts JM, and Victoria RL (eds) Amazonian deforestation and climate. Wiley, New York, pp 57–78
Horton JL, Hart SC (1998) Hydraulic lift: a potentially important ecosystem process. Trends Ecol Evol 13:232–235
Hulme M, Vilmer D (1998) A climate change scenario for the tropics. Clim Change 39:145–176
Jackson RB, Sperry JS, Dawson TE (2000) Root water uptake and transport: using physiological processes in global predictions. Trends Plant Sci 5:484–491
Jipp P, Nepstad DC, Cassel K, Carvalho CJR (1998) Deep soil moisture storage and transpiration in forests and pastures of seasonally-dry Amazônia. Clim Change 39:395–412
Kleidon A, Heimann M (1999) Deep-rooted vegetation, Amazonian deforestation, and climate: results from a modelling study. Global Ecol Biogeograph 8:397–405
Lee JE, Oliveira RS, Dawson TE, Fung I (2005) Root functioning modifies seasonal climate. Proc Natl Acad Sci USA (in review)
Ludwig F, Dawson TE et al (2003) Hydraulic lift in Acacia tortilis trees on an East African savanna. Oecologia 134(3):293–300
Ludwig F, de Kroon H, Berendse F, Prins HHT (2004) The influence of savanna trees on nutrient, water and light availability and the understory vegetation. Plant Ecol 170:93–105
Nepstad DC, Moutinho PRS, Dias-Filho MB, Davidson EA, Cardinot G, Markewitz D, Figueiredo R, Viana N, Lefebvre PA, Ray DG, Chambers JQ, Barros L, Ishida FY, Belk E, Schwalbe K (2002) The effects of rainfall exclusion on canopy processes and biogeochemistry of an Amazon forest. J Geophys Res 107:1–18
Nepstad DC, Carvalho CJR d, Davidson EA, Jipp P, Lefebvre PA, Negreiros GH, Silva ED, Stone TA, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669
Nepstad DC, Veríssimo A, Alencar A, Nobre CA, Lima E, Lefebvre PA, Schlesinger P, Potter C, Moutinho PRS, Mendoza E, Cochrane MA, Brooks V (1999) Large-scale impoverishment of Amazonian forests by logging and fire. Nature 398:505–508
Nepstad DC, Lefebvre P, Da Silva UL, Tomasella J, Schlesinger P, Solorzano L, Moutinho P, Ray D, Benito JG (2004) Amazon drought and its implications for forest flammability and tree growth: a basin-wide analysis. Global Change Biol 10:704–717
Pausch RC, Grote EE, Dawson TE (2000) Estimating water use by sugar maple trees: important considerations when using heat-pulse methods in trees with large functional sapwood volumes. Tree Physiol 20:217–227
Ribeiro JF, Walter BMT (1998) Fitofisionomias do Bioma Cerrado. In: Sano MS, Almeida SP (eds) Cerrado, Ambiente e flora. Brasília, DF EMBRAPA/CPAC pp 89–152
Richards JH, Caldwell MM (1987) Hydraulic lift—substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 73(4):486–489
Rocha HR, Goulden ML, Miller SD, Menton MC, Pinto LDVO, Freitas HC, Figueira AMS (2004) Seasonality of water and heat fluxes over a tropical forest in eastern Amazônia. Ecol Appl 14(4):22–32
Ryel RJ, Caldwell MM et al (2002) Hydraulic redistribution in a stand of Artemisia tridentata: evaluation of benefits to transpiration assessed with a simulation model. Oecologia 130(2):173–184
Ryel RJ, Caldwell MM et al (2003) Rapid soil moisture recharge to depth by roots in a stand of Artemisia tridentata. Ecology 84(3):757–764
Saleska SR, Miller SD, Matross DM, Goulden ML, Wofsy SC, da Rocha HR, de Camargo PB, Crill P., Daube BC, de Freitas HC, Hutyra L, Keller M, Kirchhoff V, Menton M, Munger JW, Pyle EH, Rice AH, Silva H (2003) Carbon in amazon forests: unexpected seasonal fluxes and disturbance-induced losses. Science 302:1554–1557
Scholz FG, Bucci SJ et al (2002) Hydraulic redistribution of soil water by neotropical savanna trees. Tree Physiol 22(9):603–612
Sternberg LDS, Moreira MZ et al (2002) Uptake of water by lateral roots of small trees in an Amazonian Tropical Forest. Plant Soil 238(1):151–158
Trenberth KE, Hoar TJ (1997) El Niño and climate change. Geophys Res Lett 24:3057–3060
Wan CG, Xu WW, Sosebee RE, Machado S, Archer T (2000) Hydraulic lift in drought-tolerant and susceptible maize hybrids. Plant Soil 219:117–126
Wan CG, Yilmaz I et al (2002) Seasonal soil-water availability influences snakeweed, root dynamics. J Arid Environ 51(2):255–264
Whitmore TC (1998) An introduction to tropical rain forests, 2nd edn. Oxford University Press, Oxford
Acknowledgments
Our research was supported by grant from the NSF to D.C.N (DEB#), the A.W. Mellon Foundation and the University of California faculty COR grants to T.E.D., and a Department of Integrative Biology Summer research award and Beim award (University of California—Berkeley) to R.S.O as well as a CNPq scholarship (200129/99-6) to R.S.O. from the Government of Brazil. We thank all the staff of the LBA office and IPAM at Santarém and at Terra Rica, especially Levinaldo Seixas for his exceptional field assistance, Dr. David Ray and Marisa Tohver, for providing background data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jim Ehleringer
Rights and permissions
About this article
Cite this article
Oliveira, R.S., Dawson, T.E., Burgess, S.S.O. et al. Hydraulic redistribution in three Amazonian trees. Oecologia 145, 354–363 (2005). https://doi.org/10.1007/s00442-005-0108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0108-2