Abstract
The hatching of diapausing eggs is a means of temporal dispersal that can provide populations with genotypes adapted to different environments. In a salinity-variable shallow lake, we predicted that the mixing of different age-classes of eggs in the sediment may yield genotypes with different salinity optima. The alternative would be the absence of local adaptation and the presence of a homogenous population of salt-tolerant genotypes with high phenotypic plasticity. We tested these alternatives by isolating Daphnia magna resting eggs from different sediment depths, exposing them to hatching cues at different salinity levels and measuring the performance of hatched individuals. Results revealed a homogeneous sediment with generally broad-tolerance genotypes and some genotypes with low salt tolerance, which supports the second hypothesis. However, the disturbed character of the sediment hampered historical reconstruction. The absence of local adaptation in the diapausing egg bank may be the result of various scenarios in the response of D. magna populations to severe salinity changes in the lake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Resting egg banks represent an accessible record of local history which can be used to understand fundamental ecological issues such as how populations adapt to changing environments (Hairston et al. 1999). Molecular and ecologically relevant markers have been applied to reconstruct evolutionary changes in the recent past of many lakes (Weider et al. 1997; Hairston et al. 1999, 2001; Kerfoot et al. 1999; Cousyn et al. 2001; Limburg and Weider 2002; Jankowski and Straile 2003; Jeppesen et al. 2001). These lakes often provide a well-documented historical record if their sediments are undisturbed and a clear-cut relationship exists between depth and age. For example, in a large lake such as Lake Constance (Weider et al. 1997), stratified sediments allow a correlation between sediment horizons and age. In that case, we may expect that (1) resting eggs are most abundant in the surficial layers and (2) egg viability and hatchability decrease with depth. In the hypothetical case of a rapid evolution in which population changes genetically and so does the hatching response through time, one can further expect (3) genetic and/or life-history differences increasing with increasingly distant layers and (4) hatching cues differing between resting eggs produced at different times. In unstratified sediments, on the contrary, there is no reason to believe that depth and age are reliably correlated. Historical reconstruction appears, therefore, limited to certain types of aquatic habitats and organisms. It may be difficult, for instance, for the cladoceran Daphnia magna, which is a key dominant organism in many small, shallow and temporary ponds with disturbed sediments. Only one study successfully achieved historical reconstruction in D. magna (Cousyn et al. 2001). Nevertheless, a mix of resting stages produced at different times and under different conditions can exert important ecological and evolutionary consequences for the population as a whole when the recruitment of old genotypes into the water column brings back genetic variability for environmental responses that might have been lost in the course of time (Ellner and Hairston 1994; Hairston et al. 1996; Hairston and Kearns 2002).
In an attempt to reconcile historical reconstruction to a wider range of habitats, we tested the four assumptions described above in a shallow, unstratified lake with a known history of drastic salinity changes over the last decades (Grosser Binnensee, Germany). D. magna was studied as it has been present in the lake for a long time. It is possible that salinity-tolerant genotypes may have prevailed in the lake during periods of high salinity and vice versa. If all genotypes contributed resting eggs to the sediment egg bank, we should be able to find the different genotypes, which may be characterised either by preferences for hatching at different salinities and/or differential survival and performance in a salinity gradient. Grosser Binnensee is shallow and wind-exposed, hence the sediments may be mixed frequently, which makes it unlikely to find a chronological order of genotypes reflecting different salinity conditions. However, if they were present in the lake, we should still find genotypes with different salinity optima in the sediment. The alternative would be the absence of local adaptation and the presence of a homogenous population of salt-tolerant genotypes with a large breadth of adaptation (Gabriel and Lynch 1992). We tested these alternatives by isolating resting eggs from different sediment depths, exposing them to hatching cues at different salinities and measuring the performance of hatched individuals.
2 Materials and methods
2.1 Study site background
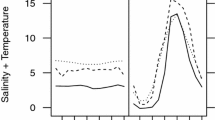
Grosser Binnensee (54°19′N, 10°38′E) is a shallow, hypertrophic coastal lake that receives water from a small stream (Kossau) and drains into the Baltic Sea. Its mean depth is 2 m and the surface area is 5.3 km2 (more information on the lake in Sommer 1989; Lampert 1991). Before 1989, it occasionally received sea-water from the Baltic Sea, depending on the wind and stream inflow, and experienced moderate short-term fluctuations of salinity (between 0 g L−1 and 3 g L−1, see Lampert 1991; Stolpe 1993). In 1989, strong storms caused the intrusion of large amounts of sea water that increased salinity levels up to ca. 20 g L−1 (Stolpe 1993). The lake had gradually been recovering its slightly brackish levels until 1994, when its connection to the sea was permanently cut off by a lock and its water became fresh (unpublished data).
Daphnia magna is a cyclical parthenogenetic cladoceran. Due to their short generation time, populations can rapidly increase during asexual reproduction, i.e. before unfavourable conditions induce the shift to sexual reproduction. This characteristic life cycle provides high opportunities for local adaptation (De Meester 1996). D. magna occurs both in freshwater and saline habitats up to 12.5 g L−1 (Lagerspetz 1955), and salinity tolerance can differ among populations or even clones (Arnér and Koivisto 1993). It has been reported in Grosser Binnensee as the dominant species over the summer before and after the sea-water intrusion, although its presence in the lake has gradually become more restricted (Lampert 1991; Jürgens and Stolpe 1995). Nowadays, presumably due to fish predation pressure, its presence is limited to a few weeks in early spring (R. Ortells, personal observations). Ephippia production and hatching has been hypothesised to be a crucial factor modifying D. magna population dynamics in this lake (Lampert 1991).
2.2 Resting egg bank quantification
Four sediment cores were collected from equidistant points at a water depth of approximately 2 m. Care was taken to prevent mixing and to minimise the disturbance of the sediment. Cores I and II were 20-cm long and 5 cm in diameter, cores III and IV were 50-cm long and 6 cm in diameter. They were stored at 4°C in a dark room for at least 20 weeks to allow a refractory period before hatching experiments commenced.
Once in the lab, 1-cm-thick slices were cut along the core, except for core IV, which was cut into 4-cm-thick slices. A subsample from the centre of each slice was taken with a plastic ring, discarding the outer 1 cm to avoid any possible transfer of ephippia between layers during the slicing process. Each slice was sieved through a 250 μm mesh. The remaining material was transferred to Petri dishes and checked under a stereomicroscope. To test for replication between cores and homogeneity along depth, sediment texture and organic matter were recorded. Ephippia were picked out with soft forceps and stored in the above conditions until the experiment was started. The length of ephippia from cores I and III was measured under a stereomicroscope connected to a computer. To start an experiment, ephippial cases were split using fine forceps and dissecting pins to remove the resting eggs. To quantify the density and viability of the resting egg bank, each egg was counted and its state registered (clearly degenerated, unhealthy and healthy).
2.3 Hatching-salinity and life-history traits
Resting eggs were incubated at different salinities to test if they showed preferences for hatching under different salt conditions. Apparently well-preserved resting eggs (potentially viable eggs, only discarding those visibly damaged) were isolated in 12-well trays with distilled water adjusted to three salinities with commercially available sea-salt. Each tray contained two salinity treatments, randomly distributed. Whenever two eggs came from the same ephippium, one was always placed at 0 g L−1 as a control, and the other at 3 g L−1 (cores I, II and III) or at 6 g L−1 (core IV). When an egg was found single within the ephippium, its salinity treatment was randomly assigned. Individual eggs were incubated at 20°C with constant light, as preliminary experiments had shown that these conditions provided optimal results (R. Ortells, personal observations). Trays were checked daily for 30 days, and the random position of the trays in the incubator was changed daily.
Newborn daphnids were transferred into 100-mL culture jars with filtered and oxygenated lake water from a mesotrophic lake (Schöhsee) adjusted to the experimental hatching-salinity levels. They were fed daily with the equivalent of 1 mg C L−1 of the green algae Scenedesmus obliquus from a chemostat culture and the medium was changed every 3 days. Daphnids were monitored daily until eggs appeared in the brood chamber. Age at maturity and clutch size were registered and used for life history comparisons. This information was also used to calculate the intrinsic rate of population increase (r) as a measure of fitness. To do so, we used an MS-DOS program (Stearns 1992). Normally, the power of this measure increases with further clutches, but a good linear relation between r values calculated with only the first clutch and with the inclusion of further clutches has been reported in D. magna from Grosser Binnensee (Teschner 1995). Statistical analyses were performed using SPSS v. 11.5 (SPSS Inc., Chicago, USA).
2.4 Genetic characterisation
In order to study genetic diversity and differentiation in the egg bank, we used the polymorphism displayed by six polymorphic microsatellite markers. Each hatchling was raised as a clonal culture in its salinity-born conditions. DNA was extracted from adult individuals of D. magna using DNeasy Tissue kit (Qiagen GmbH, Germany) and PCR-amplified for six microsatellite loci. Three of the primer pairs have been published (Dma 11, Dma 12 and Dma 14, GenBank accession numbers: AF291911, AF291912 and AF291913, respectively). The other three (S4-87; S4-144; S6-38) were developed by C. Clabby at the University of Hull, and optimised at the Catholic University of Leuven and the Max Planck Institute of Limnology in Plön. PCR reactions were carried out with final concentrations of 1 μL DNA extract, 0.2 μM of each primer, 0.25 mM of dNTP, 1x NH4 Reaction Buffer, 1.5 mM MgCl2 solution, 1% Bovine Serum Albumin and 0.75 units of Taq DNA Polymerase (Invitek, Berlin, Germany). PCR amplifications commenced with denaturation for 3 min at 94°C, followed by 27 cycles of 94°C denaturation for 1 min, annealing at 58°C for 1 min, 72°C extension for 1 min, followed by a 72°C extension for 50 min. Microsatellite alleles were separated and visualized on an ABI 3100 automated DNA sequencer (Applied Biosystems). Size scoring relative to an internal size standard (Rox 350) was performed using the software GeneScan and Genotyper (Applied Biosystems).
The software Genetix v. 4.03 (Belkhir et al. 2001) was used to examine allelic frequencies, mean number of alleles per locus, observed direct-count heterozygosity, expected heterozygosity under Hardy-Weinberg assumptions (unbiased estimate; Nei 1978) and Fis values. Additionally, it estimated global and pairwise Fst values (Weir and Cockerham 1984) among populations from sediment sections, and for hatchling groups under different salinity regimes. The program Genepop v. 3.1 (1998; updated from Raymond and Rousset 1995) was used to estimate the exact test values for deviations from Hardy-Weinberg proportions, genotypic linkage equilibrium (Fisher’s method) and genic differentiation based on Fisher’s exact tests among both depth sediment sections and salinity-differential hatchlings.
3 Results
3.1 State of the resting egg bank
The sediment of Grosser Binnensee showed a homogeneous pattern, both horizontally (among cores) and vertically (along each core). There appeared to be no sign of sediment stratification, as we found no visual differences in sediment texture or organic matter. Neither was there a change in ephippia size or shape in the sediment. A two-way ANOVA showed no significant differences in ephippia length between the two cores analysed (F 1, 74=5.90; P=0.25), along sediment depths (F 2, 74=5.36; P=0.61) or the interaction (F 1, 74=0.12; P=0.73). Due to the homogeneous character of the sediment, we pooled data from three core sections: top (0–4 cm), medium (4–8 cm) and bottom (the rest). No ephippia were found deeper than 12 cm. Despite spatially dispersed sample points, there was no significant difference between cores in the patterns of ephippia distribution, egg viability or hatching proportions (Table 1). Consequently, the four cores were pooled and analysed together further on. Figure 1 summarises egg abundance, viability and hatchability averaged along the sediment cores. The majority of the eggs was found in the upper 8 cm (86%), with highest abundances in the medium section (4–8 cm). The proportions of the total number of eggs that were viable and of the viable eggs that hatched were approximately constant across the length of the core (ca. 30%) except for the proportion (20%) of viable eggs that hatched from the layer between 8 cm and 12 cm (Fig. 1). However, neither the number of ephippia, viable eggs or hatchlings differed significantly with depth or among the four cores (Table 1), although hatching success showed a tendency to decrease in the deepest layer.
A high proportion of empty or unviable ephippia was recorded throughout the core: 60% of all ephippia found had at least one missing or unviable egg, independent of the sediment depth (Table 1). Among the ephippia found (1,122), 35% were recorded as potentially viable, and 32% of these hatched.
3.2 Salinity stress responses
A total of 127 daphnids hatched (from the 398 eggs originally incubated). The percentage of hatchlings from the “potentially viable” egg pool was 29% in the freshwater condition, and 21% in each of the brackish and saline conditions. Furthermore, the ratio between siblings hatching in fresh or brackish water was almost equal. The two-way ANOVA for repeated measures confirmed nonsignificant differences among salinity treatments (P=0.111). Furthermore, this lack of differences in hatching response to salinity was independent of sediment depth (P=0.097) and core location (P=0.228). However, we did find a significant depth × core interaction (P=0.002).
Normally, hatchlings occurred after 3 days of incubation, and most of them (93%) within the first week, although seven daphnids hatched after periods from 10 days to 15 days and one hatched on day 30 (mean: 5.08±3.36 SD). After 1 month of incubation, the remaining eggs were clearly damaged. Twenty-one individuals died before reaching maturity. Age at maturity varied between 5 days and 11 days (mean: 6.91±1.05 SD). Clutch size was very variable, from 1 eggs to 44 eggs (mean: 14.01±7.47 SD), and three females did not reproduce at all. Table 2 shows the results of the analysis of variance with depth and salinity treatment as factors. All three parameters measured (time to hatch, age at maturity and clutch size) and the estimate of fitness (r) did not differ between salinity treatments or along sediment depth, neither was there a significant salinity × depth interaction. This means that viable resting eggs hatched independently of the salinity treatment or their position in the sediment. However, the hatchlings displayed a poor survival rate in the most saline treatment. Survival 3 days after hatching was above 88% at 0 and 3 g L−1, but dropped drastically to 44% at 6 g L−1 (Fig. 2). The surviving clones however, performed similarly, displaying similar fitness optima (Table 2).
3.3 Genetic characterisation
The number of alleles per locus ranged from 3 (Dma 12) to 7 (S4-144) and all loci were polymorphic in each depth section. Table 3 shows the genetic variability in the three sections. We found Hardy–Weinberg deviations in the top and medium sediment sections due to heterozygote deficiencies in locus Dma 12 (P <0.0001), locus S4-87 (P=0.0009) and locus S4-144 (P=0.0042). Results over loci across populations showed the strongest deviations in the medium section (P<0.0001). If we group the individuals according to their salinity hatching preferences, the strongest deviations are found in locus Dma 12 (P<0.0001) and in those individuals hatching at 0 g L−1 (P<0.0001), although this result might be blurred by the different sample sizes (cf. Materials and methods). There was no physical linkage between any locus pair across the resting egg bank after correcting with Bonferroni for multiple comparisons (Rice 1989). However, due to low sample size (i.e. fixed alleles) in the bottom section, some locus pairs could not be analysed.
Genic differentiation was not significant in any locus (F=17.23; df=12; P=0.14), indicating that there were no different contributions of specific alleles across the three sediment sections. This was confirmed by using F-statistics on allele frequencies. Here, an estimation of overall Fst was −0.005, a differentiation that was not statistically significant. When individuals were grouped according to their hatching-salinity, the resulting genetic structure was also homogeneous (genic differentiation: F= 18.52; df=12; P=0.10). Consequently, Fst estimation (−0.01) was also not different from zero. Tables 4 and 5 show Fst estimates for each pair of groups. There was no genetic differentiation between depths, or between salinity-grouped hatchlings.
4 Discussion
This study revealed a homogeneous state of the sediment in terms of egg densities and hatching responses of D. magna in the shallow lake Grosser Binnensee. The hypothesis that specialist genotypes with different fitness optima existed in the lake can be rejected since the genotypes diapausing in the sediment showed broad tolerance, performing similarly in the range of salinities tested. Since the lake has been fresh for several years, it is safe to presume that most of the genotypes produced have never experienced such salinities. Therefore, D. magna in Grosser Binnensee is adapted to high salinities. Whether this adaptation is the result of selective processes in the lake or an intrinsic characteristic of the species is difficult to decide. However, despite the potential limitations for a direct historical reconstruction, resting eggs are produced in the past and thus offer valuable information about the responses of past populations to the environment. We produced four lines of evidence: (1) a homogeneous pattern of the sediment in terms of egg quality, (2) lack of hatching-salinity preferences among diapausing eggs, (3) similarity in life history traits between daphnids hatched at different salinities and (4) genetic homogeneity. By analysing the scenario from these different perspectives, we can consider possible explanations for the patterns observed.
We found a homogeneous vertical pattern of the sediment in terms of resting egg quality in the three sections of our cores. Despite lack of statistical significance, a tendency towards decreasing egg density with depth has been observed, as most eggs were accumulated in the first 8 cm and no eggs were found below 12 cm. In stratified sediments, this pattern can be explained by lower survival rates of old eggs found in deep sediment layers (Carvalho and Wolf 1989; Cáceres 1998; Keller et al. 2002; Limburg and Weider 2002). However, the fact that the ratio of viable eggs to hatchlings did not change with depth renders such an explanation unlikely for Grosser Binnensee. One possible explanation is that strong mixing produced by persistent disturbances in the lake may have produced a homogeneous mixture of old and new ephippia in the sediment, blurring any sign of stratification. In that case, an additional explanation for the highest density of ephippia in the surface layers is needed. There are two mutually nonexclusive possibilities. (1) Resting eggs produced during the last season may not have had enough time to be buried in deeper layers, as mixing will mostly occur during winter storms. In this case, surficial eggs would be younger, but the age difference (<1 year) may be too small to detect differences in viability. (2) Resuspension of the sediment by storms would also resuspend any buried egg, but as eggs are lighter and less dense than sediment particles, they would settle later and remain near the surface. This process has been empirically demonstrated by Kerfoot et al. (2004), and it would result in higher densities of resting eggs near the sediment surface but homogeneous egg quality (i.e. egg viability) through all sediment layers.
Frequent mixing of the sediments could also be an explanation for the low hatching success of surficial resting eggs in Grosser Binnensee (20%) compared to >70% reported for Lake Constance with undisturbed sediments (Weider et al. 1997). Contrary to Lake Constance, the resting eggs in Grosser Binnensee may contain a large fraction of old specimens at all sediment depths. Daphnia magna populations in Grosser Binnensee are temporary, thus ephippial hatchlings comprise the major source of recruits at the beginning of the growing season. Low hatching rates caused by the recurrent presence of old eggs and the high number of empty ephippia may be considered disadvantageous for recruitment. On the other hand, sediment mixing assures that more eggs experience the appropriate cues for hatching, and old eggs are not lost by being buried forever in the sediment (Hairston and Kearns 2002). The presence of empty ephippial cases may either indicate that many resting eggs have hatched (Carvalho and Wolf 1989) or the occurrence of ephippia predators, and that the rate of production of new ephippia is slower than the decay rate of empty cases.
An alternative hypothesis for the sediment vertical pattern found is that of a young sediment bank. If during the catastrophic event, the ephippia were completely removed, our cores might represent a sample of young, post-catastrophe populations. In that case, the degeneration of the eggs found to be unviable must have occurred in a relatively short period of time, and the presence of empty ephippia would be better explained by high predation pressure. Even in this case, given the shallow character of the lake and the strong winds, sediment disturbance can be expected. An attempt to date the sediments of Grosser Binnensee directly failed, which suggests mixing processes (M. Slusarczyk, personal communication). Due to the impossibility of ascertaining how many decades are included in our cores, neither hypothesis can be rejected.
We found no evidence for differential hatching over a range of salinities. Once a minimum dormancy period was ensured, hatching occurred simultaneously soon after exposure to light as reported in other studies (Schwartz and Hebert 1987; Carvalho and Wolf 1989; Cousyn and De Meester 1998; Bailey et al. 2003), independently of core, depth or salinity conditions. We tried to control for clonal differences in hatching by incubating sibling eggs in the different salinity treatments. Although these eggs are not identical, they share a common environment and half their genetic background. The fact that all siblings hatched at either salinity is evidence for the absence of clonal differences in salinity hatching cues.
The absence of hatching salinity preferences was further accompanied by an absence of differentiation in growth and egg production between individuals surviving and growing in the different salinity conditions. Although some variability was found for age at maturity and clutch size, this was not directly related to a salinity effect, nor was it dependent on their position in the sediment. However, survival after hatching was severely affected by salinity. Almost half of the hatchlings died at high salinity conditions before they reached maturity. The physiological constraint is probably not related to hatching but to survival (i.e. tolerance to long-term exposure). Hatching of freshwater diapausing eggs exposed to high salinity has been reported for other cladocerans (Bailey et al. 2003). All survivors in our study performed similarly. This suggests that intolerant genotypes are quickly eliminated after hatching while those that survive acquire normal functioning in a range of salinity conditions (i.e. phenotypically plastic). In this direction, when reporting high mortality of D. magna in a 8-g L−1 treatment, Baillieul et al. (1998) defined salinity as an acclimate-or-die stress, where sustained survival at suboptimal rates does not seem to be an option.
There were no genetic differences found among hatchlings of different depth sections. However, samples sizes, especially in the bottom section of the cores, might be too small to detect differences. On the other hand, we found heterozygote deficiencies within each depth section. These results again concur with the idea of a mixture of different age-classes of eggs homogeneously spread along the sediment. Contrary to lakes with genetic change across stratified sediments (Weider et al. 1997; Limburg and Weider 2002; but see Cousyn et al. 2001 for a lack of genetic differentiation), allelic frequencies did not show important shifts with sediment depth in our study. Due to the pseudo-neutral character of the genetic markers, the lack of genetic changes is not a valid argumentation against differences between genetically adapted clones, but is evidence of continuous gene flow through time. In stratified lakes that overcome selective environmental changes, populations are in disequilibrium because there is a differential hatching response through time and differentially adapted clones are not contemporaneous. This does not seem to be the case here. We found high genetic variability, which is in agreement with the idea of a sufficiently large resting egg bank. Again, the two hypotheses can be compared. If in the course of time, hatching salinity has not been a constraint, and the sediment mixing has allowed eggs from different ages to hatch, the water column population would recruit genotypes produced under different conditions, and after sexual recombination, generalist genotypes could be produced and sink back into the sediment. If the sediment has been recently produced, we would expect genetic homogeneity, unless the population has not yet reached equilibrium.
Our data suggest the existence of two groups of clones, one with low salt tolerance that can hatch but not survive at the highest salinity, and a second one with broad salt tolerance, that can perform well at all salinities up to 6 g L−1. Considerable genetic variability in potential osmoregulatory capacities has, indeed, been demonstrated in daphnid populations (Weider et al. 1997). Unfortunately, non-tolerant genotypes were too damaged at the moment of death and they could not be genetically analysed. We found no evidence of a group that can only survive at high salinity. This contradicts the hypothesis that genotypes adapted to specific salinity conditions have been selected during the drastic salinity changes occurring in the lake, unless there are more subtle life history related costs. The lack of high-salinity adapted genotypes may be a consequence of the short duration of the catastrophic event. The salinity after the intrusion of sea water was most likely beyond the capacity of any genotype present in the lake, which might have resulted in a population breakdown and no contribution to the resting egg bank. Recovery of the population must then have been initiated later by generalist genotypes, either present in the sediments or dispersed from a different lake. In the first case, these genotypes could have been produced before 1989, when salinity fluctuations in the lake were moderate, rapid and unpredictable. Such conditions select for genotypes with a broad tolerance range (Gabriel and Lynch 1992). In the second case, as no evidence of population heterogeneity has been found, a new population with broadly tolerant genotypes must have established itself in the lake. This can only be explained if during the drastic event all ephippia were erased from the sediment extinguishing the population, or if this population arrived in the lake before local hatching, and the residents were outcompeted by a higher number of invader descendants. In this case, there could not be local adaptation in the resident population to compensate for the numerical dominance (Boileau et al. 1992; De Meester et al. 2002). The problem of local adaptation could be easily solved by comparing reaction norms of this lake and a permanently freshwater one (R. Ortells and W. Lampert, unpublished results).
Under both hypotheses, the clones adapted to low-salinity might have appeared or increased in recent years after the lake became completely fresh and selection for salinity tolerance disappeared. In about the last 10 years, the situation has changed as salinity has continuously decreased, and there are no more fluctuations. We may expect genotypes with low salinity tolerance to become more abundant in the future, although there may be osmoregulatory costs in fresh water. If due to sediment mixing generalist genotypes are provided to the population with the same efficiency as specialist genotypes this process will be slow and the egg bank will be diluted very slowly. We have however, no independent proof of the validity of our interpretations. If correct, this is a potentially prominent case for slowing down microevolutionary processes by hatching from the egg bank as suggested by Hairston and De Stasio (1988).
The situation is further complicated by the fact that the reduced salinity in the lake did not only affect D. magna populations. It was accompanied by changes in many components of the ecosystem. For example, the populations of size-selective predators (fish and mysids) changed, which affected the life cycle of D. magna. While D. magna was present in the lake from May to November before 1989, its occurrence is now restricted to a spring peak and it is replaced by smaller Daphnia species during summer. The present egg bank may already reflect the new selective forces acting in the lake.
References
Arnér M, Koivisto S (1993) Effects of salinity on metabolism and life-history characteristics of Daphnia magna. Hydrobiologia 259:69–77
Bailey SA, Duggan IC, van Overdijk CDA, Jenkins PT, MacIsaac HJ (2003) Viability of invertebrate diapausing eggs collected from residual ballast sediment. Limnol Oceanogr 48:1701–1710
Baillieul M, De Wachter B, Blust R (1998) Effect of salinity on the swimming velocity of the water flea Daphnia magna. Physiol Zool 71:703–707
Belkhir K, Borsa P, Chikhi L, Rautaste N, Bonhomme F (2001) GENETIX 4.01, logiciel spus Windows pour la génétique des populations. Labratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier III, Montpellier (France)
Boileau MG, Hebert PDN, Schwartz SS (1992) Non-equilibrium gene frequency divergence: persistent founder effects in natural populations. J Evol Biol 5:25–39
Caceres CE (1998) Interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology 79:1699–1710
Carvalho GR, Wolf HG (1989) Resting eggs of lake Daphnia. 1. Distribution, abundance and hatching of eggs collected from various depths in lake-sediments. Freshwat Biol 22:459–470
Cousyn C, De Meester L (1998) The vertical profile of resting egg banks in natural populations of the pond-dwelling cladoceran Daphnia magna Strauss. Arch Hydrobiol Spec Issues Advanc Limnol 52:127–139
Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F (2001) Rapid, local adaptation of zooplankton behaviour to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci USA 98:6256–6260
De Meester L (1996) Local genetic differentiation and adaptation in freshwater zooplankton populations: patterns and processes. Ecoscience 3:385–399
De Meester L, Gómez A, Okamura B, Schwenk K (2002) The Monopolization hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol Int J Ecol 23:121–135
Ellner SP, Hairston NG (1994) Role of overlapping generations in maintaining genetic-variation in a fluctuating environment. Am Nat 143:403–417
Gabriel W, Lynch M (1992) The selective advantage of reaction norms for environmental tolerance. J Evol Biol 5:41–59
Hairston NG, De Stasio BT (1988) Rate of evolution slowed by a dormant propagule pool. Nature 336:239–242
Hairston NG, Kearns CM (2002) Temporal dispersal: ecological and evolutionary aspects of zooplankton egg banks and the role of sediment mixing. Integr Comp Biol 42:481–491
Hairston NG, Kearns CM, Ellner SP (1996) Phenotypic variation in a zooplankton egg bank. Ecology 77:2382–2392
Hairston NG, Lampert W, Caceres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer JM, Fox JA, Post DM (1999) Lake ecosystems—rapid evolution revealed by dormant eggs. Nature 401:446–446
Hairston NG, Holtmeier CL, Lampert W, Weider LJ, Post DM, Fischer JM, Cáceres CE, Fox JA, Gaedke U (2001) Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution 55:2203–2214
Jankowski T, Straile D (2003) A comparison of egg-bank and long-term planktonic dynamics of two Daphnia species, D. hyalina and D. galeata: potentials and limits of reconstruction. Limnol Oceanogr 48:1948–1955
Jeppesen E, Leavitt P, De Meester L, Jensen JP (2001) Functional ecology and palaeolimnology: using cladoceran remains to reconstruct anthropogenic impact. Trends Ecol Evol 16:191–198
Jürgens K, Stolpe G (1995) Seasonal dynamics of crustacean zooplankton, heterotrophic nanoflagelates and bacteria in a shallow, eutrophic lake. Freshwat Biol 33:27–38
Keller B, Bürgi HR, Sturm M, Spaak P (2002) Ephippia and Daphnia abundances under changing trophic conditions. Verh Int Verein Limnol 28:851–856
Kerfoot WC, Robbins JA, Weider LJ (1999) A new approach to historical reconstruction: combining descriptive and experimental paleolimnology. Limnol Oceanogr 44:1232–1247
Kerfoot WC, Budd JW, Eadie BJ, Vanderploeg HA, Agy M (2004) Winter storms: sequential sediment traps record Daphnia ephippial production, resuspension and sediment interactions. Limnol Oceanogr 49:1365–1381
Lagerspetz K (1955) Physiological studies on the brackish water tolerance of some species of Daphnia. Arch Soc Vanamo Suppl 9:138–143
Lampert W (1991) The dynamics of Daphnia magna in a shallow lake. Verh Int Verein Limnol 24:795–798
Limburg PA, Weider LJ (2002) ‘Ancient’ DNA in the resting egg bank of a microcrustacean can serve as a paleolimnological database. Proc R Soc Lond B 269:281–287
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Raymond M, Rousset F (1995) genepop: a population genetic software for exact tests and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Schwartz SS, Hebert PDN (1987) Methods for the activation of the resting eggs of Daphnia. Freshwat Biol 17:373–379
Sommer U (1989) Nutrient status and nutrient competition in a shallow hypertrophic lake. Limnol Oceanogr 34:1162–1173
Stearns SC (1992) The evolution of life histories, 1st edn. Oxford University Press, New York
Stolpe G (1993) Populationsdynamik und biotische Interaktionen von Daphnia magna im Grosser Binnensee. Diplomarbeit. Universität Kiel. Germany
Teschner M (1995) Effects of salinity on the life-history and fitness of Daphnia magna—variability within and between populations. Hydrobiologia 307:33–41
Weider LJ, Lampert W, Wessels M, Colbourne JK, Limburg P (1997) Long-term genetic shifts in a microcrustacean egg bank associated with anthropogenic changes in the Lake Constance ecosystem. Proc R Soc Lond B 264:1613–1618
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Acknowledgements
We would like to thank J. Grey, D. García-Sala, M. Volquardsen, H. Wardenga, C. Becker, L. Sivars, Y. Carotenuto, B. Seidendorf, C. Clabby and A. Wollebrants for their valuable help. We are indebted to two anonymous reviewers for helpful comments on a previous version of the manuscript and to G. Green for language improvements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl
Rights and permissions
About this article
Cite this article
Ortells, R., Reusch, T.B.H. & Lampert, W. Salinity tolerance in Daphnia magna: characteristics of genotypes hatching from mixed sediments. Oecologia 143, 509–516 (2005). https://doi.org/10.1007/s00442-005-0027-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0027-2