Abstract
Although it is now recognized that mutualistic species are common and can have stable populations, the forces controlling their persistence are poorly understood. To better understand the mechanisms that impact the stability of obligate mutualists, I conducted several field experiments within a sandy coral reef lagoon in Moorea, French Polynesia that manipulated densities of fish (gobies) that interact mutualistically with shrimp. Obligate, mutualistic partnerships of gobies and shrimp are common on Indo-Pacific coral reefs and have been shown previously to interact as follows: shrimp construct burrows in which both species reside, and gobies warn shrimp of predators through tactile communication. Augmentation of gobies by up to 100% above ambient densities within 9 m2 plots produced no change in overall density of gobies or shrimp because gobies competed intraspecifically for a limited number of shrimp burrows and smaller gobies were outcompeted by larger individuals. I used predators to assess the impact of goby removal on the stability of goby and shrimp populations. First, although surveys taken throughout the lagoon revealed no relationship between goby and predator densities, predators correlated negatively with the proportion of adult gobies and positively with the proportion of small gobies paired with large shrimp. Second, experimental augmentation of predators resulted in a dramatic reduction of adult gobies within predator-addition plots, but had no impact on overall densities as immigrants rapidly replaced the missing adult gobies. Furthermore, goby turnover resulted in an increase in the proportion of small gobies paired with large shrimp because body sizes of gobies and shrimp in a burrow were similar prior to predator introduction, and predators apparently had a greater impact on gobies than shrimp. The mechanisms that prevent expansion (intraspecific competition) and collapse (immigration) of goby-shrimp populations likely contribute to local-scale stability of mutualistic populations in other terrestrial and aquatic environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, many ecologists thought that interspecific, mutualistic interactions were rare, largely confined to benign environments, or inherently unstable (Williamson 1972; May 1973; reviewed by Boucher et al. 1982). Within the last two decades, however, scientists have begun to recognize that mutualisms are common in a variety of habitats (see reviews by Dickman 1992; Stachowicz 1999) and that mutualistic organisms can affect dramatically the function of ecosystems (Stachowicz 1999). As a consequence, it is important to elucidate the factors that affect the population dynamics of mutualistic organisms.
Mutualisms were long ignored partially because early theoretical models predicted that populations of obligate, mutualistic species were unstable and subject to either unbounded growth following an increase in the density of one or both mutualists or rapid extinction following a decline in density (Vandermeer and Boucher 1978; May 1982). Recent models, however, show that population explosion is prevented if per capita benefits (e.g., protection, nutrition) decline as densities increase (e.g., Dean 1983; Morales 2000; Holland et al. 2002). Furthermore, immigration can theoretically stabilize population sizes and prevent extinction (Hutson et al. 1985; Amarasekare 2004; Thompson 2003). Immigration may be particularly important for marine mutualists because many have bipartite life histories that include a mobile, planktonic larval phase with a high dispersal potential (Mora and Sale 2002). In light of these theoretical findings, the current challenge for ecologists is to determine empirically which processes contribute to the stability of sets of mutualistic populations.
Ecologists can test whether populations are stable by augmenting or reducing densities and assessing whether a population to returns to its original size. In cases where convergence to initial levels occurs, a deeper understanding can be gained by determining the mechanisms that promote stability (Murdoch 1994). In this study, I employ this technique to explore the stability properties (persistence and mechanisms) of a marine-based mutualism involving a goby (Ctenogobiops feroculus Lubbock and Polunin) and a shrimp (Alpheus djeddensis Coutiere). Specifically, I asked whether populations increase following experimental augmentation of gobies and if populations decline under intense predation pressure. I found that intraspecific competition among gobies prevents population expansion, whereas immigration buffers the system from extinction following the removal of gobies by predators.
Mutualistic gobies and shrimps are abundant members of coral reef communities in the Indo-West Pacific, and there are approximately 100 goby and 20 shrimp species that interact mutualistically (reviewed by Karplus 1987; A. Anker, personal communication). Their interaction is mutually beneficial because shrimp construct burrows in which gobies reside, and gobies warn shrimp of danger through tactile communication (Karplus et al. 1979). When separated from a shrimp, rates of goby mortality increase dramatically (Thompson 2003). Shrimp, by contrast, halt burrowing activity and do not emerge from burrows when gobies are absent. This reduction in activity apparently leads to a reduction in feeding and retards rates of growth (Thompson 2003).
Materials and methods
Site description
The study was conducted within the Vaipahu lagoon along the north shore of Moorea, French Polynesia (17°30′S, 149°50′W), between June and September 2001–2003 (see Thompson 2004 for map; Vaipahu lagoon is Coarse-Scale Plot 2 in Thompson 2004). Moorea is a volcanic island surrounded by a barrier reef that encloses relatively shallow lagoons (mean depth=1.82 m, width=500–1,500 m). The north shore of Moorea includes four lagoons that have extensive areas covered by sand, but also contain live and dead coral (Porites spp., Pocillopora spp., Millepora spp. and Acropora spp.)
Goby addition
To determine how goby addition affects the stability of the goby–shrimp mutualism, I conducted a field experiment in which I released marked gobies into previously unmanipulated plots. This experiment addressed four main questions: (1) Does goby addition result in a persistent increase in goby density? (2) Are shrimp commonly found without gobies in the field? (3) Do gobies compete intraspecifically for shrimp burrows? (4) If so, does goby body size influence the outcome of competitive interactions?
Prior to goby addition, I conducted spatially explicit surveys of the location and size (total length to the nearest 5 mm) of each goby within 12, 3×3 m plots separated by 5–20 m. When surveying gobies, I concurrently estimated visually the size of the shrimp (to the nearest 5 mm, measured from tail to the end of the major cheliped) that resided in the same burrow. Because shrimp never appeared in the absence of a goby, it was not possible to estimate directly the density of shrimp (see below for an indirect method of estimating the density of unpaired shrimp). I was confident that sizes were estimated accurately because there was a strong correlation between visually estimated and actual sizes of gobies and shrimp collected from non-survey sites (Thompson 2003). I surveyed plots every other day between 10 and 16 August 2001. The location of each plot was chosen based on the presence of substrate appropriate for the construction of burrows by shrimp (70% sand and 30% rubble) (see Thompson 2004 for a detailed description of goby–shrimp habitat use in Moorea).
After initial surveys, I captured gobies at least 500 m from the experimental sites, measured each goby to the nearest 1 mm TL, anesthetized them with tricaine methanesulfonate (MS-222), and tattooed each individual by injecting acrylic paint subcutaneously on its dorsal surface. After labeling, gobies were kept in an aquarium and fed TetraMin flake food ad libitum for at least 1 week to ensure that they survived the potential trauma of capture and marking.
On 16 August I introduced eight adult gobies (mean TL±SE=58.5±0.89 mm) to each of six haphazardly chosen plots and left the other six as controls on 16 August. This perturbation represented an 82% (SE=7%) increase of gobies above ambient densities (Fig. 1). I classified gobies ≥45 mm as adults because individuals of this size were occasionally observed in breeding pairs, whereas gobies ≤45 mm were never found in breeding pairs (A. Thompson, unpublished data). I qualitatively observed interactions among gobies immediately following goby addition to assess if gobies acted aggressively towards one another.
After 24 h, I surveyed the position and size of each marked and unmarked goby and the size of the shrimp associated with each goby, and then resurveyed sites on two occasions over the next 10 days. In addition, I carefully searched for marked gobies within a 20 m radius of each plot to determine if gobies emigrated from the goby-addition plots.
I determined whether the addition of gobies affected the overall density (mean density/plot/observation period) of gobies by comparing the change in density between goby addition and control plots using a t-test. Next, I ascertained whether gobies that were clearly associated with shrimp after goby addition gained access to the burrow either by excluding a resident or pairing with a shrimp that was initially without a goby. In addition, I concomitantly determined if unpaired shrimp were present prior to goby addition. Although destructive excavation could theoretically expose shrimp without gobies, this technique is empirically impractical because of the depth and complexity of shrimp burrows (Yangisawa 1984; Karplus 1987). Furthermore, burrows readily collapse when disturbed (A. Thompson, personal observation), which prevents accurate quantification even if a burrow is thoroughly dug up. Hence, I assessed indirectly if unpaired shrimp were present by examining the spatial location of gobies and shrimp before and after the addition of marked gobies. If a marked goby was found with a shrimp in a location where no shrimp or goby had been observed prior to goby addition, I concluded that the added goby paired with a shrimp that previously did not have a goby partner. If, by contrast, a marked goby was found in the same location where a shrimp and a goby were previously observed, I concluded that the invader outcompeted the resident for the burrow. If competitive exclusion clearly occurred, I then examined the location of all other gobies and shrimp to determine if a goby that was excluded from its burrow (either by a marked fish or another individual) paired with a shrimp that had not been seen prior to goby addition. If no novel shrimp were observed and the density of gobies per plot did not increase following the establishment of a marked fish, I concluded that a goby was competitively excluded from the survey area.
To ascertain whether goby body size affected the outcome of competition among gobies for burrows, I compared frequency distributions of actual differences in body size between a marked goby and the resident it displaced and the expected distribution of body size differences if marked gobies excluded residents irrespective of body size. I calculated observed differences by subtracting the body size (total length) of a resident goby that was clearly displaced from its burrow by a marked fish from the size of that marked fish. I then created a frequency histogram of observed differences by compiling the number of differences within 5 mm bins. Next, to establish a frequency distribution of expected differences, I subtracted the body size of every goby in a plot from a marked goby that had successfully paired with a shrimp in the same plot. I repeated this for each marked goby that had paired with a shrimp and tallied these differences in 5 mm bins. I then standardized the expected frequencies to match the total number of observed values and compared the two distributions with a chi-square goodness-of-fit test (Zar 1996). To increase the number of marked fish that displaced residents, I repeated the experiment in 2002 by introducing four marked fish (mean TL=60.4, SE=0.7) to each of eight plots located in the same lagoon to which gobies had been introduced in 2001. I followed the same protocol in 2002 as in 2001, but did not keep track of control plots in 2002. Hence, I used the 2002 data only to compare body sizes of displaced to added gobies, but not to compare overall changes in goby density.
Predator effects
I quantified the impact of predators on goby and shrimp populations by conducting a correlative field survey and a manipulative field experiment. For the former, I surveyed nine sites with approximately 70% sand and 30% rubble that were distributed throughout the Vaipahu lagoon (distance between adjacent sites >250 m) in July 2002. Within each site, the density and size of gobies, as well as the size of shrimp paired with gobies were quantified (to the nearest 5 mm) in three 3×3 m plots on three occasions using the same technique described in the goby addition experiment. In addition, I employed two techniques to quantify the density of predators surrounding each plot. First, while surveying each site, I recorded any predator that was inside or within 1 m of a focal 3×3 m quadrat. Predator species included the following fish I had seen consume C. feroculus on at least one occasion over the past three field seasons: six-bar wrasse (Thalassoma hardwicke), floral wrasse (Cheilinus chlorourus), groupers (Epinephelus spp.), flowery flounder (Bothus mancus), lizard fish (Saurida nebulosa), and spotted sandperch (Parapercis millepunctata). To validate these initial estimates of predator density, I recorded the identity and abundance of predators within an 81 m2 area centered on each survey plot. Because densities of predators estimated from goby surveys were highly correlated with larger scale predator counts (r2=0.90, df=8, P<0.001), I used predator estimates taken during goby surveys to characterize the density of predators throughout this paper.
Using least squares regression I assessed whether variation in total goby density, the proportion of adult (≥45 mm) gobies in a site, and the proportion of gobies paired with shrimp that were ≥15 mm larger than the goby (hereafter, small gobies paired with large shrimp) was explained by variation in the density of predators. Gobies paired with shrimp ≥15 mm larger than themselves typically depicted juvenile gobies with mature shrimp as maximal sizes of gobies (approximately 65 mm) and shrimp (approximately 60 mm) are similar. Because large gobies did not pair with small shrimp (likely due to size constraints imposed by smaller burrows), the proportion of small gobies with large shrimp reflects the proportion of body size mismatches between gobies and shrimp in the same burrow (i.e., if the proportion of small gobies with large shrimp is near 0, then body sizes of gobies and shrimp in a burrow are similar) (A. Thompson, unpublished data). I tested for normality with a Shapiro-Wilk W test and conducted an arcsin square root transformation of the proportion of small gobies paired with large shrimp. After transformation, this data set met normality assumptions at α=0.05.
I further investigated the influence of predators by experimentally increasing predator densities at sites where predators were initially scarce. Because densities of predators have been shown to correlate positively with the proportion of structurally complex habitat in Moorea (Thompson 2003), I attempted to increase densities indirectly by adding structure to sites underlain primarily by sand and rubble. Specifically, I delineated 12, 3×3 m plots (six control, six experimental) that were separated from one another by at least 5 m and recorded the density and size of all gobies, the size of shrimp associated with these gobies, and the density of predators on eight separate days in July 2002. I then placed ten concave roof tiles (length×width×height =0.45×0.2×0.09 m), which provided shelter for predators such as sandperch, floral wrasse and grouper, on the six experimental sites. After tile addition, I monitored all sites repeatedly in July and August, 2002 (n=7 observations/plot), and again in July, 2003 (n=2 observations/plot).
To determine if predator density increased in tile-addition plots relative to control plots within 2002, I performed a repeated measures ANOVA with treatment (control vs tile addition) as the independent variable and the density of predators in 2002 as the dependent variable. Between 2002 and 2003, natural sedimentation buried the roof tiles in four of the six tile-addition plots. Hence, to determine if tile effects persisted between years, I distinguished among three treatment levels (control, intact tiles, buried tiles) and again conducted a repeated measures ANOVA using predator counts taken prior to tile addition in 2002 and after tile addition in 2003.
Next, to determine if predators affected gobies or shrimp, I first regressed change in predator density within 2002 (e.g., mean predator density per plot in the period after tile addition in 2002— mean predator density per plot in the period before tile addition in 2002) against change in total goby density, proportion of large gobies, and proportion of gobies paired with relatively large shrimp within 2002. To elucidate interannual effects of predators, I regressed change in predator density between years (predator density per plot in 2003— predator density per plot prior to tile addition in 2002) against change for each variable between years. Predator density and the proportion of small gobies paired with large shrimp met normality assumptions (α=0.05) only after square root+0.5 and arcsine square root transformations, respectively.
Results
Goby addition
Although I observed 20 of the 48 added (marked) gobies in the period following goby addition, there was no difference in the change in goby density between experimental and control plots (t=0.45, df=10, P=0.66) (Fig. 1). Of these 20 marked gobies, two paired with shrimp just outside (<0.3 m) the 3×3 m plots, whereas the rest were found with shrimp within the plots. Examination of the spatial location of gobies and shrimp before and after goby addition revealed that 17 of 18 marked gobies paired with a shrimp that had previously shared a burrow with another goby, whereas one marked goby was found with a shrimp not seen in the pre-addition surveys. Furthermore, the spatial distribution of unmarked gobies and shrimp following goby addition did not reveal any additional shrimp that were not seen during initial surveys. Close scrutiny of the area within 20 m of each experimental plot failed to detect any of the other 28 marked gobies, indicating that they either emigrated >20 m from a plot or were consumed by predators.
In addition to the 16 gobies that clearly excluded a resident in 2001, I was able to discern the body size of a resident that was excluded by a marked fish for 13 of the 32 gobies that were added to plots in 2002. Comparison of the frequency of size differences between marked gobies and excluded residents with that expected if gobies indiscriminately displaced residents demonstrated that gobies did not randomly displace residents, but typically evicted gobies that were slightly smaller than themselves (χ2=22.42, V=13, P<0.05) (Fig. 2). An added goby never displaced a resident larger than itself, and the sole occasion when a marked goby displaced a resident that was >30 mm smaller than itself occurred when the resident was paired with a shrimp that was 35 mm larger than the resident.
Frequency distribution of the difference between the body size of marked gobies that gained access to a shrimp burrow by displacing a resident and the body size of the displaced resident (n=29 gobies). Expected frequency distribution represents size differences if gobies indiscriminately displaced residents
Predator effects
There was no significant relationship between the densities of gobies and predators among the nine survey sites (r2=0.18, df=8, P=0.25) (Fig. 3a). There was, however, a significant, negative relationship between the proportion of large gobies and the density of predators at a site (r2=0.79, df=8, P=0.001) (Fig. 3B). In addition, there was a significant, positive relationship between the proportion of gobies paired with shrimp that were ≥15 mm larger than the goby (i.e., small gobies paired with large shrimp) and predator density (r2=0.94, df=8, P<0.0001) (Fig. 3c).
Relationships between predator density. a Goby density per plot. b Proportion of gobies in a site that are ≥45 mm (y=−0.16x+0.80, r2=0.79). c Proportion small gobies paired with large shrimp (i.e. shrimp ≥15 mm longer than the goby) (y=0.15x+0.05, r2=0.94) (n=9 plots). The proportion of gobies with large shrimp is arcsine square root transformed
Following tile addition, predator density increased relative to control plots in all tile addition plots except one (plot 1) within 2002 (repeated measures ANOVA excluding experimental plot 1: Treatment, F1,10=13.9, P=0.0047; Time F14,140=4.6, P=0.0057; Treatment × Time F14,140=3.4, P=0.001) (Fig. 4). Tiles impacted predators between years, as densities were greater in plots where tiles remained intact (plots 5 and 6) relative to controls and those where tiles were buried (plots 1–4) (repeated measures ANOVA: Treatment, F2,9=0.06, P=0.78; Time F6,54=2.1, P=0.007; Treatment × Time F12,54=3.4, P=0.001) (Fig. 4).
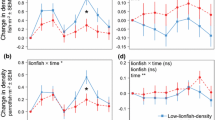
There was no significant relationship between the change in goby density and the change in predator density either within 2002 (r2=0.22, df=11, P=0.12) or between 2002 and 2003 (r2=0.13, df=11, P=0.24). By contrast, there was a significant, negative relationship between the change in the proportion of large gobies in the population and the change in predator densities both within (r2=0.60, df=11, P=0.003) and between (r2=0.37, df=11, P=0.04) years (Fig. 5a,b). Finally, the change in the proportion of gobies paired with relatively large shrimp was related positively to the change in predator density within (r2=0.59, df=11, P=0.004) and between (r2=0.84, df=11, P=0.001) years (Fig. 5c,d). Note that experimental plot 1 behaved similarly to control plots in all comparisons, thereby suggesting that predators, rather than tiles, affected goby populations.
Relationship between change in predator density. a Change in the proportion of large (≥45 mm TL) gobies within 2002 (y=−0.27x+0.003, r2=0.60). b Change in the proportion of large gobies between 2002 and 2003 (y=−0.22x+−0.04, r2=0.37). c Change in the proportion of small gobies paired with large shrimp (i.e. shrimp ≥15 mm longer than the goby) within 2002 (y=0.46x+0.01, r2=0.59). d Change in the proportion of small gobies paired with large shrimp between 2002 and 2003 (y=0.12x+0.02, r2=0.84). Squares depict control plots, solid circles experimental plots 2–6, and open circle experimental plot 1, which never attracted predators (n=12 plots). Change in the proportion of small gobies with large shrimp is arcsine square root transformed and change in predator density is square root+0.5 transformed
Discussion
My finding that intraspecific competition and immigration prevent unbounded population growth and collapse, respectively, in a mutualistic population likely applies to many systems as protection-based mutualisms similar to the shrimp–goby interaction are common. In marine environments, for example, damselfish that shelter in anemones (Schmitt and Holbrook 2003), crabs that reside in corals (Stachowicz and Hay 1999), and fish that live within corals (Meyer et al. 1983) obtain protection from the shelter provided by their mutualistic partners. In turn, these mobile mutualists stimulate the growth of their hosts by staving off predators or providing nutrients. Comparable interactions also are found in terrestrial systems where many plants are characterized by the presence of small structures (domatia) that provide shelter and protection for either mycophagous or insectivorous insects (Norton et al. 2000). These insects benefit the plant by either removing harmful fungi (Norton et al. 2000) or consuming herbivorous insects (Agrawal and Karban 1997). Hence, the results of this study should provide insight towards the dynamics of mutualistic populations in general.
Although several theoretical studies indicate that unbounded growth of mutualistic populations is prevented if benefits do not scale linearly with the number of individuals in a system (Dean 1983; Holland et al. 2002), the exact mechanism causing this saturation response is often nebulous. Results of this study indicate that intraspecific competition among gobies for shrimp burrows and the apparent scarcity of shrimp without gobies prevents population sizes from increasing following the addition of gobies (Fig. 1). That gaining access to a burrow is critical for the survival of gobies is accentuated by the finding that all marked gobies seen after the addition of gobies were paired with shrimp. While it is possible that some of the missing gobies migrated outside of the area surveyed (>20 m from the experimental plots), this is unlikely, as no gobies were seen >0.3 m from the plot to which they were released. Hence, individuals that were unable to pair with shrimp were probably consumed by predators.
Further evidence that competition for burrows plays a key role in the regulation of goby populations comes from qualitative observations of goby behavior at the time of release. Upon release, marked gobies typically attempted to enter a burrow, which often led to a confrontation with a resident goby. These confrontations often entailed vigorous gill biting and jaw locking, indicating that gobies actively defend burrows from conspecifics (A. Thompson, personal observation).
Goby body size impacted the outcome of intraspecific competition for burrows, as introduced gobies never excluded individuals larger than themselves from burrows (Fig. 2). In addition, gobies typically did not exclude individuals >30 mm smaller than themselves, probably because large gobies physically could not fit into burrows constructed by small shrimp. In fact, the lone goby that was excluded by a goby >30 mm larger than itself was paired with a shrimp that was much larger than itself. In accord with my finding that these gobies compete for shrimp burrows, Yangisawa (1982) documented aggressive interactions among shrimp gobies (Amblyeleotris japonica) in Japan and found that large gobies displaced small gobies from shrimp burrows in 87 of 90 observations of goby confrontation.
To elucidate whether intraspecific competition influenced the dynamics of C. feroculus, I increased the density of adult gobies in 9 m2 plots by up to 100%. Although I have not seen such a large influx of adults in the field, this experiment demonstrated that adults will compete for burrows if burrows are limiting. Burrow limitation likely occurs under natural circumstances for adult gobies at some point in their lives. One mechanism that could leave an adult goby without a shrimp was demonstrated in a field study on A. japonica and its shrimp partner Alpheus bellulus in Japan (Yangisawa 1984). In this system, only one shrimp and one goby occupied a burrow immediately following settlement. As shrimp matured, however, they formed permanent heterosexual bonds, thereby reducing drastically the number of burrows available for gobies. Because pair bonds between shrimp often did not form until a shrimp and its goby partner were both sexually mature (i.e., gobies were ≥45 mm TL), many gobies were forced into competition for burrows as adults (Yangisawa 1984). Adult gobies may also be incited to compete if a shrimp dies. Because burrows collapse rapidly if not maintained by shrimp, a “widowed” goby would have to evict another goby to gain access to a burrow. A third mechanism that may cause intraspecific burrow competition occurs when gobies mate. Yangisawa (1982) showed that an adult goby (A. japonica) will periodically abandon its own burrow to form a mating pair with another goby. Upon dissolution of mating pairs, which lasted for up to 7 days, a goby would seek another burrow, which often lead to competition with a conspecific (Yangisawa 1982). Given that observations have been made of aggressive, intraspecific interactions among adults of multiple species of shrimp gobies (Karplus et al. 1974; Polunin and Lubbock 1977; Yangisawa 1982, 1984; Thompson, personal observation), it is likely that competition for burrows occurs throughout the lives of shrimp gobies. Therefore, the goby augmentation experiment in this study, which showed that intraspecific competition can regulate goby population size (Fig. 1), likely reflects processes that occur in nature.
Research on the population ecology of other mutualisms also indicates that intraspecific competition can prevent unbounded population growth. For example, the density of anemone fish on anemones (Schmitt and Holbrook 2003; Buston 2003) and coral-dwelling gobies on coral (Hobbs and Munday 2004) is bounded because large fish exclude smaller conspecifics from hosts when fish densities are high. Research on ant-aphid and ant-membracid population dynamics also show that membracids and aphids compete intraspecifically for access to ants (Brenton and Addicott 1992; Morales 2000). Here, however, membracids do not directly prevent each other from obtaining access to ants. Rather, as membracid populations grow, the ratio of ants to membracids decreases, thus leaving membracid more vulnerable to predators when they are in large aggregations (Brenton and Addicott 1992; Morales 2000; Billick and Tonkel 2003). Hence, while intraspecific competition appears to be a general phenomenon affecting the dynamics of mutualistic organisms, the actual mechanisms by which competition operates apparently differs among systems.
Mutualism models that incorporate immigration predict that, at least at a local scale, immigration can stabilize population sizes and stave off extinction (Hutson 1985; Thompson et al. 2003). In agreement with theory, this study corroborated the importance of immigration on the population dynamics of mutualistic gobies and shrimp. Because gobies have pelagic larvae that can disperse widely (Sponaugle and Cowen 1994), local settlement events are likely influenced more by rates of immigration than local births. In Moorea, goby settlers (i.e., small individuals not paired with shrimp) apparently are typically in excess of available shrimp burrows. Due to their small size and cryptic coloration, these small individuals are probably less vulnerable to predators than adults and move along the benthos in search of available shrimp (Yangisawa 1982; Thompson, personal observation). These small gobies are chased away by residents when they attempt to enter a burrow occupied by a larger goby, but readily pair with shrimp that are without a goby (Yangisawa 1982; Thompson, personal observation). Hence, when predators kill large gobies, recently settled immigrants rapidly take their place, thus producing no net change in population size, but a change in the size relationship between gobies and shrimp.
Although immigration appears to be an important force affecting goby and shrimp population dynamics, it is important to note that this study was conducted on a local (10–100 m) scale and that the population of gobies and shrimp in the Vaipahu lagoon is nested within a regional metapopulation. To ultimately understand the role of immigration on mutualistic species at this larger scale, it will be necessary to identify whether immigration exceeds emigration within local patches (i.e. are patches sources or sinks? Pulliam 1988) and the degree to which individuals of both species disperse among patches. Although elucidating movement patterns of marine organisms is an active topic of research (e.g. Jones et al. 1999; Zacherl 2003), no published studies to date have attempted to track the dispersal of pairs of obligate mutualists concurrently. Understanding the dispersal dynamics of obligately mutualistic species is critical, however, as theory predicts that while immigration can rescue mutualistic sink populations, regional populations can collapse if emigration exceeds a threshold level in source populations (Amarasekare 2004).
Another factor contributing to the stability of the goby–shrimp system is that shrimp reduce their exposure to predators by curtailing burrowing activity and remaining inside burrows when gobies are absent and only resume normal burrowing activity when reunited with gobies (Cummins 1979). Therefore, the mortality of shrimp does not appear to increase dramatically when shrimp are separated from gobies. Given the importance of shrimp to goby dynamics, experiments that augment or reduce shrimp populations would likely provide further insight into the factors influencing the stability of this system. Although logistical difficulties prevented shrimp manipulation in the field, it is possible to speculate on its outcome. Based on my observation that shrimp of the same sex are highly aggressive towards one another in aquaria, it is probable that intraspecific competition for habitat affects shrimp dynamics. Furthermore, I found that shrimp burrow densities never exceeded ~3 m−2 along the north shore of Moorea (Thompson 2004) implying that competition for suitable burrow sites limits the density of shrimp in the field. Hence, augmenting shrimp densities may result in an increase in mutualist densities if habitat appropriate for burrow construction is under saturated, but may result in no change if habitat is limiting. Removing shrimp may have a negative impact on the size of populations if shrimp recruits do not immediately replace previous residents.
In conclusion, this study demonstrates that intraspecific competition, predation, and immigration contribute to the stability of the shrimp–goby mutualistic system. This research supports the findings of other recent empirical (see reviews by Connor 1995; Stachowicz 1999) and theoretical (Holland et al. 2002; Hernandez and Barrada 2003) studies which show that mutualistic populations can be highly stable. Further research into the specific mechanisms affecting mutualist population ecology will heighten our understanding of this important, but relatively poorly understood, biological interaction.
References
Agrawal AA, Karban R (1997) Domatia mediate plant-anthropod mutualism. Nature 387:562–563
Amarasekare P (2004) Spatial dynamics of mutualistic interaction. J Anim Ecol 73:128–142
Billick I, Tonkel K (2003) The relative importance of spatial versus. temporal variability in generating a conditional mutualism. Ecology 84:289–295
Boucher DH, James S, Keeler KH (1982) The ecology of mutualism. Annu Rev Ecol Syst 13:315–347
Brenton LM, Addicott JF (1992) Density-dependent mutualism in an aphid-ant interaction. Ecology 73:2175–2180
Buston P (2003) Forcible eviction and prevention of recruitment in the clown anemonefish. Behav Ecol 14:576–582
Connor RC (1995) The benefits of mutualism: a conceptual framework. Biol Rev 70:427–457
Cummins RA (1979) Ecology of Gobiid fishes associated with Alpheid shrimps. Ph.D. Dissertation, University of Sydney
Dean AD (1983) A simple model of mutualism. Am Nat 121:409–417
Dickman CR (1992) Commensal and mutualistic interactions among terrestrial vertebrates. Trends Ecol Evol 7:194–197
Hernandez M-J, Barrada I (2003) Variation in the outcome of population interactions: bifurcations and catastrophes. J Math Biol 46:571–594
Hobbs J-PA, Munday PL (2004) Intraspecific competition controls spatial distribution and social organization of the coral-dwelling goby Gobiodon histrio. Mar Ecol Prog Ser (in press)
Holland JN, DeAngelis DL, Bronstein JL (2002) Population dynamics and mutualism: functional responses of benefits and costs. Am Nat 159:231–244
Hutson V, Law R, Lewis D (1985) Dynamics of ecologically obligate mutualisms—effects of spatial diffusion on resilience of the interacting species. Am Nat 126:445–448
Jones GP, Milicich MJ, Emslie MJ, Lunow C (1999) Self-recruitment in a coral reef fish population. Nature 402:802–804
Karplus I (1987) The association between gobiid fishes and burrowing alpheid shrimps. Ocean Mar Biol 25:507–562
Karplus I, Szlep R, Tsurnamal M (1974) The burrows of alpheid shrimp associated with Gobiid fish in the Northern Red Sea. Mar Biol 24:259–268
Karplus I, Tsurnamal M, Szlep R, Algom D (1979) Film analysis of the tactile communication between Cryptocentrus steinitzi (Pisces, Gobiidae) and Alpheus purpurilenticularis (Crustacea, Alpheidae). Z Tierpsychol 49:337–351
May RM (1973) Qualitative stability in model ecosystems. Ecology 54:638–641
May RM (1982) Mutualistic interactions among species. Nature 296:803–804
Meyer JL, Schultz ET, Helfman GS (1983) Fish schools—an asset to corals. Science 220:1047–1049
Mora C, Sale PF (2002) Are populations of coral reef fish open or closed? Trends Ecol Evol 17:422–428
Morales MA (2000) Mechanisms and density dependence of benefit in an ant-membracid mutualism. Ecology 81:482–489
Murdoch WW (1994) Population regulation in theory and practice. Ecology 75:271–287
Norton AP, English-Loeb G, Gadoury D, Seem RC (2000) Mycophagous mites and foliar pathogens: leaf domatia mediate tritrophic interactions in grapes. Ecology 81:490–499
Polunin NVC, Lubbock R (1977) Prawn-associated gobies (Teleostei: Gobiidae) from the Seychelles, Western Indian Ocean: systematics and ecology. J Zool London 183:63–101
Pulliam R (1988) Sources, sinks and population regulation. Am Nat 132:652–661
Schmitt RJ, Holbrook SJ (2003) Mutualism can mediate competition and promote coexistence. Ecol Lett 6:898–902
Sponaugle S, Cowen RK (1994) Larval durations and recruitment patterns of 2 Caribbean gobies (Gobiidae)—Contrasting early-life histories in demersal spawners. Mar Biol 120:133–143
Stachowicz JJ (1999) Mutualism, facilitation, and the structure of ecological communities. BioScience 51:235–246
Stachowicz JJ, Hay ME (1999) Mutualism and coral persistence: the role of herbivore resistance to algal chemical defense. Ecology 80:2085–2101
Thompson AR (2003) Population ecology of marine mutualists. Ph.D. Dissertation, University of California, Santa Barbara
Thompson AR (2004) Habitat and mutualism affect the distribution and abundance of a shrimp-associated goby. Mar Freshwater Res 55:105–113
Vandermeer JH, Boucher DH (1978) Varieties of mutualistic interactions in population models. J Theor Biol 74:549–558
Williamson MH (1972) The analysis of biological populations. Edward Arnold, London
Yangisawa Y (1982) Social behaviour and mating system of the gobiid fish Amblyeleotris japonica. Jpn J Ichth 28:401–422
Yangisawa Y (1984) Studies on the interspecific relationship between gobiid fish and snapping shrimp. II. Life history and pair formation of snapping shrimp Alpheus bellulus. Publ Seto Mar Biol Lab 29:93–116
Zacherl DC (2003) Trace elemental fingerprinting of gastropod statoliths to study larval dispersal trajectories. Mar Ecol Prog Ser 248:297–303
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgements
I would like to thank A. Anker, S. Cooper, S. Holbrook, I. Karplus, B. Larson, P. Munday, R. Nisbet and R. Schmitt for critical discussion, N. Davies and B. Williamson for technical assistance, and S. Ferse, D. Geiger, M. Schmitt, S. Schellenberg, C. Thacker, B. Wolcott, and, especially, J. White for assistance in the field. Funding for this work was provided by R.T.G. and G.R.T. Programs in Spatial Ecology (NSF BIR94-13141 and NSF GER93-54870, both to W. Murdoch) and grants by the National Science Foundation to R Schmitt and S. Holbrook (OCE99-10677) and R. Nisbet (DEB01-08450). This paper is contribution #112 of the UC Berkeley Richard B. Gump South Pacific Research Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, A.R. Dynamics of demographically open mutualists: immigration, intraspecific competition, and predation impact goby populations. Oecologia 143, 61–69 (2005). https://doi.org/10.1007/s00442-004-1775-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1775-0