Abstract
In ant–plant symbioses, the behavior of ant inhabitants affects the nature of the interaction, ranging from mutualism to parasitism. Mutualistic species confer a benefit to the plant, while parasites reap the benefits of the interaction without reciprocating, potentially resulting in a negative impact on the host plant. Using the ant–plant symbiosis between Cordia alliodora and its ant inhabitants as a model system, I examine the costs and benefits of habitation by the four most common ant inhabitants at La Selva Biological Station, Costa Rica. Costs are measured by counting coccoids associated with each ant species. Benefits include patrolling behavior, effectiveness at locating resources, and recruitment response. I also compare the diets of the four ant species using stable isotope analysis of nitrogen (N) and carbon (C). Ants varied in their rates of association with coccoids, performance of beneficial behaviors, and diet. These differences in cost, benefit, and diet among the ant species suggest differences in the nature of the symbiotic relationship between C. alliodora and its ants. Two of the ant species behave in a mutualistic manner, while the other two ant species appear to be parasites of the mutualism. I determined that the mutualistic ants feed at a higher trophic level than the parasitic ants. Behavioral and dietary evidence indicate the protective role of the mutualists, and suggest that the parasitic ants do not protect the plant by consuming herbivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Symbiotic interactions can vary widely, ranging from truly mutualistic in which both participants benefit (Janzen 1969; Letourneau 1983; Schupp 1986; Vasconcelos 1991; Rocha and Bergallo 1992; Gaume et al. 1997; Gaume and McKey 1998) to parasitic, in which one organism gains at the expense of another (Janzen 1975; Yu and Pierce 1998; Gaume and McKey 1999; Stanton et al. 1999). A major issue in the study of symbioses is understanding how and why these interactions vary between mutualism and parasitism.
Symbioses between ants and plants have contributed greatly to our understanding of mutualism as a trade-off between the costs and benefits of an association between two organisms (Bronstein 1998). Myrmecophytes (ant plants) produce cavities called domatia that are inhabited by ants. Ant plants also often provide food resources for the ants directly in the form of extrafloral nectar or food bodies (Müller 1876, O’Dowd 1982; Fiala and Maschwitz 1992; de la Fuente and Marquis 1999; Heil et al. 2000), or indirectly via coccoid-exuded honeydew (Gullan et al. 1993; Cushman et al. 1998; Gaume et al. 1998). In return, mutualistic ants can benefit their host plant by providing protection against herbivores (Schupp 1986; Vasconcelos 1991; Madden and Young 1992; Rocha and Bergallo 1992; Fiala et al. 1994; Maschwitz and Fiala 1995; Del-Claro et al. 1996; Gaume et al. 1997; Agrawal 1998; Bronstein 1998; Gaume and McKey 1998; Moog et al. 1998), and pruning vines and encroaching vegetation (Janzen 1969; Davidson et al. 1988; Federle et al. 2002).
Most ant plants can be colonized by more than one species of ant, with one colony eventually controlling the whole plant (Wheeler 1942; Longino 1989a, b; Davidson et al. 1991; Davidson and McKey 1993; Longino 1996; Alonso 1998; Gaume and McKey 1999; Yu et al. 2001; Stanton et al. 2002). This provides a context in which the nature of the relationship between the host plant and the various ant species can be investigated. The degree to which protection against herbivores is provided may be related to the rate at which ants patrol their host plant. Ants that patrol more frequently, find herbivores faster and recruit more vigorously, are more beneficial to their host plant than ants that do not (Rocha and Bergallo 1992).
Ants might be costly to their host plants in several ways. First, myrmecophytes often produce extrafloral nectar or food bodies upon which the ants feed and these resources can be metabolically costly (Dyer et al. 2001). Second, ants often utilize honeydew from coccoids in addition to, or in lieu of, plant-produced resources (Cushman et al. 1998; Gaume et al. 1998). Feeding by homopterans represents a drain on the plant’s resources, and could represent a cost to the host plant (Vranjic and Gullan 1990; Vranjic and Ash 1997; Ozaki et al. 1999; Nava-Camberos et al. 2001; Smith and Schowalter 2001). Finally, in a few cases, the ants may actually castrate their host plant, greatly reducing that plant’s sexual reproductive capability (Yu and Pierce 1998; Stanton et al. 1999).
The relationship between Cordia alliodora (Ruiz and Pavon) and its ant inhabitants offers the opportunity to compare the costs and benefits of habitation by different ant species to the host plant. Unlike most ant plants (Wheeler 1942; Longino 1989a, b; Davidson et al. 1991; Davidson and McKey 1993; Longino 1996; Alonso 1998; Gaume and McKey 1999; Yu et al. 2001; Stanton et al. 2002), C. alliodora is commonly inhabited by multiple ant species simultaneously; I focus on four common inhabitants at La Selva biological station—Azteca pittieri (Forel), Cephalotes setulifer (Emery), Cephalotes multispinosus (Norton), and Crematogaster curvispinosa (Mayr). The first two ant species are Cordia specialists, while the latter two species are more generalized stem-nesters. Each of these ant species exhibits different levels of association with coccoids, and vary in patrolling behavior, bait location, and recruitment behavior. This allows comparisons of the costs and benefits to the host plant of habitation by these ant species.
Furthermore, I use stable isotope analysis of nitrogen (N) and carbon (C) to check for differences in diet among the four ant species from which I collected behavioral data. Stable isotope analysis is a powerful tool to track the flow of nutrients through a food web (DeNiro and Epstein 1978, 1981; Gannes et al. 1997). Biologically relevant elements, such as N and C, exist in multiple stable isotopes; the ratio of heavy to light isotope for an element can be traced from prey to consumers. Carbon values of animals reflect the C values of the plant or prey on which they feed, with only small enrichment of the heavier isotope (Michener and Schell 1994; Post 2002). Carbon is useful in determining which plant(s) is(are) the basis of a particular food web. In contrast to C, the heavier isotope of N tends to accumulate more rapidly with each trophic exchange; thus, consumers with higher N values represent higher trophic levels (DeNiro and Epstein 1981; Minagawa and Wada 1984; Peterson and Fry 1987; Cabana and Rasmussen 1996; Post 2002).
Stable isotope analysis has been previously used in explorations of ant plants to investigate the contribution of plant- or animal-based resources to ant diet (Sagers et al. 2000; Fischer et al. 2002). There is also evidence for uptake of N (Sagers et al. 2000) or both C and N (Treseder et al. 1995) by the host plant from its ant inhabitants. I focus on interspecific differences in diet among four ant inhabitants of C. alliodora, using stable isotope analysis to infer relative trophic position of the ant species.
In this system, I expect ants with more active plant patrolling, bait finding, and recruitment behavior to have isotopic values that reflect feeding at a higher trophic level than ants with lower rates of plant protective behavior. To estimate baseline isotopic values of ant dietary items, I used leaves from C. alliodora to represent plant-derived nutrition such as honeydew, and insect herbivores collected on C. alliodora represent the leaf-feeding herbivorous prey. Ants might occasionally kill and consume their trophobiotic coccoids; I was unable to directly measure N and C values for honeydew and coccoids. However, several recent studies suggest that isotopic fractionation of N in phloem feeders is greatly reduced or absent (Ostrom et al. 1997; Yoneyama et al. 1997; Oelbermann and Scheu 2002; Blüthgen et al. 2003; Davidson et al. 2003; McCutchan et al. 2003).
Materials and methods
Field site
All fieldwork was conducted in the Huertos plots at La Selva Biological Station (10°26′N, 84°00′W), Sarapiqui Canton, Heredia Province, Costa Rica. La Selva is situated in lowland tropical wet forest (mean annual precipitation: 4 m) and is administered by the Organization for Tropical Studies. Huertos is a long-term agroforestry research project and C. alliodora is one of the focal tree species. The trees used in this study were located in the 4-year rotation plots; at the time of this study they were about 2 years old and ranged in height from 4–9 m. I performed behavioral experiments and collections in three separate monoculture plots of C. alliodora.
Measuring costs
Cordia alliodora does not produce extra floral nectar or food bodies; instead, ants receive nutrition indirectly from the plant in the form of honeydew excreted by coccids and pseudococcids that cohabit the domatia. A number of different coccids and pseudococcids have been documented to live in domatia of Cordia alliodora with ants (Appendix). Two common inhabitants were Cyclolecanium hyperbaterum (Morrison) (Coccidae) and Cataenococcus larai (Williams) (Pseudococcidae) (P. Gullan and D. Kondo, personal communication). It is possible that other species of coccid and pseudococcid were present in the study plots, so I refer to the coccoids broadly as either coccids or pseudococcids.
Feeding by homopterous insects has been demonstrated to be costly to host plants in other systems, including sycamore (Dixon 1971a), lime (Dixon 1971b), Colliguaya odorifera (Molina) (Mills 1984), Eucalyptus blakelyi, (Maiden) (Vranjic and Gullan 1990; Vranjic and Ash 1997), mangroves (Ozaki et al. 1999), Douglas fir (Smith and Schowalter 2001), and cantaloupe (Nava-Camberos et al. 2001). Coccoids compete with their host plant for resources by removing sap, which can have negative fitness consequences for the plant. Furthermore, Vranjic and Ash (1997) and Smith and Schowalter (2001) found an increase in plant damage associated with escalation of attack by scales and aphids, respectively. I use the number of coccoids associated with growing colonies (i.e., domatia with brood) of each ant species as the common currency for measuring cost to the plant. Ants that cohabit with many coccoids are more costly than ants that cohabit with few coccoids.
The coccids and pseudococcids associated with the ants reside inside the domatia where they access the plant’s phloem. As domatia are discrete habitable units in this plant, I considered a comparison of coccoids per domatium among the ants to be the best relative comparison of cost. To assess the relationship between each ant species and the coccoids, I collected and dissected all domatia from nine entire Cordia alliodora trees. Before dissection, I froze the domatia to incapacitate any ant inhabitants. During dissection, I recorded the ant species and counted the number of coccids and pseudococcids inside every domatium.
I used non-parametric Kruskal-Wallis tests to check for differences among the ant species in the number of coccoids per domatium. Unequal sample sizes for each ant species necessitated the use of this non-parametric test. For the four ant species, I eliminated domatia without brood from the analysis because this typically meant that there was only a foundress queen in the domatium. At this immature stage in colony development, it is not appropriate to infer the nature of the relationship between the ant species and the coccoids; colonies that lack coccoids during their founding stages might acquire them later. I also checked for a difference in the type of coccoid (coccids or pseudococcids) with which each ant species cohabited. Due to unequal sample sizes, I used a non-parametric Mann-Whitney U-test to compare the ranks of the number of coccids and pseudococcids inside domatia inhabited by each species of ant.
Measuring benefits
I use three different measures of ant behavior to determine each species’ potential beneficial effect on Cordia alliodora. These were duration (seconds) of patrolling trips performed by individual workers, bait-finding efficacy, and recruitment response. Other studies by Rocha and Bergallo (1992) and Dejean et al. (2001a, b) demonstrate the utility of measuring these behaviors in order to quantify the beneficial effect of the ants on the host plant.
In my study, all behavioral observations were made in the mornings and afternoons when it was not raining. Repeated checks yielded no observations of activity of these ant species during rain storms or at night. Due to the destructive nature of measuring costs by collecting entire colonies, I performed all behavioral observations on colonies in 12 trees of similar size other than those included in the collections.
Patrolling
I observed domatia inhabited by each of the four ant species. When an ant left the domatium and walked on the surface of the plant, I recorded the total time that ant spent outside until she returned to her domatium. During her trip, I also recorded the amount of time the ant spent either on the stem or on the surface of a leaf; ants that frequently patrol leaves are likely to encounter leaf-eating herbivores that ants that mainly patrol the stems might miss.
I log-normalized the data for total time spent foraging outside of the domatia (due to inequality of variance) and compared the mean ranks using a Kruskal-Wallis test. I also used the Kruskal-Wallis test to compare the time each ant species spent on the stems or on the leaves. Cephalotes setulifer was never observed to forage during these observational periods, and as such, this species is not considered in the analyses for patrolling behavior.
Bait finding
I placed insect prey (termites) (Oliveira et al. 1987; Dejean et al. 2001a, b; Apple and Feener 2001; Cogni and Freitas 2002; Cogni et al. 2003) on the surface of leaves and recorded whether the bait was found in 20 min or less, and by which ant species. If the bait was not found in 20 min, the trial was terminated. A ‘find’ consisted of an ant approaching the bait and making contact either with her mandibles or antennae. A chi-square test checked for differences among the four ant species in the frequency with which they found bait.
Recruitment
After bait was found by a foraging ant, I reset the timer and recorded how long the ants recruited nestmates to the bait. I continued until the entire bait was consumed, the ants ceased recruitment, or 20 min had elapsed. I compared mean ranks of the amount of time a bait had recruits (more than one ant present) after it was found using a Kruskal-Wallis test. All analyses in the costs and benefits portion of this study were conducted using Statview (SAS Institute).
Stable isotope analysis
For stable isotope analysis, I collected ants, herbivores, and leaves from Cordia alliodora. Individual ants were placed in vials and dried, generating the following sample sizes: A. pittieri—17; Cephalotes multispinosus—4; Cephalotes setulifer—18; Cr. curvispinosa—10. The herbivores consisted of the tortoise beetles Ischnocodia annulus (Fabricius) (Chrysomelidae) and Coptocycla leprosa (Boheman) (Chrysomelidae); both are known Cordia feeders (Noguera 1988; Windsor et al. 1992; Flowers and Janzen 1997), and are very common at this site. A total of eight herbivores were collected for analysis from the same trees as the ants when possible, or from adjacent Cordia alliodora trees when necessary. Leaves, ants, and herbivores dried in ovens at 70°C until completely dry and were subsequently stored in airtight containers with desiccant until processing.
Stable isotope analysis measures the ratio of heavy to light isotopes of biologically relevant elements, such as C and N. Sample ratios are compared to an element-specific standard and reported as ‘δX’ where δX=[(R sample/R standard)−1]×1,000. R sample and R standard refer to the ratio of heavy to light isotopes of the sample and standard, respectively. Delta values are referred to as “per mil” or “‰”. I report “per mil” δ values for the 13C/12C and 15N/14N isotopic ratios of plant material, ants, and herbivorous insects. The standard for C analysis was PeeDee belemnite carbonate; atmospheric air was the standard for N analysis.
Dried specimens were lyophilized, finely ground, and weighed into tin capsules. The University of Arkansas Stable Isotope Laboratory performed the analysis using a CE Instruments NC2500 elemental analyzer coupled with a Finnigan MAT delta plus isotope ratio mass spectrometer. I used the K nearest-neighbors randomization test (Rosing et al. 1998) with P values of pairwise comparisons adjusted by using the sequential Bonferroni method (Rice 1989) to test for differences in the isotopic ratios among the plant, the ant species and the herbivores.
Results
Costs
Azteca pittieri cohabited domatia with the most coccoids, followed by Cephalotes setulifer, Cephalotes multispinosus, Crematogaster curvispinosa, and nodes with no ants also occasionally contained coccoids (Fig. 1; Kruskal-Wallis test: df=4, H corrected for ties=3016.96, P corrected for ties<0.0001). Azteca pittieri hosted significantly more coccids than pseudococcids, C. setulifer hosted more pseudococcids than coccids, domatia with no ants had significantly more pseudococcids than coccids, and C. multispinosus and C. curvispinosa showed no difference in association with coccids or pseudococcids (Table 1).
Benefits
Patrolling
Individual Crematogaster curvispinosa workers had the longest patrolling trips, followed by Cephalotes multispinosus, and A. pittieri had the shortest total patrolling trip (Fig. 2; df=2, H corrected for ties=10.93, P corrected for ties<0.01). However, when the time spent out of the domatia was divided between time spent on the surface of a leaf, and time spent on the stem, A. pittieri and C. curvispinosa did not differ, while C. multispinosus tended to spend less of its time on the leaves than the other two ant species (Fig. 3; df=2, H corrected for ties=6.20, P corrected for ties<0.05). Crematogaster curvispinosa spent significantly more of its patrolling time on the stem than A. pittieri and C. multispinosus (Fig. 3; df=2, H corrected for ties=8.75, P corrected for ties<0.05).
Bait finding
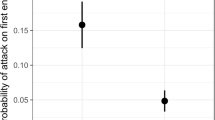
Azteca pittieri was the most effective species at finding baits (df=3, X 2=52.722, P<0.0001), successfully locating 23 of 56 baits within 20 min. In the trials I staged, the other three ant species rarely found the baits within the 20-min observation period; Crematogaster curvispinosa found 3 of 56, Cephalotes setulifer found 2 of 56, and Cephalotes multispinosus found 1 of 56.
Recruitment
Azteca pittieri had a more sustained recruitment response than the other three ant species (df=3, H corrected for ties=10.44, P corrected for ties<0.05). After A. pittieri found a bait, it recruited workers to the bait for 7.7±1.6 min, while Crematogaster curvispinosa recruited for 1.7±1.0 min; Cephalotes setulifer and Cephalotes multispinosus never recruited.
Stable isotope analysis
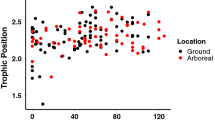
Stable isotope ratios of Cordia alliodora, the four ant species, and herbivores were significantly different (K nearest neighbors test; P<0.01; Fig. 4). Among the ants, both Cephalotes species showed no enrichment compared to C. alliodora. Azteca pittieri was significantly different from the Cephalotes species, with a δ15N value enriched by 2.6‰. Crematogaster curvispinosa was significantly different from all ants, with a δ15N value enriched by 1.5‰ above A. pittieri, and 4.1‰ above the Cephalotes species.
Discussion
The results of this study show that the nature of the relationship between Cordia alliodora and its ant inhabitants varies depending on the identity of the ant. Ants differ in their association with costly coccoids and in their beneficial protective behaviors. Stable isotopic data also suggest that there are differences in trophic level among these ant species.
The ants varied in their rate of association with coccoids, and even in the type of coccoid with which they most frequently associated. Azteca pittieri associated with the most coccoids, followed by Cephalotes setulifer, Cephalotes multispinosus, and Crematogaster curvispinosa (Fig. 1). If association with phloem-sucking trophobionts indicates relative cost to the plant for hosting each ant species, Crematogaster curvispinosa is the least expensive to host, and A. pittieri is the most expensive.
In this study, it was not feasible to directly measure the fitness consequences of coccoids on the host plant. However, numerous studies have demonstrated negative fitness consequences of feeding by homopterous insects on the host plant in a broad diversity of plant species (Dixon 1971a, b; Mills 1984; Vranjic and Gullan 1990; Vranjic and Ash 1997; Ozaki et al. 1999; Smith and Schowalter 2001). Based on these studies, it seems reasonable to propose that infestation by homopterous insects represents a cost to Cordia alliodora as well. It would be worthwhile to attempt to measure the fitness consequences of the coccids and pseudococcids on C. alliodora by conducting long-term (5–10 years) exclusion experiments. Such a study could account for the cumulative effect of coccoid feeding on C. alliodora, for the tree’s large size, and for stochastic events, such as branchfall, leading to leaf loss.
The two Cordia specialists, A. pittieri and Cephalotes setulifer, showed marked differences in association with coccids and pseudococcids. It is not clear from the present study what effect, if any, the type of coccoid has on the nature of the symbiosis. Gaume et al. (1998) found that the identity of the coccoid symbiont could have significant effects on the costs and benefits to the plant in an ant-plant system. They reported that it is more costly for Leonardoxa africana T3 to host Aphomomyrmex afer associated with coccids than with pseudococcids, and ants associated with pseudococcids had larger, and therefore more protective, colonies of ant inhabitants (Gaume et al. 1998).
Domatia without a resident colony rarely had coccoids; of those that did, significantly more had pseudococcids than coccids. This might be due to the more motile nature of the pseudococcids, which retain their legs in the adult stage. These domatia had entrance holes through which the coccoids could enter, implying that at some time, ants chewed into these cavities and subsequently abandoned them.
Of the four ant species examined, A. pittieri exhibited the most beneficial behaviors on Cordia alliodora, including the most efficient bait-finding ability and the most vigorous recruitment response. Both Crematogaster curvispinosa and Cephalotes multispinosus had longer individual patrolling trips (Fig. 2), and C. curvispinosa had similar rates of leaf patrolling as A. pittieri (Fig. 3), but this did not translate into a similar success rate for finding baits. This result is not surprising for two reasons; first, A. pittieri colonies are much larger than the other three ant species, which means more A. pittieri workers are available to forage on the plant. Second, A. pittieri spent a larger proportion of its patrolling time on the surface of leaves compared to either C. curvispinosa or C. multispinosus. Ants are more likely to encounter herbivores on leaves, which is also where I positioned the bait. Cephalotes setulifer did not perform any foraging trips during observations of its nests. However, during baiting trials, C. setulifer did encounter a bait twice. It did not attempt to recruit to the baits.
Stable isotope analysis
Stable isotope data indicate that there are considerable dietary differences among the four ant species. Azteca pittieri and Crematogaster curvispinosa appear to feed at a higher trophic level than Cephalotes setulifer and Cephalotes multispinosus. This result shows that in addition to more vigorous patrolling and recruitment behavior, A. pittieri and C. curvispinosa have a more carnivorous diet; the isotopic values of the leaf-feeding herbivores suggest that these insects might account for a portion of these two ant species’ diets. Conversely, neither Cephalotes species is enriched in δ15N compared to the host plant, indicating these two ants feed at a lower trophic level than A. pittieri and C. curvispinosa.
I was unable to analyze scale insects and honeydew from these collections. Thus, it is not possible to determine the proportion of dietary contributions from the plant via honeydew and from the leaf-feeding herbivores to the biomass of the ants. Recent work on isotopic fractionation in phloem-feeding insects (Ostrom et al. 1997; Yoneyama et al. 1997; Oelbermann and Scheu 2002; Blüthgen et al. 2003; Davidson et al. 2003; McCutchan et al. 2003) suggests that phloem-feeders and their honeydew are not enriched compared to the host plant. If this pattern holds for the coccoids on Cordia alliodora, the fact that both Cephalotes species are well below the δ15N of the leaf-feeding herbivores suggests a heavier reliance on either honeydew or phloem-feeding prey. If the ants do consume some coccoids, they do so at a rate that is low enough that they do not deplete the populations of their trophobionts.
General
These four ant species exhibit varying levels of specificity in their nesting requirements. Azteca pittieri and Cephalotes setulifer are considered Cordia alliodora obligates (but see Tillberg 2004). Cephalotes multispinosus is a live-stem generalist (J.T. Longino, personal communication), and Crematogaster curvispinosa nests in both dead, hollow sticks, or opportunistically in live stems of ant plants (Longino 2003). Based on these behavioral observations, A. pittieri is an obligate mutualist, C. setulifer is an obligate parasite, C. curvispinosa is a facultative mutualist, and C. multispinosus is a facultative parasite.
The stable isotope data are consistent with behavioral observations on the patrolling, bait finding, and recruitment behavior of these ants. Based on the dietary analyses, A. pittieri and Crematogaster curvispinosa emerge as mutualists for the plant; their diet is more carnivorous than either Cephalotes species, suggesting a heavier reliance on leaf-feeding herbivore prey. By removing insect herbivores, the ants protect the plant’s leaves (Schupp 1986; Vasconcelos 1991; Rocha and Bergallo 1992; Gaume et al. 1997; Moog et al. 1998). The diet of Cephalotes setulifer and Cephalotes multispinosus suggests they are cheaters in this mutualism. Neither incorporates a detectable amount of leaf-feeding insect prey into their diet. These two species are all cost and no benefit from the plant’s perspective.
In other cases where unprotective ants parasitize ant–plant mutualisms, the parasitic ants are the sole inhabitants of the host plant. For example, in the case of swollen-thorn Acacia inhabited by Pseudomyrmex nigropilosa, Acacia trees housing only these ants are not protected against herbivores or encroaching vines (Janzen 1975). These ants persist until they are either evicted by a more competitive species of Pseudomyrmex, or until their host plant finally dies. In the African myrmecophyte Leonardoxa africana (Leguminosae) (Gaume and McKey 1999), the plants suffer increased rates of herbivory when inhabited by non-protective Cataulacus mckeyi rather than the protective species Petalomyrmex phylax.
The symbiosis between Cordia alliodora and its ants is different from these previous examples in an important way; in the system I describe here, the parasitic species do not tend to be the dominant inhabitant of the plant. Rather, at La Selva, A. pittieri was the most abundant inhabitant, followed by Crematogaster curvispinosa, with both Cephalotes species inhabiting fewer domatia than A. pittieri and C. curvispinosa (Tillberg, unpublished work). While the Cephalotes species might represent a total loss of investment in defense for the plant, the loss may be compensated by the foraging behavior of a beneficial ant such as A. pittieri or C. curvispinosa. Thus, C. alliodora does not bear the full brunt of complete habitation by non-protective ants. Longino (1996) has aptly referred to Cephalotes setulifer as an obligate inquiline, which emphasizes its negative impact on other ant inhabitants, such as A. pittieri, by occupying domatia that these ants could otherwise inhabit. Perhaps this system remains stable because the host plant can tolerate a certain level of parasitism as long as it maintains its positive association with mutualistic ants. This proposition could be tested by comparing the health of trees artificially induced to host only parasitic ant species to unmanipulated controls.
In this study, I quantified the cost of ant habitation among potential ant inhabitants by using the common currency of coccoid association. Additionally, I compared the relative benefits of each ant species with regard to each species’ patrolling behavior, bait finding success, and recruitment response. The positive or negative effect of each ant species is corroborated by stable isotope data, indicating that mutualistic A. pittieri and Crematogaster Curvispinosa feed at a higher trophic level than the parasitic Cephalotes species. The combination of two different techniques produces a compelling case for the variable relationship between these ants and their host plant. Future studies should address how the differences in costs and benefits among these ant species translate to long-term health and reproduction of Cordia alliodora.
References
Agrawal AA (1998) Leaf damage and associated cues induced aggressive ant recruitment in a neotropical ant-plant. Ecology (Washington, DC) 79:2100–2112
Alonso LE (1998) Spatial and temporal variation in the ant occupants of a facultative ant-plant. Biotropica 30:201–213
Apple JL, Feener DH (2001) Ant visitation of extrafloral nectaries of Passiflora: the effects of nectary attributes and ant behavior on patterns in facultative ant–plant mutualisms. Oecologia 127:409–416
Beardsley JW (1959) On the taxonomy of pineapple mealybugs in Hawaii, with a description of a previously unnamed species (Homoptera: Pseudococcidae). Proc Hawaiian Entomol Soc 17:29–37
Blüthgen N, Gebauer G, Fiedler K (2003) Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137:426–435
Bronstein JL (1998) The contribution of ant-plant protection studies to our understanding of mutualism. Biotropica 30:150–161
Buckley R, Gullan P (1991) More aggressive ant species (Hymenoptera, Formicidae) provide better protection for soft scales and mealybugs (Homoptera, Coccidae, Pseudococcidae). Biotropica 23:282–286
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci USA 93:10844–10847
Cockerell TDA (1893) The West Indian species of Dactylopius. Entomologist 26:177–179
Cogni R, Freitas AVL (2002) The ant assemblage visiting extrafloral nectaries of Hibiscus pernambucensis (Malvaceae) in a mangrove forest in southeast Brazil (Hymenoptera : Formicidae). Sociobiology 40:373–383
Cogni R, Freitas AVL, Oliveira PS (2003) Interhabitat differences in ant activity on plant foliage: ants at extrafloral nectaries of Hibiscus pernambucensis in sandy and mangrove forests. Entomol Exp Appl 107:125–131
Cushman JH, Compton SG, Zachariades C, Ware AB, Nefdt RJC, Rashbrook VK (1998) Geographic and taxonomic distribution of a positive interaction: ant-tended homopterans indirectly benefit figs across southern Africa. Oecologia (Berlin) 116:373–380
Davidson DW, McKey D (1993) The evolutionary ecology of symbiotic ant-plant relationships. J Hymenoptera Res 2:13–83
Davidson DW, Longino JT, Snelling RR (1988) Pruning of host plant neighbors by ants: an experimental approach. Ecology 69:801–808
Davidson DW, Foster RB, Snelling RR, Lozada PW (1991) Variable composition of some tropical ant-plant symbioses. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 145–162
Davidson DW, Cook SC, Snelling RR, Chua TH (2003) Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300:969–972
De Lotto G (1969) On a few old and new soft scales and mealybugs (Homoptera: Coccoidea). J Entomol Soc S Afr 32:413–422
Dejean A, Solano PJ, Belin-Depoux M, Cerdan P, Corbara B (2001a) Predatory behavior of patrolling Allomerus decemarticulatus workers (Formicidae: Myrmicinae) on their host plant. Sociobiology 37:571–578
Dejean A, Solano PJ, Orivel J, Belin-Depoux M, Cerdan P, Corbara B (2001b) The spread-eagling of prey by the obligate plant-ant Pheidole minutula (Myrmicinae): similarities with dominant arboreal ants. Sociobiology 38:675–682
Del-Claro K, Berto V, Reu W (1996) Effect of herbivore deterrence by ants on the fruit set of an extrafloral nectary plant, Qualea multiflora (Vochysiaceae). J Trop Ecol 12:887–892
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Dixon AFG (1971a) Role of aphids in wood formation 1. Effect of Sycamore Aphid, Drepanosiphum platanoides (Schr) (Aphididae), on growth of Sycamore, Acer pseudoplatanus (L.). J Appl Ecol 8:165–179
Dixon AFG (1971b) Role of aphids in wood formation 2. Effect of lime Aphids, Eucallipterus tiliae L. (Aphididae), on growth of Lime, Tilia × vulgaris Hayne. J Appl Ecol 8:393–399
Dyer LA, Dodson CD, Beihoffer J, Letourneau DK (2001) Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J Chem Ecol 27:581–592
Federle W, Maschwitz U, Holldobler B (2002) Pruning of host plant neighbours as defence against enemy ant invasions: crematogaster ant partners of Macaranga protected by “wax barriers” prune less than their congeners. Oecologia 132:264–270
Fiala B, Maschwitz U (1992) Food bodies and their significance for obligate ant-associations in the tree genus Macaranga (Euphorbiaceae). Bot J Linn Soc 110:61–75
Fiala B, Grunsky H, Maschwitz U, Linsenmair KE (1994) Diversity of ant-plant interactions: protective efficacy in Macaranga species with different degrees of ant association. Oecologia (Heidelberg) 97:186–192
Fischer RC, Richter A, Wanek W, Mayer V (2002) Plants feed ants: food bodies of myrmecophytic Piper and their significance for the interaction with Pheidole bicornis ants. Oecologia 133:186–192
Flowers RW, Janzen DH (1997) Feeding records of Costa Rican leaf beetles (Coleoptera: Chrysomelidae). Fla Entomol 80:334–366
de la Fuente MAS, Marquis RJ (1999) The role of ant-tended extrafloral nectaries in the protection and benefit of a Neotropical rainforest tree. Oecologia (Berlin) 118:192–202
Gannes LZ, Obrien DM, delRio CM (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Gaume L, McKey D (1998) Protection against herbivores of the myrmecophyte Leonardoxa africana (Baill.) Aubrev. T3 by its principal ant inhabitant Aphomomyrmex afer Emery. CR Acad Sci III Sci Vie 321:593–601
Gaume L, McKey D (1999) An ant-plant mutualism and its host-specific parasite: activity rhythms, young leaf patrolling, and effects on herbivores of two specialist plant ants inhabiting the same myrmecophyte. Oikos 84:130–144
Gaume L, McKey D, Anstett M-C (1997) Benefits conferred by “timid” ants: active anti-herbivore protection of the rainforest tree Leonardoxa africana by the minute ant Petalomyrmex phylax. Oecologia (Berlin) 112:209–216
Gaume L, McKey D, Terrin S (1998) Ant-plant-homopteran mutualism: how the third partner affects the interaction between a plant-specialist ant and its myrmecophyte host. Proc R Soc Lond B Biol Sci 265:569–575
Green EE (1889) Descriptions of two new species of Lecanium from Ceylon. Entomol Mon Mag 25:248–250
Green EE (1896) Catalogue of Coccidae collected in Ceylon. Indian Mus Notes 4:2–10
Gullan PJ, Buckley RC, Ward PS (1993) Ant-tended scale insects (Hemiptera: Coccidae: Myzolecanium) within lowland rain forest trees in Papua New Guinea. J Trop Ecol 9:81–91
Heil M, Fiala B, Baumann B, Linsenmair KE (2000) Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Funct Ecol 14:749–757
Janzen DH (1969) Allelopathy by myrmecophytes: the ant Azteca as as allelopathic agent of Cecropia. Ecology 50:147–153
Janzen DH (1975) Pseudomyrmex nigropilosa: a parasite of a mutualism. Science 188:936–937
Letourneau (1983) Passive aggression: an alternative hypothesis for the Piper-Pheidole association. Oecologia 60:122–126
Linnaeus C (1758) Insecta. Hemiptera. Coccus. In: Systema naturae. Salvii, Holmiae, p 823
Longino JT (1989a) Geographic variation and community structure in an ant-plant mutualism: Azteca and Cecropia in Costa Rica. Biotropica 21:126–132
Longino JT (1989b) Taxonomy of the Cecropia-inhabiting ants in the Azteca alfari species group (Hymenoptera: Formicidae): evidence for two broadly sympatric species. Contrib Sci 412:1–16
Longino JT (1996) Taxonomic characterization of some live-stem inhabiting Azteca (Hymenoptera: Formicidae) in Costa Rica, with special reference to the ants of Cordia (Boraginaceae) and Triplaris (Polygonaceae). J Hymenoptera Res 5:131–156
Longino JT (2003) The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa 151:1–150
Madden D, Young TP (1992) Symbiotic ants as an alternative defense against giraffe herbivory in spinescent Acacia drepanolobium. Oecologia 91:235–238
Maschwitz U, Fiala B (1995) Investigations on ant-plant associations in the south-east-Asian genus Neonauclea Merr. (Rubiaceae). Acta Oecol 16:3–18
Maskell WM (1893) Further coccid notes: with descriptions of new species from Australia, India, Sandwich Islands, Demerara, and South Pacific. Trans Proc NZ Inst 25:201–252
Maskell WM (1897) Further coccid notes: with descriptions of new species and discussions of points of interest. Trans Proc NZ Inst 29:293–331
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
Michener RH, Schell DM (1994) Stable isotope ratios as tracers in marine aquatic food webs. In: Lajtha K, Michener RH (eds) Stable isotopes in ecology and environmental science. Blackwell, Cambridge
Mills JN (1984) Effects of feeding by Mealybugs (Planococcus-Citri, Homoptera, Pseudococcidae) on the growth of Colliguaya-Odorifera seedlings. Oecologia 64:142–144
Minagawa M, Wada E (1984) Stepwise enrichment of N-15 along food-chains—further evidence and the relation between delta-N-15 and animal age. Geochim Cosmochim Acta 48:1135–1140
Moog J, Drude T, Maschwitz U (1998) Protective function of the ant–plant Cladomyrma maschwitzi to its host, Crypteronia griffithi and the dissolution of the mutualism (Hymenoptera: Formicidae). Sociobiology 31:105–129
Morrison H (1929) Some neotropical scale insects associated with ants (Hemiptera—Coccidae). Ann Entomol Soc Am 22:33–60
Müller F (1876) Fritz Müller on Brazil kitchen middens, habits of ants, etc. Nature 13:304–305
Nava-Camberos U, Riley DG, Harris MK (2001) Density–yield relationships and economic injury levels for Bemisia argentifolii (Homoptera: Aleyrodidae) in cantaloupe in Texas. J Econ Entomol 94:180–189
Newstead R (1908) On a collection of Coccidae and other insects affecting some cultivated and wild plants in Java and in Tropical Western Africa. J Econ Biol 3:33–42
Newstead R (1920) Observations on scale-insects (Coccidae)—VI. Bull Entomol Res 10:175–207
Noguera FA (1988) Hispinae y Cassidinae (Coleoptera: Chrysomelidae) de Chamela, Jalisco, Mexico. Folia Entomol (Mex) 77:277–311
O’Dowd DJ (1982) Pearl bodies as ant food: an ecological role for some leaf emergences of tropical plants. Biotropica 14:40–49
Oelbermann K, Scheu S (2002) Stable isotope enrichment (delta N-15 and delta C-13) in a generalist predator (Pardosa lugubris, Araneae:Lycosidae): effects of prey quality. Oecologia 130:337–344
Oliveira PS, Oliveira-Filho AT, Cintra R (1987) Ant foraging on ant-inhabited Triplaris (Polygonaceae) in western Brazil: a field experiment using live termite-baits. J Trop Ecol 3:193–200
Ostrom PH, ColungaGarcia M, Gage SH (1997) Establishing pathways of energy flow for insect predators using stable isotope ratios: field and laboratory evidence. Oecologia 109:108–113
Ozaki K, Kitamura S, Subiandoro E, Taketani A (1999) Life history of Aulacaspis marina Takagi and Williams (Hom., Coccoidea), a new pest of mangrove plantations in Indonesia, and its damage to mangrove seedlings. J Appl Entomol-Z Angew Entomol 123:281–284
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rocha CFD, Bergallo HG (1992) Bigger ant colonies reduce herbivory and herbivore residence time on leaves of an ant-plant: Azteca muelleri vs. Coelomera ruficornis on Cecropia pachystachya. Oecologia 91:249–252
Rosing MN, Ben-David M, Barry RP (1998) Analysis of stable isotope data: aK nearest-neighbors randomization test. J Wildl Manage 62:380–388
Sagers CL, Ginger SM, Evans RD (2000) Carbon and nitrogen isotopes trace nutrient exchange in an ant–plant mutualism. Oecologia (Berlin) 123:582–586
Schupp EW (1986) Azeca protection of Cecropia: ant occupation benefits juvenile trees. Oecologia 70:379–385
Smith JP, Schowalter TD (2001) Aphid-induced reduction of shoot and root growth in Douglas-fir seedlings. Ecol Entomol 26:411–416
Stanton ML, Palmer TM, Young TP, Evans A, Turner ML (1999) Sterilization and canopy modification of a swollen thorn acacia tree by a plant–ant. Nature (Lond) 401:578–581
Stanton ML, Palmer TM, Young TP (2002) Competition-colonization trade-offs in a guild of African Acacia-ants. Ecol Monogr 72:347–363
Tillberg CV (2004) Cordia gerascanthus (Boraginaceae) produces stem domatia. J Trop Ecol 20:1–3
Treseder KK, Davidson DW, Ehleringer JR (1995) Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature (Lond) 375:137–139
Vasconcelos HL (1991) Mutualism between Maieta guianensis Aubl., a myrmecophytic melastome, and one of its and inhabitants: ant protection against insect herbivores. Oecologia 87:295–298
Vranjic JA, Ash JE (1997) Scale insects consistently affect roots more than shoots: the impact of infestation size on growth of eucalypt seedlings. J Ecol 85:143–149
Vranjic JA, Gullan PJ (1990) The effect of a sap-sucking herbivore, Eriococcus-Coriaceus (Homoptera, Eriococcidae), on seedling growth and architecture in Eucalyptus Blakelyi. Oikos 59:157–162
Wheeler WM (1942) Studies of neo-tropical ant–plants and their ants. Bull Mus Comp Zool 40:1–40
Williams DJ (1969) A new species of Cataenococcus Ferris (Hom., Coccoidea, Pseudococcidae) on banana in Costa Rica. Bull Entomol Res 59:101–104
Windsor DM, Riley EG, Stockwell HP (1992) An introduction to the biology and systematics of Panamanian Tortoise Beetles (Coleoptera: Chrysomelidae: Cassidinae). In: Quintero D, Aiello A (eds) Insects of Panama and Mesoamerica, selected studies. Oxford University Press, Oxford, pp 372–391
Yoneyama T, Handley LL, Scrimgeour CM, Fisher DB, Raven JA (1997) Variations of the natural abundances of nitrogen and carbon isotopes in Triticum aestivum, with special reference to phloem and xylem exudates. New Phytol 137:205–213
Yu DW, Pierce NE (1998) A castration parasite of an ant-plant mutualism. Proc R Soc Lond B Biol Sci 265:275–282
Yu DW, Wilson HB, Pierce NE (2001) An empirical model of species coexistence in a spatially structured environment. Ecology 82:1761–1771
Acknowledgements
I thank the Organization for Tropical Studies and the University of Colorado for financial support. The Huertos Project is funded by a grant from NSF (DEB 9975235) and the Andrew W. Mellon Foundation. Much thanks to J. Ewell, A. Reich, and everyone associated with Huertos. K. Goetz, M. Smith, S. Cooper, A. Law, R. Coggins, P. Brandauer, C. Carros, and S. White helped with the field work. N. Li assisted with sample processing. The research and manuscript were improved by helpful critiques by M. Breed, J. Longino, C. Sagers, D. Bowers, Y. Linhart, C. Bock, D. Smith, T. McGlynn, A. Suarez, M. Ben-David, and one anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Tillberg, C.V. Friend or foe? A behavioral and stable isotopic investigation of an ant–plant symbiosis. Oecologia 140, 506–515 (2004). https://doi.org/10.1007/s00442-004-1601-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1601-8