Abstract
In thermally stratified lakes with a deep chlorophyll maximum (DCM), Daphnia face a trade-off between food availability and optimum development temperatures. We hypothesize that Daphnia optimize their fitness by allocating the time spent in the different vertical habitats depending on the distribution of algal resources and the temperature gradient. We used the plankton towers (large indoor mesocosms) to study the vertical distribution of a population of Daphnia hyalina×galeata in three different temperature gradients with a DCM. Additionally, we determined the fitness of Daphnia in the epilimnion and hypolimnion by transferring water from these layers into flow-through systems where we raised Daphnia and assessed their juvenile growth rate as a measure of fitness. The fitness distribution was correlated with the vertical distribution. The vertical distribution most likely reflected the proportions of time Daphnia allocated to dwelling in the two vertical habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In thermally stratified lakes, Daphnia experience a variety of vertical gradients, e.g., gradients in abiotic factors like temperature (Watanabe 1992; Haney 1993; Winder et al. 2003a), oxygen (Hanazato and Ooi 1992; Lass et al. 2000; Winder et al. 2003a), and light (Watanabe 1992), including UV-radiation (e.g. Leech and Williamson 2001; Rhode et al. 2001). Additionally, Daphnia are affected by biotic gradients in predation risk (Gliwicz and Pijanowska 1988; De Meester et al. 1995), competitors (Leibold 1991; Winder et al. 2003b), and food. Food can vary in both quantity (Gliwicz and Pijanowska 1988; Adrian and Schipolowski 2003) and quality (Kasprzak et al. 2000; Cole et al. 2002). In nature, all of these gradients act on Daphnia jointly, which means that Daphnia have to optimize their fitness under various trade-offs, for example, trade-offs between light-dependent mortality caused by UV-radiation or fish predation, and foraging.
During summer, lakes in temperate regions are thermally stratified into a warm, mixed surface layer, the epilimnion, and a cold, deep layer, the hypolimnion. These two layers are separated by an intermediate layer, the metalimnion. Although algal biomass is usually highest in the epilimnion, many stratified lakes exhibit an algal maximum in the deep strata (meta- or hypolimnion). Such a vertical distribution of phytoplankton is called deep-water chlorophyll maximum (DCM) (Fee 1976; Williamson et al. 1996; Padisak et al. 1997). In thermally stratified lakes with a DCM (and without the interference of light-dependent mortality), Daphnia are exposed to a trade-off between food availability and temperature. In the epilimnion, the water temperature is high, which allows for fast growth and egg development (Kerfoot 1985; Dawidowicz and Loose 1992), but food is scarce, which reduces growth and fecundity (Gliwicz 1990; Kilham et al. 1997). In contrast, food is abundant in the hypolimnion, but the water temperature is low.
In recent years, the vertical distribution of Daphnia in lakes with a DCM has received increasing attention. Several studies combined observations of the vertical distributions of Daphnia populations in lakes containing a DCM with bioassays on the food quality of the algae in the different layers (Williamson et al. 1996; Cole et al. 2002; Winder et al. 2003a). Williamson et al. (1996) and Winder et al. (2003a) showed that reproductive and growth rates of Daphnia were higher when they were raised with water from the DCM than with water taken from the surface layers. However, as they did not raise Daphnia at the same temperature as in the respective layer, they did not empirically investigate the trade-off between food and temperature as such. In contrast, Cole et al. (2002) measured Daphnia growth and survival in water taken from the surface and from the DCM at corresponding and reciprocal temperatures, but as Daphnia growth was higher in the epilimnion, the daphniids in this lake were not exposed to a trade-off between food and temperature. Lampert et al. (2003) performed a laboratory experiment in large indoor mesocosms, the Plön plankton towers (Lampert and Loose 1992), where they measured the vertical distribution of Daphnia in a water column with a DCM and different temperature gradients. They calculated the fitness distribution of Daphnia in the different food and temperature gradients by incorporating the empirical food and temperature measurements into a physiological model. They found a quite good agreement between the vertical distribution and the modelled fitness distribution, but the fitness estimates in the epilimnion were too high compared to the proportion of Daphnia found in this layer. The most likely reason for this discrepancy was that Lampert et al. (2003) based their calculations of fitness on food-particle concentrations in the plankton towers, and did not take food quality into account. Consequently, they overestimated the fitness in the epilimnion where food was of poorer quality than in the hypolimnion.
In the present study, we also aimed to compare the fitness and the vertical distribution of Daphnia in a thermally stratified water column with a DCM, but in contrast to Lampert et al. (2003), we measured the fitness achieved by Daphnia directly in the different vertical habitats. We raised them in a flow-through system in water taken from the towers at the ambient temperature in each vertical habitat. Thus, we assessed the joint influence of temperature, food quantity and food quality on the fitness distribution, which was only partially considered in the model of Lampert et al. (2003). Simultaneously, we monitored the vertical distribution of a Daphnia population in the water column. The aim of the study was to demonstrate the trade-off between food and temperature and to test the hypothesis that the vertical distribution of Daphnia reflects the fitness distribution, i.e., that the vertical distribution of the Daphnia population is correlated with their fitness distribution.
Materials and methods
Organisms
The experiments were performed with the same single clone of Daphnia hyalina×galeata used by Lampert et al. (2003). The green alga Scenedesmus obliquus was used as unialgal food. Mass cultures of D. hyalina×galeata and S. obliquus were established as in Lampert et al. (2003). Inoculum populations of Daphnia were raised in 100-l containers and then transferred into the mesocosms. S. obliquus were kept in batch cultures of dilute (1:4) Z4 medium (Zehnder and Gorham 1960). S. obliquus grown under these conditions are known to provide good growth of Daphnia (Boersma 2000; Becker and Boersma 2003).
Plankton towers
The vertical distribution of Daphnia was monitored in large indoor mesocosms, the plankton towers (Lampert and Loose 1992; Lampert et al. 2003). We applied a design similar to Lampert et al. (2003). The plankton towers were thermally stratified into three different layers: the surface layer (0–2.5 m), hereafter called epilimnion, the layer underneath (2.5–5 m), referred to as hypolimnion, and the deepest layer (5–11 m). The temperature in the epilimnion was always adjusted to 20°C, while the temperature in the hypolimnion was adjusted to 10, 15, or 18°C, respectively. This resulted in three different temperature gradients, which are named according to the temperature difference between the epilimnion and the hypolimnion, i.e., 10, 5 and 2°C temperature gradient. After the temperature stratification was established, the appropriate amount of algal suspension was injected into the hypolimnion to reach a final concentration of 0.5 mg C/l. The temperature in the deepest layer was always adjusted to 8°C in order to avoid mixing of the algae into the depth. The epilimnion and hypolimnion were sampled most intensively, but food and Daphnia abundances were also checked in the deepest layer in order to verify that the food concentration was low and, at best, very few Daphnia individuals dwelled there. The diel photoperiod was set to 12:12 h light and dark. In order to determine the algal concentration in the hypolimnion, we sampled hypolimnetic phytoplankton every morning (at 3, 3.5, 4, and 4.5 m depth) and measured the particle volume of these samples in a cell counter and analyzer system (CASY 1, model TCC, Schärfe System, Reutlingen, Germany). By using a previously established calibration curve between particle volume and carbon concentration of Scenedesmus, we calculated the particulate carbon concentration in the hypolimnion. In case the carbon concentration in the hypolimnion was lower than 0.5 mg C/l, the missing amount of Scenedesmus was injected through a hose with openings at 3 and 4 m depth. Algae in the hypolimnion were allowed to mix for 4 h. Thereafter, phytoplankton was sampled in the entire water column at 12 different depths (0.1, 0.6, 1.2, 1.6, 2.1, 2.5, 3, 3.5, 4, 4.6, 5.5, and 6.5 m).
Zooplankton was sampled on the 1st, 2nd and 4th day of each series. Every sampling day, zooplankton was sampled during daytime and at night in 11 depths (0.1, 1.2, 1.6, 2.1, 2.5, 3, 3.5, 4, 4.6, 5.5, and 6.5 m) using glass traps and pumps that strained the zooplankton from 50 l of water each (Lampert and Loose 1992). All zooplankton samples were preserved with 4% sucrose formaline. The samples were counted with the bench top version of an optical plankton counter (OPC-1L, Focal Technologies, Dartmouth, Nova Scotia, Canada) that counts and sizes particles in a light beam (Kessler and Lampert 2003). Directly after the last sampling of one series (i.e., after three sampling dates), the temperature in the hypolimnion was altered to the temperature gradient of the next series. In order to allow Daphnia to acclimate to the new temperature conditions, no samples were taken for 2 days between successive series. Each series, thus, consisted of three day and three night profiles of zooplankton. The three profiles were combined and considered one replicate. Although these replicates were in part obtained with the same population of Daphnia, we considered them to be independent as the daphniids experienced two complete light cycles between the replicates, when they could redistribute. Moreover, there was turnover within the population (growth and mortality), i.e., not all the individuals were identical.
Six replicates each were obtained from the 2 and 10°C temperature gradients while the 5°C gradient was repeated seven times. Although the plankton towers cannot be kept sterile, S. obliquus was almost the only food source for Daphnia. However, unintended “surface blooms” of algae other than S. obliquus developed in some of these replicates (one in the 10°C, two in the 5°C and three in the 2°C temperature gradient), gradually increasing the carbon concentration in the epilimnion. These replicates could not be used for the analysis of the trade-off between temperature and food. In three additional replicates, with 5°C temperature difference, we deliberately created surface blooms by adding 0.5 mg C/l S. obliquus to the epilimnion. Hence, at the end we had 13 replicates with a trade-off and 9 without.
Flow-through system
In parallel to the tower experiments, Daphnia were raised in flow-through systems under food and temperature conditions identical to the epilimnion and the hypolimnion in the plankton towers. Every day, 3 h after the replenishment of the food concentration in the plankton towers, 2.5 l of water each were withdrawn from 0.5 and 1.5 m depth in the epilimnion and from 3 and 4 m depth in the hypolimnion. The water from 0.5 and 1.5 m was pooled as was the water from 3 and 4 m, strained through a 150-μm gauze to eliminate Daphnia, and then transferred into two reservoirs supplying two separate parts of a flow-through system similar to Lampert et al. (1988). Each reservoir supplied three 120-ml flow-through vessels containing ten D. hyalina×galeata. The flow-through vessels were kept in two water baths at the respective epilimnetic or the hypolimnetic temperatures of the plankton towers. The flow rate per vessel was adjusted to approximately 1.5 l day−1. We determined the epilimnetic and hypolimnetic juvenile growth rates (g j) of Daphnia that are a good proxy for fitness (Lampert and Trubetskova 1996). The juvenile growth rate was calculated as, g j=(ln W 1−ln W 0)/(t 1−t 0),where g j is the juvenile growth rate, W is the dry weight at the beginning (0) and end (1) of the experiment, while t 1−t 0 is the elapsed time between the beginning and the end of the experiment. Synchronized offspring of the third and subsequent broods of Daphnia were inserted into the flow-through system at the age of 2 days. To measure the initial dry weight, two or three times, ten randomly selected juveniles were transferred into pre-weighed aluminum containers, dried overnight at 60°C, cooled in an desiccator and weighed on a Sartorius ultramicro balance (Sartorius, Göttingen, Germany) to the nearest microgram. After 4 days, all Daphnia from one flow-through vessel were pooled and their dry mass was determined.
Statistical analysis
The statistical package NCSS 2001 (Statistical Solutions, Cork, Ireland; Hintze 2001) was used for all statistical analyses. For all vertical profiles, the proportions of the total Daphnia population dwelling in the 11 different depths were calculated and subjected to a principal component analysis (PCA), which was based on a variance-covariance matrix (for details see Lampert et al. 2003). PCA produces principal components (PC), which are linear combinations of the original dependent variables, i.e., the percentage of the population at the different depths. In order to establish linear equations of the original dependent variables, each of these variables is multiplied with a coefficient called factor loading. Sign and value of each factor loading describe its relation to the PC, i.e., the contrasts in abundance at different depths. The insertion of the measured percentages into the linear equation of a PC yields so-called factor scores. These factor scores of the PCA were subjected to a three-way ANOVA with the factors plankton tower (tower 1 and tower 2), temperature gradient (2, 5, and 10°C), and sampling time (day and night).
The juvenile growth rates were tested with one-way ANOVA, with the factor being the different combinations of food and temperature. A post-hoc comparison of the juvenile growth rates was performed with Tukey’s honest significant differences (HSD) test.
We tested the hypothesis that the habitat preference of Daphnia is correlated with the relative fitness in the different vertical strata. After calculating the total abundance of Daphnia in a tower by integrating over depth, we determined the proportion of the total population dwelling in the hypolimnion (below 2.5 m). These proportions were compared to the relative fitness in the hypolimnion as measured in the flow-through experiments. Relative hypolimnetic fitness Pg jH is defined as fitness obtained in the hypolimnion in relation to the average fitness in the plankton towers, Pg jH=(g jH×100)/[(g jE+g jH)/2], where g jE and g jH are the juvenile growth rates in epilimnion and hypolimnion, respectively.
In order to avoid the use of proportions in the statistical test, we calculated a linear regression of the untransformed abundances of Daphnia in the hypolimnion versus the total abundances in the towers. We then analysed the residuals and tested for a significant correlation of the residuals with the relative hypolimnetic fitness. The null hypothesis was that the residuals were not correlated with the relative fitness, i.e., the daphniids chose their habitat randomly.
Results
Distribution of algae
Measurement of the food distribution showed that a stable hypolimnetic algal maximum was successfully established in 13 replicates. Above the thermocline, the food concentration was on average 0.1 mg C/l, while it ranged from 0.3 mg C/l just below the thermocline to 0.5 and 0.6 mg C/l in the other depths of the hypolimnion. Epilimnetic food concentrations were variable in the additional nine replicates lacking a trade-off. These replicates were not included in the PCA, but in the general analysis of fitness and distribution.
Vertical distribution of Daphnia
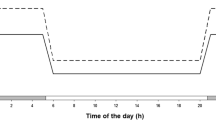
The temperature in the hypolimnion influenced the vertical distribution of the total Daphnia population (Fig. 1). The proportion of the population dwelling in the hypolimnion increased with an increasing temperature in this layer, i.e., with a decreasing temperature gradient between epilimnion and hypolimnion. Moreover, with a decreasing temperature gradient, fewer daphniids accumulated near the thermocline.
Vertical distribution of the total Daphnia population given as average proportions of the population dwelling at the respective depths (±SE) at day (unfilled bars) and night (filled bars) in the three different temperature gradients. The temperature in the epilimnion was always 20°C. The temperature differences between epilimnion and hypolimnion are given in the panels. The dashed line indicates the thermocline
These observations are confirmed by the PCA. The PCA resulted in three PC explaining 95% of the total variation (Table 1). The first PC (58.3% of total variation) contrasted the vertical distribution of Daphnia in the epilimnion, especially at port 6 (2.5 m) directly at the thermocline, with the distribution in the hypolimnion. Thus, the first PC indicated the shift of the population between these two layers. The second PC (25.4% of total variation) contrasted port 7, which is just below the thermocline, with all other depths, especially port 10, indicating the shift of the Daphnia population within the hypolimnion. The third PC (11.3% of total variation) contrasted port 6 with all other depths, especially the upper epilimnion and port 10 in the hypolimnion. Thus, the third PC represents the shift of the total population at the thermocline.
The ANOVA on the factor scores of the first PC showed that both temperature in the hypolimnion and light (day vs night) had a significant influence on the vertical distribution of Daphnia, but there was no significant effect of the plankton tower (Table 2). No significant effect of tower, temperature gradient or light was detected for the factor scores of the second PC. As the second PC indicated the shift of the population within the hypolimnion, it is not very important within the scope of this study. The factor scores of the third PC were significantly influenced by light and the interaction of temperature gradient and light (Table 2), but this PC only accounted for 11.3% of the total variation.
Fitness
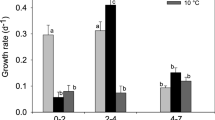
The juvenile growth rate g j was significantly influenced by the combination of temperature and food availability in the different layers and temperature gradients (1-way ANOVA, df=5, F=21.25, P<0.001, Fig. 2). The epilimnetic g j was similar in all temperature gradients, while the hypolimnetic g j increased with temperature (Fig. 2). In the 10°C temperature gradient, g j was significantly lower in the hypolimnion than in the epilimnion (Tukey’s HSD for unequal sample sizes, P=0.004), but it was significantly higher in the hypolimnion compared to the epilimnion in the 5 and 2°C temperature gradients (Tukey’s HSD for unequal sample sizes, P=0.010 and P<0.001, respectively).
Juvenile growth rates (g j) of Daphnia in epilimnetic water (unfilled bars) at 20°C and in hypolimnetic water (filled bars) at 10, 15, and 18°C. Epilimnetic and hypolimnetic g j measured within the same temperature gradient are plotted next to each other. Asterisks indicate significant differences between g j in epilimnion and hypolimnion within one temperature gradient (Tukey’s HSD post-hoc test)
Distribution and fitness
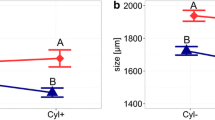
As light had a significant effect on the vertical distribution of Daphnia, we used only night profiles for the analysis of the relationship between depth distribution and fitness. The hypolimnetic (below 2.5 m) proportion of the Daphnia population was positively related to the relative fitness they could gain in the hypolimnion (Fig. 3), regardless of the reason for the fitness differences (temperature or food). To avoid running a test with proportions, we based the statistical analysis on untransformed abundances. The relationship between the total number of daphniids in the tower and the number dwelling in the hypolimnion was highly significant. In order to improve homoscedasticity, we excluded a single replicate with an unusually high total abundance, but this did not affect the parameters of the regression markedly. The regression for the remaining 21 data points was A H=0.825 A T−6941 (df=20, r 2=0.925, P<0.001), where A H is the abundance in the hypolimnion and A T is the total abundance.
Proportion of the total Daphnia population in the hypolimnion measured in the towers, and the respective relative fitness in the hypolimnion measured in the flow-through system. Different symbols represent the temperature gradient in which the data point was measured: circles represent a 2°C temperature difference between epilimnion and hypolimnion, diamonds a 5°C difference, and triangles a 10°C temperature difference. Filled symbols represent replicates measured in a water column with a deep-water algal maximum. Replicates with unintended algal blooms in the epilimnion are depicted with unfilled symbols with the shape of the corresponding temperature gradient. Unfilled squares represent experiments at a 5°C temperature gradient with addition of 0.5 mg C/l Scenedesmus to the epilimnion
We then plotted the residuals against the relative fitness in the hypolimnion. The residuals (RES) were positively correlated with the relative fitness (Pg jH):
RES = 74 Pg jH−7352 (df=20, r 2=0.238, P=0.025). Consequently, with increasing relative fitness in the hypolimnion, the number of daphniids dwelling there increases significantly more than the total population, i.e., the relationship depicted in Fig. 3 is significant.
Discussion
The results of this study support our hypothesis: In the trade-off between low food availability and high temperature on the one hand and high food availability and low temperature on the other, the vertical distribution of Daphnia is correlated with the fitness distribution. In contrast to earlier studies, we were able to demonstrate this with direct fitness measurements that confirmed the trade-off between food and temperature. This trade-off was reflected by the g j in the respective layers and in the different temperature gradients. In the epilimnion, the g j was low due to the low food concentration. As no S. obliquus was added to this layer, the epilimnetic food consisted of detritus particles (Lampert et al. 2003) of low food quality to Daphnia (Lampert 1987; Hessen et al. 2003), bacteria, and possibly small amounts of Scenedesmus transported across the thermocline by eddies and migrating Daphnia. Thus, most likely the fitness in the epilimnion was reduced by the low quantity and quality of the food. This effect might have been intensified by the higher temperature in the epilimnion which increases respiration (Cole et al. 2002). However, the juvenile growth rates were still positive, which means that, despite the poor food conditions, growth of the daphniids dwelling in the epilimnion was still possible.
In contrast, due to the daily addition of S. obliquus, the food availability in the hypolimnion was much higher than in the epilimnion. Not only was the quantity higher, the fresh Scenedesmus also provided a high-quality food (Lampert 1987). Nevertheless, g j in the hypolimnion was significantly reduced by the low temperatures. Reduced growth of Daphnia at low temperatures has been reported in many studies (Kerfoot 1985; Dawidowicz and Loose 1992; Loose and Dawidowicz 1994).
Despite the low temperatures, the hypolimnetic g j was significantly higher than the epilimnetic g j in the two shallow temperature gradients, which resulted in a deep-water fitness maximum. In these temperature gradients, the high food concentration more than compensated the effects of the small temperature difference, although it was not sufficient to compensate a 10°C difference. A deep-water fitness maximum was found in several water columns with a deep algal maximum, both in field studies (Williamson et al. 1996; Winder et al. 2003a) and in laboratory experiments (Lampert et al. 2003). When the epilimnion contained more food, during surficial algal blooms, there was no trade-off and the fitness was always highest in the epilimnion. However, the relative fitness varied as the epilimnetic food concentration and quality were not always the same. In these cases, most daphniids avoided the cold hypolimnion, but they were still distributed according to the relative fitness (cf. Fig. 3). Avoidance of low hypolimnetic temperatures in water bodies with a surface algal maximum and without fish predation has also been observed in other studies (Calaban and Makarewicz 1982; Haney 1993).
In general, the vertical distribution of Daphnia coincided well with the fitness distribution, regardless of the reasons for differences in fitness (temperature or food) in the different layers. The good match between the measured fitness distribution and the vertical distribution of the Daphnia population is somewhat surprising as one would expect all Daphnia, not only a proportion of the population, to dwell in the layer providing the highest g j. For example, if the fitness in the epilimnion is higher than in the hypolimnion, Daphnia should all stay there, regardless of the absolute difference in fitness. However, this was not observed as, depending on the costs, part of the Daphnia population was always found in the less favourable layers.
The explanation for this discrepancy is probably that Daphnia can find the optimum fitness in the vertical gradient, but not the maximum fitness they potentially could achieve. Even if they dwell in the optimum layer, there may still be a better place to be. Thus, they may leave the patch to search for a more profitable one, but the time spent outside the optimum patch must depend on the costs associated with the search. The lower the fitness gain in a certain layer is, the higher are the costs for the overall fitness, i.e., the lower is the tendency to stay there. Consequently, the time allocated to search in a certain habitat will depend on the local fitness gain, and the result will be a correlation between the Daphnia distribution in the gradient and the distribution of relative fitness.
Lampert et al. (2003) pointed out that such a distribution resembles an ideal free distribution with costs (Tyler and Gilliam 1995). Besides the low temperatures, costs may also be associated with the aggregation in a certain layer as this will increase intra-specific competition. Our fitness measurements do not take into account competition effects as we used a fixed number of Daphnia per flow-through vessel and excluded grazing losses. Hence, our measurements may not mimic the conditions in the towers perfectly. Most importantly, however, the explanation requires a dynamic distribution of the daphniids, i.e., the visible overall distribution of the population must be the result of many different migration behaviours of the individuals moving in the water column and spending various amounts of time in different layers. Evidence for a dynamic distribution in the towers was provided by Lampert and Grey (2003) using 15N-enriched hypolimnetic algae to show that all daphniids had been foraging in the hypolimnion, regardless of the depths where they had been sampled. More evidence comes from direct observations of the swimming behaviour of Daphnia in vertical perspex tubes (K. Kessler, unpublished observation) and other behavioural studies. For example, Daphnia can quickly find food patches, even over long distances, in laboratory experiments (Haney 1993; Plath 1998) and in the field (Jensen et al. 2001), and they reduce their swimming speed in patches with high food concentrations (Cuddington and McCauley 1994; Larsson 1997).
There may also be a physiological advantage of switching between layers. Daphnia may utilize the available food more efficiently if they feed in the food-rich hypolimnion and digest in the epilimnion where high temperatures accelerate food assimilation (Lampert 1977). Such a strategy would reduce the trade-off between food and temperature and consequently increase the overall fitness. This hypothesis predicts that daphniids should stay close to the thermocline to avoid long swimming distances for switching layers, in particular when the temperature gradient is steep. In fact, we observed the aggregation of Daphnia near the thermocline in the 10°C gradient (Fig. 1), and the same was reported by Lampert et al. (2003).
Individual migrations leading to a dynamic distribution must be distinguished from a synchronized diel vertical migration of a Daphnia population. Possible metabolic gains are independent of light and they should consequently not be synchronized within the population. Therefore, diel vertical migration triggered by changes in light intensity cannot be a strategy to optimize the fitness gain in a trade-off between food and temperature. Nevertheless, we observed slight but significant differences in the vertical distribution of the Daphnia population between day and night, although not of the same order as diel vertical migration induced by the presence of fish. The slight upward shift at night is probably a consequence of a genetically fixed residual response of large females to light even in the absence of fish (Kessler and Lampert 2004). We excluded this effect when we analysed only the night distributions. The advantage of our system is that the presence of fish kairomones (indicating predation threat) can be excluded, which is almost impossible in field studies. Fish predation is a powerful selection factor, forcing large daphniids into dark hypolimnetic refuges. Although the presence of a DCM can compensate the negative effect on the fitness of Daphnia caused by low temperature during diel vertical migration, synchronized migrations indicate that avoidance of light-related mortality (fish or UV-radiation) is an important factor. Hence, the combined effects of temperature-food trade-off and mortality avoidance cannot be separated in lakes with a DCM (Williamson et al. 1996; Winder et al. 2003a).
The optimal habitat choice of Daphnia is the result of many trade-offs. If the food is distributed in the epilimnion, the risk of light-dependent mortality must be balanced during the day, which will result in diel vertical migration. In the absence of light-dependent mortality, we can expect a dynamic distribution correlated with the vertical distribution of fitness. We have analysed the costs of low temperatures, but further studies should include gradients of other environmental factors, e.g. oxygen and invertebrate predators.
References
Adrian R, Schipolowski T (2003) Bacterial and protozoan mass accumulation in the deep chlorophyll maximum of a mesotrophic lake. Arch Hydrobiol 157:27–46
Becker C, Boersma M (2003) Resource quality effects on life histories of Daphnia. Limnol Oceanogr 48:700–706
Boersma M (2000) The nutritional quality of P-limited algae for Daphnia. Limnol Oceanogr 45:1157–1161
Calaban MJ, Makarewicz JC (1982) The effect of temperature and density on the amplitude of vertical migration of Daphnia magna. Limnol Oceanogr 27:262–271
Cole PC, Luecke C, Wurtsbaugh WA, Burkart G (2002) Growth and survival of Daphnia in epilimnetic and metalimnetic water from oligotrophic lakes: the effects of food and temperature. Freshwater Biol 47:2113–2122
Cuddington KM, McCauley E (1994) Food-dependent aggregation and mobility of the water fleas Ceriodaphnia dubia and Daphnia pulex. Can J Zool 72:1217–1226
Dawidowicz P, Loose CJ (1992) Metabolic costs during predator-induced diel vertical migration of Daphnia. Limnol Oceanogr 37:1589–1595
De Meester L, Weider LJ, Tollrian R (1995) Alternative antipredator defences and genetic polymorphism in a pelagic predator-prey system. Nature 378:483–485
Fee EJ (1976) The vertical and seasonal distribution of chlorophyll in lakes of the Experimental Lakes Area, northwestern Ontario, Canada: implications for primary production estimates. Limnol Oceanogr 21:767–783
Gliwicz ZM (1990) Food thresholds and body size in cladocerans. Nature 343:638–640
Gliwicz ZM, Pijanowska J (1988) Effect of predation and resource depth distribution on vertical migration of zooplankton. Bull Mar Sci 43:695–709
Hanazato T, Ooi T (1992) Morphological responses of Daphnia ambigua to different concentrations of a chemical extract from Chaoborus. Freshwater Biol 27:379–385
Haney JF (1993) Environmental control of diel vertical migration behaviour. Arch Hydrobiol Spec Issues Advan Limnol 39:1–17
Hessen DO, Andersen T, Brettum P, Faafeng BA (2003) Phytoplankton contribution to sestonic mass and elemental ratios in lakes: implications for zooplankton nutrition. Limnol Oceanogr 48:1289–1296
Jensen KH, Larsson P, Högsted G (2001) Detecting food search in Daphnia in the field. Limnol Oceanogr 46:1013–1020
Kasprzak P, Gervais F, Adrian R, Weiler W, Radke R, Jager I, Riest S, Siedel U, Schneider B, Bohme M, Eckmann R, Walz N (2000) Trophic characterization, pelagic food web structure and comparison of two mesotrophic lakes in Brandenburg (Germany). Int Rev Hydrobiol 85:167–189
Kerfoot WC (1985) Adaptive value of vertical migration: comments on the predation hypothesis and some alternatives. In: Rankin MA (ed) Migration: mechanisms and adaptive significance, vol 27. University of Texas, Port Aransas, pp 91–113
Kessler K, Lampert W (2003) Counting and sizing preserved Daphnia with the Optical Plankton Counter. Arch Hydrobiol 156:485–493
Kessler K, Lampert W (2004) Depth distribution of Daphnia in response to a deep-water algal maximum: the effect of body size and temperature gradient. Freshwater Biol 49:392–401
Kilham SS, Kreeger DA, Goulden CE, Lynn SG (1997) Effects of algal food quality on fecundity and population growth rates of Daphnia. Freshwater Biol 38:639–647
Lampert W (1977) Studies on the carbon balance of Daphnia pulex as related to environmental conditions. Part 2: the dependence of carbon assimilation on animal size temperature food concentration and diet species. Arch Hydrobiol/Algol Stud 48:310–335
Lampert W (1987) Feeding and nutrition in Daphnia. Mem Ist Ital Idrobiol 45:143–192
Lampert W, Grey J (2003) Exploitation of a deep-water algal maximum by Daphnia: a stable-isotope tracer study. Hydrobiologia 500:95–101
Lampert W, Loose CJ (1992) Plankton towers: bridging the gap between laboratory and field experiments. Arch Hydrobiol 126:53–66
Lampert W, Trubetskova I (1996) Juvenile growth rate as a measure of fitness in Daphnia. Funct Ecol 10:631–635
Lampert W, Schmitt RD, Muck P (1988) Vertical migration of freshwater zooplankton: test of some hypotheses predicting a metabolic advantage. Bull Mar Sci 43:620–640
Lampert W, McCauley E, Manly BFJ (2003) Trade-offs in the vertical distribution of zooplankton: ideal free distribution with costs. Proc R Soc Lond B 270:765–773
Larsson P (1997) Ideal free distribution in Daphnia? Are daphnids able to consider both the food patch and the position of competitors? Hydobiologia 360:143–152
Lass S, Boersma M, Spaak P (2000) How do migrating daphnids cope with fish predation risk in the epilimnion under anoxic conditions in the hypolimnion? J Plankton Res 22:1411–1418
Leech DM, Williamson CE (2001) In situ exposure to ultraviolet radiation alters the depth distribution of Daphnia. Limnol Oceanogr 46:416–420
Leibold MA (1991) Trophic interactions and habitat segregation between competing Daphnia spp. Oecologia 86:510–520
Loose CJ, Dawidowicz P (1994) Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75:2255–2263
Padisak J, Krienitz L, Koschel R, Nedoma J (1997) Deep-layer autotrophic picoplankton maximum in the oligotrophic Lake Stechlin, Germany—origin, activity, development and erosion. Eur J Phycol 32:403–416
Plath K (1998) Adaptive feeding behavior of Daphnia magna in response to short-term starvation. Limnol Oceanogr 43:593–599
Rhode SC, Pawlowski M, Tollrian R (2001) The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature 412:69–72
Tyler JA, Gilliam JF (1995) Ideal free distributions of stream fish: a model and test with minnows, Rhinichthys atratulus. Ecology 76:580–592
Watanabe Y (1992) Effects of thermal stratification on trophic linkages among plankton communities in eutrophic lakes. Adv Limnol 35:1–12
Williamson CE, Sanders RW, Moeller RE, Stutzman PL (1996) Utilization of subsurface food resources for zooplankton: implications for diel vertical migration theory. Limnol Oceanogr 41:224–233
Winder M, Boersma M, Spaak P (2003a) On the cost of vertical migration: are feeding conditions really worse at greater depths? Freshwater Biol 48:383–393
Winder M, Bürgi HR, Spaak P (2003b) Mechanisms regulating zooplankton populations in a high-mountain lake. Freshwater Biol 48:795–809
Zehnder AA, Gorham PR (1960) Factors influencing the growth of Microcystis aeruginosa Kütz. emend. Elenk. Can J Microbiol 6:645–660
Acknowledgements
We would like to thank H. Hansen, H. Deiwick, and D. Albrecht for the maintenance of the plankton towers, Y. Harder and K. Wiedenhöft for cultivation of the algae, E. Geißler, M. Volquardsen, H. Wardenga and H.J. Krambeck for valuable assistance, and R.E. Moeller for linguistic improvements. Comments by M. Kopp and two anonymous reviewers improved an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kessler, K., Lampert, W. Fitness optimization of Daphnia in a trade-off between food and temperature. Oecologia 140, 381–387 (2004). https://doi.org/10.1007/s00442-004-1592-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1592-5