Abstract
Many mechanisms have been proposed to explain broad scale spatial patterns in species richness. In this paper, we evaluate five explanations for geographic gradients in species richness, using South American owls as a model. We compared the explanatory power of contemporary climate, landcover diversity, spatial climatic heterogeneity, evolutionary history, and area. An important aspect of our analyses is that very different hypotheses, such as history and area, can be quantified at the same observation scale and, consequently can be incorporated into a single analytical framework. Both area effects and owl phylogenetic history were poorly associated with richness, whereas contemporary climate, climatic heterogeneity at the mesoscale and landcover diversity explained ca. 53% of the variation in species richness. We conclude that both climate and environmental heterogeneity should be retained as plausible explanations for the diversity gradient. Turnover rates and scaling effects, on the other hand, although perhaps useful for detecting faunal changes and beta diversity at local and regional scales, are not strong explanations for the owl diversity gradient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broad-scale patterns in species richness, especially the so-called latitudinal gradient, have been widely discussed by ecologists and biogeographers, and many mechanisms have been proposed to explain them (see Whittaker et al. 2001; Willig et al. 2003, for recent reviews). However, there is now widespread support for the general explanation that elements of contemporary climate have a strong influence on diversity gradients, particularly energy inputs and water availability (Wright 1983; Hawkins et al. 2003a, b, and references therein). Even so, it is not yet clear to what extent it is necessary to incorporate long-term, historical processes into the explanations, and this issue remains under intense debate (e.g., Currie and Paquin 1987; Latham and Ricklefs 1993; McGlone 1996; Francis and Currie 1998; Currie 2001; Hawkins and Porter 2003a). Ultimately, the answer will come from empirical analyses of diversity gradients that include aspects of both contemporary and historical factors.
Quantifying the contemporary factors affecting diversity is straightforward; including variables related to energy and water inputs usually explains large amounts of the variation in the pattern of diversity for most plant and animal groups (Whittaker et al. 2001; Hawkins et al. 2003b) and this fact can be interpreted as resulting from the responses of species to contemporary climate. This approach can also used to predict the responses of species to future climate change (Currie 2001). Incorporating historical factors, in contrast, is more difficult, and workers have used several approaches. Hawkins and Porter (2003a) recently used the spatial pattern of glacial retreat during the most recent ice age to quantify the length of time that different parts of the northern half of North America have been exposed to recolonization by mammals and birds, and they found that, although contemporary energy inputs explain the most variance, there appears to be a detectable historical signal in the diversity gradients of these groups. However, this analysis was restricted to examining history over a short time span (20,000 years) and over a restricted part of the world (where the land was completely buried under ice during the most recent glacial maximum). Other approaches are necessary to examine effects operating over much longer time periods in warmer parts of the world.
One way to examine long-term evolutionary effects is to use aspects of tree structure derived from phylogenies to determine if they are correlated with total richness patterns for that group. For example, Kerr and Currie (1999) derived indices of evolutionary development (e.g., the mean number of nodes from the root to the tips of species-level phylogenies for all species present in a cell) for cicindelid (tiger) beetles and four families of freshwater fish in North America to determine if they better described the diversity gradients of these groups than did measures of climate. They found in all cases that climatic variables consistently explained more variance than did the evolutionary indices, particularly when both were included in multiple regression models, and thus concluded that historical factors have only a minor role to play in understanding the current diversity gradient of these groups. This approach does not address any specific mechanisms that may underlie why phylogenetic patterns are the way they are, only whether or not such patterns contribute to the pattern of diversity.
Finally, there are factors beyond climate or historical contingency that might contribute to broad-scale diversity gradients. For example, at some scales, topographical variability has been shown to be associated with high diversity in a number of taxonomic groups (e.g., Richerson and Lum 1980; O’Brien et al. 2000; Rahbek and Graves 2001). It is often assumed that this represents an effect of increased habitat heterogeneity in mountains, but this has not been well documented. Turner and Hawkins (2004) have argued that topographic relief actually represents a measure of climate heterogeneity, since diversity and topography are positively associated only in warm climates, but are not (or may even be negatively correlated) in cold climates. But even through there is some question about its meaning, topography does sometimes have a relationship with species richness, so it is important to include it in analyses of gradients. If Turner and Hawkins (2004) are correct, this should be especially true for tropical, hot environments, in which range in elevation creates a highly variable complex of ‘local’ climates.
A variable rarely included in analyses (but see Flather 1996; Lyons and Willig 1999, 2002), but hypothesized to be important over very large scales, is area. Rosenzweig (1992, 1995) in particular has argued that the global-scale gradient is driven by differential speciation-extinction dynamics because the tropical ‘biome’ is much larger than temperate ‘biomes’. However, this hypothesis has a large number of untested underlying assumptions, and the predictions it makes depend critically on how biomes are defined (Hawkins and Porter 2001). But despite some potential problems with the hypothesis as currently developed, it is not unreasonable to believe that area could influence diversity gradients, and thus area can be included in analyses of diversity patterns at large scales.
In this paper, we examine simultaneously the influences of climate, phylogeny, topographic and climatic heterogeneity and area on the geographic pattern of species richness in South America, using owls as our focus group. Although two analyses of South American birds have been conducted across all taxonomic groups (Rahbek and Graves 2001; Hawkins et al. 2003a), neither included area or historical variables, so their contributions to the diversity gradient in this region remain unknown. We restrict the taxonomic range of the study due to the availability of a phylogenetic tree for this group, resolved to genus. Although this obviously limits the results of our analysis to a relatively narrow taxonomic group, an important aspect of this analysis is that we evaluate all factors at the same grain size, allowing us to use a multiple regression approach to identify and partition their relative contributions to the contemporary owl diversity pattern.
Material and methods
South America was divided into 374 equal area cells 220 km×220 km (2°×2° at the equator). Adjacent coastal cells were often combined in order to keep cell area as constant as possible (Hawkins et al. 2003a). The geographic ranges of the 43 species of owls present on the continent (Del Hoyo et al. 1999) were re-drawn over this grid, and the presence of each species in each cell was recorded.
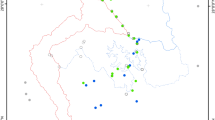
Initially, area and scaling effects were tested using the z-values of species-area curves, given by S=cAz. Species richness was calculated using nested sets of quadrats of five different sizes, centered at 23 focal cells (see Fig. 2) and with areas equal to 12,100, 24,200, 48,400, 96,800 and 193,600 km2. This restricted number of cells was selected to minimize pseudoreplication arising from the overlap of the largest quadrats. However, because of the relatively low power of this analysis, we also performed a cross-validation procedure for the correlation between z-values and richness using random samples of 12 focal quadrats, repeated 100 times.
An elevated slope (z) indicates high turnover rates and scale sensitivity in the region, such that an increase in species richness would be highly dependent on an increase in area. The z-values should be associated with historical factors and topographic heterogeneity if scale-dependent equilibrium between speciation and extinction rates or habitat heterogeneity explains variation in species richness (see Gotelli 1998). It is important to note that we are not directly evaluating the effect of cell size on our models (e.g., Rahbek and Graves 2001), because all analyses were performed using our standard cell size (48,400 km2) and z-values were used here just as a predictor of species richness at this standard cell size.
After evaluating the influence of area, the effects of the climatic and historical factors were evaluated using the complete 374-cell grid. Five climatic variables that have been shown to be associated with broad-scale richness gradients were compiled from various sources: (1) potential evapotranspiration (PET), (2) actual evapotranspiration (AET), (3) mean daily temperature in the coldest month, (4) annual mean temperature, and (5) annual rainfall (see Diniz-Filho et al. 2003; Hawkins et al. 2003a for details). We also included range in elevation, estimated to the nearest 50 m, to estimate topographic variability. Further, we incorporated an interaction term between topographic variability and minimum temperature (which we refer to as climatic heterogeneity), to capture the idea that topographic variability is only important in warm environments, creating strong environmental effects at the mesoscale (see Rahbek and Graves 2001 for a similar approach using the interaction between latitude and topographic variability). Finally, we counted the number of habitats in each cell using remotely sensed AVHRR (advanced very high resolution radiometer) landcover data (Hawkins and Porter 2003b), creating an explicit measurement of habitat diversity.
We measured the historical component of species richness based on the molecular phylogeny of Sibley and Ahlquist (1990), constructed using DNA hybridization techniques for most genera of birds worldwide. The average age of all owl taxa present in each cell was obtained from the molecular phylogeny as the time to the most recent living common ancestor at the generic level (MRCA) (similar to the “species evolutionary history” of Sechrest et al. 2002), in units of ΔT50H (DNA–DNA hybridization distances). Cells with a low average MRCA are occupied by species in recent genera, whereas cells with high average MRCA are occupied by species from old genera. However, since detailed branch lengths for South American species are not available, we used branch lengths for genera instead, as a conservative estimate of MRCA. Despite this limitation, owls are a good choice for this study because 10 of the 11 South American genera are present in the Sibley and Ahlquist (1990) phylogeny (Fig. 1).
Phylogenetic relationship among owl genera used in this paper, based on DNA-DNA hybridization analysis by Sibley and Ahlquist (1990). Numbers in parenthesis indicate number of species for each genus, and number at the nodes indicate taxa age in ΔT50H distances. The phylogenetic position of Pulsatrix was inferred based on Sibley and Monroe (1990)
Step-forward multiple and partial regression were used to assess the relative magnitude of the different effects on species richness. Spatial patterns were mapped using interpolation by a distance weighted least squares algorithm and investigated analytically by spatial autocorrelation analysis (e.g., Legendre and Legendre 1998; Diniz-Filho et al. 2003). We initially estimated the spatial autocorrelation in raw species richness and in residual richness after fitting the multiple regression model. Spatial correlograms were constructed using Moran’s I coefficients at 15 distance classes, using SAAP 4.3 (Wartenberg 1989). The spatial correlogram for the residuals indicates how much of the spatial structure was removed from data at different spatial scales (Badgley and Fox 2000; van Rensburg et al. 2002; Diniz-Filho et al. 2003). Significant unexplained spatial autocorrelation remains in the data if least one of the coefficients in a correlogram is significant at 0.05/15, where 15 is the number of distance classes.
Results
The spatial pattern of owl species richness was similar to that found for taxonomically broader groups of South American birds (Rahbek and Graves 2001; Diniz-Filho et al. 2002; Hawkins et al. 2003a), with higher species richness in the northern Andes and southeastern coast (Atlantic rainforest) (Fig. 2).
Our initial analysis found no correlation between z-values and richness (r=0.089; p=0.683) across the 23 nested quadrats (see Fig. 2). Nor were any correlations between z-values and richness significant in the 100 cross-validations. Further, the 95% confidence intervals for the correlations were −0.098 and −0.153, inconsistent with positive species-area effects. However, the spatial distribution of z-values suggests that higher turnover rates could be patterned in geographic space, with higher values in the Andes (Fig. 2). Even if this is true, since z-values are not associated with richness, we can drop them from the analysis. This allows us to improve substantially the power of the analysis of the other variables by considering the entire grid of 374 cells.
The step-forward multiple regression found that seven variables had significant partial coefficients, which in concert explained ca. 53% of the variation in species richness (Table 1). The partial regression analysis indicated that AET, which would be expected to operate over broad scales, was the most important predictor, followed by two variables expected to operate at smaller scales, climatic heterogeneity and landcover diversity. The remaining variables, although included in the model, explained very little of the variance in owl richness.
The spatial correlogram for species richness revealed a strong clinal pattern (Fig. 3), with positive spatial autocorrelation in the first distance class, generally decreasing with distance, and becoming negative in the largest distance classes (indicating higher richness in the northern half of the continent and lower richness in the south). The spatial correlogram for the residuals from the multiple regression was significant after Bonferroni correction (p<0.001), indicating a bias in the type I error rate is expected when statistically testing the partial regression coefficients. Even so, the correlogram for the residuals indicates that although some autocorrelation remains (reflecting that almost half of the variance in owl richness is not explained by the regression model), the environmental and historical predictors were able to explain the pattern across the entire continent well.
Discussion
Analyzing predictors of owl species richness based on different potential explanations at the same grain size allows us to compare simultaneously their relative magnitude in explaining variation in species richness across the continent. Our primary result is that contemporary climate, reflecting water-energy balance and spatial climatic variability at the mesoscale, as well as habitat diversity, contributes significantly to the pattern, whereas area and deep-time historical effects are weak predictors of owl richness.
Evolutionary diversity indices have been used in a conservation context (see Crozier 1997; Sechrest et al. 2002) and, more recently, by Kerr and Currie (1999) to explore historical and contemporary processes creating variation in species richness at broad spatial scales. Our results are similar to those of Kerr and Currie (1999), who found that contemporary environmental variables are much stronger predictors of the species richness of tiger beetles and three families of freshwater fish in North America. In South American owls, historical effects also appear to explain much less than current climate, at least when phylogenetic indices are used to quantify history. Of course, it is difficult to partition purely historical effects, dispersal across the continent, and the historical changes in the environment itself using only recent data and phylogenetic reconstruction of clades that include only extant species. We cannot conclude that history does not matter, only that we are unable to identify a strong signal independent of contemporary factors.
At the broad-scale analyzed in this paper, turnover rates are poorly related to species richness. However, the z-values may have a spatial pattern, being higher in the northern Andean region, as would be expected if they were associated with increased environmental heterogeneity (see Thiollay 1996; Rahbek 1997; Diniz-Filho et al. 2002; Storch et al. 2003), and much lower in the more homogeneous eastern side of the continent. Irrespective, turnover rates and scale-dependent measures, such as z-values, may be associated more with habitat changes and beta-diversity than with richness (see also Koleff and Gaston 2002).
The best single predictor of owl species richness is actual evapotranspiration, a widely recognized measure of energy-water balance. This is consistent with a study of all South American birds analyzed at the same grain size (Hawkins et al. 2003a), as well as with a large number of studies of plants and animals across the world (Hawkins et al. 2003b). But like most of these other studies, it is difficult to determine if this relationship reflects primarily the direct physiological effects of heat/cold stress and water availability on the birds themselves, or an indirect effect operating via plant production, which in turn increases resources for the small animals on which owls feed. Nevertheless, our study joins many others indicating that aspects of both contemporary energy and water inputs are important proximate determinants of broad-scale diversity patterns.
Topographic variability by itself explained very little variation in owl species richness, despite having high explanatory power for overall species richness in South American birds in the study by Rahbek and Graves (2001), at least at very coarse grain sizes. On the other hand, Hawkins et al. (2003a) found that topographic variability was not a strong predictor in South America (or anywhere else in the world), possibly because of differences in the extent and grain size of the two studies. However, we consider it likely that range in elevation is not a proxy for habitat heterogeneity as widely assumed, but rather is a measure of spatial climatic heterogeneity at the mesoscale, as argued by Turner and Hawkins (2004). In overall cold climates, elevational gradients make little difference, since it is basically cold everywhere, whereas in overall warm climates, elevation sets up a diverse range of climatic zones to which birds (and other animals) can respond (thus implicating direct climatic effects as being more important than indirect trophic level effects, see previous paragraph). Further evidence that topographical variability does not measure habitat diversity is indicated by a low association between range in elevation and remotely sensed landcover diversity (r 2=0.186). Similarly low associations between topographical variability and more direct measures of habitat diversity have been found in other studies (Rahbek and Graves 2001; Kerr et al. 2001; Hawkins and Porter 2003a). Thus, we conclude that elevation interacts strongly with regional climates, and diversity studies that encompass cold regions as well as warm ones should examine this interaction to evaluate the influence of topographic relief on diversity gradients.
We also found that landcover diversity contributes to a statistical explanation of owl richness independently of topographic relief, having virtually the same explanatory power as climatic heterogeneity, as indicated by the partial correlation coefficients. Thus, owls appear to respond not only to overall water-energy input but also to multiple measures of mesoscale heterogeneity (variability within individual cells). It is difficult to evaluate the generality of the landcover result, because although habitat diversity has sometimes been found to be associated with animal diversity at broad scales (e.g., Kerr et al. 2001 for Canadian butterflies, and Rosenweig 1995 for southeastern Australinan mammals), in other cases it has not (Hawkins and Porter 2003a for western Palearctic butterflies). Although habitat diversity is generally believed to underlie many species-area relationships, additional studies incorporating this variable are still needed to determine the general contribution of habitat heterogeneity to continental and global diversity gradients.
Finally, our paper illustrates how different mechanisms can be tested using a single framework, which can be useful for reaching more general conclusions about the determinants of species richness. Slightly more than 50% of the variation in owl species richness was explained by the combined effects of climate and their interaction with topographic variability, with perhaps a small independent contribution of historical effects measured by deep-time phylogenetic indices. Although additional studies using the standard methods we describe here are clearly necessary, our results suggest that turnover rates and scaling effects, although useful for detecting faunal changes and beta diversity, may not represent strong explanation for broad-scale patterns in species richness, especially after factoring out effects of climate and environmental heterogeneity.
References
Badgley C, Fox DL (2000) Ecological biogeography of North American mammals: species density and ecological structure in relation to environmental gradients. J Biogeogr 27:1437–1467
Crozier RH (1997) Preserving the information content of species: genetic diversity, phylogeny and conservation worth. Annu Rev Ecol Syst 28:243–268
Currie DJ (2001) Projected effects of climate change on patterns of vertebrate and tree species richness in the conterminous United States. Ecosystems 4:216–225
Currie DJ, Paquin V (1987) Large-scale biogeographical patterns of species richness of trees. Nature 329:326–327
Del Hoyo J, Elliott A, Sargatal J (1999) Handbook of the birds of the World, vol 5. Barn-owls to Hummingbirds. Lynx Edicions, Barcelona
Diniz-Filho JAF, de Sant’Ana CER, de Souza MC, Rangel TFLVB (2002) Null models and spatial patterns of species richness in South American birds of prey. Ecol Lett 5:47–55
Diniz-Filho JAF, Bini LM, Hawkins BA (2003) Spatial autocorrelation and red herrings in geographical ecology. Glob Ecol Biogeogr 12:53–64
Flather CH (1996) Fitting species accumulation functions and assessing land use impacts on avian diversity. J Biogeogr 23:155–168
Francis AP, Currie DJ (1998) Global patterns of tree species richness in moist forests: another look. Oikos 81:598–602
Gotelli N (1998) A primer of ecology. Sinauer, Sunderland, Mass.
Hawkins BA, Porter EE (2001) Area and the latitudinal diversity gradient for terrestrial birds. Ecol Lett 4:595–601
Hawkins BA, Porter EE (2003a) Relative influences of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Glob Ecol Biogeogr 12:475–481
Hawkins BA, Porter EE (2003b) Water-energy balance and the geographic pattern of species richness of western Palearctic butterflies. Ecol Entomol 28:678–686
Hawkins BA, Porter EE, Diniz-Filho JAF (2003a) Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology 84:1608–1623
Hawkins BA, Field R, Cornell HV, Currie DJ, Guégan J-F, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O’Brien EM, Porter EE, Turner JRG (2003b) Energy, water, and broad-scale geographic patterns of species richness. Ecology 84:3105–3117
Kerr JT, Currie DJ (1999) The relative importance of evolutionary and environmental controls on broad-scale patterns of species richness in North America. Ecoscience 6:329–337
Kerr JT, Southwood TRE, Cihlar J (2001) Remotely sensed habitat diversity predicts butterfly richness and community similarity in Canada. Proc Natl Acad Sci USA 98:11365–11370
Koleff P, Gaston KJ (2002) The relationship between local and regional species richness and spatial turnover. Glob Ecol Biogeogr 11:363–375
Latham RE, Ricklefs RE (1993) Global patterns of tree species richness in moist forests—energy-diversity theory does not account for variation in species richness. Oikos 67:325–333
Legendre P, Legendre L (1998) Numerical ecology, 2nd English edn. Elsevier, Amsterdam
Lyons SK, Willig MR (1999) A hemispheric assessment of scale-dependence in latitudinal gradients of species richness. Ecology 80:2483–2491
Lyons SK, Willig MR (2002) Species richness, latitude and scale-sensitivity. Ecology 83:47–58
McGlone MS (1996) When history matters: scale, time, climate and tree diversity. Glob Ecol Biogeogr Lett 5:309–314
O’Brien EM, Field R, Whittaker RJ (2000) Climatic gradients in woody plant (tree and shrub) diversity: water-energy dynamics, residual variation, and topography. Oikos 89:588–600
Rahbek C (1997) The relationship among area, elevation and regional species richness in neotropical birds. Am Nat 149:857–902
Rahbek C, Graves GR (2001) Multiscale assessment of patterns of avian species richness. Proc Natl Acad Sci USA 98:4534–4539
van Rensburg BJ, Chown SL, Gaston KJ (2002) Species richness, environmental correlates, and spatial scale: a test using South African birds. Am Nat 159:566–577
Richerson PJ, Lum K (1980) Patterns of plant species diversity in California: relation to weather and topography. Am Nat 116:504–536
Rosenzweig ML (1992) Species diversity gradients: we know more or less than we thought. J Mammal 73:715–730
Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press, Cambridge
Sechrest W, Brooks TM, Fonseca GAB, Konstant WR, Mittermeier RA, Purvis A, Ryland A, Gittleman JL (2002) Hotspots and the conservation of evolutionary history. Proc Natl Acad Sci USA 99:2067–2071
Sibley CG, Ahlquist JE (1990) Phylogeny and classification of birds. Yale University Press, New Haven
Sibley CG, Monroe BL (1990) Taxonomy and distribution of birds of the world. Yale University Press, New Haven
Storch D, Sizling AL, Gaston KJ (2003) Geometry of the species-area relationship in central European birds: testing the mechanism. J Anim Ecol 72:509–519
Thiollay JM (1996) Distributional patterns of raptors along altitudinal gradients in the northern Andes and effects of forest fragmentation. J Trop Ecol 12:533–560
Turner JRG, Hawkins BA (2004) The global diversity gradient. In: Lomolino MV, Heany LR (eds) Frontiers of biogeography: new directions in the geography of nature. Sinauer, Sunderland, Massachussets
Wartenberg D (1989) SAAP 4.3: spatial autocorrelation analysis program. Exeter Software, New York
Whittaker RJ, Willis KJ, Field R (2001) Scale and species richness: towards a general, hierarchical theory of species diversity. J Biogeogr 28:453–470
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal gradients of biodiversity: pattern, process, scale and synthesis. Annu Rev Ecol Syst 34:273–309
Wright DH (1983) Species-energy theory: an extension of species-area theory. Oikos 41:496–506
Acknowledgements
We thank Daniel Simberloff and two anonymous reviewers for suggestions that improved the paper. Our research program on macroecology, biodiversity and quantitative ecology have been continuously supported by the Conselho Nacional de Desenvolvimento Científico Tecnológico (CNPq) and by FUNAPE/UFG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diniz-Filho, J.A.F., Rangel, T.F.L.V.B. & Hawkins, B.A. A test of multiple hypotheses for the species richness gradient of South American owls. Oecologia 140, 633–638 (2004). https://doi.org/10.1007/s00442-004-1577-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1577-4