Abstract
We evaluated the effects of nutrient addition on interactions between the benthic microalgal community and a dominant herbivorous gastropod, Cerithidea californica (California horn snail), on tidal flats in Mugu Lagoon, southern California, USA. We crossed snail and nutrient (N and P) addition treatments in enclosures on two tidal flats varying from 71 to 92% sand content in a temporally replicated experiment (summer 2000, fall 2000, spring 2001). Diatom biomass increased slightly (~30%) in response to nutrient treatments but was not affected by snails. Blooms of cyanobacteria (up to 200%) and purple sulfur bacteria (up to 400%) occurred in response to nutrient enrichment, particularly in the sandier site, but only cyanobacterial biomass decreased in response to snail grazing. Snail mortality was 2–5 times higher in response to nutrient addition, especially in the sandier site, corresponding to a relative increase in cyanobacterial biomass. Nutrient-related snail mortality occurred only in the spring and summer, when the snails were most actively feeding on the microalgal community. Inactive snails in the fall showed no response to nutrient-induced cyanobacterial growths. This study demonstrated strongly negative upward cascading effects of nutrient enrichment through the food chain. The strength of this upward cascade was closely linked to sediment type and microalgal community composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Community structure is frequently influenced by complex interactions between resource availability and consumer control (Carpenter et al. 1985). In marine habitats, algal biomass and community composition are often regulated by the top-down effects of herbivory (Williams and Ruckelshaus 1993), though stimulation of primary production through a bottom-up increase in nutrients may reduce herbivore impacts (McQueen et al. 1986). This interactive relationship between nutrient supply and herbivore control can vary with primary producer community composition, consumer density, and the level of nutrient input (Hauxwell et al. 1998).
Nutrient input to coastal ecosystems from various terrestrial (e.g., runoff, groundwater) and atmospheric sources, both natural and anthropogenic, often results in higher productivity (McQueen et al. 1986; Micheli 1999; Menge 2000). Documented responses to anthropogenic increases in nutrient supply in estuaries include macroalgal and phytoplankton blooms (Valiela et al. 1997; Pinckney et al. 1999). Although less is known about benthic microalgal communities, it has been demonstrated that these assemblages, which are important components of many estuarine food webs (Sullivan and Moncreiff 1990), can increase in biomass in response to nutrients (Van Raalte et al. 1976; Pinckney et al. 1995).
Increased algal primary productivity in response to nutrient supply may have cascading effects up the food chain. These cascades can benefit higher trophic levels by increasing food supply (Posey et al. 1995; Sardá et al. 1996). However, nutrient enrichment can change primary producer community composition by favoring rapidly growing, opportunistic species such as some macroalgae (Valiela et al. 1997). Nutrient input may also have deleterious impacts on herbivores by causing shifts towards toxic species like cyanobacteria (Sommer 2001), although these negative upward effects are not well documented in estuarine habitats.
The objective of this study was to evaluate responses of an estuarine tidal flat community to nutrient enrichment. The dense benthic microalgal assemblages typical of southern California tidal flats consist mainly of diatoms and cyanobacteria (Zedler 1980), though microalgal species composition can vary among sediment types (Onuf 1987). Phototrophic bacteria assemblages, particularly purple sulfur bacteria, are commonly associated with cyanobacterial mats (Paerl et al. 1996), but their ecological role in southern California tidal flat communities is unknown. The focal herbivore in this study is the horn snail Cerithidea californica, a common epibenthic consumer that can reach densities of 1,000 m−2 (Race 1981; Armitage 2003). We predicted that benthic microalgal biomass would decrease in the presence of herbivores and increase in response to nutrient enrichment. We also expected that nutrient-induced microalgal growth would increase herbivore survivorship.
Materials and methods
This study took place at two sites within Mugu Lagoon, Naval Base Ventura County, southern California (34.06°N, 119.05°W). Both sites featured tidal flats (approx. 0.3 m above mean sea level) surrounded by stands of pickleweed (Salicornia virginica). One site (1.4 ha) was created in 1997 and was characterized by sandy sediments [91.8±0.5% sand (mean±SE) (Armitage 2003)] and will hereafter be referred to as the restored site. The second, relatively natural site was immediately adjacent to the first site and consisted of muddier sediments (71.2±2.0% sand) and will be referred to as the natural site. We conducted a four-factor experiment varying nutrient supply (± nutrients), snails (± snails), and site (natural and restored) at three time periods (summer 2000, fall 2000, spring 2001) to determine the effects of nutrient enrichment on microalgal biomass and community structure and on snail survivorship and growth and to evaluate the effects of snail herbivory on the microalgal community. We did not repeat the experiment in winter, a period of Cerithidea californica inactivity (Race 1981).
Twenty experimental units (0.5 m ×0.5 m open-topped, 30 cm tall fiberglass window screening (1.6 mm mesh) enclosures buried 2 cm in the sediment to minimize snail emigration and immigration) were installed in each site and all snails were removed from the enclosures. Enclosures were repositioned for each time period.
To allow development of the microalgal community, we established nutrient treatments 4 weeks prior to snail addition. We randomly assigned nutrient enrichment treatments (+nutrient) to half of the 20 enclosures at each site. A window-screen mesh bag containing 10 g of slow release Osmocote fertilizer (18% N, 12% P by dry weight) was secured to the center of all +nutrient enclosures and replaced after 6 weeks; empty screen bags were placed in cages with ambient nutrient (−nutrient) treatments. Biweekly additions of 2 g of granulated urea fertilizer (46% N by dry weight) supplemented the Osmocote addition. Average nutrient loading rates were 0.088 g N day−1 plot−1 and 0.024 g P day−1 plot−1 and were established based on a fertilization protocol at an adjacent salt marsh restoration project (K.E. Boyer and A.R. Armitage, unpublished data). Eight randomly located sediment cores (2 cm deep, 2.5 cm diameter) were collected from each plot at the end of the pre-experimental period (before snail addition). Sediments were dried at 60°C, sifted through a 1 mm sieve, and the homogenized samples sent to the Division of Agriculture and Natural Resources Analytical Laboratory at UC Davis for determination of TKN with a N gas analyzer, utilizing an induction furnace and thermal conductivity (Sweeney 1989).
By the end of the 4-week pre-experimental period, a dense microalgal mat was present in all enriched plots (Armitage 2003). To begin the experimental period, we randomly assigned five +nutrient and five −nutrient plots as snail addition (+snail) enclosures in each site. No snails were added to the remaining five plots of each nutrient treatment (−snail). We collected C. californica from an adjacent natural mudflat area, measured their lengths in the laboratory, and assigned them to 5 mm size classes from 5 to 35 mm (snails less than 5 mm were difficult to locate in the field and were not used). On all dates we added snails to the plots in densities (350–380 snails cage−1) and size frequency distributions that approximated natural populations (Armitage 2003). In all experiments, the snails remained in the cages for an experimental period of 8 weeks. Cages were maintained on a weekly basis by removing fouling material (mainly the green macroalga Enteromorpha intestinalis).
In the spring 2001 study, we marked snails to monitor migration rates into and out of the enclosures. Snails were cleaned with a paper towel, briefly air-dried, and marked with colored nail enamel. On a weekly basis over the experimental period, we counted the number of marked snails climbing up cage walls and outside of the enclosures and the number of unmarked snails immigrating into enclosures, and calculated the average per-plot daily rates of climbing, escape, and immigration.
To determine if the snails could detect biotic and abiotic changes created by nutrient addition and whether they moved towards or away from nutrient treatments, we established ten additional uncaged plots in each site (five per nutrient treatment per site) in spring 2001. Natural snail densities in the plots were not manipulated, but nutrient treatments were identical to those in the enclosures during both the pre-experimental and experimental periods.
Sampling protocols
To quantify total benthic microalgal biomass, we sampled benthic chlorophyll a at the end of each experimental period. We pooled three randomly located 1.5 cm diameter, 1 cm deep cores from each plot, transported them on ice in a dark cooler, and froze them at −20°C until analysis. Pigments were extracted with 90% acetone and the absorbance of the extract was determined in a Spectronic 20 Genesys spectrophotometer at 750 nm and 664 nm before and after acidification with 1 N HCl to account for phaeopigments (Lorenzen 1967).
Following qualitative observations of dark green films on the sediment surface in +nutrient plots in the first two time periods, we preserved cores (collected as above) from each plot in 6% Lugol’s solution, observed them at 200× under a light microscope, and noted the cell types present. We then quantitatively estimated the biomass of each microalgal taxonomic group using high-performance liquid chromatography at the conclusion of the spring 2001 experiment. We collected benthic microalgal samples in the same manner as the chlorophyll a cores, extracted the pigments with 100% acetone, and determined the concentrations of the major indicator pigments (fucoxanthin: diatoms, zeaxanthin: cyanobacteria, and bacteriochlorophyll a: purple sulfur bacteria) following Pinckney et al. (1999).
At the end of the 8-week experimental period, all snails were collected by sifting the top 3 cm of sediment through a 1 mm sieve (most snails were on the sediment surface). Over 70% of the snails were recovered on all dates except in summer 2000, when only 54% were recovered, but snail escape rate was similar between nutrient treatments (see below). Mortality of recovered snails was determined by the absence of an operculum, discolored tissue, or black or white bacterial films on the mouth of the shell (Byers 2000).
To evaluate natural snail movement in response to nutrient addition in the open plots, we counted the total number of snails on the sediment surface after 12 weeks of nutrient addition at the end of the experimental period.
The variances of all data were tested for homoscedasticity using the F max test and transformed if necessary to conform to the assumptions of ANOVA. Most data were analyzed with three- or four-factor ANOVA, where the factors were site, time period, nutrients, and, for pigment concentrations at the end of the experimental period, snails. Scheffe’s post hoc tests were used when there was a significant effect of time period (all other factors had only two levels, rendering post-hoc tests unnecessary). To evaluate the effects of changes in the relative abundance of each microalgal group on snail mortality, we also performed simple regressions of percent snail mortality on pigment molar ratios (Pinckney et al. 1995) for spring 2001. Climbing, escape, and immigration rates and final densities of snails in the open plots were analyzed with two-factor ANOVA, where the factors were site and nutrients.
Results
Sediment nutrients
Sediment TKN was significantly higher in the natural than the restored site, though the difference between sites was smallest in the fall (Tables 1, 2). TKN was higher in the fall than in the spring (Scheffe’s, P<0.0001) or summer (P<0.0001). TKN was similar in summer 2000 and spring 2001 (P>0.1). Nutrient addition did not affect sediment TKN.
Microalgal abundance and community structure
On all dates, a dense, dark green, laminar microalgal mat approximately 3 mm deep was present in all enriched plots after the 4 weeks of pre-experimental nutrient addition (Armitage 2003). These mats persisted throughout the experimental period. Qualitative observations of the microalgal cells indicated the presence of diatoms and filamentous, aggregate, and solitary forms of cyanobacteria. Mats in unfertilized plots were less laminate, brown in color, and appeared to have fewer cyanobacterial cells.
In each of the 8-week experimental periods, snails decreased benthic chlorophyll a concentration (Fig. 1, Table 3). The average snail-induced decrease in chlorophyll a across all time periods was from a grand mean of 441.0±26.9 (SE) mg m−2 in cages without snails to 385.5±25.3 in cages with snails. Overall, nutrients tended to increase microalgal biomass, though the significant interaction between site and nutrients suggests that the effect of nutrient addition was stronger in the restored (+nutrient 541.3±36.5, −nutrient 351.6±28.3) than in the natural site (+nutrient 399.0±33.5, −nutrient 361.0±39.1). Scheffe’s post-hoc tests indicated that the benthic chlorophyll a concentrations differed among all time periods at significance levels less than 0.01. The significant interaction between site and time period stemmed largely from similar microalgal biomass between sites in summer and fall 2000 but higher biomass in the restored than in the natural site in spring 2001.
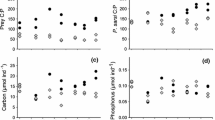
Quantification of accessory pigment concentrations in spring 2001 indicated that diatom biomass, represented by fucoxanthin, was significantly higher in the restored (55.7±5.0 mg m−2) than in the natural site (36.6±2.2; Fig. 2a, b, Table 4). There was also higher fucoxanthin biomass in nutrient addition treatments (+nutrient 52.6±5.5, −nutrient 40.5±2.8). This effect tended to be stronger in the restored site, though power is low due to small sample size. Snails did not affect diatom biomass.
Concentrations of accessory pigments in response to site, nutrient enrichment, and snail addition in spring 2001. a, b fucoxanthin (diatoms), c, d zeaxanthin (cyanobacteria), e, f bacteriochlorophyll a (purple sulfur bacteria). Significant P-values indicated (S site, N nutrients, Sn snails). Note different y -axes
Zeaxanthin concentration, an indicator of cyanobacterial biomass, was significantly higher in the restored (10.6±1.8 mg m−2) than in the natural site (4.0±0.5; Fig. 2c, d, Table 4). Zeaxanthin concentration was higher in enriched treatments (+nutrient 10.5±1.9; −nutrient 4.5±0.7). This effect tended to be stronger in the restored site, though power is low due to small sample size. Cyanobacterial biomass was lower in the presence of snails (+snails 6.0±1.5; −snails 8.7±1.6).
There was a significant interaction between site and nutrients for purple sulfur bacteria biomass, represented by bacteriochlorophyll a (Fig. 2e, f; Table 4). Bacteriochlorophyll a increased in response to nutrient addition, but only in the restored site. Snails did not affect purple sulfur bacteria biomass.
Upward cascading effects
There was a significant interaction between time period and nutrients for snail mortality, as nutrients dramatically increased snail mortality, but only in the spring and summer (Fig. 3, Table 5). In both sites, nutrient-related mortality was lower in the fall (<5% mortality) than in the summer (Scheffe’s, P=0.0007) or the spring (P =0.0001), but mortality was similar between spring and summer (P>0.1). Snail mortality tended to be higher in the restored site, but the significant interaction between site and time period suggested that the highest mortality rates in the natural site occurred in the spring and in the restored site mortality was high in both the spring and summer.
In simple linear regressions (not shown), percent snail mortality exhibited significant positive relationships to the cyanobacteria: diatom (r 2=0.334, P=0.0095) and the cyanobacteria: purple sulfur bacteria molar ratios (r 2=0.221, P=0.0421), suggesting that snail mortality increased as cyanobacteria became more common components of the microalgal community.
Final snail densities in the open plots were lower in the restored (218.8±32.2 m−2 (grand mean ±SE) than in the natural site (556.0±23.6; ANOVA, df=1, F=70.31, P<0.0001, power =1.00). Snail densities were not affected by nutrient treatment (−nutrients 363.6±66.6, +nutrients 411.2±57.9), with no significant interactions (all P>0.1, all power <0.2).
Monitoring of snail movements in the spring demonstrated that the average per-plot daily rate of the number of snails immigrating into cages (ANOVA, df=1, F=13.70, P=0.0021, power =0.95), escaping from cages (df =1, F=20.46, P=0.0005, power =0.99), and climbing cage walls (df =1, F=37.96, P<0.0001, power =1.00) was higher in the restored than in the natural site (Table 6). There was a trend of fewer snails immigrating into +nutrient enclosures (df =1, F=3.93, P=0.0661, power =0.45). Nutrient enrichment did not affect the number of escaping or climbing snails, and there were no significant interactions between factors for any snail behavior (all P>0.1, all power <0.3).
Discussion
Our study demonstrated strong effects of nutrient enrichment and weaker effects of grazing on microalgal biomass and community composition. Nutrient enrichment often stimulates primary production (e.g., Menge 1992), but upward cascading effects of nutrient addition on the biomass of higher trophic levels are variable. Meiofauna, gastropods, and bivalves have exhibited increases in biomass in response to the enhanced food quality that results from nutrient addition in freshwater wetlands (Hann et al. 2001) and lakes (Clarke et al. 1997). In contrast, a meta-analysis of food webs in pelagic habitats revealed weak linkages between nutrient loading and upper trophic levels due to complexity within trophic levels and the advection of nutrients and organisms from open marine systems (Micheli 1999). In addition, in habitats where microalgal food is abundant, such as tidal creeks, benthic consumer groups may show little response to enrichment (Wiltse et al. 1984; Posey et al. 2002), although Sardá et al. (1996) did document a long-term increase in macrofaunal biomass in tidal creeks following 15 years of nutrient addition.
The mechanism and nature of the strong upward cascading effects that we observed were different than in previous studies—the bottom-up effects were negative and were attributable more to the growth of cyanobacteria rather than to an overall increase in primary productivity. Similar nutrient-induced cyanobacterial blooms have been widely documented in phytoplankton communities and frequently contribute to deleterious conditions such as anoxia or toxicity (Paerl 1996). Harmful algal blooms, of which cyanobacteria are sometimes components, may have negative implications for upper trophic levels, as cyanobacteria are known to contain compounds toxic to consumers (Fulton and Paerl 1987; Ferrão-Filho et al. 2000). However, the upward cascading effects of cyanobacterial blooms are largely unknown in benthic estuarine habitats.
The increase in snail mortality that we observed was likely caused by consumption of nutrient-induced cyanobacterial blooms. Snail mortality was higher when cyanobacteria comprised a larger relative portion of the microalgal community. Purple sulfur bacteria closely associated with the cyanobacterial mats may have also contributed to snail mortality, although they are not generally considered to be toxic (Decho and Castenholz 1986). C. californica ingest a fluid slurry of diatoms and other surficial material (Whitlatch and Obrebski 1980), suggesting a generalized diet limited only by the size of the microalgal particles. Thus, when cyanobacteria were abundant, they probably composed a large portion of the snails’ diet. In addition, cyanobacteria was the only microalgal group that decreased in biomass in the presence of snails, a strong indication of snail consumption. The snails probably consumed diatoms as well, but higher diatom growth rates (Sommer 1997) may have masked the effects of herbivory. Alternatively, diatom migration downwards into the complex cyanobacterial mats may have provided diatoms with a refuge from herbivory (Pinckney et al. 1994; Geddes and Trexler 2003), augmenting snail consumption of cyanobacteria. Furthermore, in the fall, when the snails were less active (Race 1981) and thus consuming less microalgal biomass, mortality was much lower, despite qualitatively observed intense cyanobacterial blooms. This suggests that nutrient-related snail mortality was primarily tied to their diet rather than an abiotic side effect of nutrient addition.
C. californica consumption of a toxic food source and the absence of any tendency to avoid the cyanobacterial mats, as suggested by the lack of a snail movement response away from nutrient addition treatments in the uncaged plots and similar escape and climbing rates across nutrient treatments, present apparent contradictions of the principles of natural selection. In some cases, grazers may accept cyanobacteria as a food source despite their non-nutritive or even toxic properties because of the high nitrogen content of the cells, particularly of diazotrophic species (O’Neil 1999). This may have been particularly relevant in the nitrogen-poor sediments in the restored site. Closer evaluation of the cyanobacterial community may further account for these paradoxes. Cyanobacteria are a normal part of C. californica diet (Javor and Castenholz 1984; Page 1997), but a nutrient-induced shift in cyanobacterial species composition (Kuffner and Paul 2001; Thacker et al. 2001) may have increased the abundance of toxic or non-nutritive species and caused snail mortality. The snails may have consumed the cyanobacteria despite their toxic properties because they were not able to detect this anthropogenically influenced species-level shift within the cyanobacterial assemblage or were forced to consume it due to their confinement within the cages. Other possibilities include competitive exclusion by the invasive and competitively dominant mud snail Batillaria attramentaria (Byers 2000) that forced C. californica to consume less desirable food sources. However, B. attramentaria seldom occur as far south as Mugu Lagoon, suggesting that this was not an important interaction in our study. Alternatively, C. californica in southern California are heavily infected with trematode parasites (Lafferty 1993). Parasitism has been known to cause hosts to exhibit deleterious behaviors that contradict natural selection principles (Lafferty and Morris 1996), but this is an unlikely mechanism in our study because infected C. californica generally exhibit normal feeding behaviors (Lafferty 1993).
Although diet was probably an important cause of snail mortality, there are some possible abiotic consequences of nutrient addition that may have contributed as well. Anoxic conditions are commonly associated with eutrophication, especially in lakes or other stratified bodies of water (Childs et al. 2002). C. californica is sensitive to anoxia (Byers 2000), but the sediments in our study were highly anoxic across treatments and sites (Armitage 2003). Alternatively, the rapid nutrient-induced development of a dense, laminar cyanobacterial mat may have physically inhibited C. californica grazing by preventing the disruption and subsequent ingestion of the microalgal mat (Javor and Castenholz 1984) and by providing a barrier that prevented consumption of diatoms lower down in the mat structure (Pinckney et al. 1994; Geddes and Trexler 2003). However, C. californica exhibited the capacity to graze these cyanobacterial mats in both ambient and enriched nutrient treatments, as demonstrated by a decrease in zeaxanthin concentration in the presence of snails.
Nutrient enrichment strongly influenced the composition of our primary producer community, corroborating reports of increases in cyanobacterial biomass and shifts in microalgal community composition in response to nutrient addition in habitats ranging from coral reefs (Miller et al. 1999) to marine microbial mats (Pinckney et al. 1995). High nutrient uptake rates in cyanobacteria may give them an advantage in nutrient-replete conditions over algal groups that are better competitors in low nutrient environments (Fong et al. 1993). Another potential mechanism for this shift is that as N or P increases in supply, Si may subsequently become limiting for diatoms (Carrick and Lowe 1988), although this seems an unlikely mechanism on sandy substrates. Alternatively, cyanobacteria often produce allelopathic compounds, which may inhibit diatom growth or production (Keating 1978). In addition, those cyanobacteria that fix nitrogen may bloom in response to phosphorus additions (Pinckney et al. 1995). Although many of the cyanobacterial genera that we encountered were probably nonheterocystous (Zedler 1980), some nonheterocystous cyanobacteria can fix nitrogen by temporally and spatially separating the fixation and photosynthetic processes (Berman-Frank et al. 2001). Markedly different responses of each microalgal group to nutrient enrichment suggest that the tendency to consider the microphytobenthos as a single unit can potentially obscure the ecologically important roles of each group (Sullivan and Currin 2000). This further underscores the importance of understanding the complexity within trophic levels in evaluating the strength and nature of trophic relationships in natural systems (Micheli 1999).
Very little is known about the ecological roles of purple sulfur bacteria or their responses to eutrophication, particularly in tidal wetlands on the west coast of the USA, and our study showed that they are important components of the microphytobenthos. It has been established that phototrophic bacteria provide an important source of carbon to food webs in lakes (Overmann et al. 1999), and they are likely critical components of microbial-based food webs in coastal wetlands as well (Decho and Castenholz 1986). In addition, mats of phototrophic bacteria often form in highly eutrophic regions and may be useful indicators of nutrient input (Meyer-Reil and Köster 2000).
Although negative upward cascading effects of nutrient addition were observed in both sites, many of the interactions between site and treatment effects stemmed from between-site differences in cyanobacterial and purple sulfur bacterial biomass. Diatom, cyanobacterial, and purple sulfur bacterial biomass estimates in the natural site were similar to those in marshes on the east and Gulf coasts of USA (Pinckney et al. 1995; Goldfinch and Carman 2000), but our restored site had markedly higher cyanobacterial and purple sulfur bacterial biomass, possibly due to differences in sediment grain size structure. Onuf (1987) had previously observed higher cyanobacterial abundance on muddy than on sandy sediments at Mugu Lagoon, but Currin et al. (1996) suggest that the relatively stable nature of coarse grained sediments like those in our restored site may actually facilitate cyanobacterial survival, preventing resuspension and providing a suitable substrate for their movement. In addition, lower vegetation cover in restored marshes favors the growth of microalgal mats (Piehler et al. 1998). In one of the few studies that evaluated microalgal community composition in restored salt marshes, cyanobacterial mats dominated on compacted, drier sediments (Underwood 1997). This distribution might have been partly due to the limited mobility of cyanobacterial filaments and the subsequent inability of cyanobacteria to flourish on muddy sediments that are easily resuspended by tidal currents or bioturbation. These more muddy sediments are similar to those in our natural mudflat, where cyanobacterial biomass was lower.
Different responses to nutrient enrichment in our natural and restored sites demonstrated the close links between bottom-up trophic processes in benthic habitats and abiotic characteristics such as grain size. It is highly likely that the sandy sediments in the restored site facilitated cyanobacterial colonization and growth. In addition, the strong fertilization response in the restored site may have been augmented by the low background availability of nitrogen in the sandy sediments. The community-level effects of the use of fertilizer to aid vascular plant establishment in created wetlands (Zedler 1996) should be carefully evaluated on a case-by-case basis, as dramatic shifts in microalgal community structure in response to nutrient enrichment, especially in nutrient-poor sandy sediments, may have negative cascading effects up the food chain.
References
Armitage AR (2003) Community structure and trophic interactions in restored and natural estuarine mudflats: complex trophic cascades and positive and negative effects of nutrients. Ph.D. thesis. University of California, Los Angeles
Berman-Frank I, et al (2001) Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294:1534–1537
Byers JE (2000) Differential susceptibility to hypoxia aids estuarine invasion. Mar Ecol Prog Ser 203:123–132
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35:634–639
Carrick HJ, Lowe RL (1988) Response of Lake Michigan benthic algae to in situ enrichment with silicon, nitrogen, and phosphorus. Can J Fish Aquat Sci 45:271–279
Childs CR, Rabalais NN, Turner RE, Proctor LM (2002) Sediment denitrification in the Gulf of Mexico zone of hypoxia. Mar Ecol Prog Ser 240:285–290
Clarke KD, Knoechel R, Ryan PM (1997) Influence of trophic role and life-cycle duration on timing and magnitude of benthic macroinvertebrate response to whole-lake enrichment. Can J Fish Aquat Sci 54:89–95
Currin CA, Joye SB, Paerl HW (1996) Diel rates of N2-fixation and denitrification in a transplanted Spartina alterniflora marsh: implications for N-flux dynamics. Estuarine Coastal Shelf Sci 42:597–616
Decho AW, Castenholz RW (1986) Spatial patterns and feeding of meiobenthic harpacticoid copepods in relation to resident microbial flora. Hydrobiologia 131:87–96
Ferrão-Filho AS, Azevedo SMFO, DeMott WR (2000) Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshwater Biol 45:1–19
Fong P, Donohoe RM, Zedler JB (1993) Competition with macroalgae and benthic cyanobacterial mats limits phytoplankton abundance in experimental microcosms. Mar Ecol Prog Ser 100:97–102
Fulton RS III, Paerl HW (1987) Effects of colonial morphology on zooplankton utilization of algal resources during blue-green algal (Microcystis aeruginosa) blooms. Limnol Oceanogr 32:634–644
Geddes P, Trexler JC (2003) Uncoupling of omnivore-mediated positive and negative effects on periphyton mats. Oecologia 136:585–595
Goldfinch AC, Carman KR (2000) Chironomid grazing on benthic microalgae in a Louisiana salt marsh. Estuaries 23:536–547
Hann BJ, Mundy CJ, Goldsborough LG (2001) Snail-periphyton interactions in a prairie lacustrine wetland. Hydrobiology 457:167–175
Hauxwell J, McClelland J, Behr PJ, Valiela I (1998) Relative importance of grazing and nutrient controls of macroalgal biomass in three temperate shallow estuaries. Estuaries 21:347–360
Javor BJ, Castenholz RW (1984) Invertebrate grazers of microbial mats, Laguna Guerrero Negro, Mexico. In: Cohen Y, Castenholz RW, Halvorson HO (eds) Microbial mats: stromatolites, vol 3. Alan R. Liss, New York, pp 85–94
Keating KI (1978) Blue-green algal inhibition of diatom growth transition from mesotrophic to eutrophic community structure. Science 199:971–973
Kuffner IB, Paul VJ (2001) Effects of nitrate, phosphate and iron on the growth of macroalgae and benthic cyanobacteria from Cocos Lagoon, Guam. Mar Ecol Prog Ser 222:63–72
Lafferty KD (1993) Effects of parasitic castration on growth, reproduction and population dynamics of the marine snail Cerithidea californica. Mar Ecol Prog Ser 96:229–237
Lafferty KD, Morris AK (1996) Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77:1390–1397
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
McQueen DJ, Post JR, Mills EL (1986) Trophic relationships in freshwater pelagic ecosystems. Can J Fish Aquat Sci 43:1571–1581
Menge BA (1992) Community regulation: under what conditions are bottom-up factors important on rocky shores? Ecology 73:755–765
Menge BA (2000) Top-down and bottom-up community regulation in marine rocky intertidal habitats. J Exp Mar Biol Ecol 250:257–289
Meyer-Reil L-A, Köster M (2000) Eutrophication of marine waters: effects on benthic microbial communities. Mar Pollut Bull 41:255–263
Micheli F (1999) Eutrophication, fisheries, and consumer-resource dynamics in marine pelagic ecosystems. Science 285:1396–1398
Miller MW, Hay ME, Miller SL, Malone D, Sotka EE, Szmant AM (1999) Effects of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnol Oceanogr 44:1847–1861
O’Neil JM (1999) Grazer interactions with nitrogen-fixing marine cyanobacteria: adaptation for N-acquisition? Bull Inst Oceanogr 19:293–317
Onuf CP (1987) The ecology of Mugu Lagoon, California: an estuarine profile. 85 (7.15). U.S. Fish and Wildlife Service, Washington, D.C.
Overmann J, Hall KJ, Northcote TG, Ebenhöh W, Chapman MA, Beatty T (1999) Structure of the aerobic food chain in a meromictic lake dominated by purple sulfur bacteria. Arch Hydrobiol 144:127–156
Paerl HW (1996) A comparison of cyanobacterial bloom dynamics in freshwater, estuarine and marine environments. Phycologia 35:25–35
Paerl HW, Fitzpatrick M, Bebout BM (1996) Seasonal nitrogen fixation dynamics in a marine microbial mat: potential roles of cyanobacteria and microheterotrophs. Limnol Oceanogr 41:419–427
Page HM (1997) Importance of vascular plant and algal production to macro-invertebrate consumers in a southern California salt marsh. Estuarine Coastal Shelf Sci 45:823–834
Piehler MF, Currin CA, Cassanova R, Paerl HW (1998) Development and N2-fixing activity of the benthic microbial community in transplanted Spartina alterniflora marshes in North Carolina. Restor Ecol 6:290–296
Pinckney J, Piceno Y, Lovell CR (1994) Short-term changes in the vertical distribution of benthic microalgal biomass in intertidal muddy sediments. Diatom Res 9:143–153
Pinckney J, Paerl HW, Fitzpatrick M (1995) Impacts of seasonality and nutrients on microbial mat community structure and function. Mar Ecol Prog Ser 123:207–216
Pinckney JL, Paerl HW, Harrington MB (1999) Responses of the phytoplankton community growth rate to nutrient pulses in variable estuarine environments. J Phycol 35:1455–1463
Posey M, Powell C, Cahoon L, Lindquist D (1995) Top down vs. bottom up control of benthic community composition on an intertidal tideflat. J Exp Mar Biol Ecol 185:19–31
Posey MH, Alphin TD, Cahoon LB, Lindquist DG, Mallin MA, Nevers MB (2002) Top-down versus bottom-up limitation in benthic infaunal communities: direct and indirect effects. Estuaries 25:999–1014
Race MS (1981) Field ecology and natural history of Cerithidea californica (Gastropoda: Prosobranchia) in San Francisco Bay. Veliger 24:18–27
Sardá R, Valiela I, Foreman K (1996) Decadal shifts in a salt marsh macroinfaunal community in response to sustained long-term experimental nutrient enrichment. J Exp Mar Biol Ecol 205:63–81
Sommer U (1997) Selectivity of Idothea chelipes (Crustacea: Isopoda) grazing on benthic microlagae. Limnol Oceanogr 42:1622–1628
Sommer U (2001) Reversal of density dependence of juvenile Littorina littorea (Gastropoda) growth in response to periphyton nutrient status. J Sea Res 45:95–103
Sullivan MJ, Currin CA (2000) Community structure and functional dynamics of benthic microalgae in salt marshes. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer Academic, Dordrecht, The Netherlands, pp 81–106
Sullivan MJ, Moncreiff CA (1990) Edaphic algae are an important component of salt marsh food-webs: evidence from multiple stable isotope analyses. Mar Ecol Prog Ser 62:149–160
Sweeney RA (1989) Generic combustion method for determination of crude protein in feeds: collaborative study. J Assoc Off Anal Chem 72:770–774
Thacker RW, Ginsburg DW, Paul VJ (2001) Effects of herbivore exclusion and nutrient enrichment on coral reef macroalgae and cyanobacteria. Coral Reefs 19:318–329
Underwood GJC (1997) Microalgal colonization in a saltmarsh restoration scheme. Estuarine Coastal Shelf Sci 44:471–481
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118
Van Raalte CD, Valiela I, Teal JM (1976) Production of epibenthic salt marsh algae: light and nutrient limitation. Limnol Oceanogr 21:862–872
Whitlatch RB, Obrebski S (1980) Feeding selectivity and coexistence in two deposit feeding gastropods. Mar Biol 58:219–226
Williams SL, Ruckelshaus MH (1993) Effects of nitrogen availability and herbivory on eelgrass (Zostera marina) and epiphytes. Ecology 74:904–918
Wiltse WI, Foreman KH, Teal JT, Valiela I (1984) Effects of predators and food resources on the macrobenthos of salt marsh creeks. J Mar Res 42:923–942
Zedler JB (1980) Algal mat productivity: comparisons in a salt marsh. Estuaries 3:122–131
Zedler JB (1996) Coastal mitigation in southern California: the need for a regional restoration strategy. Ecol Appl 6:84–93
Acknowledgements
We thank Thomas Keeney and the US Navy for providing access to the research site and Brian Dolan and numerous others for field and laboratory assistance. We are indebted to James L. Pinckney and Alyce R. Lee at Texas A&M University for their generosity and time in the use of J. Pinckney’s HPLC apparatus and pigment standards. Many thanks to Richard R. Vance, Richard R. Ambrose, and two anonymous reviewers for helpful comments on an earlier version of the manuscript. This project was funded in part by a UC Coastal Environmental Quality Initiative Graduate Fellowship to A.A. and a grant from the EPA (#R827637) to P.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armitage, A.R., Fong, P. Upward cascading effects of nutrients: shifts in a benthic microalgal community and a negative herbivore response. Oecologia 139, 560–567 (2004). https://doi.org/10.1007/s00442-004-1530-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1530-6