Abstract
The fitness of non-feeding adult insects depends on energy accumulated during the larval stage. Larvae of the caddisfly Asynarchus nigriculus primarily feed on plant detritus, but supplement their diet with animal material obtained through cannibalism. Habitat drying constrains development in many populations of this species, and we hypothesized that cannibalism should accelerate development to facilitate timely metamorphosis. We manipulated larval diets in a field experiment by supplementing detritus with animal material, and in a laboratory experiment by varying animal material and detritus quality (conditioned vs unconditioned). We measured the effects of dietary manipulation on larval and pupal growth and development, the timing of metamorphosis, and adult fitness correlates. The results of the laboratory experiment suggest that this species can metamorphose with a detritus-only diet, but development is extremely protracted. In the field experiment, individuals with animal material in their diet had higher larval survival, shorter larval and pupal development times, and earlier emergence dates (7–10 days), than those without a supplement. This delay in emergence should have important effects on survival in natural populations where the difference between desiccation and successful emergence can be only a few days. Dietary supplementation also affected adult body mass (30–40% increase), female fecundity (30% more eggs), and proportional allocation to different adult body parts. Our results are consistent with recent growth-development models that predict coupled (earlier emergence and larger adults) rather than tradeoff responses (earlier emergence and smaller adults) to pre-threshold manipulation of larval diets. Many detritivorous aquatic insects supplement their diets with animal material, and our data provide evidence that this supplementation can have strong effects on fitness. This type of dietary supplementation should be especially important for taxa that do not feed as adults, and in temporary habitats that impose time constraints on larval development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannibalism has evolved in nearly all animal taxa and has important consequences for the behavioral, population, and community ecology of many species (Fox 1975; Polis 1981; Elgar and Crespi 1992). It is especially common among disparately sized individuals in size/stage-structured populations of predatory fish, amphibians, and invertebrates (e.g., arachnids, crustaceans, insects, gastropods; Polis 1988; Van Buskirk 1992; Dong and Polis 1992; Wahlström et al. 2000). In this context, cannibalism should evolve when the benefits of direct nutritional gain and elimination of a potential competitor exceed costs associated with risk of injury, parasite/disease transmission, and predation on kin (Polis 1988; Pfennig 1997; Pfennig et al. 1998). Not surprisingly, cannibalism rarely occurs between individuals of the same size, presumably because of costs associated with retaliation and/or foraging tradeoffs (e.g., long handling times; Dong and Polis 1992; Hopper et al. 1996).
Cannibalism occurs among nearly all of the predatory taxa that inhabit subalpine wetlands (e.g., beetles, water bugs, copepods, dragonflies, salamanders; Wissinger 1999b). It is especially conspicuous among the mainly detritivorous larvae of the caddisfly Asynarchus nigriculus Banks (Limnephilidae; Wissinger et al. 1996). Cannibalism in Asynarchus differs from that observed in most populations because it is a result of mobbing behavior among same-sized larvae in synchronously developing cohorts (Wissinger et al. 2003). Encounters between larvae are often agonistic, but one-on-one cannibalism is rare. However, if an individual is injured, a mob (5–15) of conspecifics attacks and devours the injured animal (Wissinger et al. 1996). Mob cannibalism in Asynarchus has at least two costs—attackers occasionally become secondary victims, and the activity of mobs attracts salamander predators that prevent this species from exploiting permanent habitats (Wissinger et al. 1999a, 2003). The purpose of this research was to investigate benefits that might explain the evolution/maintenance of cannibalism among Asynarchus larvae by studying the effects of larval nutrition on development rate, survival, and adult traits that correlate with fitness.
One hypothesis for cannibalism in this species is that it provides a high-nutrient dietary supplement that is necessary for completing development. Detritus dominates larval diets: 85–95% volume is spruce needles, bark and emergent vascular plants (Sparks 1993). Moreover, many Asynarchus habitats are oligotrophic (total N <1 µeq/l; soluble P <0.01 µeq/l; Wissinger et al. 1999b). Thus, the stochiometric nutrient content (i.e., high C:N and C:P; see Frost and Elser 2002) of this detritus is likely to be nutritionally incomplete, even after microbial colonization (Anderson and Cargill 1987; Pritchard and Berté 1987; Jacobson and Sand-Jensen 1994; Bowen et al. 1995). A second hypothesis is that cannibalism facilitates metamorphosis in the face of developmental time constraints imposed by habitat drying (sensu Ludwig and Rowe 1990; Rowe and Ludwig 1991). Most Asynarchus populations occur in temporary wetlands, and like other caddisflies, this species exhibits a suite of life history adaptations (ovarian diapause, terrestrial egg deposition, rapid development) for exploiting such habitats (Wissinger et al. 2003). Larval development in Asynarchus is remarkably rapid, and it is the only caddisfly at our study sites that emerges before extremely ephemeral temporary habitats dry. Cannibalism is especially noticeably during the latter stages of drying, and behavioral data from 32 populations of Asynarchus indicate that aggression increases as habitat duration decreases (E. Bilger and S. Wissinger, unpublished data). Our two hypotheses are not mutually exclusive, i.e., both the oligotrophic status of subalpine wetlands and time-constraints on development could contribute to the need for a high-nutrient dietary supplement.
In this paper we present the results of experiments that document the effects of diet on the survival, growth, and development of Asynarchus. Because adults do not feed, all of the nutrients available for the adult soma and reproduction are obtained before pupation. Thus, we predicted that a nutrient supplement during the larval stage should not only reduce development time, but also affect adult fitness correlates such as body size and fecundity. Understanding how events during the larval stage affect adult fitness is prerequisite for testing theory related to growth-development tradeoffs and the allocation of resources in species with complex life cycles (Nijhout and Emlen 1998; Nylin and Gotthard 1998; Day and Rowe 2002). Although the effects of larval nutrition on adult fitness have been studied in terrestrial insects (Boggs 1997), there are few such data for aquatic insects (Richardson and Baker 1997; Peckarsky et al. 2002).
Materials and methods
Field experiment
The experiment was conducted in cages placed in a temporary wetland at the Mexican Cut Nature Reserve (elevation 3,560 m) near the Rocky Mountain Biological Laboratory in central Colorado (see Wissinger et al. 1999b for habitat description). We had long-term data on the phenology of larval development (five instars), pupation, and emergence of Asynarchus in this habitat, and the relatively late drying date allowed time to follow development through to adult emergence (Wissinger et al. 2003). By working on natural substrates (vs assembling microcosms), we could be assured of natural quantities and quality of detritus, as well as natural fluctuations in temperature, photoperiod, and other factors (e.g., dissolved oxygen) that could affect development.
The experiment was designed as a randomized complete block to account for variation associated with positional effects in the pond. Five blocks of four cages (0.25-m2 surface area; 0.5-m height; 1.5-mm screen mesh on wood frames) were placed along the edge of the pond at locations with comparable depth, substrate, and slope. Survival and growth rates in these experimental enclosures were similar to those in open populations in a previous study, suggesting minimal cage effects (Wissinger et al. 1996). The bottom edges of the cages were pressed through the organic substrates to a clay mineral layer and sealed with excess clay from around the cages. Average water depth in the cages was 26.3±4.3 cm.
The four treatments (ambient, reduced, and enhanced animal material; cage control) were assigned randomly to cages within blocks. Cage controls (no caddisflies) were used to assess invertebrate abundance in the absence of caddisfly manipulations and to monitor the permeability of the cages to caddisfly immigration. There was no immigration during the experiment. Before caddisflies were added to the cages, detritus and invertebrates were removed from each cage with a D-frame aquatic net, and invertebrates were identified and counted. Detritus and invertebrates were returned to the cage controls. In the “reduced” treatments, invertebrates were removed before detritus was returned to the cages. In the “ambient” and “enhanced ” treatments, detritus and non-caddisfly invertebrates (excluding larvae of the beetle Dytiscus dauricus, a predator on caddisflies) were returned to each cage as potential animal prey. In the enhanced treatment, we supplemented the animal material in each cage weekly with three 1-cm3blocks (172.3±26.8 mg) of freeze-dried Tubifex worms (Wardley freeze dried Tubifex: minimum crude protein 50%, crude fat 8%). In a pilot study, we found that the Tubifex could be dispersed on the substrate to prevent unnatural clumping of caddisfly larvae. The experiment was initiated on 25 June by adding 50 third instar Asynarchus larvae to each cage. Larvae were selected randomly from a pool of 1,000 animals from the pond (mean larval dry wt =0.75 mg ±0.03 SE; n =10). Densities were based on historical data and were the same as those in previous experiments (Wissinger et al. 1996).
During the first three weeks of the experiment, we conducted behavioral observations following a protocol used previously to assess larval aggression (Wissinger et al. 1996). In 10-min trials conducted between 1000–1200 hours, we observed the number of encounters among larvae and the proportion that escalated into aggression (foreleg wrestling, case shaking, biting). Three observers worked simultaneously on each treatment within a block; thus, replicates were not sequential and time of day should not have biased comparisons among treatments. After each trial, we looked for evidence of pupation, and following its discovery, checked cages daily for new pupae. Each pupa was transferred to a round plastic microcosm (100.3 cm2; 500 ml pond water; natural detritus) with an overhead emergence funnel located in a portable field laboratory near the ponds. Emergence chambers were floated in a water bath with diel temperature fluctuations comparable to those in the ponds. We recorded time to pupation and emergence for each individual and froze adults for subsequent analysis. To test for cage artifacts, we monitored emergence of free-living Asynarchus with emergence traps along the shoreline of the pond. After the last pupae were removed from the field enclosures, invertebrates were censused using a D-frame net to determine if the initial prey reduction in the reduced treatment was maintained during the experiment, and to test for treatment effects (ambient, enhanced, cage control) on invertebrate abundance. Samples were dried at 50°C for 48 h and weighed on a Cahn C-31 microbalance.
Eggs were dissected and counted under a Wild-Heerbrugg M5 dissecting microscope, and body and egg masses were measured on the Cahn C-31 microbalance to the nearest 0.1 μg. Wing and leg length are correlated with male mating success in some caddisflies (e.g., Petersson 1996), thus we measured wing (metathoracic) and leg (mesofemur) length to the nearest 0.1 mm on uninjured animals with intact parts (343/355 emerging adults).
All statistical analyses were conducted with SAS Statview (SAS 1999). We used MANOVA to investigate effects of treatment on survival, time to emergence, and body mass. Significant MANOVA effects were explored with two-way (treatment × sex) and one-way protected ANOVA and Scheffé‘s a posteriori contrasts to identify which treatments differed (after Day and Quinn 1989; Scheiner 1993). We analyzed data on larval aggression on three dates with repeated-measures ANOVA. Initially, we explored the effects of blocks on the analyses. None were found for survival, larval behavior, and body size ratios (e.g., egg mass/body mass). Block effects were significant for responses related to development time (dates to pupation and emergence) and body size (mass, wing length). However, there were no interactions between treatment and block effects; thus, all ANOVA results are from models in which variances are partitioned among treatment, block, and error terms without block × treatment interactions (following Potvin 1993).
Laboratory experiment
Pilot studies revealed that development rates of individually held larvae in laboratory microcosms were markedly slower than in the pond (hence, the decision to conduct the primary experiment in the field). However, we wanted to determine the effects of larval diet on growth, development, and survival in the absence of cannibalism, and thus conducted a second experiment with isolated individuals in a portable field laboratory near the pond. We used a two × two factorial design with high and low quality detritus (unboiled and boiled for 20 min to reduce the microbial flora), and animal material supplement absent or present (10 mg dry wt Tubifex added weekly). This design enabled us to assess starvation levels under extremely low food resources (boiled detritus and no Tubifex), and to study the interactive effects of a dietary supplement and detritus quality. We initiated the experiment 1 day after the field experiment (26 June) by adding a third-instar Asynarchus larva (from the same pool of animals used for the field experiment) to each of 32 small, round microcosms (base of the emergence chambers described above) that had been randomly assigned to the four treatments (n =8). Each microcosm contained 100 mg (matted wet weight) detritus (mainly spruce bark and needles from the pond in which we conducted the field experiment), and 500 ml filtered pond water. Detritus in all treatments was replaced weekly. After the first larva pupated, we added emergence funnels to each chamber and began checking daily for pupae and adults. We used two-way ANOVA to analyze the effects of diet on time to pupation, but could not assess the effects on adult fitness correlates because many animals did not complete development.
Results
Field experiment
MANOVA and subsequent ANOVAs indicated that larval diet affected the three main response variables: survival to adulthood, time of adult emergence, and adult body mass (Tables 1, 2). Responses differed between males and females, but there was no interaction between the effects of sex and diet treatment. Sex-specific differences in development time and body weight were nearly identical to those observed in natural populations (Wissinger et al. 2003), i.e., Asynarchus females were heavier and emerged a few days later on average than males.
Larval survival and behavior
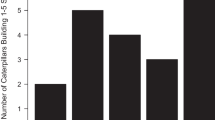
The number of larvae that emerged differed among treatments (one-way ANOVA F 2,8=15.2, P =0.002; Fig. 1). Few animals died during pupation, thus the effect on survival to emergence (Table 2) was mainly due to differences in larval mortality. More larvae emerged from the enhanced treatment (Scheffé contrast P <0.05) than from the ambient and reduced treatments, which did not differ (Scheffé contrast P >0.05; Fig. 1).
The percentage of encounters that involved agnostic behaviors during the first 3 weeks differed among treatments (Fig. 2; repeated measures ANOVA F 2,24=38.6, P <0.001). There was an interaction between treatment and date of observation because treatments differed only during week 3 (F 4,24=4.6, P =0.007), when aggressive encounters were lower in the enhanced (Scheffé contrast P >0.05) than in the reduced and ambient treatments (protected one-way ANOVA F 2,12=24.9, P =0.001). The significant date by treatment interaction was due to reduced aggression in the enhanced treatment during weeks 2 and 3 (Fig. 2).
Effects of larval diet on caddisfly aggression. Values are mean (n =5) percentage (± SE) of encounters with at least one agonistic interaction (biting, foreleg wrestling, case shaking). Asterisks indicate significant differences among treatments based on Scheffé contrasts (P >0.05) on one-way ANOVA for each date
At the conclusion of the experiment, the abundance of invertebrates (mg dry mass m-2) in the reduced (invertebrates removed) treatment (94.4±67.7 SD) was much lower (Scheffé contrast P <0.05) than in the treatments in which invertebrates were not removed (treatment effect ANOVA F 3,12=32.45, P <0.001). Invertebrate abundance did not differ among ambient (608.6±231.4 mg dry weight), enhanced (727.6±242.1 mg dry weight), and cage control (613.2±191.3 mg dry weight) treatments (all Scheffé contrasts P >0.05).
Time to pupation and emergence
Total time for larval and pupal development differed among treatments (Table 2). Time to emergence was significantly shorter in the enhanced treatment (Scheffé contrast P <0.05) than in the ambient and reduced treatments, which did not differ (Scheffé contrast P >0.05; Fig. 3). This overall effect included significant treatment differences in larval (days before pupation; protected one-way ANOVA F 2,8=10.5, P =0.006) and pupal development (days of pupation; protected one-way ANOVA F 2,8=9.4, P <0.008). In both cases, development was faster in the enhanced treatment than in the reduced and ambient treatments (Scheffé contrast P <0.05).
Adult body mass, wing length, and leg length
Larval diet had a significant effect on adult body mass (Table 2, Fig. 4). Adults emerging from the enhanced treatment were heavier (Scheffé contrast P <0.05) than those emerging from the ambient and reduced treatments, which did not differ (Scheffé contrast P >0.05). Females were significantly heavier than males in all treatments and there was no interaction between sex and treatment (Tables 1, 2). Males were about 34% heavier and females over 41% heavier in the enhanced treatment than in the ambient and reduced treatments (Fig. 4).
Wing length was greater in males (12.1±0.65 mm) than females (10.5±0.55 mm; two-way ANOVA main effect of sex F 2,24=129.1, P <0.001), and significantly greater for both sexes in the enhanced (Scheffé contrast P <0.05) than ambient and reduced treatments (Fig. 5; two-way ANOVA main effect of diet F 2,24=20.9, 1, P <0.001). ANCOVA with body weight as a covariate revealed a significant interaction between sex and the wing length response to treatment (F 1,26=27.3, P <0.001); i.e., the slope of the regression of wing length on body mass for males was significantly steeper than that for females (Fig. 5). This result was independent of increased egg mass because of the low mass of eggs relative to total mass (see later), i.e., the same result is obtained for the regression on empty (without eggs) female mass.
Relationship between body mass and wing length for males and females from the experimental treatments. Squares represent data from reduced, circles from ambient, and triangles from enhanced food treatments. Each data point represents the mean from one experimental cage. ANCOVA indicated that the slope of the relationship is steeper for males (y =8.39+1.23x; R 2 =0.91) than for females (y =7.94+0.66x; R 2 =0.92; see text)
Fecundity
Larval diet affected the fecundity of females emerging from the field enclosures. Female egg mass was significantly greater in the enhanced treatment than in the reduced and ambient treatments (Scheffé contrast P <0.05; F 2,8 =4.83, P =0.042). The mass of individual eggs did not differ among treatments (F 2,8 =0.35, P =0.72), rather females in the enhanced treatment had more eggs than in the other two treatments (Scheffé contrast P <0.05; F 2,8 =16.4, P =0.001). Females that emerged from the enhanced treatments had about 30% more eggs (72 eggs/female) than females in the reduced and ambient treatments (56 and 55 eggs/female, respectively). A significant regression of body size vs fecundity including all females from the experiment and those from the pond showed: (1) a positive relationship (r 2 =0.45) between female body mass and egg number, and (2) that fecundities of females from ambient treatments were similar to those from non-experimental animals that metamorphosed during the experiment (Fig. 6). Egg mass/body mass did not differ among treatments (reduced =0.034, ambient =0.036, enhanced =0.039; F 2,12 =1.24, P =0.34.), nor did number of eggs/body mass (F 2,12 =2.04, P =0.17).
Laboratory experiment
Larvae reared individually in the laboratory had protracted development compared to larvae in the field. Because many animals from the non-supplement treatments had not emerged by the end of the experiment in mid-September, and because several animals that did emerge were damaged, we could not evaluate the effects of treatment on adult fitness. However, two results from this experiment were useful for interpreting the field experiment. First, larvae with an enhanced diet emerged earlier than those without (main effect of supplement F 1,24=69.5, P <0.001; Fig. 7). Development time was longer with boiled than unboiled detritus (main effect of detritus F 1,24=17.9, P <0.001). The significant interaction between dietary supplement and detritus quality (F 1,24=16.4, P <0.001) reflected a relatively small difference in development time between detritus treatments with a supplement as compared to the large difference without one (Fig. 7). At the end of the experiment, larvae in the no-supplement, boiled detritus treatment had not emerged, and several had not even pupated.
The second result of interest from this experiment was that mortality did not differ among treatments. One larva died in the no supplement-detritus treatment, and two animals in each of the other treatments. We did not observe mortality during the latter stages of larval development, even in the boiled-no supplement treatment where development was extremely slow.
Discussion
Our results provide evidence that animal material in the diet of caddisfly larvae has multiple effects on fitness that are manifested in the larval (increased survival and faster development), pupal (faster development) and adult stages (increased adult size and fecundity) of the life cycle. We discuss our results in the context of (1) cannibalism and developmental time constraints in temporary habitats, (2) the nutritional ecology of detritivores, (3) allocation of larval resources to adult fitness, and (4) life history tradeoffs in growth and development in animals with complex life cycles.
Cannibalism and time-constrained development in temporary wetlands
A. nigriculus is one of several limnephilid caddisflies at our study site with life history traits that facilitate development in temporary wetlands (Wissinger et al. 2003). However, it is the only species that can complete larval and pupal development before vernal pools dry in early summer. Our results suggest that the ability of larvae to complete development under these time constraints is at least in part due to a propensity to supplement their diet with animal material. Larvae in treatments with a supplement completed development faster and emerged earlier than those with no supplement. Earlier emergence was due to both decreased larval development time, and decreased time spent pupating. The magnitude of this effect of a dietary supplement of animal material on emergence date (7–10 days earlier than without a supplement) should dramatically increase survival in vernal habitats, especially in drought years (e.g., 1996, 2002), when the difference between desiccation and successful emergence is only a few days (Wissinger et al. 2003). Experimental data, field observations, and gut contents all suggest that cannibalism is the main source of an animal- material dietary supplement in natural populations of Asynarchus (Wissinger et al. 1996, 2003).
Curiously, Asynarchus appears to rarely prey on other invertebrate taxa. Invertebrate abundances at the end of the field experiment in the ambient treatment (with Asynarchus) did not differ from those in the cage control (without Asynarchus). Survival, emergence times, adult body size, and fecundity were the same in the ambient (with invertebrates) and reduced treatments (without invertebrates). One explanation for the apparently minor role of other invertebrates in Asynarchus diets is that cannibalism has the dual benefits of direct nutritional gain and the elimination of potential competitors (Polis 1988). Low encounter rates with other taxa that are either infaunal (oligochaetes, chironomids) or in the water column (cladocerans, copepods, hemipterans, beetles; Wissinger et al. 1999b) might also contribute to the absence of interspecific predation. A third explanation is that cannibalism is an outcome of aggression associated with case-grazing and case defense. Larvae frequently crawl on each other‘s cases, apparently grazing on case silk and/or biofilm (Bergey and Resh 1994). Silk is energetically expensive and highly nutritious (Huryn and Wallace 2000), and interactions between case owners and case grazers often escalate into agonistic interactions. Case grazing, by increasing encounter rates, could be one of the proximate mechanisms that led to the evolution of cannibalism in this species.
An unanticipated outcome of this study was that larval survival in the field experiment was higher with than without a supplement. Several types of evidence suggest that this survival effect was due to decreased cannibalism. First, after 3 weeks of food supplementation, levels of aggression were lower among larvae with (15% of encounters included agonistic behaviors) than without (30–40%) the supplement. Second, starvation was unlikely in the field, given its absence in the no supplement-boiled detritus treatment in the laboratory. Food quantity has often been cited as a factor that affects the propensity for cannibalism (Fox 1975; Polis 1981; Kretier and Wise 2001). In our study, a small quantity of a high quality food supplement appears to reduce cannibalism.
Diet supplementation and detritivory
Although dietary supplementation clearly increases development rates in Asynarchus, it appears from our laboratory data that at least some individuals can complete development without a supplement. However, development in detritus-only treatments in the laboratory was so slow that some individuals emerged more than 5 weeks later than has been observed in any natural populations at our study sites (Wissinger et al. 2003). Our results are consistent with those of Anderson (1976) who found that the limnephilid caddisfly, Clistoronia magnifica, was either unable to emerge, or did so late and with damaged wings, when reared on a solely detrital diet. Larvae in our study could not complete development on a diet of sterilized detritus, emphasizing the importance of microbial conditioning to the nutritional ecology of detritivores (Webster and Benfield 1986, Arsuffi and Suberkropp 1989; Jacobsen and Sand-Jensen 1994; Graca et al. 2001). Development time in the laboratory did not differ between the two detritus treatments with a protein supplement, suggesting that a small amount of animal material can compensate for a reduction in detrital quality. Although dietary supplementation has been documented for other detritivores (Winterbourn 1971; Anderson 1976; Iverson 1979; Berté and Pritchard 1986; Giller and Sangpredub 1993, Jacobsen and Friberg 1995; MacNeil et al. 1997; Mihuc 1997), we know of no previous studies that link this phenomenon to cannibalism.
Larval diet and adult fitness correlates
For insects that feed as adults, understanding patterns of resource allocation requires integrating the energy and nutrient budgets of larvae and adults (as in Boggs 1981, 1997). However, many aquatic insects do not feed after pupation and, thus, all energy and nutrients required by adults must be obtained during larval development. In this study, the body size of males and females was 34% and 41% larger with than without a larval food supplement, and females that received a supplement as larvae had 30% higher fecundity than those that did not. The positive relationship that we observed between female body size and fecundity (no. eggs/female) is similar to that observed in many other insects (Honek 1993). Although it is often assumed that energetic allocation to reproduction in insects is typically realized through increases in the numbers of eggs, some studies have found systematic changes in the size of eggs (Parker and Begon 1986; Corkum et al. 1997). We found no effect of diet on egg size, although small differences would have been difficult to detect because Asynarchus eggs are immature at emergence (Wissinger et al. 2003).
Interpreting the increased body size of males as a result of diet supplementation is less straightforward than that for females in the absence of information on mating system. It is often assumed that body size in male insects should have a positive effect on fitness by increasing dispersal ability, longevity, male-male competition, and/or mate choice (Choe and Crespi 1997; Sokolovska et al. 2000). However, selection for body size can differ in males and females, depending on cost-benefit tradeoffs between sexual and natural selection (Blankenhorn 2000).
Theoretically, the availability of increased resources during larval development should be preferentially allocated to body parts that have the strongest effects on fitness (Nijhout and Emlen 1998). Thus, the increased proportional allocation of additional resources to wing length and leg length that we observed in males might suggest that these structures play some role in intra- and/or intersexual selection (Emlen 2000). Wing length and leg length have been linked to male mating success in caddisflies, although Asynarchus males do not appear to swarm like those in previous studies (e.g., Petersson 1996). Understanding how body size, wing length, and leg length affect the mating success of males of this species will require additional study of the mating system.
Larval diet and growth-development tradeoffs
Many studies on taxa with complex life cycles find that larval food levels and diet affect size and age at maturity or metamorphosis (Nylin and Gotthard 1998; Hentschel and Emlet 2000; Morey and Reznick 2000). In some cases, there is a tradeoff between growth and development (smaller and earlier vs larger and later), whereas in others increased food levels result in a coupled response (larger and earlier). Day and Rowe (2002) propose a general model to resolve the critical factors that might explain differences among empirical studies in growth-development responses. Their model predicts that different responses are expected depending on whether changes in resource levels occur before or after a developmental threshold for transforming to the next stage. Growth can be decoupled from development after such a threshold; thus, pre-threshold manipulations result in earlier metamorphosis and larger size, and those after the threshold in growth-development tradeoffs. Our results are consistent with Day and Rowe‘s model in that we observed increased development rates and increased body size in response to pre-threshold (during 3rd–5th larval instars) manipulation of diet. A developmental threshold in Asynarchus would have to occur during the last instar, given that limnephilid caddisflies have determinate development with five molts before pupation (Wiggins 1996). In taxa with indeterminate development (variable number of instars), thresholds can occur before the final larval stage, resulting in the earlier decoupling of growth and development. For example, mayflies that emerge early to avoid fish predation are smaller than those that extend larval development in the absence of fish (Peckarsky et al. 2001, 2002). Similarly, damselflies that emerge early under time constraints are smaller than those that emerge later (Johansson et al. 2001). We suspect the difference between our results (“larger and earlier”; also Stevens et al. 2000) and those of Peckarsky et al. and Johansson et al. (“smaller and earlier”) reflects a general pattern in how determinate and indeterminate species respond to food levels or other stresses during early development. Type of metamorphosis could also affect development-growth tradeoffs. In holometabolous taxa (e.g., caddisflies), allocation to adult tissues occurs during pupation when there is competition between different body parts for a common pool of energy (Nijhout and Emlen 1998). In hemimetabolous taxa (e.g., mayflies and damselflies), individuals can incrementally adjust resource allocation to growth and development during the gradual transformation to the adult form. There should be a close correspondence between the size of final instar larvae and adults in mayflies, but that correspondence could be weaker in caddisflies depending on the energetics of allocation to different types of tissues during pupation. Resolving differences among studies in growth-development tradeoffs will require threshold models (as in Day and Rowe 2002) that account for developmental differences along at least three gradients: (1) type of metamorphosis (hemi- to holometabolous; Truman and Riddiford 1999), (2) developmental plasticity during the larval stage (determinate to indeterminate), and (3) the extent to which adult feeding complements the resources obtained during larval development (e.g., Boggs 1997).
References
Anderson NH (1976) Carnivory by an aquatic detritivore, Clistorina magnifica (Trichoptera Limnephilidae). Ecology 57:1081–1085
Anderson NH, Cargill AS (1987) Nutritional ecology of aquatic detritivorous insects. In: Slansky F, Rodriquez JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York, pp 903–925
Arsuffi TL, Suberkropp K (1989) Selective feeding by shredders of leaf colonized stream fungi: comparison on macroinvertebrate taxa. Oecologia 79:30–37
Bergey EA, Resh VH (1994) Interactions between a stream caddisfly and the algae on its case: factors affecting algal quality. Freshw Biol 31:153–163
Berté SB, Pritchard G (1986) The life histories of Limnephilus externus Hagen, Anabolia bimaculata (Walker) and Nemotaulius hostilis (Hagen) (Trichoptera, Limnephilidae) in a pond in southern Alberta, Canada. Can J Zool 64:2348–2356
Blankenhorn WU (2000) The evolution of body size: what keeps animals small? Q Rev Biol 75:385–407
Boggs CL (1981) Nutritional and life history determinants of resource allocation in holometabolous insects. Am Nat 117:692–709
Boggs CL (1997) Dynamics of reproductive allocation from juvenile and adult feeding: radiotracer studies. Ecology 78:190–202
Bowen SH, Lutz EV, Ahlgren MO (1995) Dietary protein and energy as determinants of food quality: trophic strategies compared. Ecology 76:899–907
Choe JC, Crespi BJ (1997) The evolution of mating systems in insects and arachnids. Cambridge University Press, Cambridge
Corkum LD, Ciborowski JJH, Poulin RG (1997) Effects of emergence data and maternal size on egg development, sizes of eggs, and first-instar nymphs of a semelparous aquatic insect. Oecologia 111:69–75
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
Day T, Rowe L (2002) Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am Nat 159:339–350
Dong Q, Polis GA (1992) The dynamics of cannibalistic populations: a foraging perspective. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, New York,pp 12–37
Elgar MA, Crespi BJ (eds) (1992) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, New York
Emlen DJ (2000) Integrating development with evolution: a case study with beetle horns. Bioscience 50:403–418
Fox LR (1975) Cannibalism in natural populations. Annu Rev Ecol Syst 6:87–106
Frost PC, Elser JJ (2002) Growth responses of littoral mayflies to the phosphorus content in their food. Ecol Lett 5:232–240
Giller PS, Sangpredub N (1993) Predatory foraging behavior and activity patterns of larvae of two species of limnephilid cased caddis. Oikos 67:351–357
Graca MAS, Cressa C, Gessner MO, Feio MJ, Callies KA, Barrios C (2001) Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshw Biol 46:947–957
Hentschel BT, Emlet RB (2000) Metamorphosis of barnacle nauplii: effects of food variability and a comparison with amphibian models. Ecology 81:3495–3508
Honek A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Hopper KP, Crowley PH, Kielman D (1996) Density dependence, hatching synchrony, and within-cohort cannibalism in young dragonfly larvae. Ecology 77:191–200
Huryn AD, Wallace JB (2000) Life history and production of stream insects. Annu Rev Entomol 45:83–110
Iverson TM (1979) Laboratory energetics of larvae of Sericostoma personatum (Trichoptera). Holarctic Ecol 2:1–5
Jacobsen D, Friberg N (1995) Food preference of the trichopteran larva Anabolia nervosa from two streams with different food availability. Hydrobiologia 308:139–144
Jacobsen D, Sand-Jensen K (1994) Growth and energetics of a trichopteran larva feeding on fresh submerged and terrestrial plants. Oecologia 97:412–418
Johansson F, Stoks R, Rowe L, De Block J (2001) Life history plasticity in a damselfly: effects of combined time and biotic constraints. Ecology 82:1857–1869
Kreiter NA, Wise DA (2001) Prey availability limits fecundity and influences the movement pattern of female fishing spiders. Oceologia 127:417–424
Ludwig D, Rowe L (1990) Life-history strategies for energy gain and predator avoidance under time constraints. Am Nat 135:686–707
MacNeil C, Dick JTA, Elwood RW (1997) The trophic ecology of freshwater Gammarus spp. (Crustacea:Amphipoda): problems and perspectives concerning the functional feeding group concept. Biol Rev 72:349–364
Mihuc TB (1997) The functional trophic role of lotic primary consumers: generalist versus specialist strategies. Freshw Biol 37:455–462
Morey S, Reznick D (2000) A comparative analysis of plasticity in larval development in three species of spadefoot toads (Anura: Pelobatidae: Scaphiopus). Ecology 81:1736–1749
Nijhout HF, Emlen DH (1998) Competition among body parts in the development and evolution of insect morphology. Proc Natl Acad Sci 95:3685–3689
Nylin S, Gotthard K (1998) Plasticity in life history traits. Annu Rev Entomol 43:68–83
Parker GA, Begon M (1986) Optimal egg size and clutch size: effects of environment and maternal phenotype. Am Nat 128:573–592
Peckarsky BL, Taylor BW, McIntosh AR, McPeek MA, Lytle DA (2001)Variation in mayfly size at metamorphosis as a developmental response to risk of predation. Ecology 82:740–757
Peckarsky BL, McIntosh AR, Taylor BW, Dahl J (2002) Predator chemicals induce changes in mayfly life history traits: a whole-stream manipulation. Ecology 83:612–618
Petersson E (1996) Male load-lifting capacity and mating success in the swarming caddisfly Athripsodes cinereus. Physiol Entomol 20:66–70
Pfennig DW (1997) Kinship and cannibalism. Bioscience 47:667–675
Pfennig DW, Ho SG, Hoffman EA (1998) Pathogen transmission as a selective force against cannibalism. Anim Behav 55:1255–1261
Polis GA (1981) The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst 12:225–251
Polis GA (1988) Exploitation competition and the evolution of interference, cannibalism, and intraguild predation in age/size-structured populations. In: Ebenman B, Persson L (eds) Size-structured populations. Springer, New York Berlin Heidelberg, pp 183–202
Potvin C (1993) ANOVA: experiments in controlled environments. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 46–68
Pritchard G, Berté SB (1987) Growth and food choice by two species of limnephilid caddis larvae given natural and artificial foods. Freshw Biol 18:529–535
Richardson JML, Baker RL (1997) Effect of body size and feeding on fecundity in the damselfly Ischnura verticalis (Odonata: Coenagrionidae). Oikos 79:477–483
Rowe L, Ludwig D (1991) Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology 72:413–427
SAS (1999) StatView, version 5.0. SAS Institute, Cary, N.C.
Scheiner SM (1993) MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 94–112
Sokolovska N, Rowe L, Johansson F (2000) Fitness and body size in mature odonates. Ecol Entomol 25:239–248
Sparks GB (1993) Competition and intraguild predation between two species of caddisfly (Trichoptera) larvae in permanent and semi-permanent high elevation ponds. B.Sc. Thesis, Allegheny College, Meadville, Pa.
Stevens DJ, Hansell MH, Monaghan P (2000) Developmental trade-offs and life histories: strategic allocation of resources in caddisflies. Proc R Soc Lond 267:104:1511–1515
Truman JW, Riddiford LM (1999) The origins of insect metamorphosis. Nature 401:447–452
Van Buskirk J (1992) Competition, cannibalism, and size class dominance in a dragonfly. Ecology 65:455–464
Wahlström E, Persson L, Diehl S, Byström P (2000) Size-dependent foraging efficiency, cannibalism, and zooplankton community structure. Oecologia 123:138-148
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst 17:567–597
Wiggins GB (1996) Larvae of the North American caddisflies (Trichoptera), 2nd edn. University of Toronto Press, Toronto
Winterbourn MJ (1971) The life histories and trophic relationships of the Trichoptera of Marion Lake, British Columbia. Can J Zool 49:623–635
Wissinger SA, Sparks GB, Rouse GL, Brown WS, Steltzer H (1996) Intraguild predation and cannibalism among larvae of detritivorous caddisflies in subalpine wetlands. Ecology 77:2421–2430
Wissinger SA, Whiteman HH, Sparks GB, Rouse GL, Brown WS (1999a) Tradeoffs between competitive superiority and vulnerability to predation in caddisflies along a permanence gradient in subalpine wetlands. Ecology 80:2102–2116
Wissinger SA, Bohonak AJ, Whiteman HH, Brown WS (1999b) Subalpine wetlands in central Colorado: habitat permanence, salamander predation, and invertebrate communities. In: Batzer DP, Rader RR, Wissinger SA (eds) Invertebrates in freshwater wetlands of North America: ecology and management. Wiley, New York, pp 757–790
Wissinger SA, Brown WS, Jannot JE (2003) Caddisfly life histories along permanence gradients in high-elevation wetlands in Colorado (USA). Freshw Biol 48:255–270
Acknowledgements
We are grateful to the Rocky Mountain Biological Laboratory and The Nature Conservancy for access to the Mexican Cut Nature Reserve. Thanks to Suzanne Kilby for field assistance. An earlier version of the manuscript was improved greatly by discussions with Bobbi Peckarsky, Jason Jannot, Sara Mattie, and the Freshwater Ecology Research Group at the University of Canterbury (especially Angus McIntosh, Michael Winterbourn, Jon Harding, Nicholas Dunn, Leanne O‘Brien, Jane Goodman, and Hans Eikaas). This study was funded by the National Science Foundation (DEB 9407856).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wissinger, S., Steinmetz, J., Alexander, J.S. et al. Larval cannibalism, time constraints, and adult fitness in caddisflies that inhabit temporary wetlands. Oecologia 138, 39–47 (2004). https://doi.org/10.1007/s00442-003-1397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1397-y