Abstract
The photosynthetic pathway composition (C3:C4 mixture) of an ecosystem is an important controller of carbon exchanges and surface energy flux partitioning, and therefore represents a fundamental ecophysiological distinction. To assess photosynthetic mixtures at a tallgrass prairie pasture in Oklahoma, we collected nighttime above-canopy air samples along concentration and isotopic gradients throughout the 1999 and 2000 growing seasons. We analyzed these samples for their CO2 concentration and carbon isotopic composition and calculated C3:C4 proportions with a two-source mixing model. In 1999, the C4 percentage increased from 38% in spring (late April) to 86% in early fall (mid-September). The C4 percentages inferred from ecosystem respiration measurements in 2000 indicate a smaller shift, from 67% in spring (early May) to 77% in mid-summer (late July). We also sampled daytime CO2 concentration and carbon isotope gradients above the canopy to determine ecosystem discrimination against 13CO2 during net uptake. These discrimination values were always lower than corresponding nighttime ecosystem respiration isotopic signatures would suggest. After accounting for the isotopic disequilibria between respiration and photosynthesis resulting from seasonal variations in the C3:C4 mixture, we estimated canopy photosynthetic discrimination. The C4 percentage calculated from this approach agrees with the percentage determined from nighttime respiration for sampling periods in both growing seasons. Isotopic imbalances between photosynthesis and respiration are likely to be common in mixed C3:C4 ecosystems and must be considered when using daytime isotopic measurements to constrain ecosystem physiology. Given the global extent of such ecosystems, isotopic imbalances likely contribute to global variations in the carbon isotopic composition of atmospheric CO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grassland ecosystems of the North American Great Plains contain mixtures of C3 and C4 plants that vary spatially and temporally. The C4 percentage of annual production increases along a diagonal from the northwest to the southeast, reaching a maximum in the tallgrass prairies of eastern Kansas and north-central Oklahoma (Tieszen et al. 1997). In the shortgrass prairie ecosystem, C3 grasses generally grow in spring and early summer, while C4 grasses predominate during mid- and late summer (Kemp and Williams 1980). Similarly, in upland mixed grass prairies, C3 grasses grow in spring and fall, while C4 grasses dominate in summer (Ode et al. 1980). Such patterns in photosynthetic mixtures have been explained as differential responses to temperature exhibited by these plant types (Ehleringer 1978; Ehleringer et al. 1997; Collatz et al. 1998; Sage et al. 1999). In the third main grassland type of the Great Plains, the tallgrass prairie ecosystem, C4 grasses co-occur with C3 grasses, some forbs (non-graminoid herbs), and occasional streamside deciduous trees (Freeman 1998; Knapp et al. 1998).
The original tallgrass prairies have been largely plowed under and replaced by crops. However, there are still patches of pure tallgrass prairie, and pasturelands throughout several of the Great Plains states contain many of the original tallgrass species, including the characteristic perennial C4 tall grasses: big and little bluestem (Andropogon gerardii and Schizachrium scoparius), indiangrass (Sorghastrum nutans), and switchgrass (Panicum virgatum). One of these pastures in north-central Oklahoma was the site of intensive measurements with an eddy covariance system and associated instrumentation from late 1996 to late 2000 (Suyker and Verma 2001). The focus of that study was to characterize the annual carbon balance of this ecosystem remnant and better understand the influence of climate variations and land use decisions on carbon storage.
As part of this larger effort, we collected air, soil, and plant samples and analyzed them for their carbon concentration and isotopic composition, with the goal of better understanding the physiological controls on carbon exchanges. Because the dominant functional types in this ecosystem are C3 and C4 plants, our approach was to use the distinction in photosynthetic carbon isotope fractionation between these plant types to estimate the contribution of each to gas and organic matter samples. Specifically, we sampled soil organic matter, leaf biomass, soil and plant respiration, nighttime ecosystem respiration, and daytime ecosystem photosynthesis. The nighttime ecosystem respiration and daytime ecosystem photosynthesis measurements are particularly useful in a system like this with a great deal of vegetation heterogeneity. Since these measurements integrate a large spatial area and represent the flux-weighted contribution of C3 and C4 plants, they complement more intensive vegetation composition surveys and spot measurements of soil respiration and its carbon isotopic composition.
Site description
Measurements were taken in a tallgrass pasture that is located (36°56′N, 96°41′W) within a matrix of land uses and land covers, including sparse tree cover, some crops, and other pastures and grasslands. The maximum green leaf area index (LAI) of the pasture is ~3.0, maximum daytime net ecosystem exchange (NEE) is –30–35 μmol CO2 m−2 s−1, and the average nighttime NEE is –4–6 μmol CO2 m−2 s−1 (Suyker and Verma 2001). The site is burned annually in early April in accordance with the management regime of the region, and it has not been grazed since late 1996. The measured integral of NEE between 1997 and 1998 was 268 g C m−2; when the carbon lost in the annual burn was factored in, annual NEE was estimated to be almost zero (Suyker and Verma 2001). The plant species composition at the site is dominated by C4 grasses, which constituted at least 78% of the species present in a vegetation cover survey conducted in 1997 (Suyker and Verma 2001).
Materials and methods
Isotopic approach
The distinction between C3 and C4 plants in photosynthetic isotope fractionation provides a useful tracer of the carbon fluxes associated with each photosynthetic pathway. Because of inherent biochemical and anatomical differences between these plant types, they discriminate against 13CO2 to different degrees during photosynthesis (the discrimination or fractionation is denoted Δ13) (Farquhar et al. 1989). Given isotopic composition values for each photosynthetic type (δ13C3 and δ13C4), it is possible to estimate the contribution of each to measured (δ13CM) samples. The percentage contribution from C4-derived carbon is given by the following equation:
See Table 1 for a complete list of symbols and variables used in this paper.
Leaf biomass and soil organic carbon isotope analyses
Sunlit, upper canopy leaves from a variety of plant species were collected throughout the 1999 growing season. The leaf samples were dried for 48–72 h at 75°C, ground into powder, and sub-sampled for isotope and elemental analysis at the Carnegie Institution of Washington, Department of Plant Biology (CIW). Samples were combusted in an elemental analyzer (Carlo-Erba), the CO2 was separated by chromatography and directly injected into a continuous-flow Isotope Ratio Mass Spectrometer (Finnigan Delta S). Standards were run every ten samples. The standard deviation of the analysis was always below 0.1‰.
Soil cores were collected at the following depth increments in July 2000: 0–2 cm, 2–5 cm, 5–10 cm, and 10–20 cm. They were dried for 48–72 h at 70°C, sieved to remove roots, ground into powder, decarbonated with sulfurous acid, and prepared for isotope and elemental analysis at the Stanford University Stable Isotope Laboratory. Litter samples were not collected, since the site is burned annually and there is minimal buildup.
Nighttime respiration collection and analysis
Air samples were collected at four heights above the surface (0.5 m, 1.5 m, 2.5 m, and 4.5 m) during nighttime periods when both autotrophic and heterotrophic respiration (i.e., ecosystem respiration) were occurring. We designed and built an automated system for near-simultaneous trapping of air from all heights. Sampling tubes from each of the four heights on the tower led to a 4-port manifold. At pre-selected times, the system activated itself and tested the lowest and highest levels by alternately opening the solenoid valve on the manifold corresponding to that level. Air was then pumped at ~1 l min−1 through 4-l buffer volumes, a water trap (magnesium perchlorate), multi-port valve (Valco ST configuration E 16 position valve), sampling flask (100-ml glass flasks, Kontes Custom Glass Shop), and infrared gas analyzer (Li-Cor 6200). If the gradient in CO2 concentration between the lowest and highest levels were sufficient (usually a minimum of 15–20 ppm), the system would trap air from each level in a sampling flask until all flasks were filled (up to four samples/level with our 16-port valve). Flasks were not pressurized. Each sample was collected over a 2-min interval, so that all levels were sampled over a short time interval. During the 2000 growing season, we sampled nighttime gradients several times throughout the night and treated each sampling of four levels as one measurement. Samples from late 1998 and 1999 were collected over longer periods, and in April and June of 1999 separate sample collections were bundled together. Air samples were collected and stored in the sampling flasks and returned to the CIW for concentration and isotope analysis. This analysis was conducted using a novel system for simultaneously obtaining CO2 concentration and isotope ratios of small air samples. The measurement precision for CO2 concentration is 0.4–0.7 ppm, while the isotope precision for δ13C and δ18O is 0.03–0.05‰. Other details of this measurement system are described in Ribas-Carbo et al. (2002).
With the concentration and carbon isotope ratios of the samples, we constructed 'Keeling plots' to estimate the isotopic composition of ecosystem respiration. The intercept of a linear regression between δ13C and the reciprocal of the concentration provides an estimate of the isotopic composition of the source (δ13CR) (Keeling 1958, 1961; Flanagan and Ehleringer 1998; Yakir and Sternberg 2000). The linear regression for each Keeling plot was a model II, or geometric mean, regression that incorporates errors in both the concentration and isotope ratios (i.e., the independent variable also has errors associated with it) (Friedli et al. 1987; Flanagan et al. 1996; Sokal and Rohlf 1995; Bowling et al. 1999; Harwood et al. 1999; Pataki et al. 2003). The errors for our ecosystem respiration Keeling plots were calculated assuming model I regression formulas, however, as there is controversy within the statistical literature about the appropriate errors to ascribe to geometric mean regressions (Sokal and Rohlf 1995; Laws 1997).
To calculate the C4 percentage contribution to ecosystem respiration using Eq. 1 requires isotopic end members for each photosynthetic type, as well as the regression intercept. These end members were estimated from the isotopic composition of bulk leaf matter collected from representative C3 and C4 plants as previously described.
Soil respiration and plant dark respiration
We measured the isotopic composition of soil respiration on two occasions: September 1999 and July 2000. We used a Li-Cor LI 6400 photosynthesis system and LI 6400–09 soil CO2 flux chamber to estimate this composition. The soil chamber was placed on open patches of soil and air was sampled in 100-ml pre-evacuated flasks (Kontes Custom Glass Shop) during the concentration buildup that resulted from soil respiration. To avoid any pressure decline in the soil chamber during air collections, a balloon was placed on an inlet open to atmospheric air. As the sample was being collected into the pre-evacuated flasks, atmospheric air was filling the balloon, thus maintaining constant pressure inside the soil chamber. The flasks were analyzed for CO2 concentrations and isotope ratios and the Keeling plot intercept calculated with a geometric mean regression. In September 1999, soil patches adjacent to clumps of either C3 or C4 grasses were chosen on a single day (two Keeling plots total). In July 2000, multiple soil patches were chosen, and the samples from each patch were bundled together for each Keeling plot. This was repeated a second day, for a total of two soil Keeling plots in July 2000.
We sampled plant dark respiration and estimated its isotopic composition in a similar fashion. For these collections in July and September 1999, several leaves from representative C3 or C4 plants were clipped and placed in the LI 6400–09 soil CO2 flux chamber. The chamber was sealed and the ensuing concentration buildup was sampled with flasks connected to the chamber's outflow. The flasks were analyzed for CO2 concentrations and isotopic ratios and the Keeling plot intercept was calculated with a geometric mean regression. In some cases, other plant organs (i.e., stems and flowers) were collected and their respiration sampled.
Leaf-level discrimination measurements
We obtained leaf-level discrimination against 13CO2 during leaf gas exchange measurements with representative grass and forb leaves. We sampled air in the inlet and outlet streams of the LI 6400 leaf chamber. Samples were taken under steady-state conditions, which typically occurred within several minutes after a leaf was sealed in the chamber. Following Evans et al. (1986), discrimination values were calculated based on the difference in CO2 concentration and isotopic ratio between the two air streams.
Daytime gradient sampling
To assess the photosynthetic composition of the canopy during daytime net carbon uptake, we sampled CO2 concentration and δ13C gradients between the highest (4.5 m-denoted 'hi') and lowest (0.5 m-denoted 'lo') tower levels during the 1999 and 2000 measurement campaigns. The 0.5 m level was just above the prairie canopy at peak growth in July. The flow diagram for air sample collection was the same as that used for nighttime collections, except only two levels were sampled for each measurement.
Net ecosystem discrimination against 13CO2, which includes the influence of ecosystem respiration, was calculated from the following equation (derived in the Appendix),
where the components are average CO2 and δ13C of air sampled at 4.5 m (hi) and 0.5 m (lo). This formulation is similar to an equation developed by Lloyd et al. (1996) to quantify ecosystem discrimination using measurements of the concentration and isotopic composition of one-way fluxes into a forest canopy. Equation 2 predicts slightly larger discrimination values than the Lloyd et al. equation, but the same values as the Evans et al. (1986) equation to describe leaf-level discrimination during gas exchange. For each ecosystem discrimination calculation, we used average CO2 concentration and δ13C values sampled at each height during a 1–3 h period. On a typical day, only 5–7 gradient pairs with sufficient concentration and isotope gradients could be sampled due to the strong winds at the site. An example of the average CO2 and δ13C at 4.5 m and 0.5 m collected during mid-day on 6 June 2000 is displayed in Fig. 1.
The daytime canopy photosynthetic composition (C4 percentage) was determined in the same fashion as in the nighttime respiration approach (using Eq. 1), except the component terms were Δ13 values instead of δ13C values. Two sets of end members were used for these calculations: one set based on the bulk leaf biomass, and the other based on discrimination during leaf gas exchange measurements. For the bulk-leaf biomass end member set, we estimated each discrimination end member as (Farquhar et al. 1989; Buchmann and Ehleringer 1998),
where the subscript 3/4 represents the quantity for C3 or C4 vegetation sampled at the site. The value of δ13Catm, which is the isotopic composition of the background atmosphere, was taken as the troposphere average of –8.0‰. In all cases, the canopy C4 percentage was calculated using the corrected photosynthetic discrimination (Eq. 4—next section), not the net ecosystem discrimination calculated from Eq. 2.
Correction to canopy discrimination calculations and implications for daytime Keeling plots
A correction must be applied to Eq. 2 to calculate canopy photosynthetic discrimination, since this equation represents ecosystem discrimination during net carbon uptake, and thus includes the influence of both soil and plant fluxes. For a system in isotopic balance, i.e., the isotopic composition of respiration is the same as that of photosynthesis, photosynthetic and ecosystem discrimination are equal and no correction need be applied. However, most if not all systems that contain C3 and C4 plants, as well as systems dominated by C3 plants that experience large climate stresses and thus discrimination variations, are out of isotopic balance for parts of the year and between years.
A disequilibrium in isotope fluxes should be expected in our system due to the different seasonal activities of C3 and C4 plants. For example, the dominance of C3 grasses and forbs in the cooler spring would bias the soil organic carbon (SOC) and litter towards lower (i.e., more depleted) δ13C values, and this bias might carry forward into the summer when C4 vegetation becomes dominant. This would produce a disequilibrium in isotope fluxes because the heterotrophic respiration would have more of a C3 signal than the net photosynthetic flux. In this scenario, if the net ecosystem discrimination calculated with Eq. 2 is taken as the canopy photosynthetic discrimination, the C4 percentage would be overestimated. Each 1‰ error in canopy discrimination corresponds to a ~6% error in the inferred C4 percentage from Eq. 1.
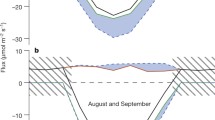
The effect of fluxes with different isotope signatures and opposite signs (i.e., carbon uptake versus carbon release) on the ambient or background atmosphere is illustrated with a vectorial approach in Fig. 2. The axes denote concentration and isotopic differences relative to a background value, in this case, air sampled at 4.5 m. Respiration adds CO2 to the atmosphere that is relatively depleted in 13CO2, thus producing a positive CO2 deviation and a negative δ13C deviation from the background; photosynthesis does just the opposite. For example, soil heterotrophic respiration with a δ13C signature of –26‰ would produce a vector with a slope of −0.05‰ ppm−1. The total 'isotopic forcing' is a function of the flux magnitude and the difference in isotopic composition between the background atmosphere and the flux. As discrimination increases, the vector slope (and isotopic forcing) increases. In Fig. 2, vectorial addition of the soil respiration and photosynthetic vectors yields a resultant vector whose length is the change in background CO2 concentration resulting from net carbon exchange and whose slope is lower than either of the component vectors.
A vectorial approach for demonstrating the influence of photosynthetic (dash-dot arrow) and respiratory (solid arrow) fluxes that differ in isotopic composition on the discrimination measured with daytime above-canopy gradients. The axes denote concentration and isotope differences relative to a background atmospheric value (i.e., the deviation of an air sample from a background or ambient value because of photosynthetic or respiratory fluxes). The slope of each component vector is a function of the isotopic composition of the photosynthetic or respiratory flux; a more negative isotopic value corresponds to a greater slope. The length of each component vector is a function of the flux magnitude. Addition of the photosynthetic and respiratory component vectors produces the resultant vector (net dashed arrow), which has a lower slope than either of the components
The impact of isotopic imbalances must be considered for daytime Keeling plot analyses as well. In general, daytime Keeling plot intercepts in systems that are out of isotopic balance can give counterintuitive results. For example, in a system where soil respiration is isotopically heavier than canopy photosynthesis (the opposite of the situation plotted in Fig. 2), a Keeling plot of air samples collected along a concentration and isotope gradient above the canopy will give an intercept that is lighter (more depleted in 13C) than either the photosynthetic or respiratory flux. Yakir and Wang (1996) sampled daytime above-canopy gradients in both a corn and wheat field to partition NEE into its photosynthetic and respiratory components. To accomplish this partitioning, the isotopic composition of the component fluxes must differ. Both situations (respiration lighter and heavier than photosynthesis) and the resultant impact on their ecosystem Keeling plot intercepts are apparent in Table 1 of Yakir and Wang (1996).
The example in Fig. 2 is a specific case of isotopic disequilibria between incoming and outgoing carbon fluxes, in which only heterotrophic respiration is out of isotopic balance with net autotrophic uptake. However, in ecosystems with large turnover times in the biomass and rapid discrimination changes such as forests (Ekblad and Hogberg 2001; Bowling et al. 2002; Randerson et al. 2002a), it is also possible for autotrophic respiration to have a different isotopic composition from photosynthesis. Mixed C3:C4 ecosystems might experience isotopic imbalances from autotrophic fluxes as well. For example, if the C3 vegetation in our tallgrass prairie pasture is contributing a proportionally larger amount to canopy respiration than to canopy photosynthesis during a gradient measurement, an isotope disequilibrium will result.
Because the daytime gradients we sampled were susceptible to isotopic imbalances (from both heterotrophic and autotrophic respiration), we corrected them by accounting for the influence of ecosystem respiration on the measured net ecosystem discrimination. Ideally, this correction requires two pieces of information: the isotopic composition of ecosystem respiration (δ13CR), and its magnitude (R). This is apparent in the equation we used to correct our net ecosystem discrimination values from Eq. 2 to canopy photosynthetic discrimination (Δ13 can) values,
where A is gross photosynthesis (net photosynthesis plus all autotrophic respiration), and δ13CA is its isotopic composition. This equation is very similar to a correction derived with equation 11 from Lloyd et al. (1996), although their A is the sum of foliage photosynthesis and respiration. Also, they approximated ecosystem and canopy photosynthetic discrimination as \( {\Delta ^{{13}}_{{{\rm{eco}}}} \cong \delta ^{{13}} {\rm{C}}_{{{\rm{atm}}}} - \delta ^{{13}} {\rm{C}}_{{{\rm{eco}}}} } \) and \( {\Delta ^{{13}}_{{{\rm{can}}}} \cong \delta ^{{13}} {\rm{C}}_{{{\rm{atm}}}} - \delta ^{{13}} {\rm{C}}_{{{\rm{can}}}} } \), whereas Eq. 4 is derived with the full discrimination formulation (e.g., Eq. 3) for both terms. Eq. 4 produces discrimination values that are only slightly larger (less than 0.5%) than those predicted by the equation derived from equation 11 in Lloyd et al. (1996). A derivation of Eq. 4 is presented in the Appendix.
We assigned values for δ13CR from the average of contemporaneous nighttime Keeling plot intercepts with CO2 gradients exceeding 20 ppm. Because of the 1,000 multiplier, the Aδ13CA and Rδ 13CR terms in Eq. 4 have a small influence on the discrimination correction. δ13CA and δ13CR were set to δ13CR. Daytime R was predicted with a NEE regression formulation developed for the site by Suyker and Verma (2001). We estimated A from the difference between R and the daytime NEE measured by the adjacent eddy flux system. In reality, the canopy photosynthetic discrimination includes leaf respiration, which we have removed in this approach. However, since leaf respiration is typically less than 10% of the maximum photosynthetic rate for tallgrass C4 plants (Colello et al. 1998), this distinction has a very small impact on the corrected canopy photosynthetic discrimination.
The Suyker and Verma (2001) model is based on measured soil temperature at 10 cm, air temperature at 0.5 m, and canopy green LAI, and includes separate terms for soil respiration and plant respiration. Soil moisture was found to have no significant influence on predicted nighttime NEE by Suyker and Verma (2001). Their model accurately predicted measured nighttime respiration on nights before and after our daytime gradient sampling.
The correction to photosynthetic discrimination in Eq. 4 is a function of the sensitivity to the R and (δ13Catm−δ13CR) terms. As the relative magnitude of respiration (i.e., R/A) increases, the (δ13Catm−δ13CR) term becomes more important. For example, in September 1999 when respiration was 41% of photosynthesis, each 1‰ change in the (δ13Catm−δ13CR) term corresponds to a 0.4‰ change in the inferred gross discrimination; in May 2000 R/A was 28% and the sensitivity decreased to 0.3‰. The latter sensitivity is more typical since the rest of our measurements earlier in the growing season were during periods with lower R/A ratios. Also, in September the magnitude of the (δ13Catm−δ13CR) term should be the smallest since the primary growth period of most C3 plants at this site is spring and early summer.
The sensitivity to R in the Eq. 4 is more variable than to the (δ13Catm−δ13CR) term. Again, the largest sensitivity is in September 1999 at the end of the growing season when photosynthesis is very low. Each 1 μmol m−2 s−1 increase in R increased the canopy photosynthetic discrimination by 0.7‰ for this period. Earlier in the growing season, the sensitivity was 0.2–0.4‰/μmol m−2 s−1.
Error propagation
Gaussian error propagation was used to estimate the uncertainty associated with calculations using Eqs. 1, 2 and 4. For example, the standard error (SE) in Eq. 1 was calculated by adding the SEs of the components in quadrature for the numerator and denominator. The errors in the numerator and denominator were then propagated through the calculation by adding their fractional errors (i.e., \( {{{{\rm{SE}}(x)} \over {\bar{x}}}} \), where x is the numerator or denominator and the overbar is the average of that quantity) in quadrature (Daniels et al. 1962; Morgan and Henrion 1990):
Although Eqs. 5a, 5b, 5c assume no correlation among component variables in an equation (Morgan and Henrion 1990), Eqs. 2 and 4 do contain correlated variables. A formulation that includes the correlation among component terms should be used. However, it is not obvious what correlation coefficients to employ for such a calculation, such as the relationship between δ13C measured at 4.5 m and at 0.5 m, or net discrimination and net photosynthesis. Thus, the Gaussian approximation of Eqs. 5a, 5b, 5c was also employed to propagate the errors in the component terms of the discrimination calculations in Eqs. 2 and 4.
Results
Plant biomass and SOC analyses
The δ13C and carbon: nitrogen (C:N) values of leaf biomass for representative C3 and C4 grasses and forbs are displayed in Table 2; the δ13C, C4 percentage, and C:N values for bulk soil organic carbon (SOC) in the upper soil layers are listed in Table 2. The δ13C values of C3 and C4 plants at this site are fairly consistent within each grouping, and the carbon isotope offset between the groupings is large (~16‰). The C:N elemental ratios of each photosynthetic pathway, by contrast, are less distinct. In general, C4 plants should have higher C:N ratios because of the higher amounts of Rubisco protein in C3 plants (Long 1999).
The δ13C values of SOC increase (become heavier) with depth. It is possible that this trend reflects changes in root competition between C3 and C4 plants with depth, reflecting a higher percentage of C4-derived carbon. However, a similar trend is apparent in soils from many ecosystems around the world, reflecting the decrease in atmospheric δ13C due to fossil fuel and biomass burning over the last 200 years, and some combination of microbial fractionation during decomposition, microbial preference for different components of soil organic matter, and enrichment of microbial and fungal products at depth (Ehleringer et al. 2000). It is therefore difficult to unambiguously attribute the δ13C trend at our site to changes in the C3 or C4 contribution with depth.
Isotopic composition of soil and plant respiration
The δ13C values of soil respiration collected on two occasions are presented in Table 3, along with inferred C4 percentages and 95% confidence intervals. The δ13C values of dark respiration from C3 and C4 plant organs (leaves, stems and flowers) are presented in Table 4. Because of the vegetation heterogeneity in this pasture, the δ13C of soil respiration varied considerably, as is apparent for the 1999 sampling. Sampling on multiple soil patches and bundling the results together in 2000 reduced this variability.
Seasonal and interannual changes in the isotopic composition of ecosystem respiration
The Keeling plots for each of our nighttime respiration sampling periods are displayed in Figs. 3a, b. The 1999 measurements (Fig. 3a—note October 1998 as well) have a larger variability in slopes than the 2000 measurements (Fig. 3b). Figure 4 is a plot of the C4 percentage contribution to respiration from October 1998 to July 2000 (solid circles with SE). The apparent contribution of C4-derived carbon to ecosystem respiration increases from ~40% in early 1999 to over 80% in late 1999, whereas during 2000 the C4 percentage is always greater than 65%. There appears to be an increase in the C4 percentage in 2000 compared to the corresponding 1999 period. The C4 percentage in June and late July of 1999 is close to 50%, whereas in similar periods in 2000 it is greater than 70%. This may represent a real change in the photosynthetic mixture between years.
a Keeling plots of nighttime collections for sampling campaigns in late-1998 and throughout the 1999 growing season, with sampling month given. The intercepts of these plots represent the isotopic composition of ecosystem respiration. b Keeling plots of nighttime collections for sampling campaigns in the 2000 growing season, with sampling month given. The intercepts of these plots represent the isotopic composition of ecosystem respiration
The C4 percentage (SE) of nighttime respiration and daytime photosynthesis plotted together for the period from October 1998 to July 2000. Solid circles denote values derived from nighttime respiration measurements, open circles represent values derived from daytime photosynthesis measurements. Years are separated by vertical gray lines
However, there were slight differences in the methodology of sample collections in April and June of 1999 versus late 1999 and all of 2000 that might affect this interpretation. In April and June of 1999, samples collected throughout a night were treated as one measurement (i.e., were bundled together for one regression). During the other periods, each collection was treated as its own measurement, and the intercepts throughout the night averaged together. The April and June 1999 samples were treated as one measurement because the spatial gradients in CO2 between 0.5 m and 4.5 m during each collection throughout individual nights were not sufficient to create robust Keeling plots. However, the temporal gradient in CO2 concentration was sufficient (i.e., buildup throughout the night) for a single 'bulked' plot. The basic difference between the April–June 1999 plots and the remaining plots can be characterized by the distinction of 'Keeling plots in time' (either collected along gradients or from a single level) versus 'Keeling plots in space' (collected at the same time over a spatial gradient). However, even with this methodology consideration, there appears to still be a real difference in C4 percentage between 1999 and 2000. The intercept from the July 1999 Keeling plot, which was treated the same as the 2000 collections (i.e., point-in-time through space), is more depleted than the average of corresponding 2000 plots by about 5‰.
Treating collections in the former fashion will tend to bias the regression intercept towards a more regional value of isotopic composition because the concentration changes are largely driven by air that has been advected over heterogeneous surfaces upwind of the sampling point. If those surfaces are of the same photosynthetic composition as the vegetation surrounding the pasture, no bias will be introduced. However, our site is surrounded by a matrix of land covers and land uses that introduces large variations in regional (tens to hundreds of km2) photosynthetic composition. The areas to the west of our site are largely planted in winter wheat, to the south and southeast are tree-grass savannahs, and to the north and northeast lie the Flint hills which contain the largest extent of remaining tallgrass prairie in North America. Therefore, unless the wind was consistently from the north-northeast, a grouping of samples through time will introduce more of a C3 regional signal into the regression.
We did see some evidence of wind-related variations in the δ13C signature of nighttime respiration. During the night of 18–19 July 2000, we collected air samples at 9:37 p.m., 12:20 a.m. and 3:30 a.m. The first two δ13C intercepts (standard error) were –14.9‰ (0.6) and –14.7‰ (0.7), while the 3:30 a.m. δ13C intercept was –16.5‰ (0.4). The period between the 12:20 a.m. and 3:30 a.m. collections is marked by large variations in horizontal wind speed and wind direction at 4.5 m measured at the adjacent flux tower. The δ13C variation over this time period might be related to footprint effects, but it is difficult to eliminate other mechanisms. Roughly concurrent with the 3:30 a.m. sampling was a positive spike in the canopy CO2 storage flux and very large values of nighttime respiration. However, these values were recorded at wind speeds just above the 2 m s−1 cutoff often employed in eddy flux studies (Suyker and Verma 2001). If the storage and respiration fluxes are real, they suggest a burst of CO2-rich air that had been stored below the sonic anemometer and was carried past the sensor by an updraft of air.
Seasonal changes in the isotopic composition of photosynthesis
The ecosystem net and canopy gross discrimination values, as well as C4 percentages and associated 95% confidence limits, are presented in Table 6. Our statistical confidence in the calculated C4 percentage for several of the measurement periods (June 1999 in particular) is small for two reasons. First, to resolve meaningful isotopic differences between the two heights, we needed a minimum concentration gradient of 5 ppm (corresponding to an expected δ13C difference of ~0.06‰ for a C4 canopy and ~0.25‰ for a C3 canopy, or 2–8 times the measurement precision of our mass spectrometer). However, we rarely encountered such standing gradients due to the near-continuous winds at the site, so we collected a relatively small number of flasks during each sampling period (Table 6). While the standard errors were reasonable for most measurements, the values from the t-distribution were large because of the small number of sample pairs, producing broad confidence limits. In 2000, the confidence limits were smaller because of the larger number of sample pairs we collected. The second reason for our low statistical confidence is because each C4 percentage calculation requires error propagation through three equations (Eqs. 1, 2, and 4), and the errors add at each step.
In contrast to the nighttime respiration measurements, the C4 percentage through time from the daytime measurements is less variable seasonally, and is generally higher than the corresponding fraction from the respiration measurements, though they are not statistically separated at the 95% confidence level (Fig. 4—open circles with SE).
Discussion
Mass-weighted versus flux-weighted measures of isotopic composition
Our nighttime Keeling plot intercepts represent the autotrophic respiration of C3 and C4 live biomass, as well as heterotrophic metabolism of litter and soil organic matter derived from both plant types. Therefore, these intercepts represent the flux-weighted amount of C3-derived and C4-derived carbon respired by the entire ecosystem. However, we are using the δ13C values of representative C3 and C4 sunlit leaf biomass samples as end members in Eq. 1 to estimate the C4 contribution to ecosystem respiration. This is a mass-weighted measure of isotopic composition, not a flux-weighted measure. The potential discrepancy between the two measures is apparent when examining the isotopic composition of plant dark respiration. While the leaf biomass samples are separated by ~16‰ on average and there is relatively little variation within each pathway (Table 2a), the δ13C values of respiration from leaves and other plant organs are not so homogeneous and distinct. This is apparent in the scatter of values in Table 4. Assuming no fractionation during autotrophic respiration (Lin and Ehleringer 1997; but see Duranceau et al. 1999 and Ghashghaie et al. 2001), this scatter suggests heterogeneity in plant composition, which requires fractionations during biosynthetic reactions. This heterogeneity has been discussed previously (e.g., Farquhar et al. 1989). For example, the relatively depleted carbon isotopic composition of lipids is well known and would contribute to such within-plant heterogeneity (Benner et al. 1987).
These data point out the discrepancy between mass-weighted versus flux-weighted measures of isotopic composition. This discrepancy is also apparent in the δ13C values of upper SOC (top 5 cm) and soil-respired CO2, which are separated by some 2‰. One reason for this discrepancy might be the lack of root tissue in the SOC analysis, while root respiration contributes to the soil-respired CO2 isotope signature. Also, the lower SOC layers (10–20 cm) are very similar to the isotopic composition of soil-respired CO2, and they might disproportionately contribute to the respired flux. On the other hand, differences between the isotope signatures of SOC and soil-respired CO2 are common in systems with C3:C4 spatial and temporal mixtures (Buchmann and Ehleringer 1998; Ehleringer et al. 2000).
We also calculated C4 percentages from the daytime gradients measurements using discrimination measurements from leaf-level gas exchange (flux-weighted end members) with representative C3 and C4 plants as end members. These percentages are given in Table 6 under the 'online' column. In general, they are very similar to the percentages inferred with leaf biomass δ13C end members, although the uncertainties (not shown) are much larger due to the larger standard errors of online discrimination measurements. Miranda et al. (1997) also discuss the use of leaf biomass δ13C end members versus online leaf-level discrimination end members in calculating the C4 percentage from ecosystem respiration Keeling plots.
Impact of C3 and C4 discrimination variations
We did not explicitly examine the influence of seasonal changes in C3 or C4 discrimination on our respiration δ13C trends and inferred C4 percentages. The study of Mole et al. (1994) suggests a trend toward lower C3 discrimination in prairie grasses as the growing season progresses, which would look like more C4 production in our analysis. However, the seasonal variations in discrimination observed by Mole et al. (1994) are not larger than 1–2‰. Our data for online leaf-level discrimination in C3 plants also suggest a decrease in the 2000 growing season (Table 5), although the uncertainties are too large to say this definitively. Because an examination of the seasonal variations in C3 discrimination was not the focus of our study, we collected relatively few samples of air from leaf gas exchange for isotope analysis.
Similarly, although there is evidence of variations in C4 discrimination (Peisker and Henderson 1992; Buchmann et al. 1996), the magnitude is expected to be small during unstressed conditions. For example, Buchmann et al. (1996) showed a sharp increase in \( \Delta _4^{13} \) as light levels dropped below 700 μmol m−2 s−1. However, when weighted by carbon assimilation, this effect should be small as uptake will be decreasing as well. Buchmann et al. (1996) also present evidence of increasing \( \Delta _4^{13} \) during water stress. As Henderson et al. (1992) discuss, short-term online discrimination measurements in C4 species correlate poorly with discrimination inferred from leaf δ13C biomass analyses. Our sparse leaf-level, online discrimination measurements on C4 grasses suggest small seasonal variations (Table 5); however, the mean of all these measurements is close to the discrimination inferred from C4 leaf biomass δ13C data (Table 2).
Diurnal, seasonal and interannual isotopic disequilibria
Only a few studies have investigated the carbon isotopic composition of respiration from ecosystems that contain both C3 and C4 plants. Miranda et al. (1997) collected nighttime ecosystem respiration above a Brazilian savanna during the wet and dry season and calculated the C4 contribution with a mixing model whose end members were based on leaf organic δ13C analyses of representative tree (C3) and grass (C4) species. The C4 contribution to respiration was higher in the dry season (42% vs 30%) at this site, but Miranda et al. (1997) note that the interpretation is complicated by non-steady state vegetation conditions. These conditions would favor a higher C4 contribution than is likely present because some of the C3 carbon fixation is shunted to slowly respiring wood biomass, suggesting that this ecosystem was not in isotopic balance.
Another study by Buchmann and Ehleringer (1998) examined the carbon isotopic composition of ecosystem respiration from both C3 (alfalfa) and C4 (corn) crop canopies planted on soils with rotation histories that alternated between both photosynthetic types. Thus, the SOC at these sites included inputs from the aboveground vegetation at the time of the study, as well as vegetation inputs (of varying photosynthetic pathways) from past crop rotations. In addition to vegetation biomass, they also measured the isotopic composition of SOC and soil respiration at both sites. The δ13C of soil respired-CO2 was the same at both sites, even though the δ13C of SOC was different by up to 8‰. The δ13C values of ecosystem-respired CO2 were also statistically indistinguishable, reflecting the dominant contribution of soil respiration to the overall signature. As a result, these crop sites were not in isotope equilibrium due to their rotation histories.
Finally, in the Yakir and Wang (1996) partitioning study they had to assume a difference in the isotopic composition of photosynthetic and respiratory fluxes over a corn (C4) field and a wheat (C3) field. They were able to measure the δ13C of net uptake directly with a Keeling plot approach, but they used the δ13C of leaf biomass as a proxy for their respiration flux.
Our only opportunity to compare daytime Keeling plot intercepts with nighttime Keeling plot intercepts was in June 2000. Table 6 presents the intercept of a daytime Keeling plot collected on the afternoon of 6 June 2000. The intercept is ~1‰ heavier than the average of nighttime intercepts sampled the previous night, suggesting an isotopic imbalance between canopy photosynthesis and ecosystem respiration, but not at the 95% confidence level. Additionally, the isotope signature of soil respiration was more depleted than that of nighttime ecosystem respiration by ~1‰ in July 2000, implying that canopy respiration was isotopically more enriched than soil respiration. However, the large spatial heterogeneity in δ13C of soil respiration (Table 3) makes it difficult to compare the two approaches. This heterogeneity is also reflected in the δ13C of clipped biomass taken from several locations in the pasture (data not shown).
Another possible explanation for an isotopic imbalance between daytime and nighttime carbon fluxes is different sampling footprints between day and night. Although we have not calculated footprints for this site, to explain the isotope disequilibrium between photosynthesis and respiration due to a footprint effect there would have to be sharp gradients in the photosynthetic composition of the vegetation surrounding the tower (for 'point-in-time' daytime sampling). The daytime horizontal footprint that the 4.5 m level "samples" is ~450 m, or about the same distance that pasture extends from the tower in any direction before a road is encountered. This footprint can be much smaller if sensible and latent heat fluxes are large and there are strong vertical movements of air. Within this pasture, the vegetation is fairly heterogeneous. In addition, the area within several kilometers of the tower is largely pasturelands. It seems unlikely, therefore, that the disequilibrium can be explained entirely by a footprint effect. In general, differences can arise when comparing 'point-in-time' measurements to 'point-in-space' measurements (e.g., sampling temporal concentration gradients during the night and spatial gradients during the day).
It is useful to conduct daytime gradient measurements in combination with nighttime respiration collections to examine possible disequilibria between daytime photosynthesis and nighttime respiration. If this imbalance in isotope fluxes is widespread, it would be notable for two reasons. First, although isotopic disequilibria are incorporated into global inversions that make use of 13CO2 data, they are entirely model-based. There are very few studies that have attempted to measure terrestrial isotopic disequilibria of any sort, and none has demonstrated definitively a carbon isotope disequilibrium between photosynthetic and respiratory fluxes at a site. Second, the modeled terrestrial disequilibrium is based on the observed atmospheric trend in δ13C over the last 150 years, which has been driven by fossil fuel and deforestation fluxes (the Suess effect). Because these fluxes are originally derived from C3 vegetation, they are isotopically lighter than the background atmospheric CO2 and have decreased its δ13C by ~1.4‰ over this period. When combined with large mean residence times in terrestrial ecosystems that induce a lag between the age of carbon fixed and released by ecosystems in a given year, this trend in δ13C produces an isotope disequilibrium flux (Ciais et al. 1995).
The Suess effect disequilibrium is unlikely to vary much on a seasonal or interannual basis. The mean residence time of soil carbon in grassland ecosystems is relatively short (Raich and Schlesinger 1992), and thus any disequilibrium driven by the depletion of atmospheric δ13C resulting from fossil fuel fluxes is likely to be small. Isotopic disequilibria in this tallgrass ecosystem, however, would be driven by seasonal and possibly interannual variations in photosynthetic composition and thus discrimination superimposed on a Suess effect disequilibrium. Such photosynthetic variations will inevitably produce disequilibria fluxes that are independent from disequilibria driven by the trend in atmospheric δ13C. This discrimination disequilibria will be a function of the interplay between recalcitrant and labile pools of carbon and the aboveground photosynthetic mixture. Not only will photosynthetic mixture variations impact the magnitude of the total terrestrial isotope disequilibrium, it is possible they are responsible for some of the interannual variability in δ13C measured at remote monitoring stations as alternating C3:C4 mixtures will produce disequilibria that enrich or deplete the atmosphere in 13C (an interannual trend from more C3 to more C4 will deplete the atmosphere, whereas the reverse will enrich it).
However, this is likely to be true only if the C3:C4 mixtures of tropical savannas change inter-annually, as mixed grasslands like tallgrass prairie are mostly confined to regions of the Great Plains and small parts of Asia and Africa, whereas savannas cover some 17 million km2. Given the large climate variability, direct human disturbance, and high fire frequency that savannas experience, interannual variations in photosynthetic mixtures are likely. Since the net primary production of mixed C3:C4 ecosystems (savannas and grasslands) is ~25 Pg C year−1 (Still et al. 2003), an annual average isotopic imbalance in these systems of 0.2‰ (or –0.2‰ in the case of a C3➔C4 trend) would correspond to an isotope forcing of 5 Pg C ‰ year−1 (−5 Pg C ‰ year−1). This forcing is important in the context of the global 13C budget, as the total land disequilibrium from the Suess effect is estimated to be 20–25 Pg C ‰ year−1. Introducing a C3:C4 disequilibrium of 5 Pg C ‰ year−1 into the standard 13C budget equation with a global terrestrial discrimination of 18‰ would increase the inferred annual ocean sink by 0.3 Pg C (at the expense of the land sink). Thus, these new disequilibria should be investigated to determine their contribution to interannual variations in the land versus ocean partitioning inferred from 13C-based global inversions.
Conclusions
The C3:C4 composition of this ecosystem clearly exhibits seasonal variations in response to climate. As expected, the C4 percentage increases as the temperature increases and the precipitation decreases. The isotopic composition of respiration (Fig. 4, solid circles) provides the best proxy for this transition between photosynthetic types, given its April–September coverage and smaller associated uncertainties (relative to the photosynthesis approach). At our site, the C3:C4 balance is also partly determined by spring fires. A high fire frequency will tend to increase the cover of C4 vegetation, as has been demonstrated at the Konza Prairie LTER site (Collins et al. 1998). This study demonstrates the use of isotopic tracers to provide a strong constraint on seasonal and interannual dynamics in photosynthetic composition, although care must be taken in interpreting daytime measurements due to the potential for isotopic disequilibria between photosynthesis and respiration.
Our measurements are also useful in the context of global carbon cycle research. Although the global coverage of mixed C3:C4 grasslands and savannas are significant, there are very few isotopic measurements in these regions. Because carbon isotopes in atmospheric CO2 are used to constrain the land versus ocean partitioning of the carbon sink (Ciais et al. 1995; Francey et al. 1995; Keeling et al. 1995; Battle et al. 2000) and infer discrimination and thus plant physiology at large scales (Randerson et al. 2002b), basic information on the isotopic composition of carbon fluxes in mixed C3:C4 ecosystems is very useful.
References
Battle M, Bender ML, Tans PP, White JWC, Ellis JT, Conway T, Francey RJ (2000) Global carbon sinks and their variability inferred from atmospheric O2 and δ13C. Science 287:2467–2470
Benner R, Fogel ML, Sprague EK, Hodson RE (1987) Depletion of C-13 in lignin and its implications for stable carbon isotope studies. Nature 329:708–710
Bowling DR, Baldocchi DD, Monson RK (1999) Dynamics of isotope exchange of carbon dioxide in a Tennessee deciduous forest. Global Biogeochem Cycles 13:903–922
Bowling DR, McDowell NG, Bond BJ, Law BE, Ehleringer JR (2002) C-13 content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 131:113–124
Buchmann N, Ehleringer JR (1998) CO2 concentration profiles, and carbon and oxygen isotopes in C3 and C4 crop canopies. Agric For Meteorol 89:45–58
Buchmann N, Brooks JR, Rapp KD, Ehleringer JR (1996) Carbon isotopic composition of C4 grasses is influenced by light and water supply. Plant Cell Environ 19:392–402
Ciais P, Tans PP, Trolier M, White JWC, Francey RJ (1995) A large northern hemisphere terrestrial CO2 sink indicated by the 13C/12C ratio of atmospheric CO2. Science 269:1098–1102
Colello GD, Grivet C, Sellers PJ, Berry JA (1998) Modeling of energy, water, and CO2 flux in a temperate grassland ecosystem with SiB2: May–October 1987. J Atmos Sci 55:1141–1169
Collatz GJ, Berry JA, Clark JS (1998) Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: present, past, and future. Oecologia 114:441–454
Collins SL, Knapp AK, Briggs JM, Blair JM, Steinauer EM (1998) Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 280:745–747
Daniels F, Williams JW, Bender P, Alberty RA (eds) (1962) Experimental physical chemistry. McGraw-Hill, New York, USA, pp 393–417
Duranceau M, Ghashghaie J, Badeck F, Deleens E, Cornic G (1999) Delta C-13 of CO2 respired in the dark in relation to delta C-13 of leaf carbohydrates in Phaseolus vulgaris L-under progressive drought. Plant Cell Environ 22:515–523
Ehleringer JR (1978) Implications of quantum yield differences to the distributions of C3 and C4 grasses. Oecologia 31:255–267
Ehleringer JR, Cerling TE, Helliker BR (1997) C-4 photosynthesis, atmospheric CO2 and climate. Oecologia 112:285-299
Ehleringer JR, Buchmann N, Flanagan LB (2000) Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10:412–422
Ekblad A, Hogberg P (2001) Natural abundance of C-13 in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127:305–308
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher-plants. Aust J Plant Physiol 13:281–292
Farquhar GD, Ehleringer JR, Hubick KT (1989). Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Mol Biol 40:503–537
Flanagan LB, Ehleringer JR (1998) Ecosystem-atmosphere CO2 exchange: interpreting signals of change using stable isotope ratios. Trends Ecol Evol 13:10–14
Flanagan LB, Brooks JR, Varney GT, Berry SC, Ehleringer JR (1996) Carbon isotope discrimination during photosynthesis and the isotope ratio of respired CO2 in boreal forest ecosystems. Global Biogeochem Cycles 10:629–40
Francey RJ, Tans PP, Allison C, Enting IG, White JWC, Trolier M (1995) Changes in oceanic and terrestrial carbon uptake since 1982. Nature 373:326–330
Freeman CC (1998) The flora of Konza prairie: a historical review and contemporary patterns. In: Knapp AK, Briggs JM, Hartnett DC, Collins SL (eds) Grassland dynamics. Oxford University Press, New York, pp 69–80
Friedli H, Siegenthaler U, Rauber D, Oeschger H (1987) Measurements of concentration, 13C/12C and 18O/16O ratios of tropospheric carbon dioxide over Switzerland. Tellus 39B:80–88
Ghashghaie J, Duranceau M, Badeck FW, Cornic G, Adeline MT, Deleens E (2001) Delta C-13 of CO2 respired in the dark in relation to delta C-13 of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ 4:505–515
Harwood KG, Gillon JS, Roberts A, Griffiths H (1999) Determinants of isotope coupling of CO2 and water vapour within a Quercus petraea forest canopy. Oecologia 119:109–119
Henderson SA, von Caemmerer S, Farquhar GD (1992) Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19:263–285
Keeling CD (1958) The concentrations and isotope abundances of atmospheric carbon dioxide in rural areas. Geochem Cosmochim Acta 13:322–334
Keeling CD (1961) The concentrations and isotope abundances of atmospheric carbon dioxide in rural and marine air. Geochem Cosmochim Acta 24:277–298
Keeling CD, Whorf TP, Wahlen M, Vanderplicht J (1995) Interannual extremes in the rate of rise of atmospheric carbon-dioxide since 1980. Nature 375:666-670
Kemp PR, Williams GJ (1980) A physiological basis for niche separation between Agropyron smythii (C3) and Bouteloua gracilis (C4). Ecology 61:846–858
Knapp AK, Briggs JM, Blair JM, Turner CL (1998) Patterns and controls of aboveground net primary production in tallgrass prairie, In: Knapp AK, Briggs JM, Hartnett DC, Collins SL (eds) grassland dynamics. Oxford University Press, New York, pp193-221
Laws E (1997) Mathematical methods for oceanographers. Wiley, New York
Lin GH, Ehleringer JR (1997) Carbon isotopic fractionation does not occur during dark respiration in C-3 and C-4 plants. Plant Physiol 114:391-394
Lloyd J, Kruijt B, Hollinger DY, Grace J, Francey RJ, Wong SC, Kelliher FM, Miranda AC, Farquhar GD, Gash JHC, Vygodskaya NN, Wright IR, Miranda HS, Schulze ED (1996) Vegetation effects on the isotopic composition of atmospheric CO2 at local and regional scales: theoretical aspects and a comparison between rain forest in Amazonia and a boreal forest in Siberia. Aust J Plant Physiol 23:371–399
Long SP (1999) Environmental responses. In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, New York, pp 215–249
Miranda AC, Miranda HS, Lloyd J, Grace J, Francey RJ, McIntyre JA, Meir P, Riggan P, Lockwood R, Brass J (1997) Fluxes of carbon, water and energy over Brazilian cerrado: an analysis using eddy covariance and stable isotopes. Plant Cell Environ 20:315–328
Mole S, Joern A, O'Leary MH, Madhaven S (1994) Spatial and temporal variation in carbon isotope discrimination in prairie graminoids. Oecologia 97:316–321
Morgan MG, Henrion M (1990) Uncertainty: a guide to dealing with uncertainty in quantitative risk and policy analysis. Cambridge University Press, Cambridge
Ode DJ, Tieszen Ll, Lerman JC (1980) The seasonal contribution of C-3 and C-4 plant-species to primary production in a mixed prairie. Ecology 61:1304-1311
Pataki DE, Ehleringer JR, Flanagan LB, Yakir D, Bowling DR, Still CJ, Buchmann N, Berry JA (2003) The application and interpretation of Keeling plots in terrestrial carbon cycle research. Global Biogeochem Cycles (in press)
Peisker M, Henderson SA (1992) Carbon: terrestrial C4 plants. Plant Cell Environ 15:987–1004
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–99
Randerson JT, Collatz GJ, Fessenden JE, Munoz AD, Still CJ, Berry JA, Fung IY, Suits N, Denning AS (2002a) A possible global covariance between terrestrial gross primary production and 13C discrimination: consequences for the atmospheric 13C budget and its response to ENSO. Global Biogeochem Cycles 16:1136
Randerson JT, Still CJ, Balle JJ, Fung IY, Doney SC, Tans PP, Conway TJ, White JWC, Vaughn B, Suits N, Denning AS (2002b) Carbon isotope discrimination of arctic and boreal biomes inferred from remote atmospheric measurements and a biosphere-atmosphere model. Global Biogeochem Cycles 16:1028
Ribas-Carbo M, Still CJ, Berry JA (2002) An automated system for simultaneous analysis of δ13C, δ18O, and CO2 concentration in small air samples. Rapid Commun Mass Spectrom 16:339–345
Sage RF, Wedin DA, Li M (1999) The biogeography of C4 photosynthesis: patterns and controlling factors In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, New York, pp 313–373
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. W.H. Freeman, New York
Still CJ, Berry JA, Collatz GJ, DeFries RS (2003) Global distribution of C3 and C4 vegetation: Carbon cycle implications. Global Biogeochem Cycles 17:1006
Suyker AE, Verma SB (2001) Year-round observations of the net ecosystem exchange of carbon dioxide in a native tallgrass prairie. Global Change Biol 7:279–289
Tieszen LL, Reed BC, Bliss NB, Wylie BK, DeJong DD (1997) NDVI, C3 and C4 production, and distributions in Great Plains grassland land cover classes. Ecol Appl 7:59–78
Yakir D, Sternberg LDL (2000) The use of stable isotopes to study ecosystem gas exchange. Oecologia 123:297–311
Yakir D, Wang XF (1996) Fluxes of CO2 and water between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 380:515–517
Acknowledgements
This research was supported by the Office of Science, Biological and Environmental Research Program (BER), U.S. Department of Energy, through the Great Plains Regional Center of the National Institute for Global Environmental Change (NIGEC) under Cooperative Agreement No. DE-FC03–90ER61010. This research was also supported by an EPA STAR fellowship to C.J.S. We thank S. Verma, G. Burba, and A. Suyker for access to the eddy flux and meteorological data collected at the site. Comments by K. Tu, W. Riley, and anonymous reviewers improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Derivation of discrimination equations
We present a derivation of Eq. 2 for calculating net ecosystem discrimination given average CO2 concentrations and δ13C at 4.5 m (hi) and 0.5 m (lo) above the surface. First, define the isotopic composition of net ecosystem uptake:
Now, define ecosystem discrimination,
and set δ13Catm=δ13Clo.
Substitute and rearrange terms to get Eq. 8:
A derivation of Eq. 4 for correcting net ecosystem discrimination to canopy photosynthetic discrimination follows. First, define the isotopic composition of net ecosystem uptake (with terms defined in the text):
Next, substitute Eq. 9 into Eq. 7 to get this equation:
Now, define canopy photosynthetic discrimination:
Finally, substitute Eq. 11 into Eq. 10 and solve for canopy photosynthetic discrimination:
Rights and permissions
About this article
Cite this article
Still, C.J., Berry, J.A., Ribas-Carbo, M. et al. The contribution of C3 and C4 plants to the carbon cycle of a tallgrass prairie: an isotopic approach. Oecologia 136, 347–359 (2003). https://doi.org/10.1007/s00442-003-1274-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1274-8