Abstract
Populations of northern small rodents have previously been observed to fluctuate in spatial synchrony over distances ranging from tens to hundreds of kilometers between sites. It has been suggested that this phenomenon is caused by common environmental perturbations, mobile predators or dispersal movements. However, very little focus has been given to how the physical properties of the geographic area over which synchrony occurs, such as landscape composition and climate, affect spatial population dynamics. This study reports on the spatial and temporal properties of vole population fluctuations in two areas of western Finland: one composed of large interconnected areas of agricultural farmland interspersed by forests and the other highly dominated by forest areas, containing more isolated patches of agricultural land. Furthermore, the more agricultural area exhibits somewhat milder winters with less snow than the forested area. We found the amplitude of vole cycles to be essentially the same in the two areas, suggesting that the relative amount of predation on small rodents by generalist versus specialist predators is similar in both areas. No seasonal differences in the timing of synchronization were observable for Microtus voles, whereas bank vole populations in field habitats appeared to become synchronized primarily during winter. Microtus populations in field habitats exhibited smaller spatial variation and a higher degree of synchrony in the more continuous agricultural landscape than in the forest-dominated landscape. We suggest that this inter-areal difference is due to differences in the degree of inter-patch connectivity, with predators and dispersal acting as the primary synchronizing agents. Bank vole populations in field habitats were more synchronized within the forest-dominated landscape, most likely reflecting the suitability of the inter-patch matrix and the possibility of dispersal. Our study clearly indicates that landscape composition needs to be taken into account when describing the spatial properties of small rodent population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations of small mammal species in northern regions commonly exhibit pronounced multi-annual cyclic fluctuations in density (Elton 1924; Kalela 1962; Krebs and Myers 1974; Hansson and Henttonen 1985; Stenseth and Saitoh 1998). In the boreal zone of Fennoscandia, these cycles typically have a period of 3–5 years and are manifested most pronouncedly in populations of the vole genera Microtus and Clethrionomys (Hansson and Henttonen 1985, 1988; Korpimäki et al. 1991; Hanski et al. 2001). Characteristic for these cycles is that they often occur in temporal synchrony across various species and taxa, such as shrews, mice, and voles (Hansson 1984; Henttonen 1985; Korpimäki 1986). Furthermore, cyclic small mammal populations commonly fluctuate in spatial synchrony over distances of tens or even hundreds of kilometers (Myrberget 1973; Mackin-Rogalska and Nabaglo 1990; Steen et al. 1996; Ranta and Kaitala 1997; Ims and Andreassen 2000).
The cyclic behavior of Fennoscandian vole populations is currently most commonly thought to be brought about by the combined effects of mortality by specialist predators (Korpimäki and Norrdahl 1998; Hanski et al. 2001; Korpimäki et al. 2002) and winter food limitation (Klemola et al. 2000; Hansson 2002; Huitu et al. 2003). Predation has also been proposed to be the mechanism causing the observed interspecific temporal synchrony in small mammal cycles (Angelstam et al. 1984; Norrdahl and Korpimäki 2000). Factors inducing spatial synchrony in vole population fluctuations, on the other hand, have remained more elusive. This phenomenon has been suggested to potentially arise by three mechanisms: correlated environmental perturbations, dispersal, or predation (reviewed by, e.g., Bjørnstad et al. 1999a; Koenig 1999).
Fluctuating populations with similar structures of density dependence are expected to become synchronized under the influence of spatially correlated environmental disturbances, such as weather or climatic fluctuations. This type of effect, later coined the Moran effect (Royama 1992), was first described by Moran (1953) as an explanation for spatial synchrony in the Canadian lynx (Lynx canadensis) cycle. The Moran effect has since been invoked as a synchronizing mechanism in a number of population studies, both theoretical and empirical (e.g., Hanski and Woiwod 1993; Ranta et al. 1997, 1999; Grenfell et al. 1998; Myers 1998; Cattadori et al. 2000; Benton et al. 2001; Post and Forchhammer 2002). Dispersal has been shown to be able to induce spatial synchrony in population fluctuations through the movement of individuals between populations, albeit at a much more restricted scale than the Moran effect (Blasius et al. 1999; Schwartz et al. 2002). At a scale intermediate to the Moran effect and dispersal, predation by mobile, most often avian, predators has also been suggested to be capable of synchronizing animal population fluctuations (Ydenberg 1987; Korpimäki and Norrdahl 1989; Ims and Steen 1990; Heikkilä et al. 1994; Norrdahl and Korpimäki 1996; Bjørnstad et al. 1999a; Ims and Andreassen 2000). The aforementioned three mechanisms are not mutually exclusive and may act in concert, at different scales, in causing geographically distinct populations to fluctuate in synchrony (Lande et al. 1999; Paradis et al. 1999; Kendall et al. 2000; Ripa 2000).
Although research on the spatial aspect of population dynamics has been extensive, still very little is known about the effects of the actual physical properties of the spatial domain in question on the outcome of population dynamics, especially spatial synchrony. Hansson and Henttonen (1985) analyzed Fennoscandian small mammal trapping series and found a latitudinal gradient in density variations, such that increasing snow cover from south to north was associated with increasingly pronounced small mammal cycles. The observed differences were explained by the relative abundances of generalist and specialist predators of voles, such that southern localities would be able to support more generalists, which in turn are capable of stabilizing vole population dynamics (Andersson and Erlinge 1977; Erlinge et al. 1983; Hansson 1987; Hanski et al. 1991; Korpimäki et al. 2002).
Subsequent studies have indicated that also an increase in the proportion of agricultural land in the landscape predicts a decrease in the amplitude of population cycles (Martinsson et al. 1993; Hansson 1999, 2002). This decrease has likewise been linked to increasing predation pressure by generalist predators, such as corvids and red foxes (Vulpes vulpes), which are suggested to thrive in agricultural surroundings due to an abundance of alternative prey types (Angelstam et al. 1984; Andrén et al. 1985; Martinsson et al. 1993). Hansson (2002) compared the dynamics of small rodents in two different landscape types, both types situated in two regions with differing climatic conditions, and concluded that regional effects override smaller-scale landscape effects in shaping population fluctuations. Along the lines of Hansson and Henttonen (1985), however, the results of Hansson (2002) were also best explained by regional differences in snow cover, which in turn influences the relative rates of specialist and generalist predation.
Landscape structure has also been shown to influence spatial dynamics of small mammal populations. Adler (1994) studied populations of a frugivorous rodent in Panama and concluded that tropical forest fragmentation and habitat isolation increased the level of asynchrony among population fluctuations. Another clear indication of the significance of landscape structure is provided by Sherratt et al. (2002), who reported on the direction of travelling waves in vole population fluctuations being guided by landscape features. Hansson (1999) further postulated that vole population dynamics are influenced to a great extent by human-induced alterations in boreal landscapes, namely, forestry practices, such that the most profitable habitats for small rodents are spatially and temporally all but constant.
The causal mechanism inducing spatial synchrony among a group of populations is commonly interpreted from a measure of how rapidly the degree of synchrony declines with increasing distance between populations (e.g., Hanski and Woiwod 1993; Ranta et al. 1995a; Steen et al. 1996; Cattadori et al. 1999). More often than not, this approach has not taken into account the structure of the space over which the populations are observed. The effects of dispersal, for example, in inducing spatial synchrony are expected to be magnified in homogeneous landscapes comprised of uniform suitable habitat, as compared to landscapes comprised of isolated patches of suitable habitat embedded in a hostile matrix. A number of studies exist indicating that the interchange of individuals between habitat patches decreases with a decrease in their connectivity (e.g., Lawrence 1988; Holyoak and Lawler 1996; Ims and Yoccoz 1997; Andreassen and Ims 1998), but this has not been firmly linked to effects on spatial synchrony. However, Holyoak (2000) demonstrated experimentally with ciliates that a negative association does exist between inter-patch distance and spatial synchrony among populations of both prey and their predators.
This study reports on the characteristics of temporal and spatial dynamics of cyclic vole populations in two areas of western Finland, which differ both in landscape structure and, to some degree, also in climate. Our aim is to address the following questions:
-
1.
Are there inter-areal differences in the temporal properties of the vole cycles?
-
2.
Are there landscape-related inter-areal differences in the spatial dynamics of vole populations?
-
3.
Are there interspecific and habitat-specific differences in spatial population dynamics between Microtus voles (pooled field voles, M. agrestis, and sibling voles, M. rossiaemeridionalis) and bank voles (Clethrionomys glareolus)?
Materials and methods
Study areas and vole trapping
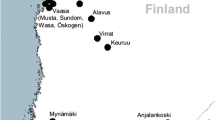
The study area is centered at 63°N, 23°E in western Finland and encompasses ca. 150×120 km (Fig. 1). The area was divided into two (hereafter western and eastern area) on the basis of landscape composition and climatic conditions (FMI 1994; Solantie et al. 1996; Solantie 2000) (Fig. 1). The western area is composed of large interconnected areas of agricultural farmland interspersed by forests and exhibits relatively mild winters and little snow. The eastern area, on the other hand, is highly dominated by forests imbedded with more isolated patches of agricultural farmland and exhibits somewhat colder winters, with a thicker and longer-lasting snow cover (Tables 1 and 2). With respect to predator communities, the species composition and number of breeding owls have both been shown to differ between the landscapes, such that more vole-specialized owls inhabit the western area than the eastern area (Korpimäki 1987).
Location of the western and eastern study areas in western Finland, as defined by the large circles. Dark gray coloration indicates forest, and light gray indicates agricultural field areas and white water bodies. Small circles indicate trapping sites of the western area, and diamonds indicate sites of the eastern area. K and Ä denote the meteorological data collection sites of Kauhava and Ähtäri, respectively (see Table 2)
The small mammal trapping data were collected in six municipalities per area by members of the Ornithological Society of Suomenselkä. Trapping was conducted in one field habitat site and one forest habitat site per municipality, except in Kauhava in the western area, where trapping was carried out in two field habitat sites and one forest habitat site. Field and forest sites within the municipalities were separated by 2–10 km. Trappings were conducted biannually (May and late September–early October) between the years 1979 and 2000, with the length of the trapping series per site ranging from 15 trapping occasions to a maximum of 44 trapping occasions (Table 1). At each site, 20–100 mouse snap traps (Finnish commercial metal traps) were placed in 2–10 rows, with a distance of 10 m between traps. Traps were set for 3–4 nights and checked, and reset if necessary, once a day. Bread was used as bait. All trapping series used in the calculations are derived from trapping indices expressed as individuals trapped per 100 trap nights. All trapping series exhibit cyclic dynamics, with a period of 3–4 years (Fig. 2). Furthermore, long-term trapping data from the western area shows that Microtus voles and bank voles fluctuate in close temporal synchrony (cross-correlation coefficient with no time lag = 0.69; E. Korpimäki, K. Norrdahl, O. Huitu, T. Klemola, unpublished).
Analyses
We characterized the composition of the landscape in the western and eastern areas from the smallest possible non-overlapping circles that encompass all trapping sites (Fig. 1). This was done by calculating by division the total ratio of agricultural land to forest (hereafter field-forest ratio) within the encircled areas (Table 1). We also calculated the municipality-wise field-forest ratio from municipalities where trappings were conducted, to demonstrate the relative evenness in the distribution of agricultural land and forests within the two regions (Table 1). The municipality Kuortane (westernmost site in eastern circle) is grouped into the eastern area, despite exhibiting a field-forest ratio more similar to municipalities in the western area (Table 1). The climatic conditions and general topography of Kuortane, however, are more similar to the eastern than to the western sites (E. Korpimäki, K. Norrdahl, personal observation). Furthermore, the degree of connectivity between Kuortane and the other eastern sites is comparable to the overall degree of connectivity between the eastern sites (Fig. 1). In addition, trial analyses with Kuortane included into the western area indicated that vole dynamics in Kuortane are more similar to the eastern sites than to the western sites. This strongly supports the inclusion of Kuortane into the eastern area. Field and forest area data for the municipalities were obtained from Tomppo et al. (1998) and http://fennica.net.
We compiled separate, municipality-wise trapping series for Microtus voles and bank voles, for both agricultural habitat trappings and forest trappings, thus yielding four sets of trapping series per municipality (six for Kauhava; see above). Both spring and autumn trapping indices were used in the series. Since field voles and sibling voles were rarely caught in the forests, all series for Microtus in the forest trappings were excluded from the analyses. For the remaining series, all analyses were performed separately for each species in both trapping habitats. To identify whether the amplitude of vole cycles differs between the two areas, we computed a coefficient of variation [\( CV = ({\rm{SD}}/\bar X) \times 100 \)] for each trapping series (see Hansson and Henttonen 1985). These were then compared between areas with a standard analysis of variance, using the maximum likelihood method [procedure MIXED, SAS (Littell et al. 1996)].
For analyses of seasonal and landscape effects on spatial variation in population dynamics, all trapping series were ln (x+1)-transformed and standardized to mean = 10 and SD=1, to reduce correlation between the mean and the variance of the series and to remove trapping site-specific differences possibly caused by differences in trapping protocols (Ranta et al. 1995b; Koenig 1999). We adopted a twofold approach to the identification of whether spatial synchronization occurs primarily during the winter or during the summer season and whether this is affected by the structure of the landscape.
First, we calculated coefficients of spatial variation (see formula above) for all separate spring and autumn trapping indices across trapping sites, separately for both areas. We predict that if spatial synchronization were to take place primarily during winter seasons, the spatial variation in the trapping indices would be smaller in the spring trapping sessions than in the autumn trappings, and vice versa. In addition, seasonal effects on synchronization may have impacts of different magnitudes in the two landscape types. Second, we calculated population growth rates r t for each biannual trapping interval (summer and winter) for each trapping series using the formula r t =ln (N t+1/N t ), where N t is the trapping index at time t. Similar to our first approach, we then calculated an index of variation V r for all separate summer and winter growth rates across trapping sites, separately for both areas. Since the mean population growth rate per area was negative between several trapping sessions, and zero between a few, we calculated V r using the formula \( V_r = \left[ {{\rm{SD}}/\left( {|\bar X| + 1} \right) \times 100} \right] \), to avoid obtaining negative indices of variation and to avoid division by zero. Again, we predict that if spatial synchronization were to take place primarily during winter seasons, the spatial variation in population growth rates would be smaller in winter than in summer. In both approaches, differences in the indices of variation were analyzed using an ANOVA with season and area as explanatory variables.
For the analysis of landscape effects on spatial synchrony, we used the biannual population growth rate (r t ; see above for formula) data calculated from the ln (x+1)-transformed trapping series which were standardized to mean = 10 and SD=1. Pairwise cross-correlation analyses (Chatfield 1989) were performed on these series of growth rates between sites, in both areas separately (procedure ARIMA, SAS). The procedure calculates cross-correlation coefficients at various time lags, with high positive values at lag = 0 indicating synchronously fluctuating populations (Ranta et al. 1995b). Although pairwise cross-correlation coefficients calculated from a group of populations are commonly considered statistically non-independent of each other (Ranta et al. 1995b; Koenig 1999), we chose to include all cross-correlation coefficients at lag zero into analyses of covariance, with distance between sites and area as explanatory variables, for descriptive purposes. The assumptions of all analyses of covariance were met, as no interactions were observed between the inter-site distance and area.
Results
The mean (±SE, n=6) field-forest ratio was nearly four times higher in the trapping municipalities of the western area (0.33±0.05) than in those of the eastern area (0.09±0.02) (t -test: t=4.5, P=0.001). The difference in field-forest ratio was even greater on a regional scale, within the area of the circles (west: 0.68, east: 0.14). The mean ratio of trapped Microtus voles over bank voles was roughly 3:1 in the field habitats in the western area and roughly 1:1 in the eastern area. Bank voles were slightly more numerous in forest trappings in the east than in the west (Table 3). No differences were found in the coefficients of variation of the trapping series of either species between the eastern and the western areas (F<2.3, and P>0.2 in all cases), indicating that the amplitude of small rodent cycles was essentially the same in both areas (Table 3).
Microtus voles in field habitats exhibited substantially less spatial variation in trapping indices in the western area than in the eastern area, in both the spring and autumn trapping sessions (Table 4, Fig. 3a). Bank voles in field habitats, on the other hand, exhibited less spatial variation in trapping indices in the spring than in the autumn, regardless of landscape type (Table 4, Fig. 3b). Additionally, the growth rates of Microtus populations in field habitats varied spatially much less in the western than in the eastern area during both seasons (Table 5, Fig. 4a). The spatial variation in the growth rates of bank vole populations in field habitats, on the other hand, did not differ between areas or seasons (Table 5, Fig. 4b). No effects of area or season were detected on the spatial variation in bank vole population indices (Table 4, Fig. 3c) or population growth rates (Table 5, Fig. 4c) in the forest habitats.
The spatial synchrony, as calculated by cross-correlation without time lag, in population fluctuations of Microtus voles in field habitats was higher in the agriculturally more uniform western area than in the more fragmented eastern area (Farea 1,33=78.7, P<0.0001) (Fig. 5a). The degree of synchrony in both areas tended to decline with increasing distance between sites (Fdistance 1,33=4.0, P=0.053). Pairwise cross-correlation coefficients in the western area, however, remained clearly positive at distances of more than 75 km, whereas those in the eastern area did not differ from zero throughout the range of observations (t=0.6, P >0.5) (Fig. 5a). In contrast to Microtus voles, bank vole populations in field habitats exhibited a higher degree of spatial synchrony within the more forested eastern area, as compared to the agricultural western area (Farea 1,33=10.7, P=0.003) (Fig. 5b). The occurrence of bank voles in field habitats did not differ between the areas; one or more bank voles were trapped in exactly 50% of all trapping sessions in both the eastern and the western areas. The degree of spatial synchrony in bank vole populations in forest habitats was clearly positive throughout the spatial range of observations and did not differ between areas (bank voles: Farea 1,27=3.3, P=0.08, Fdistance 1,27 <0.1, P=0.84) (Fig. 5c).
The degree of synchrony among vole population fluctuations in relation to the pairwise distance between trapping sites in two regions of western Finland. The degree of synchrony between two populations is expressed as a cross-correlation coefficient (with time lag = 0), calculated from biannual population growth rates obtained from ln (x+1)-transformed trapping series, which were standardized to mean=10 and SD=1
Discussion
We found the amplitude of vole cycles to be essentially the same in two areas of western Finland: one area composed of large interconnected areas of agricultural farmland interspersed by forests and the other highly dominated by forest areas, containing more isolated patches of agricultural land. The degree of spatial variation and synchrony in the fluctuations of vole populations in field habitats differed markedly between the landscapes. Populations of Microtus voles in their preferred habitats, grassy open areas (Hansson 1971), exhibited substantially less spatial variation in their numbers and dynamics in the western, more continuous agricultural area than in the eastern, more fragmented agricultural area. Bank voles in field habitats exhibited a similar degree of spatial variation in population numbers and growth rates in both areas. However, bank vole populations, being generally more confined to forest habitats (Hansson 1971), fluctuated spatially more synchronously within field habitats in the eastern area than in the western area. Bank voles within forest habitats fluctuated spatially relatively synchronously and similarly in both areas.
Amplitude of vole cycles
We found no differences in the amplitude of vole cycles between the western agricultural area and the eastern forested area. Agriculturally dominated landscapes have previously been shown to exhibit more stable vole dynamics than forest-dominated landscapes because of higher numbers of generalist predators, which may subsist year-round in agricultural habitats on abundant alternative prey types (Erlinge et al. 1983; Angelstam et al. 1984; Andrén et al. 1985; Martinsson et al. 1993). The fact that the agricultural landscape in this study did not exhibit more stable vole dynamics than the forested landscape cannot be explained by a lack of generalist predators in the western area. The species composition in communities of owls, commonly classified as functionally generalized predators (Hanski et al. 1991; see also Korpimäki and Krebs 1996), has been found to differ between the areas, such that the proportion of species within the community that feed primarily on voles [e.g., the long-eared owl (Asio otus), the short-eared owl (A. flammeus), and Tengmalm's owl (Aegolius funereus)], as well as the total number of their nesting pairs, increases from east to west (Korpimäki 1987). The agricultural landscape also supports substantial populations of other generalist-type predators, such as Eurasian kestrels (Falco tinnunculus), corvids (magpies [Pica pica]), hooded crows (Corvus corone), and, although not highly numerous, red foxes (Korpimäki and Norrdahl 1991; Norrdahl and Korpimäki 2002). Nonetheless, the abundance of generalist predators was apparently not sufficient enough to prevent the cyclic dynamics exhibited by vole populations in the western area (Fig. 2)
Although meteorological data indicate a decrease in snow depth and duration of snow cover from the eastern area to the western area (FMI 1994; Solantie et al. 1996; Solantie 2000), we believe that the sheltering effect of snow for voles is not markedly different between the two areas. The most stable year-round prime habitats for small rodents in agricultural landscapes are grassy edges of ditches and barns, which are spared from annual plowings (Norrdahl and Korpimäki 1993). In vast open landscapes, such as in the western study area, wind-driven snow rapidly fills these open ditches and the edges of barns during the onset of the winter season, while little snow cover accumulates on the agricultural fields themselves or on sites where snow-depth data is collected. The snow cover in ditches often accumulates to the brim, and very rarely melts away during warmer spells, when most field areas become bare. This holds also for spring, as ditches may retain their snow cover for up to a few weeks longer than the surrounding field areas. Furthermore, due to the effect of wind, the snow cover in the ditches has a very hard crust for the greatest part of the winter, making predation on voles by supranivean, especially avian, predators virtually impossible. In effect, voles are as, if not more, sheltered from predation in the western, more agricultural landscape than in the forested east, regardless of the relative numbers of generalist and specialist predators present.
Spatial variation in population sizes and growth rates
The spatial dynamics of Microtus voles in field habitats were consistently, throughout the year, more uniform in the western agricultural landscape than in the eastern forested landscape. We could not, therefore, identify either summer or winter as the main season of synchronization for Microtus. The observed inter-areal differences in the degree of spatial variation were more likely related to landscape composition than to differences in climate. Based on meteorological data alone, we might have expected stronger wintertime synchronization of Microtus population fluctuations within the western area, with less snow, than in the eastern area. However, for reasons mentioned above, vole populations in both areas appeared to be relatively equally sheltered by snow cover during the winter from the synchronizing impact of either recurrent environmental perturbations or mobile predators. We therefore suggest that the smaller degree of spatial variation in Microtus population dynamics in the western area is to a great extent brought about by a higher degree of connectivity between trapping sites, enabling more effective and regular movements of both the voles and their predators (Holyoak and Lawler 1996; Blasius et al. 1999).
Bank vole populations in field habitats exhibited a higher degree of spatial variation in autumn trapping indices than in spring trapping indices in both areas. No inter-areal or seasonal differences were observed in the spatial variation of population growth rates. This may imply that bank vole populations in field habitats become primarily synchronized during winter months. If so, this effectively precludes mobile avian predators as the main synchronizing agent of these populations, as the majority of vole-eating birds of prey migrate out of Northern Europe for the winter months (Mikkola 1983; Sonerud 1986; Korpimäki 1992) and vole populations become protected by snow cover. If synchronization of bank vole populations were to take place primarily during the winter, also dispersal as a causal mechanism would have to be largely dismissed (Lomolino 1989; see also Mihok 1984), thus indicating either a Moran effect or mammalian predation in action as a synchronizing mechanism. The most important mammalian predators of voles in the study area, least weasels (Mustela nivalis) and stoats (M. erminea) (Norrdahl and Korpimäki 1995), have been shown to concentrate their hunting efforts into agricultural habitats (Klemola et al. 1999), and their potential influence on bank vole populations during winter can be considered substantial.
The observations of the seasonal differences in bank vole synchronization must, however, be treated with caution, as autumn trappings were generally carried out towards the end of their breeding season, when territorial behavior begins breaking down before winter (Ylönen and Viitala 1985, 1991). An increase in movement associated with this process predisposes bank voles to snap traps, possibly resulting in inflated population indices. If the breakdown in territoriality occurs asynchronously within an area, depending, for instance, on vole density, the spatial variation in autumn bank vole indices would become temporarily abnormally elevated. However, this explanation is challenged by the fact that bank vole populations in forest habitats, where the breakdown in territoriality occurs equally, did not exhibit the aforementioned seasonal difference in spatial variation.
Spatial population synchrony
Populations of Microtus voles in field habitats fluctuated in closer synchrony in the western agricultural landscape than in the eastern forested landscape. Pairwise cross-correlation coefficients for the western area remained above roughly 0.5 through the range of observations, at distances of up to 80 km (Fig. 5). Synchrony between populations in the eastern area, on the other hand, was effectively nonexistent. Both areas exhibited a slight tendency for the degree of synchrony to decline with increasing distance between sites (Fig. 5a). Previously, the degree of synchrony among vole populations in Finland has been suggested to be high over distances of even hundreds of kilometers (Kalela 1962; Henttonen et al. 1977), which suggests a Moran effect as a likely synchronizing mechanism (Ranta et al. 1995a). Elsewhere, more detailed studies at a smaller scale have indicated that synchrony among populations of arvicoline rodents may prevail at distances of up to only 50 km between sites (Steen et al. 1996; Lambin et al. 1998; Bjørnstad et al. 1999b; MacKinnon et al. 2001). Such an extent of synchrony most strongly supports dispersal as a causal agent (Steen et al. 1996).
Our results suggest that neither a Moran effect nor dispersal is alone adequate for causing the observed patterns of spatial synchrony between Microtus populations in field habitats. Based on the degree and extent of synchrony in the western area, a Moran effect would be a plausible causal agent, as synchrony remains remarkably high at distances nearing 80 km between sites, with only a slight decrease in strength with increasing distance. This extent clearly exceeds previous estimates of the potentially synchronizing effect of dispersal movements in arvicoline rodents (30–50 km) (Steen et al. 1996; Bjørnstad et al. 1999b). In the eastern area, however, at comparable distances, Microtus populations in field habitats did not fluctuate synchronously (Fig. 5a). Although the two areas were defined partly on the basis of climatic features, we do not believe that the areas differ to such a great extent in the frequency and magnitude of environmental perturbations as the differences in synchrony might suggest. In the case of a pure Moran effect synchronizing populations in our study area, we would expect to observe more similar patterns of synchrony between the western and the eastern areas. Because we do not, we believe that landscape composition, and the degree of connectivity between trapping sites, plays a major role in producing the observed patterns in spatial synchrony.
Avian predators have been suggested to be capable of inducing regional synchrony among small rodent populations (Ydenberg 1987; Korpimäki and Norrdahl 1989; Ims and Steen 1990; see also Petty et al. 2000); this suggestion also has received experimental support (Norrdahl and Korpimäki 1996; Ims and Andreassen 2000). Heikkilä et al. (1994) also suggested that stoats might be capable of synchronizing vole population fluctuations. The synchronizing effect of these predators is naturally dependent upon their scale of operation, as well as on their preferences of habitat. Furthermore, landscape composition may influence synchronizing effects. In a spatially relatively homogeneous environment, such as one comprised of agricultural fields, landscape structures cannot be considered to greatly hinder movements of predators between hunting grounds. In effect, the costs of travel for predators in this kind of environment are small (Bernstein et al. 1991). In a spatially heterogeneous environment, predators would be expected to forage in prey patches and subsequently migrate between patches whenever the energetic gain in their current patch declines to a level lower than that of the region-wide average (Bernstein et al. 1999). Bernstein et al. (1991) have shown that when the costs of between-patch travel for predators are increased, e.g., in the form of increasing distance between patches or increasing unsuitability of the habitat in between prey patches, predators tend to become more sedentary and unwilling to leave their current patch, resulting in an uneven distribution of predators in relation to prey abundance.
We suggest that this may be a plausible explanation for the observed inter-areal differences in synchrony among field-habitat Microtus populations in our study area. As shown earlier (Korpimäki and Norrdahl 1991; Klemola et al. 1999), most vole-eating predators in our study area prefer agricultural farmland habitats for hunting. The western continuous agricultural landscape enables predators to distribute their hunting efforts relatively evenly among field habitats, thus yielding substantial potential for synchronization. The eastern landscape, however, confines predators more rigidly to the individual isolated agricultural fields, with high travel costs between patches. This applies to both mustelids and birds of prey, particularly during the reproductive season, when the predators must act as central place foragers. This spatial aggregation in turn may result in spatially more independent predator-prey interactions and, consequently, the observed lower degree of synchrony between sites in the eastern area.
Bank vole populations in field habitats exhibited a higher degree of spatial synchrony in the eastern, forest-dominated landscape than in the western, more agricultural area. Field trapping sites in the eastern area are surrounded by a matrix of forests, the main habitat of bank voles (Hansson 1971), which harbor few Microtus, the dominant competitor of the bank vole (Hansson 1983; Hanski and Henttonen 1996). Field trapping sites in the west, on the other hand, are part of, and to a great extent surrounded by, vast expanses of agricultural farmland, mostly inhabited by Microtus voles. This effectively decreases the connectivity of the trapping sites for bank voles. Furthermore, due to the cyclic nature of vole populations in our study area (e.g., Korpimäki et al. 1991, 2002), bank voles often become periodically extirpated from field habitats. Although bank voles occurred equally frequently in field trappings in both areas, recolonization by voles into fields following these extirpations is most likely both temporally and spatially more irregular in the western area. This may be due to either the competitive effect of the presence of Microtus or a high risk of predation in the fields (Klemola et al. 1999). Recolonization by bank voles into fields in the eastern area is conversely expected to be more predictable due to larger source populations in the surrounding forests and relatively low numbers of competing resident Microtus.
The spatial synchrony among bank vole populations in forest habitats was positive throughout the range of observations but did not differ between the two landscapes. The overall level of bank vole synchrony in the forests was similar to the eastern field sites. Judging from the extent of synchrony (strong positive correlations at distances of up to 90 km), bank vole populations in forests throughout our study area may be synchronized by the action of a Moran-type effect, such as weather.
Conclusions
We found that the composition of the landscape greatly affects the spatial, but not the temporal, properties of vole cycles. The cyclic dynamics of vole population fluctuations did not appear to become more stable with increasing amounts of agricultural habitat in the landscape, as has previously been suggested. However, the spatial dynamics of these fluctuations showed clear landscape-related differences between the areas. Microtus voles exhibited a higher degree of spatial synchrony in a more agricultural landscape, as compared to a forested landscape. The spatial dynamics of bank voles displayed an opposite pattern. Bank voles also exhibited a seasonal difference in the timing of synchronization, with winters having a stronger effect than summers. The observed inter-areal differences in spatial population dynamics can most likely be attributed to the structure of the landscape and, through this, the degree of connectivity between habitat patches. Our results therefore indicate that landscape composition needs to be taken into account when describing patterns and causes of spatial synchrony in animal population fluctuations. More research, aimed specifically at untangling the relative influences of predators, dispersal, and climate on synchrony, is needed. This can be achieved by large-scale monitoring schemes in various environments that enable the controlling of each possible mechanism separately.
References
Adler GH (1994) Tropical forest fragmentation and isolation promote asynchrony among populations of a frugivorous rodent. J Anim Ecol 63:903–911
Andersson M, Erlinge S (1977) Influence of predation on rodent populations. Oikos 29:591–597
Andreassen HP, Ims RA (1998) The effects of experimental habitat destruction and patch isolation on space use and fitness parameters in female root vole Microtus oeconomus. J Anim Ecol 67:941–952
Andrén H, Angelstam P, Lindström E, Widén E (1985) Differences in predation pressure in relation to habitat fragmentation: an experiment. Oikos 45:273–277
Angelstam P, Lindström E, Widen P (1984) Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 62:199–208
Benton TG, Lapsley CT, Beckerman AP (2001) Population synchrony and environmental variation: an experimental demonstration. Ecol Lett 4:236–243
Bernstein C, Kacelnik A, Krebs CJ (1991) Individual decisions and the distribution of predators in a patchy environment. II. The influence of travel costs and structure of the environment. J Anim Ecol 60:205–225
Bernstein C, Auger P, Poggiale JC (1999) Predator migration decisions, the ideal free distribution, and predator-prey dynamics. Am Nat 153:267–281
Bjørnstad ON, Ims RA, Lambin X (1999a) Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol Evol 11:427–432
Bjørnstad ON, Stenseth NC, Saitoh T (1999b) Synchrony and scaling in dynamics of voles and mice in northern Japan. Ecology 80:622–637
Blasius B, Huppert A, Stone L (1999) Complex dynamics and phase synchronization in spatially extended systems. Nature 399:354–359
Cattadori IM, Hudson PJ, Merler S, Rizzoli A (1999) Synchrony, scale and temporal dynamics of rock partridge (Alectoris graeca saxatilis) populations in the Dolomites. J Anim Ecol 68:540–549
Cattadori IM, Merler S, Hudson PJ (2000) Searching for mechanisms of synchrony in spatially structured gamebird populations. J Anim Ecol 69:620–638
Chatfield C (1989) The analysis of time series: an introduction. Chapman & Hall, London
Elton CS (1924) Periodic fluctuations in numbers of animals: their causes and effects. Br J Exp Biol 2:119–163
Erlinge S, Göransson G, Hansson L, Högstedt G, Liberg O, Nilsson IN, Nilsson T, von Schantz T, Sylvén M (1983) Predation as a regulating factor in small rodent populations in southern Sweden. Oikos 40:36–52
FMI (1994) Monthly climate observations in Finland. Finnish Meteorological Institute, Helsinki
Grenfell BT, Wilson K, Finkenstädt BF, Coulson TC, Murray S, Albon SD, Pemberton JM, Clutton-Brock TH, Crawley MJ (1998) Noise and determinism in synchronized sheep dynamics. Nature 391:674–677
Hanski I, Henttonen H (1996) Predation on competing vole species: a simple explanation of complex patterns. J Anim Ecol 65:220–232
Hanski I, Woiwod IP (1993) Spatial synchrony in the dynamics of moth and aphid populations. J Anim Ecol 62:656–668
Hanski I, Hansson L, Henttonen H (1991) Specialist predators, generalist predators, and the microtine rodent cycle. J Anim Ecol 60:353–367
Hanski I, Henttonen H, Korpimäki E, Oksanen L, Turchin P (2001) Small-rodent dynamics and predation. Ecology 82:1505–1520
Hansson L (1971) Small rodent food, feeding and population dynamics: a comparison between granivorous and herbivorous species in Scandinavia. Oikos 22:183–198
Hansson L (1983) Competition between rodents in successional stages of taiga forests: Microtus agrestis vs. Clethrionomys glareolus. Oikos 40:258–266
Hansson L (1984) Predation as the factor causing extended low densities in microtine cycles. Oikos 43:255–256
Hansson L (1987) An interpretation of rodent dynamics as due to trophic interactions. Oikos 50:308–318
Hansson L (1999) Intraspecific variation in dynamics: small rodents between food and predation in changing landscapes. Oikos 86:159–169
Hansson L (2002) Dynamics and trophic interactions of small rodents: landscape or regional effects on spatial variation? Oecologia 130:259–266
Hansson L, Henttonen H (1985) Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia 67:394–402
Hansson L, Henttonen H (1988) Rodent dynamics as community processes. Trends Ecol Evol 3:195–200
Heikkilä J, Below A, Hanski I (1994) Synchronous dynamics of microtine rodent populations on islands in Lake Inari in northern Fennoscandia: evidence for regulation by mustelid predators. Oikos 70:245–252
Henttonen H (1985) Predation causing extended low densities on microtine cycles: further evidence from shrew dynamics. Oikos 44:156–157
Henttonen H, Kaikusalo A, Tast J, Viitala J (1977) Interspecific competition between small rodents in subarctic and boreal ecosystems. Oikos 29:581–590
Holyoak M (2000) Habitat patch arrangement and metapopulation persistence of predators and prey. Am Nat 156:378–389
Holyoak M, Lawler SP (1996) The role of dispersal in predator-prey metapopulation dynamics. J Anim Ecol 65:640–652
Huitu O, Koivula M, Korpimäki E, Klemola T, Norrdahl K (2003) Winter food supply limits growth of northern vole populations in the absence of predation. Ecology (in press)
Ims RA, Andreassen HP (2000) Spatial synchronization of vole population dynamics by predatory birds. Nature 408:194–196
Ims RA, Steen H (1990) Geographical synchrony in microtine population cycles: a theoretical evaluation of the role of avian predators. Oikos 57:381–387
Ims RA, Yoccoz NG (1997) Studying transfer in metapopulations: Emigration, migration and colonization. In: Hanski I, Gilpin M (eds) Metapopulation biology: ecology, genetics and evolution. Academic Press, London, pp 247–265
Kalela O (1962) On the fluctuations in the numbers of arctic and boreal small rodents as a problem of production biology. Ann Acad Sci Fenn (Ser A IV B) 66:1–38
Kendall BE, Bjørnstad ON, Bascompte J, Keitt TH, Fagan WF (2000) Dispersal, environmental correlation, and spatial synchrony in population dynamics. Am Nat 155:628–636
Klemola T, Korpimäki E, Norrdahl K, Tanhuanpää M, Koivula M (1999) Mobility and habitat utilization of small mustelids in relation to cyclically fluctuating prey abundances. Ann Zool Fenn 36:75–82
Klemola T, Koivula M, Korpimäki E, Norrdahl K (2000) Experimental tests of predation and food hypotheses for population cycles of voles. Proc R Soc Lond B 267:351–356
Koenig WD (1999) Spatial autocorrelation of ecological phenomena. Trends Ecol Evol 14:22–26
Korpimäki E (1986) Predation causing synchronous decline phases in microtine and shrew populations in western Finland. Oikos 46:124–127
Korpimäki E (1987) Composition of owl communities in four areas in western Finland: importance of habitats and interspecific competition. Acta Regiae Soc Sci Litt Gothob Zool 14:118–123
Korpimäki E (1992) Population dynamics of Fennoscandian owls in relation to wintering conditions and between-year fluctuations of food. In: Galbraith CA, Taylor IR, Percival S (eds) The ecology and conservation of European owls. Joint Nature Conservation Committee (UK Nature Conservation, No 5), Peterborough, pp 1–10
Korpimäki E, Krebs CJ (1996) Predation and population cycles of small mammals: a reassessment of the predation hypothesis. BioScience 46:754–764
Korpimäki E, Norrdahl K (1989) Predation of Tengmalm's owls: numerical responses, functional responses and dampening impact on population fluctuations of microtines. Oikos 54:154-164
Korpimäki E, Norrdahl K (1991) Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology 72:814–826
Korpimäki E, Norrdahl K (1998) Experimental reduction of predators reverses the crash phase of small-rodent cycles. Ecology 79:2448–2455
Korpimäki E, Norrdahl K, Rinta-Jaskari T (1991) Responses of stoats and least weasels to fluctuating food abundances: is the low phase of the vole cycle due to mustelid predation? Oecologia 88:552–561
Korpimäki E, Norrdahl K, Klemola T, Pettersen T, Stenseth NC (2002) Dynamic effects of predators on cyclic voles: field experimentation and model extrapolation. Proc R Soc Lond B 269:991–997
Krebs CJ, Myers JH (1974) Population cycles in small mammals. Adv Ecol Res 8:267–399
Lambin X, Elston DA, Petty SJ, MacKinnon JL (1998) Spatial asynchrony and periodic travelling waves in cyclic populations of field voles. Proc R Soc Lond B 265:1491–1496
Lande R, Engen S, Saether B-E (1999) Spatial scale of population synchrony: environmental correlation versus dispersal and density regulation. Am Nat 154:271–281
Lawrence WS (1988) Movement ecology of the red milkweed beetle in relation to population size and structure. J Anim Ecol 57:21–35
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SASSystem for Mixed Models. SAS Institute, Cary, NC
Lomolino MV (1989) Bioenergetics of cross-ice movements by Microtus pennsylvanicus, Peromyscus leucopus and Blarina brevicauda. Holarct Ecol 12:213–218
MacKinnon JL, Petty SJ, Elston DA, Thomas CJ, Sherrat TN, Lambin X (2001) Scale invariant spatio-temporal patterns of field vole density. J Anim Ecol 70:101–111
Mackin-Rogalska R, Nabaglo L (1990) Geographic variation in cyclic periodicity and synchrony in the common vole, Microtus arvalis. Oikos 59:343–348
Martinsson B, Hansson L, Angelstam P (1993) Small mammal dynamics in adjacent landscapes with varying predator communities. Ann Zool Fenn 30:31–42
Mihok S (1984) Life history profiles of boreal meadow voles (Microtus pennsylvanicus). Winter ecology of small mammals. Spec Publ Carnegie Mus Nat Hist 10:91–102
Mikkola H (1983) Owls of Europe. T & A D Poyser, Carlton
Moran PAP (1953) The statistical analysis of the Canadian lynx cycle. I. Structure and prediction. Aust J Zool 1:163–173
Myers JH (1998) Synchrony in outbreaks of forest Lepidoptera: a possible example of the Moran effect. Ecology 79:1111–1117
Myrberget S (1973) Geographical synchronism of cycles of small rodents in Norway. Oikos 24:220–224
Norrdahl K, Korpimäki E (1993) Predation and interspecific competition in two Microtus voles. Oikos 67:149–158
Norrdahl K, Korpimäki E (1995) Mortality factors in a cyclic vole population. Proc R Soc Lond B 261:49–53
Norrdahl K, Korpimäki E (1996) Do nomadic avian predators synchronize population fluctuations of small mammals? A field experiment. Oecologia 107:478–483
Norrdahl K, Korpimäki E (2000) Do predators limit the abundance of alternative prey? Experiments with vole-eating avian and mammalian predators. Oikos 91:528–540
Norrdahl K, Korpimäki E (2002) Seasonal changes in the numerical responses of predators to cyclic vole populations. Ecography 25:428–438
Paradis E, Baillie SR, Sutherland WJ, Gregory RD (1999) Dispersal and spatial scale affect synchrony in spatial population dynamics. Ecol Lett 2:114–120
Petty SJ, Lambin X, Sherratt TN, Thomas CJ, Mackinnon JL, Coles CF, Davison M, Little B (2000) Spatial synchrony in field vole Microtus agrestis abundance in coniferous forest in northern England: the role of vole-eating raptors. J Appl Ecol 37:136–147
Post E, Forchhammer MC (2002) Synchronization of animal population dynamics by large-scale climate. Nature 420:168–171
Ranta E, Kaitala V (1997) Travelling waves in vole population dynamics. Nature 390:456
Ranta E, Kaitala V, Lindström J, Lindén H (1995a) Synchrony in population dynamics. Proc R Soc Lond B 262:113–118
Ranta E, Lindström J, Lindén H (1995b) Synchrony in tetraonid population dynamics. J Anim Ecol 64:767–776
Ranta E, Kaitala V, Lundberg P (1997) Population variability in space and time: the dynamics of synchronous population dynamics. Science 278:1621–1623
Ranta E, Kaitala V, Lindström J (1999) Spatially autocorrelated disturbances and patterns in population synchrony. Proc R Soc Lond B 266:1851–1856
Ripa J (2000) Analysing the Moran effect and dispersal: their significance and interaction in synchronous population dynamics. Oikos 90:175–187
Royama T (1992) Analytical population dynamics. Chapman & Hall, London
Schwartz MK, Mills LS, McKelvey KS, Ruggiero LF, Allendorf FW (2002) DNA reveals high dispersal synchronizing the population dynamics of Canada lynx. Nature 415:520–522
Sherratt JA, Lambin X, Thomas CJ, Sherratt TN (2002) Generation of periodic waves by landscape features in cyclic predator-prey systems. Proc R Soc Lond B 269:327–334
Solantie R (2000) Snow depth on January 15th and March 15th in Finland 1919–98, and its implications for soil frost and forest ecology. Meteorological Publications no 42, Finnish Meteorological Institute, Helsinki
Solantie R, Drebs A, Hellsten E, Saurio P (1996) Timing and duration of snow cover in Finland during 1961–1993. Meteorological Publications no 34, Finnish Meteorological Institute, Helsinki
Sonerud GA (1986) Effect of snow cover on seasonal changes in diet, habitat, and regional distribution of raptors that prey on small mammals in boreal zones of Fennoscandia. Holarct Ecol 9:33–47
Steen H, Ims RA, Sonerud GA (1996) Spatial and temporal patterns of small-rodent population dynamics at a regional scale. Ecology 77:2365–2372
Stenseth NC, Saitoh T (1998) The population ecology of the vole Clethrionomys rufocanus: a preface. Res Popul Ecol 40:1–3
Tomppo E, Katila M, Moilanen J, Mäkelä H, Peräsaari J (1998) Kunnittaiset metsävaratiedot 1990–94. Folia For 4B:619–839
Ydenberg RC (1987) Nomadic predators and geographical synchrony in microtine population cycles. Oikos 50:270–272
Ylönen H, Viitala J (1985) Social organization of an enclosed winter population of the bank vole Clethrionomys glareolus. Ann Zool Fenn 22:353–358
Ylönen H, Viitala J (1991) Social overwintering and food distribution in the bank vole Clethrionomys glareolus. Holarct Ecol 14:131–137
Acknowledgements
We would like to thank the members of the Ornithological Society of Suomenselkä, who conducted the extensive fieldwork and provided us with the data used in this study. In particular, we want to acknowledge the contributions of J. Ryssy, O. Ihantola, J. Kolari, J. Koskela, J. Löytömäki, E. Rajala, E. Rautiainen, P. Sulkava, R. Sulkava, R. Viitasaari, and T. Yks-Petäjä. Harri Hakkarainen, Tero Klemola, and Janne Sundell provided valuable comments on the manuscript. This study was financially supported by the Turku University foundation and the Graduate School of Evolutionary Ecology (grants to O.H.) and the Academy of Finland (grants to E.K.; project no. 63525, 64542, 69014, 71110, and 74131).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huitu, O., Norrdahl, K. & Korpimäki, E. Landscape effects on temporal and spatial properties of vole population fluctuations. Oecologia 135, 209–220 (2003). https://doi.org/10.1007/s00442-002-1171-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-002-1171-6