Abstract
We evaluated the hypothesis that CO2 uptake by a subalpine, coniferous forest is limited by cool temperature during the growing season. Using the eddy covariance approach we conducted observations of net ecosystem CO2 exchange (NEE) across two growing seasons. When pooled for the entire growing season during both years, light-saturated net ecosystem CO2 exchange (NEEsat) exhibited a temperature optimum within the range 7–12°C. Ecosystem respiration rate (R e), calculated as the y-intercept of the NEE versus photosynthetic photon flux density (PPFD) relationship, increased with increasing temperature, causing a 15% reduction in net CO2 uptake capacity for this ecosystem as temperatures increased from typical early season temperatures of 7°C to typical mid-season temperatures of 18°C. The ecosystem quantum yield and the ecosystem PPFD compensation point, which are measures of light-utilization efficiency, were highest during the cool temperatures of the early season, and decreased later in the season at higher temperatures. Branch-level measurements revealed that net photosynthesis in all three of the dominant conifer tree species exhibited a temperature optimum near 10°C early in the season and 15°C later in the season. Using path analysis, we statistically isolated temperature as a seasonal variable, and identified the dynamic role that temperature exhibits in controlling ecosystem fluxes early and late in the season. During the spring, an increase in temperature has a positive effect on NEE, because daytime temperatures progress from near freezing to near the photosynthetic temperature optimum, and Re values remain low. During the middle of the summer an increase in temperature has a negative effect on NEE, because inhibition of net photosynthesis and increases in R e. When taken together, the results demonstrate that in this high-elevation forest ecosystem CO2 uptake is not limited by cool-temperature constraints on photosynthetic processes during the growing-season, as suggested by some previous ecophysiological studies at the branch and needle levels. Rather, it is warm temperatures in the mid-summer, and their effect on ecosystem respiration, that cause the greatest reduction in the potential for forest carbon sequestration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forested ecosystems cover a large expanse of the global terrestrial surface area, represent a large global sink for CO2, and strongly influence the dynamics of the global carbon cycle (Houghton 1991; Wisniewski and Lugo 1992; Schimel 1995; Pacala et al. 2001; Schimel et al. 2001). Predictions from models of forest activity suggest that this CO2 sink may not be maintained at its current level in a warmer climate (Schimel et al. 1994; Grace and Rayment 2000). In light of the current increase in atmospheric CO2 and the potential for forest carbon sequestration to provide a negative feedback on this trend, the development of a mechanistic understanding of the controls over forest activity is crucial (Rosenberg et al. 1983; Schimel 1995; Sellers et al. 1996; Schlesinger 1997; Keeling and Whorf 1998; Delucia et al. 1999; Field 1999; Law et al. 1999, 2001; Canadel et al. 2000). Studies of forest CO2 uptake have increased in number recently, especially following the establishment of tower flux networks and their emphasis on long-term continuous monitoring using the eddy covariance technique (Wofsy et al. 1993; Black et al. 1996; Goulden et al. 1996, 1997; Greco and Baldocchi 1996; Baldocchi et al. 1997; Jarvis et al. 1997; Hollinger et al. 1998; Malhi et al. 1998; Anthoni et al. 1999; Aubinet et al. 2000; Goldstein et al. 2000; Granier et al. 2000; Law et al. 2001; Monson et al. 2002).
The development of ecosystem-level perspectives on net CO2 exchange is relatively new to the field of plant ecophysiology, and this new perspective has not been fully integrated with past studies that were commonly conducted at the leaf and branch scales. For example, in the subalpine, coniferous forest ecosystem, past studies of branch-level photosynthesis resulted in the hypothesis that low temperatures throughout the spring and summer constrain plant performance (DeLucia and Smith 1987; Smith and Knapp 1990; Day et al. 1991; Teskey et al. 1995). Mid-summer rates of needle net CO2 fixation at ambient temperature have been reported to be 50–60% of the maximum (Smith and Carter 1988), with the implication that an increase in ambient temperature would result in increased net photosynthesis. In fact, a strong case has been made for the adaptive nature of the morphology of subalpine conifer shoots in maintaining needle temperatures substantially above air temperature (Smith and Brewer 1994). In this study, we have used 2 years of continuous measurements of net ecosystem CO2 exchange at the Niwot Ridge Ameriflux site to test the hypothesis that cool temperatures during the growing season, and their effect on photosynthetic processes, represent a primary constraint on forest carbon sequestration. If this hypothesis could not be supported, we aimed to elucidate the alternative constraint(s) on forest carbon sequestration. In testing this hypothesis, we particularly focused on whether a consistent line of insight into the role of temperature as a constraint on ecosystem-level carbon sequestration could be obtained from studies at the scales of individual branches and the entire ecosystem.
Materials and methods
Study site
This study was conducted at the Niwot Ridge Ameriflux site, in a subalpine forest at approximately 3,050 m elevation above sea level (40°1′58″N, 105°32′47″W). The forest is approximately 100 years old, having regenerated following clear-cut logging in the early 1900s, and it consists primarily of lodgepole pine (Pinus contorta), Engelmann spruce (Picea engelmannii), and subalpine fir (Abies lasiocarpa). The average canopy height is 11.4 m and the average mid-summer leaf-area index is 4.2 m2 m-2.
Ecosystem-level CO2 fluxes
Net ecosystem CO2 exchange (NEE) was measured using the eddy covariance technique, with instruments mounted at 21.5 m on a 26-m scaffolding tower. Details of the eddy covariance measurements have been provided in two separate papers, including co-spectral analyses of the fluxes, dependence of fluxes on above-canopy friction velocity, gap-filling procedures, statistical screening for stationarity, and a complete analysis of the ecosystem energy budget, including efforts at energy budget closure (Monson et al. 2002; Turnipseed et al. 2002). Briefly, fluxes of CO2 and H2O were measured using a triaxial sonic anemometer (SWS-211/3 K, Applied Technologies, Boulder, Colo., USA) and a closed-path infrared gas analyzer (Li-Cor 6262, Li-Cor, Lincoln, Neb., USA). Measurements were aligned with the mean wind streamlines (Kaimal and Finnigan 1994), and standard density corrections (Webb et al. 1980) were applied. Beneath-canopy CO2 storage (primarily a nighttime and early-morning factor) was determined by vertical integration of six profile stations located on the tower, and added to the eddy-flux measurement to compute the overall NEE as described in Goulden et al. (1996). Methods of gap filling are described in Monson et al (2002), but no gap filled data were used in the statistical relationships described in this paper. In the micrometeorological literature, CO2 fluxes are assigned a mathematical sign, which by convention is referenced to the atmosphere; negative CO2 fluxes (carbon assimilation by the system) represent CO2 loss from the atmosphere and positive CO2 fluxes (net respiration) represent CO2 gain by the atmosphere. In this paper, we have reversed this convention since the focus of the paper is on the ecosystem CO2 balance, not the atmospheric CO2 balance. Thus, net photosynthesis by the ecosystem is assigned a positive value, and net respiration is assigned a negative value.
Photosynthetic photon flux density (PPFD) and temperature were measured at 21.5 m using a cosine-corrected quantum sensor (Li-Cor, Lincoln, Neb., USA) and a fine-wire platinum resistance thermometer attached to a datalogger (Campbell Scientific, model CR23X, Logan, Utah, USA). Within canopy air temperature was measured with a platinum resistance thermometer at 8 m height. Data were collected at 1 Hz frequency but are presented here and analyzed as 30-min averages. Rainfall was measured with a tipping bucket rain gauge located above the canopy. Long-term monthly averages of air temperature (maximum, minimum and mean) were taken from a nearby meteorological site operated by NOAA, and archived in the LTER.
Branch-level CO2 fluxes
The photosynthetic and respiratory response to temperature was determined with a portable gas-exchange system (Li-Cor, model 6400, Lincoln, Neb., USA) using branches from the mid-canopy of three trees of each species within 40 m of the flux tower in May and July 2001. Plants were accessed by three 12-m canopy-access towers. The Li-Cor 6400 was modified to use a custom-made integrating sphere cuvette with light provided through two fiberoptic cables by a halogen-lamp light source (Walz, Effeltrich, Germany). PPFD in the cuvette was measured with the standard quantum sensor provided with the Li-Cor 6400. This cuvette configuration provided a uniformly diffuse PPFD across the photosynthetic surfaces of these conifer shoots. Temperature of the cuvette was controlled with a circulating water bath. Needle temperature was monitored with a single fine-wire thermocouple pressed to the surface of one needle.
Branches were cut and transported to the photosynthesis system, which was housed in a trailer, approximately 800 m from the trees. Branches were re-cut under water and placed in the cuvette, where they were acclimated to high light and a needle temperature of 15°C. After 30 min, we measured the light response of the branch using ten PPFD values (at least five on the linear portion of the curve), progressing from high to low light. A stable respiratory signal was determined in complete darkness following the lowest PPFD level, and the process was repeated at 10°C and 5°C (in July we also included 20° and 25°C). At each temperature we used a 30-min acclimation period prior to data collection after the needles had reached the appropriate temperature. Following the gas-exchange measurements, branches were harvested, and the needles were collected and oven dried at 70°C for 100 h. Hemispherical needle surface area was determined from regressions of needle mass to surface area (determined on a large sample of needles by the volume displacement method, see Chen 1997).
In a separate sample of cut branches (n=3), we ran continuous measurements of net CO2 exchange and stomatal conductance at a single, light-saturating PPFD for 3 h to assess whether there were any artifacts due to the long measurement period in the gas-exchange cuvette; no significant effects were observed. In July 2000, we also compared photosynthesis rates of cut branches with those left intact on the trees, and found no significant effects due to cutting.
Analysis of NEE
Data from ecosystem fluxes were used in a statistical model to assess temperature effects at different light levels across the day and season. Half-hour flux data were separated into nine temperature intervals (<0, 0–5, 5–7.5, 7.5–10, 10–12.5, 12.5–15, 15-17.5, 17.5–20, >20°C) for 1999 and 2000. The light response of canopy CO2 flux for each temperature interval in each year was fit to the following equation by non-linear, least-squares regression using SigmaPlot (SPSS, Chicago, Ill., USA):
where αe′ is the apparent quantum yield of the ecosystem, NEEsat is the net CO2 exchange at light saturation, and R e is the mean respiratory net CO2 exchange at PPFD =0 (Ruimy et al. 1995). Gross ecosystem CO2 exchange (GEE) was calculated as the sum of NEEsat and R e. Standard errors for the mean of each parameter at each temperature interval were determined from the least-squares regression. For the determination of GEE, error terms were combined for NEEsat and R e as the square root of their sum squared. The majority of the PPFD-response curves at the nine temperature increments were significantly different from each other when evaluated by the method of Potvin et al. (1990), but the differences between years were not as consistent. Because of an earlier spring warm up and longer growing season in 2000, a greater temperature range was available for analysis compared to 1999.
To evaluate more completely the photosynthetic responses of the canopy, we estimated the temperature response of the ecosystem quantum yield. The αe′ determined from a best-fit model of the photosynthetic response to the entire range of PPFD (Eq. 1) often underestimates the true value. By using a linear regression model and only evaluating the photosynthetic response to PPFD levels less than 125 μmol m-2 s-1, while also excluding PPFD values of zero, we estimated a "true" quantum yield (αe). We pooled temperature increments to only four value ranges (<5°C, 5–10°C, 10–15°C, >15°C). Standard errors for each mean were determined from the least-squares regressions in SigmaPlot. From this linear analysis, we also estimated the ecosystem light compensation point for both 1999 and 2000, determined as the point at which CO2 flux is zero from the regression analysis.
Path analysis evaluation of ecosystem CO2 flux
To evaluate the dependence of NEE on several climate parameters, we used path analysis (Li 1981; Sokal and Rohlf 1981; and Schemske and Horvitz 1988) with eddy covariance data from 1999 and 2000. Path analysis is similar to multiple regression and is especially useful as an analysis tool when a priori causal or correlative information is known about the relationship among variables (Li 1981). This is the case for climate effects on leaf-level photosynthesis, and in this paper we make the same assumption for ecosystem-level photosynthesis. Path analysis is most effective in evaluating data where independence is not certain, making the more common multiple regression techniques inappropriate.
We used a conceptual model similar to that used by Bassow and Bazzaz (1998) in their evaluation of the control of leaf-level gas exchange within the complexities of a deciduous forest. Our model was designed to evaluate the seasonal shifts in primary factors that were important in controlling NEE. In this model we assumed that temperature, PPFD and vapor pressure deficit (VPD) are important in driving or constricting photosynthetic carbon fixation, and their interrelated paths provide the means to evaluate direct and indirect responses (primarily of PPFD). To construct the path values for each component, we conducted four multiple regressions (SPSS, 7.5.1, SPSS, Chicago, Ill., USA) on data collected between 0700 and 1700 hours Mountain Standard Time (MST) during rain-free periods that did not violate conditions of statistical stationarity in the measured turbulent fluxes. We used standardized partial-regression coefficients to describe the path strengths generated from the following analyses: (1) NEE regressed on ecosystem evapotranspiration rate, above-canopy PPFD, and within-canopy air temperature; (2) ecosystem evapotranspiration rate regressed on above-canopy PPFD, within-canopy air temperature, and above-canopy VPD; (3) within-canopy air temperature regressed on above-canopy PPFD and above-canopy air temperature; and (4) above-canopy VPD regressed on above-canopy air temperature.
We generated path diagrams for each month of the year that net CO2 uptake of the forest was positive (May–September). From these path diagrams we evaluated the following paths and their control over NEE: (1) the direct effects of PPFD; (2) the indirect effects of PPFD (e.g., through influences on within-canopy air temperature); (3) the indirect effects of above-canopy air temperature (e.g., through influences on above-canopy VPD); and (4) the indirect effects of above-canopy VPD (e.g., through influences on within-canopy air temperature). The direct effects of PPFD were taken to be the standardized partial-regression coefficient from the first multiple regression between NEE and PPFD. The indirect effects were take to be the product of the standardized partial-regression coefficients summed across all possible paths from our parameter of interest (PPFD, above-canopy air temperature, VPD) and NEE.
Results
Meteorological conditions
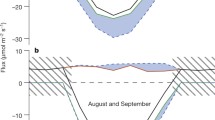
Mean monthly temperatures during the growing season range from ~0° in April to a peak of 10.5°C during July (Fig. 1A). Mean maximum air temperature is greater than 5°C from April through October; however, freezing temperatures are possible at any time of the year and the mean minimum temperature is consistently less than 5°C. Precipitation falls mainly as snow in winter, but late summer rain events are also important (Fig. 1B). The 2 years used in this study both had total precipitation amounts (757 mm in 1999 and 654 mm in 2000) that were slightly lower than the long-term average (810 mm). Both years were considered "La Niña" years, although the summer of 2000 was considerably drier, especially from April through September.
Meteorological data from the Niwot Ridge long-term ecological research site. A The 15-year temperature (°C) averages (mean, maximum and minimum) for each month of the growing season at 3,050 m (taken from the NOAA C1 Climate Station located approximately 500 m north of the Ameriflux site). B precipitation (mm) over the 1999 and 2000 growing seasons
Seasonal pattern of NEE
During 1999 and 2000, maximum carbon uptake was observed during the early season in late-May and early-June, when air temperatures were relatively cool (mean 7–10°C) and the ground was still covered with several centimeters of snow (Fig. 2). Mid-summer NEE was considerably lower than the early season maximum, and there was a secondary increase in NEE during the late season in September and October, when air temperatures had cooled once again.
Temperature response of CO2 flux at the ecosystem and needle scale
There was significant variation in the temperature response of NEEsat, as determined from the parameters generated from the least-squares regression model for the 1999 and 2000 growing seasons (Fig. 3a). Maximum NEEsat occurred between 8 and 12°C for both years, but the maximum was slightly higher in the wetter 1999 (18.4±0.9 μmol m-2 s-1), as compared to the drier 2000 (16.3±0.5 μmol m-2 s-1), growing season. For both years, even at temperatures between 0–5°C, NEEsat was relatively high (between 68 and 73% of the maximum). When temperatures increased from the optimum (8–12°C) to 15°C, NEEsat decreased to 60–75% of the maximum. Respiration at the ecosystem level (determined as the respiration parameter from the regression model R e) increased significantly with increasing air temperature in both 1999 and 2000 (Fig. 3b). Even at air temperatures below 0°C, R e was relatively high.
Top panel Light-saturated net ecosystem CO2 exchange (NEEsat) for 1999 and 2000 at the Niwot Ridge Ameriflux Site as a function of air temperature. Values for NEEsat were determined from the non-linear least-squares regression evaluation of light response of the canopy. Regression lines are: [y=z−0.69(x 2)+1.45(x)+9.87; r 2=0.92] for 1999 and [y=−z0.71(x 2)+1.48(x)+7.88; r 2=0.96] for 2000. Middle panel Ecosystem respiration (R e) for 1999 and 2000 as a function of air temperature. This parameter was generated from eddy covariance data categorized by temperature and fit to the non-linear least squares regression
Because of the broad range of temperatures in the respiration response for the ecosystem, we were able to calculate a Q 10 of R e for each year (see Giardina and Ryan 2000). From the data presented in Fig. 3, values in 1999 reflected a Q 10 of 1.87, while those in 2000 reflected a Q 10 1.53. The Q 10 of R e had significant seasonal variation throughout both years, as a peak Q 10 is seen during August and July, for 1999 and 2000 respectively (Table 1). Early and late in the growing season, Q 10 was much lower, and there appeared to be differences in the pattern of seasonal change in Q 10 of R e between 1999 and 2000, with values slightly lower early in the season in 1999 as compared to 2000. This is most likely due to differences in early season temperature, and the later initiation of the growing season (Fig. 1, 2).
The αe′ of NEE derived from the least-squares model did not vary significantly with temperature (Fig. 4a). Values during 1999 were generally greater than during 2000. Using a linear regression model to calculate the slope of the NEE response to PPFD between 0 and 125 μmol m-2 s-1, and using measurements of the canopy-gap fraction (0.17, see Monson et al. 2002) (to adjust the incident PPFD to eliminate photons that fell between tree crowns) and an estimated foliar absorptance (0.85), we calculated the αe as 0.04–0.06 mol CO2 mol-1 photons (Fig. 4b). The αe was highest at low temperatures (2.5–7.5°C). In 1999, the derived light compensation point was lower than in 2000, and in both cases, there was a clear effect of increasing temperatures (Fig. 4c). At an air temperature of 15°C, the light compensation point was 3–5 times that observed at 5°C.
Top panel The apparent quantum yield (αe′), derived from the eddy covariance data fit to a non-linear least squares regression as a function of air temperature. Middle panel An estimate of 'true' quantum yield (αe), adjusted for the absorbance and gap fraction of the forest, determined from the slope of the fit to a non-linear least squares regression considering PPFD values <125 μmol m-2 s-1 and expressed as a function of air temperature. Bottom panel The light compensation point of the ecosystem (LCP), determined as the point at which CO2 flux is zero from the non-linear least squares regression, expressed as a function of air temperature
The photosynthetic-temperature response of isolated branches at light saturation was relatively flat between 5 and 15°C for all three species at most time periods of the growing season (Fig. 5). Needle dark respiration rates increased significantly in all three species as temperature was increased above 5°C (Fig. 5).
Needle level photosynthetic and respiratory responses to temperature in the three dominant conifer species. Data are taken from branches harvested at mid-canopy. Presented variables are net CO2 assimilation at light saturation (A n) and needle respiration rate (R n). Data are plotted for each species for May and July of 2000. Vertical bars represent ±1 SE, n=3
The ratio of R e to GEE decreased with increasing temperature in a curvilinear fashion (Fig. 6a). As temperatures increased from 8°C to 15°C, gross ecosystem CO2 uptake decreased by 3 μmol m-2 for every μmol m-2 of CO2 lost by respiration, which represents a 15% reduction in the capacity for the forest to assimilate CO2. The relationship between respiration and gross photosynthesis appeared similar at the needle scale as compared to the whole ecosystem, except for temperatures less than 5°C and greater than 15°C. While the shape of the curve was similar, the ratio was less negative at the needle scale than at the ecosystem scale for relatively cooler temperatures, but similar at higher temperatures Thus, the inclusion of the soil respiration component most likely results in a ratio that is more negative at the ecosystem scale.
Top panel The ratio of ecosystem respiration (R e from the non-linear least-squares regression evaluation of the light response of the ecosystem) to light saturated GEE (NEEsat minus R e from the same analysis) for 1999 and 2000. The regression line is [y=−0.0011(x 2)+0.016(x)z−0.02; r 2=0.95]. Bottom panel the ratio of needle respiration to light saturated gross photosynthetic rate (determined from the light response curves) for 1999 and 2000. The regression line is [y=−0.0004(x 2)−0.004(x)−0.03; r 2=0.88]. In the bottom panel, filled symbols are from May 2000, and open symbols are from July 2000
Path analysis evaluation of ecosystem CO2 flux
The path analysis revealed a number of minor relationships among variables that will not be discussed in detail here (Fig. 7). The effects of temperature and PPFD were clearly dominant. The effect of temperature changed from a strong positive effect (higher temperature causes higher NEE) during May to a strong negative effect (higher temperature causes lower NEE) throughout the remainder of the summer. The direction of the effect of PPFD was consistently positive throughout the season (increased PPFD caused increased NEE).
Path diagram that illustrates the changes in effects on different parameters related to NEE. Standardized correlation coefficients are plotted as variation in the thickness of each arrow (see legend on each figure). The data represent the pooled values for all daytime measurements during the growing seasons of 1999 and 2000, illustrated by month
From the path analysis, we calculated the direct and indirect effects of PPFD, the direct and indirect effects of temperature, and the indirect effects of VPD (Fig. 8). Across the growing season the indirect effects of VPD did not significantly explain variance in NEE. In some cases, the indirect effects of PPFD were significant, such as the strong positive effect in August 1999, when high PPFD appears to have contributed to high NEE, through indirect effects of water flux and canopy temperature. However, for the most part, the indirect effects of PPFD were also unimportant as a control over variance in NEE. Differences in the path values between years indicated that NEE during 2000 was considerably less sensitive to PPFD and more sensitive to the indirect effects of temperature, compared to NEE during 1999 (Fig. 9). This is likely related to the mid-summer drought of 2000 (see Fig. 1).
The direct and indirect effects of above canopy PPFD, air temperature and VPD on NEE as a function of month for the primary growing seasons in 1999 and 2000. Since the path values presented in this figure are the standardized partial correlation coefficients, they can be evaluated relative to each other (and a larger value represents a stronger effect). Positive effects, such as that of PPFD throughout the season in both years, suggests that an increase in NEE occurred with an increase in this parameter. Negative values indicate a decrease in NEE with an increase in any parameter
The cumulative path values for the 1999 and 2000 season (data pooled across date) for NEE. The parameters and interpretation follows the description in Fig. 6 and described in the text
Discussion
We examined the emerging hypothesis that low temperatures during the growing season represent a primary limitation to productivity, and assumedly restrict, NEE in subalpine forests (Smith and Knapp 1990; Teskey et al. 1995). We expanded the scale of this issue by looking across needles and branches to the whole ecosystem to integrate responses attributed to both autotrophic and heterotrophic components of the system, and thus we gained a more comprehensive view of the limitations to NEE than has been conducted in the past for this ecosystem. Our analysis revealed that this subalpine forest is optimally suited for net CO2 assimilation at relatively low temperatures early in the growing season (Figs. 2, 3). The high capacity for CO2 assimilation at low temperature was also observed in individual branches (Fig. 5), and suggests that the photosynthetic biochemistry of the dominant trees in this forest is also optimally suited for function at relatively cool growing-season temperatures. Measurements across broad scales thus are consistent in revealing near-optimal function by the plant components of this ecosystem at cool temperatures.
As seasonal temperatures are increased above the early-season optimum, increases in R e caused significant reductions in NEE, a pattern that cannot be simply predicted from branch level measurements (Fig. 6). Past studies at the Niwot Ridge site have also determined that R e is not an important factor in NEE dynamics early in the growing season when snow still covers the ground (Monson et al. 2002). It is only after snow melt, when the soil is free of the insulating effects of the snow, that soil respiration increases and causes R e to be a dominant variable in driving dynamics in NEE. Soil respiration measured by chambers is quite responsive to temperature throughout the course of the season, but within any one time period, spatial differences in respiration rate do not correlate with spatial differences in temperature or soil moisture (Scott-Denton et al. 2002). Differences between spatial locations appear to be driven by variation in soil organic matter content.
The temperature sensitivity of respiration is often measured by the Q 10. Compared to other studies, our estimates of the respiratory Q 10 are low. For a Canadian boreal black spruce forest, Goulden et al. (1997) present a Q 10 of 2.3, for a European beech forest Valentini et al. (1996) present a value of 2.2, and for a slash pine forest in Florida. Clark et al. (1999) report a value of 2.0. The lower values for our study (1.87 for a wet year and 1.53 in a dry year) may be due to the fact that our forest may be associated with relatively low growth rates associated with the time since recovery from disturbance in this forest ecosystem.
The temperature-dependence of the light compensation point of the ecosystem may be a useful tool in understanding temporal aspects of how respiration constrains NEE. In our analysis, the observed light compensation point was low until temperatures above 15°C, when it increased and became comparable with those reported in other studies (between 100 and 250 μmol m-2 s-1; Ruimy et al. 1995; Clark et al. 1999). The lower compensation point values measured in this study were likely a function of the relatively high rate of photosynthesis, and low rate of soil respiration, during early season periods when cool air temperatures are conducive to high rates of photosynthesis and snow-covered soils are conducive to low rates of respiration. Higher rates of ecosystem respiration might be a more consistent component of seasonal dynamics in the light compensation point of other forest ecosystems that lack extended snow cover during the growing season.
To better understand the role of temperature as a driver of seasonal patterns in NEE, we used a path analysis model. The model was designed to evaluate seasonal shifts in factors that were important in controlling (in a correlative or causal sense) NEE. The results of the analysis revealed that during the spring, increased temperature is associated with increased CO2 flux into the ecosystem (positive NEE). The temperature response did not appear to operate through influences on H2O flux; rather, temperature most likely operated on rates of biochemistry. Later in the growing season, increases in temperature are associated with decreases in CO2 flux into the ecosystem (negative NEE). This is attributed to the increased control of soil respiration on NEE, and its response to temperature. Chamber measurements of soil respiration were higher later in the season as compared to earlier for both 1999 and 2000 (Scott-Denton et al. 2002). The presence or absence of the snow pack in this ecosystem appears to act as the switch that changes the direction of the path analysis relationship with regard to temperature.
The temperature optimum for photosynthesis in needles of high-elevation, North American conifer species has been determined by a number of different researchers in the past 40 years at a number of different locations (Smith 1985; Delucia 1986; Delucia and Smith 1987; Teskey et al. 1995). Smith and Knapp (1990) suggest that freezing air temperatures constrain photosynthetic carbon gain to between 80% and 100% of maximum capacity during the early and late season (periods when freezing nights occur). During the mid-summer period, instantaneous measurements of net CO2 fixation at ambient temperatures have been reported to be 50–60% of the maximum rate (Smith and Carter 1988). When taken together, these studies support the emerging hypothesis that low temperatures are the primary limitation to productivity in these forests. The case for inhibition of productivity by mid-season frosts, and subsequent recovery, appears clear; we have not addressed frost damage in the current study. However, the case for low, but non-freezing temperatures limiting productivity is not supported by our observations.
In this study, we make the case that, depending on the analysis used, CO2 uptake by these forests may be less limited by low growing season temperatures than previously suggested. Furthermore, we argue that limitations to CO2 uptake, as have been deduced through plant ecophysiological studies, should not be used in isolation to deduce limitations to ecosystem CO2 exchange. It is clear from our studies that ecosystem respiration, particularly that from the soil, is crucial to understanding the response of ecosystem CO2 fluxes to seasonal and interannual temperature dynamics. This perspective is consistent with the growing realization that a clear view of ecosystem carbon sequestration must include an accurate estimate of respiratory activity (see Giardina and Ryan 2000; Valentini et al. 2000).
References
Anthoni PM, Law BE, Unsworth MH (1999) Carbon and water vapor exchange of an open-canopied ponderosa pine ecosystem. Agric For Meteorol 95:151–168
Aubinet M, Grelle A, Ibrom A, Rannik U, Moncrieff J, Foken T, Kowalski P, Martin P, Berbigier P, Bernhofer C, Clement R, Elbers J, Granier A, Grunwald T, Morgenster K, Pilegaard K, Rebmann C, Snijders W, Valentini R, Vesala T (2000) Estimates of the annual net carbon and water exchanges of European forests: the EUROFLUX methodology. Adv Ecol Res 30:113–174
Baldocchi DD, Vogel CA, Hall B (1997) Seasonal variation of carbon dioxide exchange rates above and below a boreal jack pine forest. Agric For Meteorol 83:147–170
Bassow SL, Bazzaz FA (1998) How environmental conditions affect canopy leaf-level photosynthesis in four deciduous tree species. Ecology 79:2660–2675
Black TA, DenHartog G, Neumann HH, Blanken PD, Yang PC, Russell C, Nesic Z, Lee X, Chen SG, Staebler R, Novak MD (1996) Annual cycles of water vapour and carbon dioxide fluxes in and above a boreal aspen forest. Global Change Biol 2:219–229
Canadell JG, Mooney HA, Baldocchi DD, Berry JA, Ehleringer JR, et al (2000) Carbon metabolism of the terrestrial biosphere: a multi-technique approach for improved understanding. Ecosystems 3:115–130
Chen JM, Rich PM, Gower ST, Norman JM, Plummer S (1997) Leaf area index of boreal forests: theory, techniques, and measurements. J Geophys Res 102:29429–29443
Clark KL, Gholz HL, Moncrieff JB, Cropley F, Loescher HW (1999) Environmental controls over net exchanges of carbon dioxide from contrasting Florida ecosystems. Ecol Appl 9:936–948
Day TA, Heckathorn SA, DeLucia EH (1991) Limitations of photosynthesis in Pinus taeda L. (loblolly pine) at low temperatures. Plant Physiol 96:1246–1254
Delucia EH (1986) Effect of low root temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engelmann spruce (Picea engelmannii Parry ex Engelm.) seedlings. Tree Physiol 2:143–154
DeLucia EH, Smith WK (1987) Air and soil temperature limitations on photosynthesis in Engelmann spruce during summer. Can J Bot 17:527–533
Delucia EH, Hamilton JG, Naidu SL, et al (1999) Net primary production of a forest ecosystem with experimental CO2 enrichment. Science 284:1177–1179
Field CB (1999) Diverse controls on carbon storage under elevated CO2: toward a synthesis. In: Luo Y, Mooney HA (eds) Carbon dioxide and environmental stress. Academic Press, New York, pp 373–392
Giardina CP, Ryan MG (2000) Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404:858–861
Goldstein AH, Hultman NE, Fracheboud JM, Bauer MR, Panek JA, Xu M, Qi Y, Guenther AB, Baugh W (2000) Effects of climate variability on the carbon dioxide, water and sensible heat fluxes above a ponderosa pine plantation in the Sierra Nevada (CA). Agric For Meteorol 101:113–129
Goulden ML, Munger JW, Fan S-M, Daube BC, Wofsy SC (1996) Measurements of carbon sequestration by long-term eddy covariance: Methods and a critical evaluation of accuracy. Global Change Biol 2:169–182
Goulden ML, Daube BC, Fan SM, Sutton DJ, Bazzaz FA, Munger JW, Wofsy SC (1997) Physiological responses of black spruce forest to weather. J Geophys Res 102:28987–28996
Grace S, Rayment M (2000) Respiration in the balance. Nature 404:819–820
Granier A, Ceschia E, Damesin C, Dufrene E, Epron D et al (2000) The carbon balance of a young beech forest. Funct Ecol 14:312–325
Greco S, Baldocchi DD (1996) Seasonal variations of CO2 and water vapor exchange rates over a temperate deciduous forest. Global Change Biol 2:183–197
Hollinger DY, Kelliher FM, Schulze ED, Bauer G, Arneth A et al (1998) Forest-atmosphere carbon dioxide exchange in eastern Siberia. Agric For Meteorol 90:291–306
Houghton JT (1991) The role of forests in affecting the greenhouse gas composition of the atmosphere. In: Wyman RL (ed) Global climate change and life on earth. Chapman & Hall, New York, USA, pp 43–56
Jarvis PG, Massheder JM, Hale SE, Moncrieff JB, Rayment M, Scott SL (1997) Seasonal variation of carbon dioxide, water vapor and energy exchanges of a boreal black spruce forest. J Geophys Res 102:28953–28966
Kaimal JC, Finnigan JJ (1994) Atmospheric boundary layer flows, their structure and measurement. Oxford University Press, New York
Keeling CD, Whorf TP (1998) Atmospheric CO2 records from sites in the SIO air sampling network. In: Trends: a compendium of data on global change carbon dioxide. Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, Tenn.
Law BE, Ryan MG, Anthoni PM (1999) Seasonal and annual respiration of a ponderosa pine ecosystem. Global Change Biol 5:169–182
Law BE, Godstein AH, Anthoni PM, Unsworth MH, Panek JA, Bauer MR, Fracheboud JM, Hultman N (2001) Carbon dioxide and water vapor exchange by young and old ponderosa pine ecosystems during a dry summer. Tree Physiol 21:299–308
Li CC (1981) Path analysis: a primer, 3rd edn. Boxwood, Pacific Grove, Calif.
Malhi Y, Nobre AD, Grace J, Kruijt B, Pereira MGP, Culf, Scott S (1998) Carbon dioxide transfer over a central Amazonian rain forest. J Geophys Res 103:31593–31612
Monson RK, Turnipseed AA, Sparks JP, Harley PC, Scott-Denton LE, Sparks K, Huxman TE (2002) Carbon sequestration in a high elevation subalpine forest. Global Change Biol 8:459–478
Pacala SW, Hurtt GC, Baker D, Peylin P, Houghton RA, Birdsey RA, Heath L, Sundquist ET, Stallard RF, Ciais P, Moorcroft P, Caspersen JP, Shevliakova E, Moore B, Kohlmaier G, Holland E, Gloor M, Harmon ME, Fan SM, Sarmiento JL, Goodale CL, Schimel D, Field CB (2001) Consistent land- and atmosphere-based US carbon sink estimates. Science 292:2316–2320
Potvin C, Lechowicz MJ, Tardif S (1990) The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology 71:1389–1400
Rosenberg NJ, Blad BL, Verma S (1983) Microclimate: the biological environment. Wiley, New York
Ruimy A, Jarvis PG, Baldocchi DD, Saugier B (1995) CO2 fluxes over plant canopies and solar radiation: a review. Adv Ecol Res 26:1–68
Schemske DW, Horvitz C (1988) Plant animal interactions and fruit production in a neotropical herb: a path analysis. Ecology 69:1128–1137
Schimel DS (1995) Terrestrial ecosystems and the carbon cycle. Global Change Biol 1:77–91
Schimel DS, Brawell BH, Holland EA, McKeown R, Ojima DS, Painter TH, Parton WJ, Townsend AR (1994) Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Global Biogeochem Cycles 8:279–293
Schimel DS, House JI, Hibbard KA, et al (2001) Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 414:169–172
Schlesinger WH (1997) Biogeochemistry: an analysis of global change. Academic Press, San Diego, Calif.
Scott-Denton LE, Sparks KL, Monson RK (2002) Spatial and temporal controls over soil respiration rate in a high-elevation, subalpine forest. Soil Biol Biochem (in press)
Sellers PJ, Dickinson RE, Randall DA, et al (1996) Comparison of radiative and physiological effects of doubled atmospheric CO2 on climate. Science 271:1402–1406
Smith WK (1985) Environmental limitations of leaf conductance in central Rocky Mountain conifers, USA. In: Turner W, Tranquillini T (eds) Establishment and tending of subalpine forest: research and management. Eidg Anst Forstl Vers Ber 270:95–101
Smith WK, Brewer CA (1994) The adaptive importance of shoot and crown architecture in conifer trees. Am Nat 143:528–532
Smith WK, Carter GA (1988) Shoot structural effects on needle temperature and photosynthesis in conifers. Am J Bot 75:496–500
Smith WK, Knapp AK (1990) Ecophysiology of high elevation forests. In: Osmond CB, Pitelka LF, Hidy GM (eds) Plant biology of the basin and range. Springer, Berlin Heidelberg New York, pp 87–142
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, San Francisco, Calif.
Teskey RO, Sheriff DW, Hollinger DY, Thomas RB (1995) External and internal factors regulating photosynthesis. In: Smith WK, Hinkley TM (eds) Resource physiology of conifers. Academic Press, San Diego, Calif., pp 105–140
Turnipseed AA, Blanken PD, Anderson DE, Monson RK (2002) Energy budget above a high-elevation subalpine forest in complex topography. Agric For Meteorol 110:177–201
Valentini R, DeAngelis G, Matteucci G, Monaco R, Dore S, Scarascia Mugnozza GE (1996) Seasonal net carbon dioxide exchange of a beech forest with the atmosphere. Global Change Biol 2:199–207
Valentini R, Matteucci G, Dolman AJ, et al (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865
Webb EK, Pearman GI, Leuning R (1980) Correction of flux measurement for density effects due to heat and water vapor transfer. Q J R Meteorol Soc 106:85–100
Wisniewski J, Lugo AE (1992) Natural sinks of CO2. Kluwer Academic, Dordrecht
Wofsy SC, Goulden ML, Munger JW, Fan SM, Bakwin PS, Daube BC, Bassow SL, Bazzaz FA (1993) Net exchange of CO2 in a mid-latitude forest. Science 260:1314–1317
Acknowledgements
The authors are grateful for the support and assistance of Laura Scott-Denton, Kim Sparks, William Bowman, Dave Bowling, Dan Hereid, and Brant Backland. Comments on an earlier version of the manuscript by two referees helped to improve the manuscript. This research was funded by the South Central Section of the National Institute for Global Environmental Change (NIGEC) through the U.S. Department of Energy (Cooperative Agreement No. DE-FC03–90ER61010). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the DOE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huxman, T.E., Turnipseed, A.A., Sparks, J.P. et al. Temperature as a control over ecosystem CO2 fluxes in a high-elevation, subalpine forest. Oecologia 134, 537–546 (2003). https://doi.org/10.1007/s00442-002-1131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-002-1131-1