Abstract
Olfactory marker protein (OMP) was first described as a protein expressed in olfactory receptor neurons (ORNs) in the nasal cavity. In particular, OMP, a small cytoplasmic protein, marks mature ORNs and is also expressed in the neurons of other nasal chemosensory systems: the vomeronasal organ, the septal organ of Masera, and the Grueneberg ganglion. While its expression pattern was more easily established, OMP’s function remained relatively vague. To date, most of the work to understand OMP’s role has been done using mice lacking OMP. This mostly phenomenological work has shown that OMP is involved in sharpening the odorant response profile and in quickening odorant response kinetics of ORNs and that it contributes to targeting of ORN axons to the olfactory bulb to refine the glomerular response map. Increasing evidence shows that OMP acts at the early stages of olfactory transduction by modulating the kinetics of cAMP, the second messenger of olfactory transduction. However, how this occurs at a mechanistic level is not understood, and it might also not be the only mechanism underlying all the changes observed in mice lacking OMP. Recently, OMP has been detected outside the nose, including the brain and other organs. Although no obvious logic has become apparent regarding the underlying commonality between nasal and extranasal expression of OMP, a broader approach to diverse cellular systems might help unravel OMP’s functions and mechanisms of action inside and outside the nose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When studying the basic science of olfaction, there is one protein that, one might argue, represents the complexity of the peripheral olfactory system: olfactory marker protein (OMP). This protein is expressed in olfactory receptor neurons (ORNs), the neurons that detect odorants and convert their presence into action potentials. The story of OMP is filled with the trials and tribulations of trying to understand the function of the protein in general and in ORNs in particular, even nearly 50 years since its discovery (Margolis 1972). OMP is still the subject of many research papers, and it continues to reveal surprises, though not necessarily its function(s), whose complexity is still blurred.
Since the first seminal paper, by Frank L. Margolis in 1972 (Margolis 1972), OMP has been showing remarkable features:
-
1.
Specificity: it is abundantly and predominately expressed in the chemosensory systems (Hartman and Margolis 1975; Monti-Graziadei et al. 1977).
-
2.
Phylogenetically conserved: it displays similar expression patterns in the chemosensory organs of many vertebrate species (Keller and Margolis 1975).
OMP was first described as a protein of low molecular weight found in a fraction of soluble proteins from mouse brain extract (Margolis 1972). At the time, OMP was detected exclusively in the olfactory bulb (OB), leading Margolis to hypothesize that its expression site could be in the nasal cavity, and therefore peripheral to the OB, where the ORNs that innervate the bulb are located. Thus, the protein found in the brain extract could be from ORN axons that synapse onto second-order neurons in the glomeruli of the OB.
Margolis’s hypothesis turned out to be correct, and OMP was then localized in the olfactory epithelium (OE) and the vomeronasal organ using biochemical methods. Margolis and Tarnoff (1973) could not exclude OMP expression in other, non-neuronal cell types in the OE, they concluded “The existence of a protein uniquely associated with a specific neuron type, synapsing in an area of the brain which modulates sexual, social, and nutritional behavior, will facilitate meaningful studies of biochemical correlates of specific behavioral parameters at the level of the primary sensory input.” Later work described OMP expression in the other two chemosensory organs in the nasal cavity: the septal organ of Masera, located at the base of the septum (Breipohl et al. 1989; Weiler and Farbman 2003), and the Grueneberg ganglion in the rostrodorsal part of the nasal cavity (Fleischer et al. 2006).
Margolis and colleagues made an antibody directed against OMP (Keller and Margolis 1975; Margolis 1972; Monti-Graziadei et al. 1977), and since then, its use has become almost a rite of passage—it has been used probably at least once by any scientist working on the basic aspects of the peripheral olfactory system. Over the years, the Margolis lab has sent the antibody to 400 labs in more than 30 countries (personal communication with Dr. Margolis) and OMP’s peculiar expression pattern was determined using the antibody and immunostaining. In the OE, OMP is expressed only in fully mature ORNs (Barber et al. 1982; Hartman and Margolis 1975; Monti-Graziadei et al. 1977). This finding gave scientists an important tool to study chemosensory systems from the peripheral organ to the first synapse in the OB, by using OMP not only to identify mature ORNs but also to drive or delete genes of interest using Cre-mediated approaches. Since then, much progress in understanding OMP function and the chemical senses has been made. However, OMP’s potential role, or even multiple roles, in ORN transduction, axonal targeting, and behavior remain to be fully elucidated.
Following the cloning of OMP (Rogers et al. 1987) and the first transgenic mouse model expressing Thy-1.1 as a reporter gene in mature ORNs under the OMP promoter (Danciger et al. 1989), several studies have used OMP to characterize the OE in different species and experimental conditions (e.g., regeneration studies, identification via co-localization of other proteins expressed in ORNs). In the meantime, an increasing number of olfactory transduction proteins were described and their cellular localization determined. These proteins involved in odorant transduction are abundantly expressed in the cilia/knob of ORNs and, at best, at very low levels in the cell body, while OMP, as a cytoplasmic protein, is present in all the cellular compartments, from cilia to axonal endings in the OB. This made hypothesizing a single role, or potentially several locally and developmentally dependent roles, for OMP a challenging endeavor.
Cell types of the olfactory epithelium
The OE is an epithelium with several cell types with different functions and morphologies (Fig. 1a). Finding markers to help distinguish among them all is crucial. The main cell type in the OE is the ORN, a bipolar neuron with its soma located in the middle portion of this pseudostratified epithelium. ORNs extend their axons behind the basal membrane to form the olfactory nerve that reaches the OB. On the other end of their soma, a single unbranched dendrite departs and reaches the apical portion of the OE, where it ends in a knob from which several cilia protrude into the nasal cavity (Morrison and Costanzo 1992). Olfactory knobs have a round, spherical shape, 1–2 μm in diameter. The number of cilia varies; usually 10–25 processes extend from each knob with different lengths, depending on the species (some extend over 50 µm) (Moran et al. 1982b; Morrison and Costanzo 1990). Cilia contain all the proteins required to transduce an odor stimulus into a generator potential that triggers action potentials (APs) in the cell body of ORNs, which then travel via the ORN axons to the OB. The ORNs are the cells that express OMP in the OE. Although several other cellular markers have been described for ORNs, OMP is still widely used, and it serves in several transgenic animal models to drive expression of or to delete genes of interest specifically in mature ORNs.
The olfactory epithelium and olfactory transduction. a Schematic representation of the olfactory epithelium (OE). The OE is a pseudostratified epithelium consisting of several cell types, both neuronal (mature and immature ORNs) and non-neuronal. Mature ORNs are ciliated bipolar neurons extending an apical dendrite to the surface of the epithelium and a basal axon to the olfactory bulb. Non-neuronal cells in the OE include sustentacular cells (columnar supporting cells with apical microvilli), microvillar cells (a diverse population of pear-shaped cells extending an apical process with microvilli into the nasal cavity), globose basal cells (progenitors of ORNs), and horizontal basal cells (attached to the basal lamina and mitotically inactive in the uninjured OE). The OE also has Bowman’s glands (ducts projecting through the OE that release mucus). Note that the olfactory cilium and dendritic knob of ORNs are represented again in b and c, respectively, to highlight the olfactory transduction events related to OMP occurring at each location. b Schematic representation of the olfactory transduction cascade occurring within the cilium of an ORN and suggested roles of olfactory marker protein (OMP). OR, olfactory receptor; Gαolf, β, and γ, subunits of the olfactory G protein; AC3, adenylyl cyclase 3; CNG, cyclic nucleotide-gated channel; Ano2, Ca2+-activated Cl− channel anoctamin 2; NCKX4, K+-dependent Na+/Ca2+ exchanger 4; PDE1C, phosphodiesterase 1C. OMP is hypothesized to act upstream of the production of cAMP. Dashed arrows indicate the speculative effect of OMP on various elements of the olfactory transduction cascade. The OMP structure presented in this figure is a β-clamshell formed by two β sheets based on the work by Smith et al. (2002). c Schematic representation of the hypothesized role of OMP in the dendritic knob of ORNs. An OMP dimer forms a complex with brain-expressed X-linked protein 1 (Bex1) that binds to the plasma membrane and can interact with Ca2+/calmodulin (CaM) and an Na+/Ca2+ exchanger (NCX). Images created with Biorender
In addition to its complement of ORNs, the OE contains several other cell types (Fig. 1a): sustentacular (Sus) supporting cells (Andres 1966; Graziadei and Graziadei 1979), microvillar cells (a heterogeneous population with respect to phenotype and function) (Asan and Drenckhahn 2005; Genovese and Tizzano 2018; Hegg et al. 2010; Kusumakshi et al. 2015; Montani et al. 2006; Moran et al. 1982a; Morrison and Costanzo 1992), and two populations of basal cells, dark or horizontal basal cells (HBCs) and light or globose basal cells (GBCs) (Graziadei and Graziadei 1979). The OE also contains the Bowman’s duct glands that secrete mucus. None of these other OE cell types express OMP.
Sus cells are tall and columnar shaped, extending throughout the entire layer of the OE. Their apical surface bears microvilli (Engstrom et al. 1989; Menco 1980; Moran et al. 1982b). They are closely associated with ORNs, almost entirely wrapping the ORN cell bodies and dendrites extending to the epithelial surface. Markers that uniquely identify Sus cells are typical of epithelial cells (cytokeratin 8 [K8] and K18) (Holbrook et al. 2011).
HBCs are quiescent cells and have been described as stem cells of the adult OE (Carter et al. 2004; Graziadei and Graziadei 1979), but now are considered to be the “reserve stem cells” of the OE, activated only in case of severe damage of the OE (Schwob et al. 2017). Markers for this cell population are K5 and K14 and the transcription factor p63. GBCs are the progenitors of ORNs in the OE. They constitute a heterogeneous population of cells and share some markers with HBCs, such as Pax6 and Sox2 (Peterson et al. 2019; Schwob et al. 2017). Following damage of the OE (e.g., bulbectomy), ORNs derive from differentiating GBCs (Caggiano et al. 1994; Schwob et al. 1994). They are considered immature ORNs, not expressing OMP but instead the markers GAP-43 and the G protein subunit gamma 8 (Tirindelli and Ryba 1996; Verhaagen et al. 1989). Interestingly, these immature ORNs express multiple types of olfactory receptors (ORs), and their single OR choice will be stabilized during later stages of maturation (Hanchate et al. 2015).
Does a protein expressed everywhere in ORNs participate in olfactory transduction?
The answer to this question came when a knockout (KO) mouse model for OMP was first generated by Buiakova et al. (1996). By recording electro-olfactograms (EOGs; odorant-induced field potential changes) from the ciliary layer of the OE of OMP-KO animals, they found a reduced response to odors, slowed response kinetics (Fig. 2a), and reduced ability to respond to a second stimulation in a paired-pulse adaptation protocol compared with wild-type (WT) mice. EOGs record the summated generator potential from the surface of the OE (Scott and Scott-Johnson 2002). It is therefore thought to represent the signal transduction events happening at the cilia/knob region of many ORNs in the vicinity of the recording site. As Buiakova et al. (1996) stated, “These observations provide strong support for the conclusion that OMP is a novel modulatory component of the odor detection/signal transduction cascade.” Probably at the time, it was also surprising that the OE and OB of OMP-KO mice appeared grossly normal, without any obvious histological changes. Interestingly, after adenoviral-mediated expression of OMP in an OMP-KO mouse, the EOG looked similar to those of WT mice (Fig. 2a; see also Ivic et al. 2000). This also suggests that, at least for the odorant-induced response, OMP is not required during development to exert its function at maturity. However, these results raise questions about how OMP modulates olfactory transduction and through which other transduction protein(s) it exerts its action.
Odorant-induced responses in wild-type and OMP-knockout mice. a EOG recordings showing that the absence of OMP causes slower kinetics of the olfactory response, here in response to amyl acetate. EOG traces were normalized to 100% to aid comparison of the response time course and recorded from control mice (OMP+/+), OMP-knockout mice (OMP−/−), and OMP−/− mice rescued with OMP adenoviral infection (using CMV-OMP-IRES-EGFPAdV) at day 1 postinfection (short RES OMP−/−) and at day 3 postinfection (RES OMP−/−). Gray traces represent the actual EOG recordings; black curves are EOG recordings fitted with double exponential functions. Figure adapted from Ivic et al. (2000) with permission from Nature Neuroscience. b, b’ Suction pipette recordings showing that the absence of OMP causes slower kinetics of the olfactory transduction current in single ORNs. Both traces are recorded in response to a 2-s stimulation with cineole (100 µM). b A control mouse (OMP+/+). b’ An OMP-knockout mouse (OMP−/−). Figure adapted from Reisert et al. (2007), with permission from the Journal of Physiology
Transduction starts upon binding of an odorant molecule to an OR, a member of the large olfactory family of G protein-coupled receptors that are abundantly expressed in the ciliary membrane of ORNs (Fig. 1b). This then activates an alpha stimulatory subunit (Golf) of a trimeric G protein to stimulate adenylyl cyclase type III (AC3) to produce cyclic adenosine monophosphate (cAMP) which opens olfactory cyclic nucleotide-gated (CNG) channels, allowing Na+ and Ca2+ to enter the ciliary lumen. This current begins to depolarize the ORN to generate APs to be sent to the OB. The Ca2+ that enters via the CNG channel is responsible for various mechanisms, including activation of Ca2+-activated Cl− channels (TMEM16B or anoctamin 2 [Ano2]) that leads to Cl− exiting the cilium, further depolarizing the cell (Billig et al. 2011; Boccaccio and Menini 2007; Dibattista et al. 2017; Pietra et al. 2016; Rasche et al. 2010; Reisert and Zhao 2011; Stephan et al. 2009). The depolarizing action of Ano2 shortens the spike train and the duration of AP firing by causing quick inactivation of voltage-dependent Na+ channels, which leads to cessation of AP generation. Both the number of APs and the AP train duration might be required to correctly encode for the right odorant concentration and stimulus duration, generating the appropriate flow of information that ultimately arrives in the OB.
The transduction events triggered by the activation of the ORs need to be terminated. In particular, at this stage, cAMP and Ca2+ are at high concentrations in the ciliary lumen. cAMP is hydrolyzed by two phosphodiesterases (PDEs), PDE1C in the cilia and PDE4A in the remaining parts of the ORN. Although expression of PDE1C and PDE4A is highly compartmentalized and mutually exclusive, their individual role in response termination is probably dispensable since the diffusion of cAMP from the cilia into the knob is sufficient to mediate a rapid response termination (Cygnar and Zhao 2009). Ca2+ removal via Ca2+ exchangers leads to closure of Ano2 and hence response termination. Since the 1990s, the major players responsible for terminating the olfactory response in ORNs were suggested to be the Na+/Ca2+ exchangers (Antolin and Matthews 2007; Jung et al. 1994; Noe et al. 1997; Reisert and Matthews 1998). The precise molecular identity of NCXs responsible for ciliary Ca2+ extrusion remained somewhat elusive until Stephan et al. (2012) showed that the K+-dependent Na+/Ca2+ exchanger 4 (NCKX4) is present in olfactory cilia and functions to extrude Ca2+ from the ciliary lumen.
The odorant response and OMP
Because EOG recordings in OMP-KO mice showed differences in the kinetics of odorant responses, it was suggested that OMP may interact with proteins involved in signal transduction. However, because EOG experiments measure the response of a population of ORNs, it was hard to pinpoint a potential target protein of OMP in the olfactory transduction pathway. Suction electrode recordings from single mouse ORNs (Fig. 2 b and b’), a loose patch-clamp method that records the odorant-induced current originating from the cilia confirmed that the odorant response kinetics were slowed in OMP-KO mice (Reisert et al. 2007), as shown previously in EOG recordings. Adaptation to a paired-pulse protocol was also impaired in OMP-KO mice, and the response failed to recover from the first stimulus, thus showing a much reduced amplitude to the second stimulation. Later experiments with ORs tagged with green fluorescent protein (GFP) in ORNs lacking OMP found prolonged response onset, slower rising phase, and slower response termination of the odorant-induced response (Dibattista and Reisert 2016), in agreement with previous reports (Lee et al. 2011; Reisert et al. 2007). In addition, because two different mouse lines were used that expressed GFP in ORNs either expressing the mOR-EG OR and the M71 OR, dose-response experiments could be performed with the cognate ligands for these two ORs. This revealed that OMP contributes to defining the dynamic range of odorant sensitivity in ORNs. In particular, in OMP-KO mice, the dose-response curve was flattened and broadened in response to lower odorant concentrations, compared with WT mice, indicating that ORs can be activated by odorants at much lower concentrations than those usually reported. OMP suppresses the responses at low odorant concentrations and thus narrows the dose-response relation. This mechanism allows ORNs to retain a well-defined dynamic response range to be able to encode meaningful concentration ranges (Dibattista and Reisert 2016).
Youngentob et al. (2003) imaged the medial face of the OE using voltage-sensitive dyes and found that the spatiotemporal pattern of activation was degraded in the absence of OMP. Mature ORNs of adult OMP-KO mice seem to lose their odorant selectivity profile, as if they expressed more than one OR type. Indeed, recording from GFP-tagged ORNs expressing mOR-23, Lee et al. (2011) showed that OMP-KO ORNs, similar to ORNs in newborn WT mice, failed to be selective for lyral, the best-known agonist for this OR, in contrast to adult WT ORNs. Instead, single mOR-23-expressing ORNs still responded to lyral but also responded to nonoverlapping sets of additional odorants known not to be mOR-23 ligands. These observations could explain the significant shift toward equal responsivity across the OE in OMP-KO mice observed using voltage-sensitive dyes (Youngentob et al. 2003).
Most likely in this scenario, in mature ORNs lacking OMP, the complex regulatory machinery that typically leads to the expression of one OR type per neuron has been altered (Magklara et al. 2011). Another possibility is that OMP acts directly on ORs to increase their sensitivity to odorants other than their main ligand, although this might be less likely given the above-described broadening of the ORN response profile to different sets of odorants. What is also unknown is whether virally mediated expression of OMP in an adult OMP-KO mouse (Ivic et al. 2000) restores not only the response kinetics, as mentioned above, but also the narrower response profile and dose response of an ORN.
Ciliary cAMP dynamics and OMP
The observed changes in response kinetics in OMP-KO ORNs suggested that the two underlying messengers of olfactory transduction, cAMP and Ca2+, should have altered kinetics. cAMP is generated by AC3 in response to OR basal activity or odorant stimulation (Connelly et al. 2013; Reisert 2010), raising the questions of if and how these two aspects of ORN physiology might be controlled by OMP. In the absence of odorants, ORNs show varying levels of “noise” (membrane potential fluctuations) driven by cAMP and generated by the given constitutive activity of the expressed OR (Connelly et al. 2013; Nakashima et al. 2013; Reisert 2010). This in turn drives G protein and AC3 activation to open the CNG and then Ano2 channels. These cAMP fluctuations are partly controlled and mitigated by cAMP-degrading PDEs, as application of the PDE blocker 3-isobutyl-1-methylxanthine (IBMX) generates responses that depend on the basal activity of the ORs (Reisert 2010). Since IBMX blocks PDEs downstream of the OR, Golf, and AC3, the resulting current reveals that it is the OR’s constitutive activity that drives the following transduction events (Reisert 2010).
Suction pipette experiments showed that OMP is indeed involved in cAMP dynamics inside the cilia. Unlike responses to odorants, ORNs lacking OMP have response kinetics similar to WT ORNs when stimulated with IBMX (Reisert et al. 2007). This indicates that OMP does not regulate the activity of CNG or Ano2 channels; rather, it acts upstream of cAMP production by AC3, hence on OR, Golf, and/or AC3 (see Fig. 1b). During low extracellular Ca2+ conditions aimed at reducing Ca2+ influx through CNG channels and to eliminate the activation of Ano2, OMP-KO ORNs still displayed slower kinetics in response to an odorant stimulation but no changes in response to IBMX. This suggests that the action of OMP does not require or is not regulated by dynamic changes in ciliary Ca2+.
Interestingly, while no change in the kinetics of IBMX responses has been observed in OMP-KO ORNs, the magnitude of IBMX responses increased and the number of ORNs responding to IBMX nearly doubled compared with WT ORNs (Reisert et al. 2007). The responses became bigger in ORNs expressing ORs with lower basal activity, as has been shown in mOR-EG-expressing GFP-tagged ORNs whose responses to IBMX were particularly larger compared with WT responses (Dibattista and Reisert 2016). It appeared that removing OMP was like removing a “brake,” but a brake on what? One hint came from the observation that AC3 expression is twofold higher in OMP-KO ORNs (Lee et al. 2011). A second hint came from the IBMX responses in mOR-EG-expressing ORNs, which lack basal OR-driven noise in WT mice (Dibattista and Reisert 2016), thus indicating that cAMP increases in OMP-KO ORNs are not predominantly driven by basal activity of mOR-EG. Instead, OMP might act as a brake on basal AC3 activity (and potentially also its expression level), such that the constitutive activity of a given OR becomes the dominant source of noise (Dibattista and Reisert 2016). Hence, OMP coordinates OR type and AC3 activity to lower cAMP levels in the absence of a stimulus. In this way, OMP controls basal activity so that the basal noise driven by the OR can play different instructive roles in ORN physiology in the olfactory system (e.g., axonal guidance to glomeruli in the OB).
Ca2+ dynamics and OMP
Altered cAMP dynamics in OMP-KO mice will alter Ca2+ influx through the CNG channel and potentially also Ca2+ efflux. A proposed site of action of OMP is the NCX (see Fig. 1c and Kwon et al. 2009). Ca2+ imaging of the dendritic knobs of ORNs showed that, in the absence of OMP, the extrusion of Ca2+ generated by the activation of cAMP signaling was slowed down in OMP-KO ORNs compared with WT. By manipulating Na+ concentration (intracellularly and extracellularly) and by pharmacological blockage, Kwon et al. (2009) showed that NCX activity was reduced both in the Ca2+ efflux mode and in the reverse Ca2+ entry mode of NCX in OMP-KO animals.
In addition, molecular analysis suggested a possible protein-protein interaction (Kwon et al. 2009) between a complex formed by OMP, brain-expressed X-linked protein 1 (Bex1), Ca2+/calmodulin, and NCX. This finding that OMP controls Na+/Ca2+ exchange in the dendritic knob seems to contradict the finding that OMP does not affect any protein downstream of AC3, which includes ciliary NCKX4. But the underlying assumption here would be that OMP has the same function in all parts of the ORN, which is actually not resolved. Instead, OMP might act differentially in different cellular compartments. Ca2+ dynamics in ORNs contributes to many aspects of response kinetics, and Ca2+ dynamics in the dendritic knob can be altered by disabling mitochondrial Ca2+ sequestration, thus prolonging the odorant response and steepening the dose-response curve (Fluegge et al. 2012). In ORNs, mitochondria are present in the knob/dendrite region and not in the cilia, so it is possible that the reported interaction between OMP and NCX happens exclusively in this cellular region, thus not excluding a different role of OMP in the cilia.
Action potential firing in ORNs and OMP
By regulating odorant-induced and basal levels of cAMP, OMP also influences odorant-induced and spontaneous AP firing. The odorant-induced current depolarizes ORNs and leads to the firing of APs (Getchell and Shepherd 1978; Mathews 1972; O'Connell and Mozell 1969). Properties of APs such as frequency, number of spikes, and latency to first spike encode several aspects of odorant responses and are translated into specific behaviors later on in the brain (Doving 1965; Harrison and Scott 1986; Kauer 1974; Kauer and Shepherd 1977; Meredith 1986; Reisert and Matthews 1999). OMP-KO ORNs show longer latency to first spike and longer AP trains (Nakashima et al. 2020; Reisert et al. 2007). The longer train of APs might be OR specific since M71-expressing ORNs fire more APs in the absence of OMP, whereas the pattern of APs firing in mOR-EG-expressing ORNs is not affected by OMP (Dibattista and Reisert 2016).
A pattern of odorant delivery that resembles a breathing frequency of 2 Hz showed that ORNs can fire APs in response to each stimulation and are able to follow the stimulation pattern (Ghatpande and Reisert 2011). However, when stimulated with a higher frequency (5 Hz), this reliability is lost. OMP-KO ORNs are not able to fire APs during either 2 Hz or 5 Hz stimulation (Dibattista and Reisert 2016; Nakashima et al. 2020). This can be explained by the fact that, in the presence of OMP, the termination phase of the response is faster. OMP facilitates cellular repolarization and enables the ORN to be ready to fire APs at the next odorant stimulation to reliably send odorant information to the OB (Dibattista and Reisert 2016). Hence, OMP enables ORNs to act as low-pass filters of the stimulation frequency as they generate APs reliably at 2 Hz but fail to do so at higher stimulus frequency (5 Hz).
OMP binding partners
An alternative approach to elucidating the function of OMP, compared with addressing physiological changes in an OMP-KO animal, is to identify potential binding partners of OMP, through which OMP might exert its effects or through which OMP itself might be regulated. Behrens et al. (2003) used phage-display screening of a cDNA library obtained from mouse olfactory tissue to identify proteins interacting with OMP. Interestingly, they did not find OMP interacting with the usual suspects of olfactory transduction proteins. Instead, OMP interacted with brain-expressed X-linked protein 2 (Bex2), which belongs to a family of five proteins in the mouse, Bex1-4 and Bex6 (Alvarez et al. 2005). Bex proteins, with molecular weights around 15 kDa and hence of size roughly comparable with OMP (19 kDa), are expressed in a wide range of tissues, including the brain and play roles in both tumor progression (Bex2) and tumor suppression (Bex1 and 3) (Kazi et al. 2015; Naderi 2019). In situ hybridization of Bex1-3 demonstrated their RNA presence in the OE, in both mature and immature ORNs, unlike OMP RNA, which was localized only in mature ORNs at the more apical portion of the OE. Perplexingly, antibody staining for both OMP and Bex1/2 entirely overlapped, indicating that Bex1 was actually not present at appreciative levels in immature ORNs (Koo et al. 2005). This discrepancy is currently not understood. Bex1-3 were also found in OMP-positive neurons in the vomeronasal organ, and it remains unknown if Bex proteins are also present in the septal organ of Masera or the Grueneberg ganglion.
Because Bex proteins are expressed prior to OMP (but see caveat mentioned above) in the developing ORN, and given their role in cell death in other systems, this raises the possibility that their roles lie in regulating maturation or cell turnover of ORNs. Because Bex proteins remain expressed in mature ORNs, they might play multiple roles, depending on their localization in developing and mature ORNs (Behrens et al. 2003). This notion is supported by observations in HEK cells heterologously expressing Bex, where it is found in both cytoplasm and nucleus. When OMP was co-transfected with Bex, it was also observed in the nucleus (Behrens et al. 2003). Immunogold labeling and electron microscopy confirmed that OMP can exist in the nucleus, predominantly in the nucleoplasm (Koo et al. 2004). These findings were somewhat unexpected since OMP was normally thought of as a strictly cytoplasmic protein.
To further address the interaction of Bex and OMP, Koo et al. (2004) performed cross-linking and co-immunoprecipitation experiments with Bex and OMP. When heterologously expressed, and when in mouse olfactory tissue, OMP could be observed both as a monomer and as a dimer, with the dimer much less abundant in native tissue. Intriguingly, it is the dimer that interacts with both Bex1 and Bex2 (Fig. 1c), while little interaction could be observed between the OMP monomer and Bex proteins. When fractionating CHO cells transfected with OMP, the OMP monomer was found in the cytoplasmic and nuclear fractions, while the dimer was enriched in the membrane and cytoskeletal fractions. Generation of the dimer requires de novo protein synthesis, and the dimer is degraded (either entirely or into monomers) to around 50% within 30 min; thus, it is relatively short-lived. The monomer turnover is approximately every 5–6 days (Kream and Margolis 1984).
Both the nuclear magnetic resonance and the crystal structure of OMP have been determined. OMP forms a β-clamshell-like structure from two β sheets comprising four β strands each. OMP takes a mostly convex shape, which does not display obvious small-molecule binding sites (Baldisseri et al. 2002; Smith et al. 2002; Wright et al. 2005). The cleft formed by the β sheets interacts with Bex1, in particular residues 50–75, with a Kd of around 150 µM for the OMP-Bex1 interaction (Baldisseri et al. 2002), although it remains unclear if this interaction occurs in the monomer or dimer form of OMP. While OMP has very little similarity with other proteins at the amino acid level, it has one highly conserved amino acid sequence that forms an Ω loop next to the Bex1 interaction site. This loop is structurally homologous to the binding domain of the ephrin B2 receptor (EphB2) (Baldisseri et al. 2002; Smith et al. 2002), but its role as a cytoplasmic protein versus in a transmembrane-located receptor tyrosine kinase has not yet been determined. Interestingly, Bex1 contains a potential isoprenylation site at its C terminus that could target Bex1 and therefore anchor the bound OMP dimer to the plasma membrane (Fig. 1c) (Baldisseri et al. 2002). While EphA receptors are known to play a role in axonal targeting of ORNs (Sakano 2020), the roles for EphB receptors are less clear but might be involved in prenatal development of the olfactory system (St John and Key 2001).
Recently, OMP has been proposed to directly bind cAMP, and its role might be to buffer changes in ciliary cAMP during the odorant response (Nakashima et al. 2020) using heterologously expressed CNG alpha 2 subunit (CNGA2) channels and OMP. While this is an interesting possibility, it remains to be verified if OMP can indeed buffer cAMP at a concentration range relevant to the sensitivity of the native olfactory CNG channel, or if the OMP present in the small ciliary volume has sufficient buffering capacity. During repeated stimulation, such a scenario would require either rapid unbinding of cAMP from OMP (which would inadvertently prolong the response, again due to cAMP released from OMP) or continuous free OMP to buffer newly generated cAMP with each successive stimulation. Such a buffering scenario would also suggest that in OMP-KO ORNs, the responses to the PDE inhibitor IBMX should be slowed and smaller similar to odorant responses, but they are not—responses are actually larger (Reisert et al. 2007). Although structures of OMP exist and studies have been carried out to determine interactors, none has previously described potential cAMP binding sites on OMP.
In the ORN axon, OMP mRNA co-localizes with ribonucleoprotein particles that form the fragile X complex composed of RNA-binding proteins and the mRNAs with which they associate (Akins et al. 2012). Fragile X mental retardation protein (FMRP) belongs to the fragile X complex, and it regulates local translation. Interestingly, when FMRP is deleted in a KO model, axonal OMP levels are increased in the axon, not only compared with the WT mice but also relative to dendrite and soma levels (Akins et al. 2017). This is consistent with FMRP regulating axonal OMP expression by local translations within axons. Since local cAMP dynamics have been shown to exist in ORN axons (Maritan et al. 2009), it could be that, during synaptic plasticity and activity-dependent synaptic formation, the fragile X complex targets OMP to modulate cAMP dynamics. This would provide a local feedback mechanism for circuitry formation and/or maintenance in the olfactory bulb (Korsak et al. 2017).
Organization of the olfactory bulb
The main olfactory bulb (OB) is a paired structure of right and left hemispheres situated in the anterior part of the forebrain and separated from the nasal cavity by the cribriform plate (Fig. 3a). The OB not only collects the information from the innervating ORNs but also mediates the afferent odorant-driven signal (Buck 1996; Nagayama et al. 2014; Shepherd 1972). From the OE in the nasal cavity, the axons of ORNs reach the OB after passing through the cribriform plate.
Organization of the olfactory bulb in the presence and absence of OMP. a Representation of a sagittal section of a mouse head. From the olfactory epithelium (OE), ORN axons pass through the cribriform plate and terminate in the glomeruli in the olfactory bulb (OB). b Terminals of ORNs expressing the same OR converge to the same isofunctional glomeruli (shown as blue, green, or red), organized in a mirroring pattern on the medial and lateral surface of each OB hemisphere. Compared with WT and OMP+/− mice (left), the OB of OMP-KO mice (right) is characterized by a high frequency of heterogeneous glomeruli, showing functional microdomains, each representing the terminals of ORNs expressing different ORs. GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; IPL, internal plexiform layer; GCL, granule cell layer. Image created with Biorender
The axons of ORNs expressing the same OR converge in discrete synaptic units called glomeruli (Mombaerts 2006; Ressler et al. 1993; Vassar et al. 1994), dense synaptic neuropils exceptionally well conserved across different species and phyla (Ache and Young 2005; Hildebrand and Shepherd 1997). Glomeruli are organized in a mirroring pattern in each OB hemisphere: typically two isofunctional glomeruli, which are targeted by ORNs that express the same OR are medially and laterally localized on the surface of the OB (Mombaerts 2006; Ressler et al. 1993; Vassar et al. 1994), with more or less stereotyped locations across bulbs and mice (Fig. 3b, left bulb). Mitral and tufted cells constitute the principal output neurons of the OB; their primary dendrites ramify in a dense tuft of branches in only one glomerulus, making synaptic contact with multiple ORN terminals and reciprocal dendrodendritic synapses with periglomerular interneurons (Schoppa and Urban 2003).
Odorants activate specific ensembles of glomeruli, which depend on the identity of the odorant and its concentration (Friedrich and Korsching 1998; Schoppa and Urban 2003; Stewart et al. 1979; Wachowiak and Cohen 2003). Due to its innervation pattern and function, each glomerulus can be considered an OR-specific functional unit (Wachowiak and Shipley 2006; Xu et al. 2000). When odorants enter the nasal cavity, they activate different sets of ORNs that express ORs activated by particular odorants. This then corresponds to the activation of a specific set of glomeruli, as the glomerular circuits produce a representation of the incoming signal from the ORNs with enhanced contrast (Gire and Schoppa 2009; Linster and Cleland 2009). Such modifications are driven mainly by intra- and interglomerular microcircuits driven by bulbar interneurons, modulating the activity of mitral and tufted cells within the same glomerulus (Belluscio and Katz 2001; Friedrich and Stopfer 2001; Soucy et al. 2009; Uchida et al. 2000) and the activity of different glomeruli in odor-specific patterns (Schoppa and Urban 2003; Wachowiak et al. 2004; Whitesell et al. 2013).
Glomerular organization in the absence of OMP
Olfactory coding in the OB intrinsically relies on the homogeneity of projections to each glomerulus, maintaining the “one OR per glomerulus” rule. Even though each glomerulus can be seen as a functional unit, it is possible to identify within each glomerulus distinct microdomains: interdigitating compartments constituted by segregated ORN axon terminals and OB neuron dendrites (Chao et al. 1997; Kasowski et al. 1999; Kosaka et al. 1997). However, these microdomains are functionally equivalent (Wachowiak et al. 2004). Ca2+ imaging of the presynaptic activity within a glomerulus shows that sensory inputs with the same odorant at different concentrations elicit the same intraglomerular map; the only variation is the response amplitude for each microdomain. Similarly, the presynaptic Ca2+ signal pattern in the same glomerulus evoked by olfactory stimuli of different duration, concentration, and identity appears to be indistinguishable (Wachowiak et al. 2004), suggesting that only ORNs expressing the same OR target the same glomerulus (Fig. 3b).
Glomeruli targeted by ORNs expressing different ORs are considered to be rare in adult mice, but they have been observed in adolescent mice (Lodovichi and Belluscio 2012; Royal and Key 1999). Ca2+ signal maps unravel the nature of more broadly responsive glomeruli in adolescent mice, which are characterized by different functional microdomains within each glomerulus, each activated by a set of different odorants. This suggests that each microdomain represents the axon terminals of ORNs expressing different ORs. Similar to mice in early developmental stages, adult OMP-KO mice show increased numbers of heterogeneous glomeruli (Fig. 3b) and a bulbar innervation pattern that is not fully refined (Albeanu et al. 2018; Kass et al. 2013a). OMP-KO mice do not show alteration of the OB macrostructure compared with OMP+/− or WT mice (Fig. 4). However, contrary to adult WT mice, they show a high ratio of heterogeneous glomeruli, which are targeted by ORNs expressing different ORs. Thus, a given glomerulus could now be responsive to a broader range of odorants. Indeed, a single odorant activates almost double the number of glomeruli, on average, in OMP-KO mice compared with OMP+/− mice (Fig. 5), with glomeruli responding to the same set of odorants located in close proximity (Albeanu et al. 2018; Kass et al. 2013b).
Glomerular macro-organization in OMP-KO mice is not altered. a Wide field view of the resting spH fluorescence in the dorsal OB surface of an OMP−/− mouse. Scale bar, 500 µm. b Quantification of the number of glomeruli in a central coronal section of the OB in OMP+/− and OMP−/− mice. n.s., not statistically significant. c, c’ Glomerular area in a central coronal section of the OB for OMP+/− (c) and OMP−/− (c’) mice, showing no significant difference overall. Modified from Albeanu et al. (2018), with permission from Nature Communications
OMP-KO mice show altered glomerular responses to odorants. a Resting light images of the dorsal surface of the OB in OMP+/− (top) and OMP−/− (bottom) mice: response maps evoked by three different concentrations of butyl acetate (BA). Levels of glomerular activation are represented in false color (see bar in the bottom). OMP-KO mice show an overall higher number of glomeruli activated by BA at each concentration. However, there was no significant difference in maximal responses evoked at each concentration between the two mouse lines. From Kass et al. (2013a), with permission from PLoS ONE. (b, c) Number of odorants able to activate a single glomerulus (b, c) and number of glomeruli responding to a single odorant (b’, c’) in OMP+/− (b, b’) and OMP−/− (c, c’) mice. In OMP-KO mice, given glomeruli respond to an increased number of odorants (c, OMP−/− 9.7 ± 0.6; OMP+/− 8.5 ± 0.5) and the number of glomeruli activated by a single odorant is almost doubled (c’, OMP−/− 16.2 ± 0.6; OMP ± 8.2 ± 0.5). Mean ± SEM, Kolmogorov-Smirnov test). Modified from Albeanu et al. (2018), with permission from Nature Communications
Another feature that OMP-KO mice share with WT mice in early developmental stages is the presence of ORN axonal projections overshooting into the external plexiform layer (St John and Key 2005). In WT mice, such projections completely disappear by postnatal day 12, while in OMP+/− and OMP-KO mice, they can still be detected up to 8 months postnatally. Overshooting axons are significantly longer and more frequent in OMP-KO than in OMP+/− mice, but in both, they are progressively reduced in number during life (St John and Key 2005).
Does OMP have a role in axon targeting and refinement of the OB sensory map?
The development and refinement of the glomeruli and the OB sensory map are regulated by multiple factors, including gradients of axon guidance molecules and neuronal activity (Imai and Sakano 2008; Lodovichi and Belluscio 2012; Lorenzon et al. 2015). The exact contribution provided by each type of mechanism is still unknown. Two different pathways have been identified in the regulation of axonal targeting during development. One is agonist independent, relying on OR spontaneous activity, mediated by the G protein Gs predominantly expressed in immature ORNs. The other is agonist dependent (i.e., odorant-evoked activity) mediated by Golf. A common and crucial role in both pathways is played by cAMP, which is regulated by OMP (Piper et al. 2007; Reisert et al. 2007).
Role of odorant-independent OR activity in development in the glomerular refinement
In immature ORNs, activity-independent cAMP signaling affects the position of their projections along the anterior-posterior axis of the OB. Agonist-independent OR activation of Gs increases local levels of cAMP through the stimulation of AC3. Higher cAMP levels activate the protein kinase A pathway, determining the expression levels of targeting molecules neuropilin 1 (Nrp1) and plexin A1 (PlxnA1) (Imai and Sakano 2008; Imai et al. 2006; Nakashima et al. 2013; Piper et al. 2007). Higher levels of cAMP shift ORN projections posteriorly; lower levels shift them anteriorly (Imai et al. 2006). In the OB, Nrp1 and PlxnA1 are expressed in ORN terminals, determining the projection's position based on the complementary gradient of these two proteins (Imai and Sakano 2008; Imai et al. 2006). Nrp1 has been analyzed in OMP-KO mice, showing no alterations in expression levels or distribution across the OB compared with WT mice (Dibattista and Reisert 2016). This result is not surprising since glomerular targeting mediated by agonist-independent cAMP signaling is characteristic of immature ORNs, which do not yet express OMP (Ihara et al. 2016; Nakashima et al. 2013; Rodriguez-Gil et al. 2015). However, other mechanisms could be promoted by spontaneous OR activity and contribute to formation of the OB sensory map. Agonist-independent OR activation might play a role in the survival, strengthening, and stabilization of synapses within the glomerulus after their formation.
The role of spontaneous activity in the glomerular formation and maintenance has been studied using a transgenic mouse line in which ORNs overexpress the inwardly rectifying potassium channel Kir2.1. These mice showed a drastic reduction of spontaneous ORN activity but no changes in odor-evoked activity compared with WT mice (Lorenzon et al. 2015; Yu et al. 2004). Kir2.1 mice also showed delayed formation of the glomeruli at birth, with a reduced number of ORN terminals in the glomerular layer compared with WT mice (Yu et al. 2004). Although in Kir2.1 mice, ORN terminals reached the designated isofunctional glomeruli on the medial and lateral sides, they showed more diffusely distributed targeting compared with WT mice, innervating several other adjacent glomeruli (Lorenzon et al. 2015; Yu et al. 2004).
This phenotype strongly resembles the one described above in OMP-KO mice, with the persistence of heterogeneous glomeruli (Albeanu et al. 2018; Kass et al. 2013b). As in Kir2.1 mice, and also in OMP-KO mice, ORNs show reduced spontaneous firing rates compared with WT mice (Dibattista and Reisert 2016), supporting the hypothesis that disruption of spontaneous ORN activity causes the presence of broader glomerular innervation, with an increased number of heterogeneous glomeruli. The frequency of spontaneous events differs among different ORs (Connelly et al. 2013; Dibattista and Reisert 2016), which might explain why, in both OMP-KO and Kir2.1 mice, different subpopulations of ORNs show different levels of mistargeting to multiple sparsely distributed glomeruli, due to reduction of their basal activity (Albeanu et al. 2018; Lorenzon et al. 2015; Yu et al. 2004).
Role of odorant-dependent OR activity and OMP in the refinement of the OB circuitry
Activity-dependent cAMP signaling, resulting in the activation of the canonical olfactory signal transduction cascade, regulates the expression of homophilic adhesion proteins, such as Kirrel2 and Kirrel3, involved in segregation of ORN axons and refinement of the glomerular map (Imai and Sakano 2008; Imai et al. 2006; Serizawa et al. 2006). Therefore, alterations of ORN activity levels can affect the expression of Kirrel2/Kirrel3 and induce changes to axonal segregation. In olfactory signal transduction, OMP acts upstream of cAMP production, and its loss changes cAMP kinetics as described above. Heterogeneous glomeruli in OMP-KO mice could therefore be explained by changes in Kirrel2/Kirrel3 expression. However, Kirrel2 and Kirrel3 are not the only molecules guiding the refinement of the glomerular map. Different axon-sorting molecules are finely regulated by ORN activity. ORNs show different response patterns in the absence of odorant exposures and during long odorant exposures (Nakashima et al. 2019; Reisert and Matthews 2001; Ukhanov et al. 2019). Such patterns are OR-dependent (Reisert 2010), leading to a mosaic expression of axon-sorting molecules uniquely linked to the OR expressed by a given ORN (Nakashima et al. 2019). Focusing on cAMP dynamics, however, might not fully explain the lack of refinement of the OB map in the absence of OMP. The slower cAMP kinetics in OMP-KO ORNs delays odor-evoked responses (Dibattista and Reisert 2016; Kass et al. 2013a; Lee et al. 2011); thus, the AP output in response to stimulation might be an alternative factor, or the determining factor, for refinement (Nakashima et al. 2019).
Beyond the glomeruli?
External tufted cells (ETCs) are located in the external plexiform layer of the OB, and they make direct synaptic contact with ORNs within a single glomerulus. Their axons project to the internal plexiform layer on the opposite OB site, targeting granule cells, which in turn synapse onto the lateral dendrites of mitral cells and ETCs, serving as a link between medial and lateral isofunctional glomeruli (Belluscio et al. 2002; Hayar et al. 2005). The disruption of ORN spontaneous firing in Kir2.1 mice affects the synaptic organization of the internal plexiform layer; specifically, ETCs present broader axonal projections, contacting an increased number of glomeruli (Lorenzon et al. 2015). No studies have addressed changes in interglomerular projections in OMP-KO mice. However, OMP-KO mice and Kir2.1-overexpressing mice share the lack of glomerular refinement, resulting in a more extensive set of glomeruli activated by the same olfactory stimulus compared with WT mice (Albeanu et al. 2018; Lorenzon et al. 2015). Therefore, we can speculate that in OMP-KO mice, as in Kir2.1 mice, ETCs might present broader projections in the internal plexiform layer, contacting a higher number of glomeruli, such that the perturbation of axonal targeting to the glomeruli in OMP-KO mice reverberates onward into the bulbar circuitry.
Changes in bulbar odor representation due to the lack of OMP
It is interesting to evaluate how OMP impacts OB coding. Due to OMP’s role in olfactory transduction, the lack of OMP affects odorant-evoked activity, slowing down the response kinetics, with delayed odor-response onset, rising phase and termination, and broadening dose-response relations, as described above. These changes in signal transduction are reflected in OB activation: in vivo odor-evoked synaptic release in OMP-KO mice shows that the odor response map develops on a longer time scale compared with OMP+/− mice, with lower neurotransmitter release in the early phases of odor presentation (Kass et al. 2013a).
Odor perception is thought to be based on spatiotemporal activity patterns. Based on identity and concentration, each odorant activates a specific subpopulation of ORNs, to generate a perceptually meaningful combination of activated glomeruli, with a precise spatial identity and temporal latency (Carey et al. 2009; Chong et al. 2020; Spors and Grinvald 2002; Spors et al. 2006). OMP contributes to both spatial identity and temporal latency of the glomerular odor representation (Albeanu et al. 2018; Kass et al. 2013a, 2013b), but how it affects olfactory coding overall is not clear yet. In OMP-KO mice, the increased and broader set of heterogeneous glomeruli being activated by a single odorant could lead to reduced numbers of possible odor representations, reducing the ability of the olfactory system to recognize and discriminate among different odorants and concentrations (Kass et al. 2013b; Perez-Orive et al. 2002; Willmore and Tolhurst 2001).
OMP and odor-driven behavior
Detecting and identifying odorants facilitates a broad range of behaviors, such as searching for food and assessing its hedonic quality; avoiding potential toxins, pathogens, and predators; sexual and reproductive behaviors; and social interactions (Firestein 2001; Tirindelli et al. 2009). Remarkably, even though olfactory behaviors are routinely assessed, very little is known about the neural mechanisms underlying detection, threshold, adaptation, or discrimination. We can gather a few pieces of the puzzle from gene studies that link components of the olfactory transduction pathway to animal behavior.
Pups’ behavior is olfactory driven, since their hearing and vision do not develop until postnatal days 12 and 14, respectively (Bouslama et al. 2005). Pups lacking Golf or the CNGA2 channel subunit have a high mortality rate, probably because of their impaired ability to smell their mother, resulting in decreased suckling behavior (Belluscio et al. 1998; Brunet et al. 1996). CNGA4−/− mice have a higher odor detection threshold and are less sensitive to new odorants (Kelliher et al. 2003). CNGB1 subunit (CNGB1)-null mice and Ano2-KO mice took longer than WT animals to locate food pellets (Michalakis et al. 2006; Neureither et al. 2017; Pietra et al. 2016), while AC3-null mice failed to detect odorants in an odorant-associated passive-avoidance test and failed to find an odorant-associated food reward (Wong et al. 2000).
OMP is involved in many physiological aspects of the odor response, so how does it alter behavioral outcomes? The behavioral repercussions of lack of OMP are subtle. Pups lacking OMP do not appear to be anosmic and can find nipple location, attach, and suckle (Buiakova et al. 1996). However, unlike WT pups, when presented with their biological mother versus an unfamiliar lactating female, OMP-null pups show no preference in suckling or huddling to their mother (Lee et al. 2011).
Olfactory habituation and discrimination can be measured using a noninvasive olfactory habituation/cross-habituation paradigm (Cleland et al. 2002; Coronas-Samano et al. 2016; Fletcher and Wilson 2001). In this paradigm, the rodent is exposed to the same odorant multiple times before being presented with a novel odorant, while the experimenter measures exploration time or the more accurate sniffing frequency. Rodents typically recognize and identify when a novel odorant is presented, as they spend more time investigating or sniffing the odorant source. The lack of OMP delays identification of the first odorant presented but does not affect the time spent investigating the odorant (Kass et al. 2013a). Repeated exposure to the same stimulus causes habituation (Rankin et al. 2009; Thompson and Spencer 1966), which manifests as a decreased behavioral response, decreased exploration time, or decreased resting sniffing frequency. OMP seems to impair habituation since the investigation time across multiple presentations of the same odorant remains the same in OMP-null animals (Kass et al. 2013a). When a different odorant is presented, cross-habituation typically occurs: the rodent increases its sniffing frequency or exploration time, indicating the ability to discriminate between the repeated and novel stimuli. However, since OMP-KO mice do not habituate, it is difficult to ascertain that cross-habituation occurs. Furthermore, when OMP-KO animals were tested for habituation/cross-habituation, odors were presented for 50 s, separated by intertrial intervals of 5 min, which complies with long-term habituation mediated by the OB in WT mice (McNamara et al. 2008); however, in OMP-KO animals, the OB is neuroanatomically different compared with their WT counterparts, and OMP-KO mice may habituate/cross-habituate at different timescales.
In addition to olfactory habituation/cross-habituation, which is a spontaneous behavioral test that requires no training, trained olfactory-guided behaviors can be used to assess olfaction, such as the odor-reward association test. When WT and OMP-KO water-restricted mice were trained to associate a reward (sucrose water) with an odorant (limonene(+)), in room air or in the presence of background limonene at low levels, OMP-KO mice took longer to locate the odor-associated reward and spent less time exploring the odor source (Nakashima et al. 2020). In addition, food-restricted OMP-KO mice rarely located a food reward (Nakashima et al. 2020). In contrast, an earlier study showed that the lack of OMP does not hinder mice’s ability to find a buried food pellet (Buiakova et al. 1996).
Other aspects of olfactory behavior are modulated differently by OMP. In OMP-null mice, odorant threshold is increased. In a go/no-go olfactory discrimination paradigm, OMP-null mice can acquire the task similarly to WT mice but are 50 times less sensitive to odorants (Youngentob and Margolis 1999). Olfactory sensitivity was restored in OMP-null mice after viral delivery of the OMP gene, demonstrating a link between OMP and olfactory threshold (Youngentob et al. 2004). However, simply training mice to identify five different odors does not seem to be dependent on OMP (Youngentob et al. 2001). These behavioral data seem to concur with imaging and electrophysiological recordings since lack of OMP alters the spatial pattern of activity elicited by an odorant (Dibattista and Reisert 2016; Lee et al. 2011; Youngentob et al. 2003).
Taken together, these data indicate that OMP, present in mature ORNs and by regulating ORN physiology, contributes to the fine-tuning of olfactory behavior. How to attribute these changes in odorant-driven behaviors to changes in, for example, odorant responses in ORNs or to broader targeting to axons to glomeruli remains to be determined.
Co-localization of OMP with ORN transduction components outside of the nose
One commonality of OMP expression in the nasal cavity is that OMP is expressed in neurons that mediate chemosensation, with the addition of cold temperatures in case of Grueneberg ganglion neurons (Schmid et al. 2010) and pressure changes by ORNs (Grosmaitre et al. 2007). While this might hint at a role preferentially in chemodetection, it does not provide a simple lead to OMP’s function, as signal transduction mechanisms for ORNs, vomeronasal neurons, and Grueneberg ganglion neurons are quite different and thus do not suggest a simple, common target. Nevertheless, it evokes the questions of whether OMP co-localizes with, in particular, olfactory transduction proteins outside of the nasal cavity and whether it contributes to neuronal functions outside of the nose. One of the early studies to address extranasal expression of OMP in rat, mouse, and hamster, focusing mainly on the brain, showed expression in several brain regions that varied across the species, but in all three species, OMP expression was observed in the preoptic region and the hypothalamus (Baker et al. 1989), the latter co-expressing Bex1 in the same neurons (Koo et al. 2005). Unlike in the OE, where OMP is expressed in ORNs at a high density, OMP expression in the brain was often limited to small clusters of just a few neurons. No clear pattern of expression was observed, for example, exclusively in motor or sensory regions.
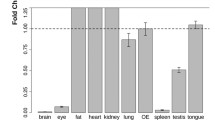
Focusing on tissues outside of the brain, Kang et al. (2015b) described expression of OMP in the bladder, thyroid, thymus, heart, and testis. Somewhat contradictorily, using an OMP-Cre mouse line to drive tdTomato expression in OMP-expressing cells, Riera et al. (2017) did not observe tdTomato expression in the thymus, thyroid, or bladder, for reasons that are unclear. In the bladder and the thymus, OMP-positive cells also expressed the olfactory transduction components Golf and AC3, whereas in the thyroid, only AC3 co-localized with OMP (Kang et al. 2015b). No immunofluorescence signal could be detected for Golf and AC3 in the heart and testis. More detailed analysis revealed that OMP localizes to calcitonin-producing parafollicular cells in the thyroid. Interestingly, three types of ORs were also expressed in OMP-positive cells in the bladder and thyroid. In a similar manner, OMP co-localizes with Golf and AC3 in α-cells in mouse pancreatic islands, as well as with several ORs (Kang et al. 2015a). In particular, the expression of the OR Olfr544 mediates responsiveness to a dicarboxylic acid (azelaic acid) to facilitate intracellular Ca2+ increases and regulates glucagon secretion. The kidney also expresses Golf and AC3, which help regulate the glomerular filtration rate, and expresses a defined set of ORs (Pluznick et al. 2009), but it remains uncertain if OMP is also expressed in kidney. OMP is expressed in the outer layer of the cornea, and Golf is expressed mostly in cells in the middle layer, with some overlap between these two sets of cells, which suggests they might have mostly independent functions in the cornea (Pronin et al. 2014). Recently, OMP was also found to co-localize with AC3 and Golf in spermatozoa (Makeyeva et al. 2020).
While these studies show that, indeed, OMP can be expressed with or without other olfactory transduction proteins outside of ORNs, its functional role remains unaddressed. Prolactin-producing lactotrophs in the human and mouse pituitary gland both express OMP. Using small interfering RNA against OMP in pituitary cell lines and OMP-KO mice, Kang et al. (2018) demonstrated that OMP controls and reduces basal levels of prolactin. Lack of OMP leads to increased levels of intracellular Ca2+ in the GH4 pituitary cell line, preventing any further Ca2+ modulation by thyrotropin-releasing hormone, which normally stimulates prolactin release. OMP-KO mice have twice the amount of circulating prolactin compared with WT control mice, while mice heterozygous for OMP have intermediate levels.
Overall, these data suggest a complex mosaic expression of olfactory transduction components outside of ORNs but also indicate that other G proteins or effector proteins beyond AC3 might be utilized in OMP-expressing cells. Conversely, OMP is expressed in cells that do not express Golf and AC3 (similar to, e.g., in the vomeronasal organ), suggesting that it might have targets other than the canonical olfactory transduction proteins. To make matters more complex, dolphins do not express OMP at all, suggesting that no functions associated with OMP in other species require OMP in dolphins (Springer and Gatesy 2017). The latter might lead one to suggest that water-based species might not require OMP for some reason. But a further interesting twist of the expression of OMP is that in the vertebrate species mentioned so far, one OMP is expressed in a unique neuronal population (mature ORNs), but this is not the case in teleost and frogs. In teleosts, two OMP genes have been identified, OMP1 and OMP2, with a mutually exclusive staining pattern probably representing different ORN subpopulations and OMP2 also expressed in the retina (Suzuki et al. 2015). Two different OMPs have also been described in Xenopus also (Rössler et al. 1998): XOMP1 and XOMP2. They are expressed in ORNs detecting water-borne and air-borne odorant molecules, respectively. While XOMPs’ roles are still elusive, it seems safe to speculate that they mark two distinct subpopulations of ORNs with XOMP2 being expressed in a heterogeneous population of cAMP-dependent, forskolin-sensitive ORNs (Syed et al. 2013).
Conclusions and future perspectives
The endeavor to understand the function of OMP started 50 years ago, following its discovery by Frank Margolis (Margolis 1972). It emerged that OMP is expressed in the neurons of the chemosensory organs in the nasal cavity of vertebrate species. At first glance, such a limited examination of the OMP expression pattern might offer easy insights into its function; however, somewhat paradoxically, it also might have hindered our ability to understand its function by providing no insight into potential functions in other tissues. Recently, studies of the function of OMP outside of the chemosensory systems have seen progress. Furthermore, we now know that cytosolic localization allows OMP to be distributed in different cellular compartments, where it could function differentially, not only in space but also in developmental time, as OMP is expressed only in mature ORNs but might be expressed at other stages of development in other cells.
In the cilia of ORNs, OMP contributes to signal transduction by controlling and speeding up cAMP dynamics. The precise mechanics are still elusive. Our work and that of others suggest a possible interaction with or upstream of AC3 (i.e., expression levels or enzymatic activity) (Dibattista and Reisert 2016; Lee et al. 2011; Reisert et al. 2007) or potentially directly by buffering cAMP (Nakashima et al. 2020). In ORN axons, OMP localizes all the way up to the glomeruli in the OB, where it contributes to refinement of the glomerular map and to axon pruning (Albeanu et al. 2018; Kass et al. 2013a; St John and Key 2005). Furthermore, ORNs lacking OMP have a broader response profile to odorants, indicating that more than one OR might be expressed (Lee et al. 2011). In the maturing ORN, ORs begin to be expressed 1–2 days prior to expression of OMP (Rodriguez-Gil et al. 2015), suggesting that OMP might play a role not in OR choice per se but in stabilization of the OR choice, possibly via interaction with elements involved in cAMP dynamics (i.e., AC3).
These diverse roles of OMP ask the question if OMP actually has one particular function that alters ORN physiology such that these changes reverberate through the system and manifest as described above. A logical starting point for this hypothesis would be altered expression of ORs in the absence of OMP, which could alter the response profile and perturb glomerular targeting in a reasonably straightforward manner. Yet, reconciling altered OR expression with the observed slowed response kinetics in OMP-KO mice is not as easily accomplished. Alternatively, OMP might have multiple interaction partners in different parts of the cell. While such a free-for-all scenario would allow for spatial and temporal flexibility, there is currently no experimental support for or against it, nor is it particularly elegant or imaginative as an explanation. On the other hand, given that OMP is more broadly expressed than only in ORNs, this might argue for OMP having multiple functions and interaction partners.
In this review, we described how OMP in the periphery of the olfactory system contributes to physiology from the single cell to animal behavior. How OMP’s role in single-cell physiology contributes to animal behavior is an interesting aspect that deserves further attention. Animals have different sniffing strategies during odorant sampling behavior, and OMP, expressed predominantly in the OE, could help us understand how odor-elicited activation patterns in ORNs drive behavioral outputs. As previously discussed, OMP-KO ORNs entirely lose their ability to act as low-pass filters to encode odorant stimulation patterns because of slower ORN kinetics and altered AP firing. This means that rodents that can reach sniffing frequency of around 10 Hz might need to change their sampling strategies. Therefore, it could be interesting to investigate whether altered kinetics in the ORN response could alter the precise coordination of the sniff cycle, an issue relevant to odorant coding in the bulb (Iwata et al. 2017).
Several questions mentioned during this review remain open: because OMP does not seem to have a role in ORN maturation, how do ORNs lacking OMP retain some of the features of immature ORNs? Do ORNs that lack OMP and show altered selectivity for odorants express more than one OR type, and if so, what is the underlying mechanism? How does OMP control response kinetics and axonal targeting? And how is the expression of OMP itself controlled? These questions also potentially apply to other nasal chemosensory systems, in which the role of OMP is even less understood.
Since its discovery, and with the advent of our modern era of molecular biology techniques, OMP has been widely used as a tool to time, spatially restrict, or enable the expression and/or deletion of several different genes to mature ORNs. As such, OMP has greatly driven our understanding of how the peripheral olfactory system works while, somewhat paradoxically, the function of OMP itself remains elusive. In recent years, OMP has become less enigmatic, slowly revealing its function and providing new insights into its roles. Understanding OMP will help us decipher a key piece of the current olfactory puzzle.
Abbreviations
- AC3:
-
Adenylyl cyclase type III
- Ano2:
-
Anoctamin 2
- AP:
-
Action potential
- Bex1-6:
-
Brain-expressed X-linked protein 1–6
- cAMP:
-
Cyclic adenosine monophosphate
- CNG:
-
Cyclic nucleotide-gated
- CNGA2, CNGA4:
-
cyclic nucleotide-gated alpha 2 or 4 subunit
- CNGB1:
-
Cyclic nucleotide-gated beta 1 subunit
- EOG:
-
Electro-olfactogram
- EphA, EphB:
-
ephrin A or B receptor
- ETC:
-
External tufted cell
- FMRP:
-
Fragile X mental retardation protein
- GBC:
-
Globose basal cell
- GFP:
-
Green fluorescent protein
- Golf :
-
Olfactory G protein
- Gs :
-
G protein
- HBC:
-
Horizontal basal cell
- IBMX:
-
3-isobutyl-1-methylxanthine
- K1-18:
-
Cytokeratin 1–18
- Kir2.1:
-
Inwardly rectifying potassium channel
- KO:
-
Knockout
- M71:
-
Olfactory receptor type
- mOR-23:
-
Olfactory receptor type
- mOR-EG:
-
Olfactory receptor type
- NCKX4:
-
K+-dependent Na+/Ca2+ exchanger 4
- NCX:
-
Na+/Ca2+ exchanger
- Nrp1:
-
Neuropilin 1
- OB:
-
Olfactory bulb
- OE:
-
Olfactory epithelium
- OMP:
-
Olfactory marker protein
- OR:
-
Olfactory receptor
- ORN:
-
Olfactory receptor neuron
- PDE:
-
Phosphodiesterases
- PlxnA1:
-
Plexin A1
- Sus:
-
Sustentacular
- WT:
-
Wild-type
References
Ache BW, Young JM (2005) Olfaction: diverse species, conserved principles. Neuron 48:417–430
Akins MR, Berk-Rauch HE, Kwan KY et al (2017) Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains. Hum Mol Genet 26:192–209
Akins MR, Leblanc HF, Stackpole EE et al (2012) Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J Comp Neurol 520:3687–3706
Albeanu DF, Provost AC, Agarwal P et al (2018) Olfactory marker protein (OMP) regulates formation and refinement of the olfactory glomerular map. Nat Commun 9:5073
Alvarez E, Zhou W, Witta SE et al (2005) Characterization of the Bex gene family in humans, mice, and rats. Gene 357:18–28
Andres KH (1966) The fine structure of the olfactory region of macrosmatic animals. Z Zellforsch Mikrosk Anat 69:140–154
Antolin S, Matthews HR (2007) The effect of external sodium concentration on sodium-calcium exchange in frog olfactory receptor cells. J Physiol 581:495–503
Asan E, Drenckhahn D (2005) Immunocytochemical characterization of two types of microvillar cells in rodent olfactory epithelium. Histochem Cell Biol 123:157–168
Baker H, Grillo M, Margolis FL (1989) Biochemical and immunocytochemical characterization of olfactory marker protein in the rodent central nervous system. J Comp Neurol 285:246–261
Baldisseri DM, Margolis JW, Weber DJ et al (2002) Olfactory marker protein (OMP) exhibits a beta-Clam fold in solution: implications for target peptide interaction and olfactory signal transduction. J Mol Biol 319:823–837
Barber PC, Jensen S, Zimmer J (1982) Differentiation of neurons containing olfactory marker protein in adult rat olfactory epithelium transplanted to the anterior chamber of the eye. Neurosci 7:2687–2695
Behrens M, Margolis JW, Margolis FL (2003) Identification of members of the Bex gene family as olfactory marker protein (OMP) binding partners. J Neurochem 86:1289–1296
Belluscio L, Gold GH, Nemes A, Axel R (1998) Mice deficient in Golf are anosmic. Neuron 20:69–81
Belluscio L, Katz LC (2001) Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci 21:2113–2122
Belluscio L, Lodovichi C, Feinstein P et al (2002) Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature 419:296–300
Billig GM, Pal B, Fidzinski P et al (2011) Ca2+-activated Cl- currents are dispensable for olfaction. Nat Neurosci 14:763–769
Boccaccio A, Menini A (2007) Temporal development of cyclic nucleotide-gated and Ca2+-activated Cl- currents in isolated mouse olfactory sensory neurons. J Neurophysiol 98:153–160
Bouslama M, Durand E, Chauviere L et al (2005) Olfactory classical conditioning in newborn mice. Behav Brain Res 161:102–106
Breipohl W, Naguro T, Walker DG (1989) The postnatal development of Masera’s organ in the rat. Chem Senses 14:649–662
Brunet LJ, Gold GH, Ngai J (1996) General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17:681–693
Buck LB (1996) Information coding in the mammalian olfactory system. Cold Spring Harb Symp Quant Biol 61:147–155
Buiakova OI, Baker H, Scott JW et al (1996) Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci USA 93:9858–9863
Caggiano M, Kauer JS, Hunter DD (1994) Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron 13:339–352
Carey RM, Verhagen JV, Wesson DW et al (2009) Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol 101:1073–1088
Carter LA, MacDonald JL, Roskams AJ (2004) Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci 24:5670–5683
Chao TI, Kasa P, Wolff JR (1997) Distribution of astroglia in glomeruli of the rat main olfactory bulb: exclusion from the sensory subcompartment of neuropil. J Comp Neurol 388:191–210
Chong E, Moroni M, Wilson C et al (2020) Manipulating synthetic optogenetic odors reveals the coding logic of olfactory perception. Science 368
Cleland TA, Morse A, Yue EL et al (2002) Behavioral models of odor similarity. Behav Neurosci 116:222–231
Connelly T, Savigner A, Ma M (2013) Spontaneous and sensory-evoked activity in mouse olfactory sensory neurons with defined odorant receptors. J Neurophysiol 110:55–62
Coronas-Samano G, Ivanova AV, Verhagen JV (2016) The habituation/cross-habituation test revisited: guidance from sniffing and video tracking. Neural Plast 2016:9131284
Cygnar KD, Zhao H (2009) Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat Neurosci 12:454–462
Danciger E, Mettling C, Vidal M et al (1989) Olfactory marker protein gene: its structure and olfactory neuron-specific expression in transgenic mice. Proc Natl Acad Sci USA 86:8565–8569
Dibattista M, Pifferi S, Boccaccio A et al (2017) The long tale of the calcium activated Cl- channels in olfactory transduction. Channels (Austin) 11:399–414
Dibattista M, Reisert J (2016) The odorant receptor-dependent role of olfactory marker protein in olfactory receptor neurons. J Neurosci 36:2995–3006
Doving KB (1965) Studies on the responses of bulbar neurons of frog to different odour stimuli. Rev Laryngol Otol Rhinol (Bord) 86(Suppl):845–854
Engstrom B, Ekblom A, Hansson P (1989) The olfactory and respiratory epithelium in rhesus and squirrel monkeys studied with freeze-fracture technique. Acta Otolaryngol 108:259–267
Firestein S (2001) How the olfactory system makes sense of scents. Nature 413:211–218
Fleischer J, Schwarzenbacher K, Besser S et al (2006) Olfactory receptors and signalling elements in the Grueneberg ganglion. J Neurochem 98:543–554
Fletcher M, Wilson DA (2001) Ontogeny of odor discrimination: a method to assess novel odor discrimination in neonatal rats. Physiol Behav 74:589–593
Fluegge D, Moeller LM, Cichy A et al (2012) Mitochondrial Ca2+ mobilization is a key element in olfactory signaling. Nat Neurosci 15:754–762
Friedrich RW, Korsching SI (1998) Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. The Journal of Neuroscience 18:9977–9988
Friedrich RW, Stopfer M (2001) Recent dynamics in olfactory population coding. Curr Opin Neurobiol 11:468–474
Genovese F, Tizzano M (2018) Microvillous cells in the olfactory epithelium express elements of the solitary chemosensory cell transduction signaling cascade. PLoS ONE 13:e0202754
Getchell TV, Shepherd GM (1978) Responses of olfactory receptor cells to step pulses of odour at different concentration in the salamander. J Physiol 282:521–540
Ghatpande AS, Reisert J (2011) Olfactory receptor neuron responses coding for rapid odor sampling. J Physiol 589:2261–2273
Gire DH, Schoppa NE (2009) Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29:13454–13464
Graziadei PP, Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8:1–18
Grosmaitre X, Santarelli LC, Tan J et al (2007) Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci 10:348–354
Hanchate NK, Kondoh K, Lu Z et al (2015) Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis. Science 350:1251–1255
Harrison TA, Scott JW (1986) Olfactory bulb responses to odor stimulation: analysis of response pattern and intensity relationships. J Neurosci 56:1571–1589
Hartman BK, Margolis FL (1975) Immunofluorescence localization of the olfactory marker protein. Brain Res 96:176–180
Hayar A, Shipley MT, Ennis M (2005) Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25:8197–8208
Hegg CC, Jia C, Chick WS et al (2010) Microvillous cells expressing IP3 receptor type 3 in the olfactory epithelium of mice. Eur J Neurosci 32:1632–1645
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631
Holbrook EH, Wu E, Curry WT et al (2011) Immunohistochemical characterization of human olfactory tissue. Laryngoscope 121:1687–1701
Ihara N, Nakashima A, Hoshina N et al (2016) Differential expression of axon-sorting molecules in mouse olfactory sensory neurons. Eur J Neurosci 44:1998–2003
Imai T, Sakano H (2008) Odorant receptor-mediated signaling in the mouse. Curr Opin Neurobiol 18:251–260
Imai T, Suzuki M, Sakano H (2006) Odorant receptor-derived cAMP signals direct axonal targeting. Science 314:657–661
Ivic L, Pyrski MM, Margolis JW et al (2000) Adenoviral vector-mediated rescue of the OMP-null phenotype in vivo. Nat Neurosci 3:1113–1120
Iwata R, Kiyonari H, Imai T (2017) Mechanosensory-based phase coding of odor identity in the olfactory bulb. Neuron 96:1139–1152
Jung A, Lischka FW, Engel J et al (1994) Sodium/calcium exchanger in olfactory receptor neurons of Xenopus laevis. NeuroReport 5:1741–1744
Kang CW, Han YE, Lee MK et al (2018) Olfactory marker protein regulates prolactin secretion and production by modulating Ca2+ and TRH signaling in lactotrophs. Exp Mol Med 50:15
Kang N, Bahk YY, Lee N et al (2015) Olfactory receptor Olfr544 responding to azelaic acid regulates glucagon secretion in alpha-cells of mouse pancreatic islets. Biochem Biophys Res Commun 460:616–621
Kang N, Kim H, Jae Y et al (2015) Olfactory marker protein expression is an indicator of olfactory receptor-associated events in non-olfactory tissues. PLoS ONE 10:e0116097
Kasowski HJ, Kim H, Greer CA (1999) Compartmental organization of the olfactory bulb glomerulus. J Comp Neurol 407:261–274
Kass MD, Moberly AH, McGann JP (2013) Spatiotemporal alterations in primary odorant representations in olfactory marker protein knockout mice. PLoS ONE 8:e61431
Kass MD, Moberly AH, Rosenthal MC et al (2013) Odor-specific, olfactory marker protein-mediated sparsening of primary olfactory input to the brain after odor exposure. J Neurosci 33:6594–6602
Kauer JS (1974) Response patterns of amphibian olfactory bulb neurons to odour stimulation. J Physiol 243:695–715
Kauer JS, Shepherd GM (1977) Analysis of the onset phase of olfactory bulb unit responses to odour pulses in the salamander. J Physiol 272:495–516
Kazi JU, Kabir NN, Rönnstrand L (2015) Brain-expressed X-linked (BEX) proteins in human cancers. Biochem Biophys Acta 1856:226–233
Keller A, Margolis FL (1975) Immunological studies of the rat olfactory marker protein. J Neurochem 24:1101–1106
Kelliher KR, Ziesmann J, Munger SD et al (2003) Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice. Proc Natl Acad Sci USA 100:4299–4304
Koo JH, Gill S, Pannell LK et al (2004) The interaction of Bex and OMP reveals a dimer of OMP with a short half-life. J Neurochem 90:102–116
Koo JH, Saraswati M, Margolis FL (2005) Immunolocalization of Bex protein in the mouse brain and olfactory system. J Comp Neurol 487:1–14
Korsak LIT, Shepard KA, Akins MR (2017) Cell type-dependent axonal localization of translational regulators and mRNA in mouse peripheral olfactory neurons. J Comp Neurol 525:2202–2215
Kosaka K, Toida K, Margolis FL et al (1997) Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb–II. Prominent differences in the intraglomerular dendritic arborization and their relationship to olfactory nerve terminals. Neurosci 76:775–786
Kream RM, Margolis FL (1984) Olfactory marker protein: turnover and transport in normal and regenerating neurons. J Neurosci 4:868–879
Kusumakshi S, Voigt A, Hubner S et al (2015) A binary genetic approach to characterize TRPM5 cells in mice. Chem Senses 40:413–425
Kwon HJ, Koo JH, Zufall F et al (2009) Ca extrusion by NCX is compromised in olfactory sensory neurons of OMP mice. PLoS ONE 4:e4260
Lee AC, He J, Ma M (2011) Olfactory marker protein is critical for functional maturation of olfactory sensory neurons and development of mother preference. J Neurosci 31:2974–2982
Linster C, Cleland TA (2009) Glomerular microcircuits in the olfactory bulb. Neural Net 22:1169–1173
Lodovichi C, Belluscio L (2012) Odorant receptors in the formation of the olfactory bulb circuitry. Physiology 27:200–212
Lorenzon P, Redolfi N, Podolsky MJ et al (2015) Circuit formation and function in the olfactory bulb of mice with reduced spontaneous afferent activity. J Neurosci 35:146–160
Magklara A, Yen A, Colquitt BM et al (2011) An epigenetic signature for monoallelic olfactory receptor expression. Cell 145:555–570
Makeyeva Y, Nicol C, Ledger WL et al (2020) Immunocytochemical localization of olfactory-signaling molecules in human and rat spermatozoa. J Histochem Cytochem 68:491–513
Margolis FL (1972) A brain protein unique to the olfactory bulb. Proc Natl Acad Sci USA 69:1221–1224
Margolis FL, Tarnoff JF (1973) Site of biosynthesis of the mouse brain olfactory bulb protein. J Biol Chem 248:451–455
Maritan M, Monaco G, Zamparo I et al (2009) Odorant receptors at the growth cone are coupled to localized cAMP and Ca2+ increases. Proc Natl Acad Sci USA 106:3537–3542
Mathews DF (1972) Response pattern of single neurons in the tortoise olfactory epithelium and olfactory bulb. J Gen Physiol 60:166–180
McNamara AM, Magidson PD, Linster C et al (2008) Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem 15:117–125
Menco BP (1980) Qualitative and quantitative freeze-fracture studies on olfactory and nasal respiratory structures of frog, ox, rat, and dog. I. A general survey Cell Tissue Res 207:183–209
Meredith M (1986) Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurosci 56:572–597
Michalakis S, Reisert J, Geiger H et al (2006) Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping. J Biol Chem 281:35156–35166
Mombaerts P (2006) Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol 22:713–737
Montani G, Tonelli S, Elsaesser R et al (2006) Neuropeptide Y in the olfactory microvillar cells. Eur J Neurosci 24:20–24
Monti-Graziadei GA, Margolis FL, Harding JW et al (1977) Immunocytochemistry of the olfactory marker protein. J Histochem Cytochem 25:1311–1316
Moran DT, Rowley JC 3rd, Jafek BW (1982) Electron microscopy of human olfactory epithelium reveals a new cell type: the microvillar cell. Brain Res 253:39–46
Moran DT, Rowley JC 3rd, Jafek BW et al (1982) The fine structure of the olfactory mucosa in man. J Neurocytol 11:721–746
Morrison EE, Costanzo RM (1990) Morphology of the human olfactory epithelium. J Comp Neurol 297:1–13
Morrison EE, Costanzo RM (1992) Morphology of olfactory epithelium in humans and other vertebrates. Microsc Res Tech 23:49–61
Naderi A (2019) Molecular functions of brain expressed X-linked 2 (BEX2) in malignancies. Exp Cell Res 376:221–226
Nagayama S, Homma R, Imamura F (2014) Neuronal organization of olfactory bulb circuits. Front Neural Circuits 8:98
Nakashima A, Ihara N, Shigeta M et al (2019) Structured spike series specify gene expression patterns for olfactory circuit formation. Science 365(6448):eaaw5030
Nakashima A, Takeuchi H, Imai T et al (2013) Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell 154:1314–1325
Nakashima N, Nakashima K, Taura A et al (2020) Olfactory marker protein directly buffers cAMP to avoid depolarization-induced silencing of olfactory receptor neurons. Nat Commun 11:2188
Neureither F, Stowasser N, Frings S et al (2017) Tracking of unfamiliar odors is facilitated by signal amplification through anoctamin 2 chloride channels in mouse olfactory receptor neurons. Physiol Rep 5(15):e13373
Noe J, Tareilus E, Boekhoff I et al (1997) Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int 30:523–531
O’Connell RJ, Mozell MM (1969) Quantitative stimulation of frog olfactory receptors. J Neurophysiol 32:51–63
Perez-Orive J, Mazor O, Turner GC et al (2002) Oscillations and sparsening of odor representations in the mushroom body. Science 297:359–365
Peterson J, Lin B, Barrios-Camacho CM et al (2019) Activating a reserve neural stem cell population in vitro enables engraftment and multipotency after transplantation. Stem Cell Rep 12:680–695
Pietra G, Dibattista M, Menini A et al (2016) The Ca2+-activated Cl- channel TMEM16B regulates action potential firing and axonal targeting in olfactory sensory neurons. J Gen Physiol 148:293–311
Piper M, van Horck F, Holt C (2007) The role of cyclic nucleotides in axon guidance. Adv Exp Med Biol 621:134–143
Pluznick JL, Zou DJ, Zhang X et al (2009) Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106:2059–2064
Pronin A, Levay K, Velmeshev D et al (2014) Expression of olfactory signaling genes in the eye. PLoS ONE 9:e96435
Rankin CH, Abrams T, Barry RJ et al (2009) Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92:135–138
Rasche S, Toetter B, Adler J et al (2010) Tmem16b is specifically expressed in the cilia of olfactory sensory neurons. Chem Senses 35:239–245
Reisert J (2010) Origin of basal activity in mammalian olfactory receptor neurons. J Gen Physiol 136:529–540
Reisert J, Matthews HR (1998) Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol 112:529–535
Reisert J, Matthews HR (1999) Adaptation of the odour-induced response in frog olfactory receptor cells. J Physiol 519:801–813
Reisert J, Matthews HR (2001) Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol 534:179–191
Reisert J, Yau KW, Margolis FL (2007) Olfactory marker protein modulates the cAMP kinetics of the odour-induced response in cilia of mouse olfactory receptor neurons. J Physiol 585:731–740
Reisert J, Zhao H (2011) Perspectives on: Information and coding in mammalian sensory physiology: response kinetics of olfactory receptor neurons and the implications in olfactory coding. J Gen Physiol 138:303–310
Ressler KJ, Sullivan SL, Buck LB (1993) A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell 73:597–609
Riera CE, Tsaousidou E, Halloran J et al (2017) The sense of smell impacts metabolic health and obesity. Cell Metab 26(198–211):e195
Rodriguez-Gil DJ, Bartel DL, Jaspers AW et al (2015) Odorant receptors regulate the final glomerular coalescence of olfactory sensory neuron axons. Proc Natl Acad Sci USA 112:5821–5826
Rogers KE, Dasgupta P, Gubler U et al (1987) Molecular cloning and sequencing of a cDNA for olfactory marker protein. Proc Natl Acad Sci USA 84:1704–1708
Rössler P, Mezler P, Breer H (1998) Two olfactory marker proteins in Xenopus laevis. J Comp Neurol 395:273–280
Royal SJ, Key B (1999) Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-lacZ transgenic mice. J Neurosci 19:9856–9864
Sakano H (2020) Developmental regulation of olfactory circuit formation in mice. Dev Growth Differ 62:199–213
Schmid A, Pyrski M, Biel M et al (2010) Grueneberg ganglion neurons are finely tuned cold sensors. J Neurosci 30:7563–7568
Schoppa NE, Urban NN (2003) Dendritic processing within olfactory bulb circuits. Trends Neurosci 26:501–506
Schwob JE, Huard JM, Luskin MB et al (1994) Retroviral lineage studies of the rat olfactory epithelium. Chem Senses 19:671–682
Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, Hewitt Coleman J (2017) Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J Comp Neurol 525:1034–1054
Scott JW, Scott-Johnson PE (2002) The electroolfactogram: a review of its history and uses. Microsc Res Tech 58:152–160
Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H (2006) A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell 127:1057–1069
Shepherd GM (1972) Synaptic organization of the mammalian olfactory bulb. Physiol Rev 52:864–917
Smith PC, Firestein S, Hunt JF (2002) The crystal structure of the olfactory marker protein at 2.3 a resolution. J Mol Biol 319:807–821
Soucy ER, Albeanu DF, Fantana AL et al (2009) Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci 12:210–220
Spors H, Grinvald A (2002) Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron 34:301–315
Spors H, Wachowiak M, Cohen LB et al (2006) Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci 26:1247–1259
Springer MS, Gatesy J (2017) Inactivation of the olfactory marker protein (OMP) gene in river dolphins and other odontocete cetaceans. Mol Phylogenet Evol 109:375–387
St John JA, Key B (2001) EphB2 and two of its ligands have dynamic protein expression patterns in the developing olfactory system. Brain Res Dev Brain Res 126:43–56
St John JA, Key B (2005) Olfactory marker protein modulates primary olfactory axon overshooting in the olfactory bulb. J Comp Neurol
Stephan AB, Shum EY, Hirsh S et al (2009) ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci USA 106:11776–11781
Stephan AB, Tobochnik S, Dibattista M et al (2012) The Na+/Ca2+ exchanger NCKX4 governs termination and adaptation of the mammalian olfactory response. Nat Neurosci 15:131–137
Stewart WB, Kauer JS, Shepherd GM (1979) Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol 185:715–734
Suzuki H, Nikaido M, Hagino-Yamagishi K et al (2015) Distinct functions of two olfactory marker protein genes derived from teleost-specific whole genome duplication. BMC Evol Biol 15:245
Syed AS, Sansone A, Nadler W et al (2013) Ancestral amphibian v2rs are expressed in the main olfactory epithelium. Proc Natl Acad Sci USA 110:7714–7719
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
Tirindelli R, Dibattista M, Pifferi S et al (2009) From pheromones to behavior. Physiol Rev 89:921–956
Tirindelli R, Ryba NJP (1996) The G-protein gamma-subunit G gamma 8 is expressed in the developing axons of olfactory and vomeronasal neurons. Eur J Neurosci 8:2388–2398
Uchida N, Takahashi YK, Tanifuji M et al (2000) Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci 3:1035–1043
Ukhanov K, Bobkov YV, Martens JR et al (2019) Initial characterization of a subpopulation of inherent oscillatory mammalian olfactory receptor neurons. Chem Senses 44:583–592
Vassar R, Chao SK, Sitcheran R et al (1994) Topographic organization of sensory projections to the olfactory bulb. Cell 79:981–991
Verhaagen J, Oestreicher AB, Gispen WH et al (1989) The expression of the growth associated protein B50/GAP43 in the olfactory system of neonatal and adult rats. J Neurosci 9:683–691
Wachowiak M, Cohen LB (2003) Correspondence between odorant-evoked patterns of receptor neuron input and intrinsic optical signals in the mouse olfactory bulb. J Neurophysiol 89:1623–1639
Wachowiak M, Denk W, Friedrich RW (2004) Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging. Proc Natl Acad Sci USA 101:9097–9102
Wachowiak M, Shipley MT (2006) Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol
Weiler E, Farbman AI (2003) The septal organ of the rat during postnatal development. Chem Senses 28:581–593
Whitesell JD, Sorensen KA, Jarvie BC et al (2013) Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci 33:1552–1563
Willmore B, Tolhurst DJ (2001) Characterizing the sparseness of neural codes. Network 12:255–270
Wong ST, Trinh K, Hacker B et al (2000) Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27:487–497
Wright NT, Margolis JW, Margolis FL et al (2005) Refinement of the solution structure of rat olfactory marker protein (OMP). J Biomol NMR 33:63–68
Xu F, Greer CA, Shepherd GM (2000) Odor maps in the olfactory bulb. J Comp Neurol 422:489–495
Youngentob SL, Kent PF, Margolis FL (2003) OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol 13:13
Youngentob SL, Margolis FL (1999) OMP gene deletion causes an elevation in behavioral threshold sensitivity. NeuroReport 10:15–19
Youngentob SL, Margolis FL, Youngentob LM (2001) OMP gene deletion results in an alteration in odorant quality perception. Behav Neurosci 115:626–631
Youngentob SL, Pyrski MM, Margolis FL (2004) Adenoviral vector-mediated rescue of the OMP-null behavioral phenotype: enhancement of odorant threshold sensitivity. Behav Neurosci 118:636–642
Yu CR, Power J, Barnea G et al (2004) Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron 42:553–566
Funding
This work was supported by NIH/NIDCD grants R01DC016647 (JR) and R21DC018358 (FG). DAK is supported by NIH/NIDCD-funded Monell Institutional Training Grant T32DC000014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dibattista, M., Al Koborssy, D., Genovese, F. et al. The functional relevance of olfactory marker protein in the vertebrate olfactory system: a never-ending story. Cell Tissue Res 383, 409–427 (2021). https://doi.org/10.1007/s00441-020-03349-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03349-9