Abstract
Lead (Pb), one of the pervasive and protracted environmental heavy metals, is believed to affect the female reproductive system in many species. The Nrf2 and NF-κB are the two key transcriptional factors regulating cellular redox status and response against stress and inflammation respectively, showing an interaction between each other. The aim of this study is to investigate the effect of Pb on bovine granulosa cells (GCs) and its association with the regulation of Nrf2 and NF-κB pathways. For this, bovine GCs were cultured in vitro and exposed to different doses of Pb for 2 h. Cellular response to Pb insult was investigated 24 h post treatment. Results showed that exposure of GCs to Pb-induced ROS accumulation and protein carbonylation. Additionally, GCs exhibited reduction in cell viability and decrease in the expression of cell proliferation marker genes (CCND2 and PCNA). This was accompanied by cell cycle arrest at G0/G1 phase. Moreover, Pb downregulated both Nrf2 and NF-κB and their downstream genes. Lead increased the expression of endoplasmic reticulum (ER) stress marker genes (GRP78 and CHOP) and the proapoptotic gene (caspase-3) while the antiapoptotic gene (BCL-2) was reduced. Our findings suggest that Pb-driven oxidative stress affected GCs proliferation, enhances ER stress, induces cell cycle arrest and mediates apoptosis probably via disruption of Nrf2/NF-κB cross-talk. However, further functional analysis is required to explain different aspects of Nrf2 and NF-κB interactions under metal challenge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a widespread naturally occurring toxic heavy metal that has a broad spectrum of industrial applications (Sanders et al. 2009). The extensive use of Pb extended its abundance in the environment due to its non-biodegradable nature. Accordingly, Pb constitutes a persistent public health problem especially in developing countries, despite extensive efforts to reduce its use (Ahamed and Siddiqui 2007). The reproductive and developmental toxicity of Pb have been demonstrated in both males and females of experimental animals and human (Qureshi et al. 2010; Vigeh et al. 2011). It can accumulate in testes, epididymis and seminal vesicle inducing adverse effects on spermatogenesis, prostatic function, sperm count and motility (Chowdhury 2009; Shan et al. 2009). However, the impact of Pb on female reproduction is more profound, as it has been documented to induce infertility, miscarriage, pregnancy hypertension, premature delivery and preeclampsia (Seyom et al. 2015; Bayat et al. 2016). Previous experimental studies have detected Pb in the follicular fluid of many species, including human (Paksy et al. 2001), cattle (Swarup et al. 2005), sheeps (Bires et al. 1995) and mouse (Taupeau et al. 2001), which in turn irreversibly impaired folliculogenesis. Exposure to Pb caused a decline in buffalo oocyte maturation and embryo development in vitro as shown by Nandi et al. (2010). The effect of Pb as an endocrine disruptor has also been documented, as it interrupts several processes along the hypothalamic–pituitary–gonadal (HPG) axis, inducing disruption of the gonadal function and reproductive hormones (Doumouchtsis et al. 2009). Furthermore, occupationally Pb-exposed women exhibited menstrual abnormalities, including hypermenorrhea and early menopause (Eum et al. 2014).
Although Pb toxicity cannot be linked to a single mechanism, oxidative stress is implicated in the pathophysiology of Pb toxicity (Flora et al. 2012). Moreover, oxidative stress arises when the balance between pro-oxidants and antioxidants is disrupted (Luderer 2014). This in turn activates a variety of transcriptional factors (TFs), which proved to have great significance in the etiology of reproductive diseases (Lu et al. 2018; Bolisetty and Jaimes 2013). The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and nuclear factor-kappaB (NF-κB) are the two key redox-sensitive TFs regulating the cellular redox status in response to intracellular and environmental signals (Wardyn et al. 2015). The Nrf2 mediates transcription of phase II antioxidant genes, which are responsible for the clearance of ROS, providing protection against the accumulation of toxic metabolites (Zhang et al. 2013). Deletion of Nrf2 was reported to reduce the number of ovarian follicles and accelerate ovarian aging in mice (Lim et al. 2015). The NF-κB is a pleiotropic factor, which plays an important role in immunity and inflammation. Moreover, it is also involved in the regulation of other processes, including development, cell growth, survival and proliferation (Bellezza et al. 2010). NF-κB has antiapoptotic effects on rat corpus luteum and inhibits progesterone (P4) catabolism in ovarian cells (Wang et al. 2002). Lim et al. (2015) reported that NF-κB along with FSH regulates porcine ovarian functions such as proliferation, secretory activities and apoptosis. Furthermore, pharmacological animal studies showed a potential cross-talk between Nrf2 and NF-κB pathways and its contribution to cellular response to different environmental insults (Wardyn et al. 2015; Lu et al. 2018).
It is known that ovarian granulosa cells (GCs) are sensitive to reactive oxygen species (ROS) and can protect oocytes through their own antioxidant system during maturation of oocytes (Tripathi et al. 2013). The integrity of GCs is hence crucial for oocytes maturation, competency and response to gonadotropins (Jahromi et al. 2015). Although the properties of Pb have been extensively studied, the articles related to the effect of Pb on ovarian functionality in mammals are still limited. Besides, evidence revealed the possibility to use bovine as a reliable model for human in in vitro reprotoxicity studies (Santos et al. 2014). So the objective of the current study is to investigate the effect of Pb exposure on bovine GCs and its association with the regulation of Nrf2 and NF-κB pathways. We postulate that Pb exposure affects in vitro cultured bovine GCs proliferation via perturbation of Nrf2/ NF-κB.
Materials and methods
Collection of ovaries and granulosa cells
Bovine ovaries were collected from a local slaughter house and transported to the laboratory in a thermo flask containing warm physiologic saline solution (0.9% NaCl) within 2 h of collection. Ovaries were processed as described previously by Gebremedhn et al. (2015).
Granulosa cell culture
Granulosa cells (2 × 105 cells per well) were seeded in a 24-well plate (Starlab, Hamburg, Germany) with 600 μl of culture media per well comprising DMEM/F-12 Ham (Roth, Karlsruhe, Germany) supplemented with 10% FBS, 1% penicillin-streptomycin and 1% amphotericin (Sigma–Aldrich, Germany). After 70% confluency at 37 °C under 5% CO2 in humidified air, cells were exposed to lead acetate (Sigma–Aldrich, Germany) at different concentrations (1, 2, 3, 5 and 10 μg/ml), adapted from the early study of Nandi et al. (2010), for 2 h under culture condition. Based on phenotypic evaluation of treated cells, three concentrations (1, 2 and 3 μg/ml) were chosen for further investigation in addition to untreated cells (control). Cultured GCs were harvested using 0.25% trypsin-EDTA (Sigma–Aldrich, Germany) 24 h post treatment and kept under − 80 °C for further use.
Cell proliferation assay
A total of 2 × 104 of GCs were cultured in 96-well plates containing 100 μl of medium and after sub-confluency, cells were treated with Pb. Cell viability of both treated and untreated cells was assessed using Cell Counting Kit-8 (CCK-8) (Dojindo EU GmbH, Germany) according to the manufacturer’s instructions. The optical density (OD) of released formazan dye, which is proportional to the number of living cells was measured at 450 nm using Synergy™ H1 Multi-Mode Reader (BioTek Germany, Germany).
Detection of intracellular ROS accumulation
The intracellular level of ROS was measured by loading GCs with H2-dichlorofluorescein diacetate (H2DCF-DA) (Life Technologies, Germany) according to the method previously mentioned by Alemu et al. (2018). Cells were imaged using a Leica DM IRB inverted microscope (Leica, Bensheim, Germany). Images were then analyzed using imageJ 1.48v (National Institutes of Health, Bethesda, MD, USA).
Analysis of cell cycle with flow cytometry
Cultured GCs were harvested 24 h after treatment and washed twice with × 1 PBS. The relative numbers of cells in different phases of the cell cycle was determined by LSRFortessa™ Flow cytometer (BD Biosciences, USA) using the method adapted from Gebremedhn et al. (2015). Results were analyzed by ModFit LT software (http://www.vsh.com/products/mflt/index.asp) and the percentages of cells were calculated in the G0/G1, S and G2/M phases of the cell cycle.
Annexin V and propidium iodide (PI) staining
The apoptotic cells were distinguished from viable or necrotic cells by the combined application of annexinV-APC and propidium iodide (PI) using Cell Meter™ APC-Annexin V Binding Apoptosis Assay Kit (Biomol, USA), according to the manufacturer’s recommendations with modifications according to van Engeland et al. (1996). Cells were analyzed with LSRFortessa™ Flow cytometer (BD Biosciences, USA), and data were processed using FACSDiva™ 6.1.3 software (Becton Dickinson).
Extraction of total RNA and cDNA synthesis
Total RNA was isolated using miRNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction. The RNA was then reverse transcribed using cDNA Synthesis Kit (Thermo Fisher scientific, Germany) according to the manufacturer’s instruction. The resultant cDNA samples were stored in − 20 °C until further use.
Real-time quantitative PCR
The qRT-PCR was performed using iTaq™ Universal SYBR® Green Supermix (Bio-Rad Laboratories GmbH, Germany) in Applied Biosystem® StepOnePlus™ (Applied biosystems, CA, USA). Gene-specific primers were designed using Primer3web version 4.0.0 (http://bioinfo.ut.ee/primer3/) and Primer blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). A total of 16 genes: oxidative stress response genes (Nrf2, Keap-1, SOD, CAT, HO-1, Thrx, NF-κB, IKK, TNF-α), cell proliferation related genes (CCDN2 and PCNA), endoplasmic reticulum–related genes (GRP78 and CHOP) proapoptotic (BAX and Caspase-3) and antiapoptotic (BCL-2)-related genes were quantified in GCs. The expression of mRNA data was analyzed by using comparative Ct (2−ΔΔCt) methods (Livak and Schmittgen 2001) and the expression levels of β-actin and GAPDH housekeeping genes were used for normalization.
Western blot analysis
Whole cell protein lysate was prepared from bovine granulosa cell samples using × 1 passive lysis buffer (Promega GmbH, Mannheim, Germany). Equal amounts of protein lysate (30 μg) were resolved with 4–18% gradient SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane (Whatman–Protran, Germany). For blocking, the membranes were incubated in × 1 Roti-Block solution (Carl Roth, Germany) for 1 h at room temperature. They were then incubated overnight at 4 °C with diluted primary antibodies of anti-PCNA rabbit polyclonal antibody (1:200 dilution; sc-7907, Santa Cruz Biotechnology, Germany), anti-BAX rabbit polyclonal antibody (1:200 dilution; sc-493, Santa Cruz Biotechnology, Germany), anti-GRP78 goat polyclonal antibody (1:250 dilution; sc-1050, Santa Cruz Biotechnology, Germany), anti-catalase rabbit polyclonal antibody (1:500 dilution; LS-B1441) and anti-β-actin mouse monoclonal antibody (1:500 dilution; Santa Cruz Biotechnology, Germany). After washing, the membranes were incubated with horseradish peroxidase (1:5000 dilution; Santa Cruz Biotechnology, Germany), goat anti-rabbit, donkey anti-goat, or goat anti-mouse secondary antibodies for 1 h at room temperature. Protein bands were visualized with enhanced chemiluminescence (ECL) substrate (Bio-Rad, Germany) and acquired using Gel Doc XRS+ imaging system (Bio-Rad, Germany). The density of protein blots was quantified by densitometry using ImageJ 1.48v (National Institutes of Health, Bethesda, MD, USA). Density normalization was achieved by dividing the protein by β-actin density values.
Oxyblot procedure
The Oxidized Protein Western Blot Detection Kit (ab178020) was used for detection of carbonyl groups introduced into proteins by oxidative reactions, according to the manufacturer’s instructions. The images were acquired using Gel Doc XRS+ imaging system (Bio-Rad, Germany). Due to the sample processing procedure for protein carbonylation analysis, it was not possible to re-probe the membranes for a loading control. Therefore, equal protein loading of samples was verified with the use of Panceau S stain.
Immunofluorescence detection of Nrf2 and NF-κB proteins
Immunohistochemistry was performed to detect and localize Nrf2 and NF-κB proteins in GCs. Briefly, cells were fixed for 30 min with 4% paraformaldehyde then permeabilized with 0.5% (v/v) Triton-X100 (Sigma–Aldrich) in PBS for 15 min at room temperature. The samples were incubated in 3% bovine serum albumin (BSA) (Sigma–Aldrich) in PBST for 1 h at room temperature, followed by incubation with specific primary antibodies against Nrf2 (1:100 dilution, orb11165, Biorbyt), or NF-κB (1:100 dilution, orb11118, Biorbyt, UK) overnight at 4 °C. After washing, cells were incubated for 2 h with Alexa Fluor 568-conjugated goat anti-rabbit secondary antibody (1:300 dilution, A-11011, Life Technologies). After counterstaining and mounting, samples were visualized with a CLSM LSM-780 confocal laser scanning microscope (Zeiss, Germany; Scale bar: 50 μm), and analyzed using imageJ 1.48v.
Statistical analysis
Data obtained from all groups were compared statistically by an ANOVA test followed by a Tukey–Kramer multiple comparison test. Statistical analysis was performed using GraphPad Prism Software (version 5, San Diego, CA, USA). Values of P < 0.05 were considered for statistical significance. All values represent the mean ± SEM from three independent experiments.
Results
In order to investigate the dose-dependent effect of Pb treatment on GCs, different doses of Pb namely, 1, 2, 3, 5 and 10 μg/ml were used to treat the GCs for 2 h. In literature, most threshold values of Pb are related to human. And owing to the limited data on the effect of Pb exposure on bovine GCs, we relied on a previous study by Nandi et al. (2010), where a dose range from 0.005 to10 μg/ml of lead acetate was investigated on buffalo oocytes in vitro, where the starting ovotoxic dose of Pb was found to be 1 μg/ml while doses less than 1 μg/ml induced no significant change. In our work, a morphological observation 24 h post treatment showed that there were no significant differences between doses of 1, 2 and 3 μg/ml and the control untreated cells. On the other hand, cells exposed to 5 μg/ml and 10 μg/ml showed cellular shrinkage and detachment from the plate, respectively (Fig. 1a, b).
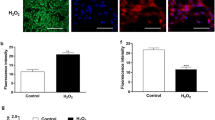
Effect of lead on the morphology of bovine granulosa cells: cells were exposed to Pb at concentrations of 1, 2, 3, 5 and 10 μg/ml for 2 h and morphologically assessed 24 h after treatment (a–b′′). The confluency of the monolayer and cellular contacts was still kept at lower doses as compared to the untreated control (a′–b), while at higher doses, shrinkage of cells and detachment from the plate were noted (b′–b′′). a: control group; a′: 1 μg/ml group; a′′: 2 μg/ml group; b: 3 μg/ml group; b′: 5 μg/ml group; b′′: 10 μg/ml group. Lead-induced intracellular reactive oxygen species (ROS) accumulation in bovine granulosa cells: fluorescent photomicrographs of bovine GCs stained with 2′, 7′-dichlorofluorescin diacetate (H2DCFDA) were shown in the untreated control and Pb-exposed groups (c, d). c: control group; c′: 1 μg/ml group; c′′: 2 μg/ml group; c′′′: 3 μg/ml group. Quantitative analysis of relative fluorescence emission (d). Values are expressed as mean ± SEM (n = 3). Lowercase letters a and b indicate statistically significant differences (P < 0.05). Scale bar; black: 50 μm, white: 100 μm
Exposure of bovine GCs to lead-elevated intracellular ROS accumulation
The relative fluorescence emissions were higher within GCs exposed to Pb than the control group, confirming that Pb-induced intracellular ROS in GCs (Fig. 1c, d). The doses of 5 and 10 μg/ml showed lower fluorescence signals revealing that these doses may cause cytotoxicity (Supplementary Fig. 1a, b). Since the aim of our study is to investigate the cellular response to the stressful not the lethal effect of Pb, doses of 1, 2 and 3 μg/ml were chosen for further investigations.
Lead-inhibited proliferation of bovine granulosa cells
The impact of Pb on GCs proliferation was assessed using cell proliferation assay and it was observed that all doses of Pb significantly reduced GCs viability compared to the control (Fig. 2a and Supplementary Fig. 1c). Cell proliferation marker genes CCND2 and PCNA were further investigated and it was found that the mRNA expression of both markers was downregulated under Pb exposure (Fig. 2b, c). However, nonsignificant reduction was found in the PCNA protein level (Fig. 2d, e).
Effect of lead on bovine granulosa cell proliferation and expression of proliferation marker genes: cell proliferation assay (a), mRNA expression of CCND2 (b), PCNA (c) and protein expression of PCNA (d) in bovine GCs co-cultured with Pb and their corresponding control. The density of PCNA protein bands was quantified by ImageJ and normalized against β-actin (e). β-actin and GAPDH were used to normalize the expression of targets genes. Data represent means ± SEM (n = 3). Lowercase letters a and b indicate statistically significant differences (P < 0.05)
Exposure of cells to lead alter the expression of Nrf2 and NF-κB in bovine granulosa cells
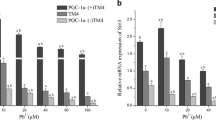
As illustrated in Fig. 3, results showed that there was significant attenuation of Nrf2 and Keap-1 genes in GCs exposed to all doses of Pb compared to the control group (Fig. 3a, b). Among Nrf2 downstream antioxidant genes, namely SOD, CAT, Thrx and HO-1, only the expression of SOD and CAT were significantly reduced (Fig. 3c–f). In addition, the protein analysis of CAT showed a marked reduction in its expression (Fig. 3g, h). Another striking finding was the significant decrease in the expression of NF-κB concomitantly with no change in the expression of IKK (Fig. 4a, b). We further investigated the TNF-α gene to evaluate whether the inflammatory response could be in involved in Pb toxicity. Consequently, we found a significant reduction in TNF-α expression (Fig. 4c).
Expression levels of genes associated with the Nrf2 pathway: mRNA expression level of Nrf2 (a), its inhibitor Keap-1 (b) and its downstream candidate genes: HO-1, SOD, CAT, and Thrx (c–f) and protein expression of CAT (g) in bovine GCs exposed to Pb and their corresponding control. The density of CAT protein bands was quantified by ImageJ and normalized against β-actin (h). β-actin and GAPDH were used to normalize the expression of targets genes, values are expressed as mean ± SEM (n = 3). Lowercase letters a and b indicate statistically significant differences (P < 0.05)
Expression levels of genes associated with the NF-κB pathway: mRNA expression level of NF-κB (a), IKK (b) and TNF-α (c) in bovine GCs exposed to Pb and their corresponding control. β-actin and GAPDH were used to normalize the expression of targets genes, values are expressed as mean ± SEM (n = 3). Lowercase letters a, b and c indicate statistically significant differences (P < 0.05)
Furthermore, the protein expression level of Nrf2 showed significant elevation only in 1 and 2 μg/ml compared to the control group (Supplementary Fig. 2a, b), while the level of NF-κB tends to be high in Pb-treated groups, being significant at the highest dose (Supplementary Fig. 3a, b).
Lead exposure elevates protein carbonyl content in bovine granulosa cells
In comparison with the untreated control group, Pb-treated samples displayed an evident increment in the intensity of the carbonylated protein bands as shown in the immunostained gel, ranging from about MW 180 KDa to 17 KDa (Supplementary Fig. 4).
Lead exposure induces apoptosis in bovine granulosa cells
We determined whether apoptosis was induced in GCs due to Pb exposure; the expression of proapoptotic- (BAX and caspase-3) and antiapoptotic (BCL-2)-related genes were investigated. Results indicated that the transcript level of BCL-2 was significantly downregulated under Pb exposure as compared to the control untreated group (Fig. 5a). There was a nonsignificant increase in the BAX mRNA level (Fig. 5b). The same pattern was observed in the protein level of BAX being significant at the dose of 3 μg/ml (Fig. 5d, e). Moreover, caspase-3 showed upregulation at the dose of 3 μg/ml in comparison to the untreated group (Fig. 5c).
Lead-induced apoptosis in bovine granulosa cells: mRNA expression levels of BCL-2 (a), BAX (b) and caspase-3 (c) and protein level of BAX (d) in bovine granulosa cells exposed to Pb and their corresponding control. The density of BAX protein bands was quantified by ImageJ and normalized against β-actin (e). Data shown as means ± SEM (n = 3). Lowercase letters a, b and c indicate statistically significant differences (P < 0.05). Expression of β-actin and GAPDH acted as internal control for gene expression
Apoptosis was further assessed by flow cytometric analysis using the annexin V-APC/PI stain. As shown in Supplementary Fig. 5, the percentage of apoptotic granulosa cells (early and late apoptotic cells in Quadrant 4 and Quadrant 2, respectively) was higher in the groups subjected to Pb than the control untreated group.
Lead-altered cell cycle transition in bovine granulosa cells
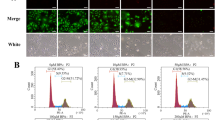
The cell cycle was evaluated in GCs by FACScan flow cytometry. As depicted in Fig. 6 (a, b), the exposure of GCs to Pb increased the percentage of cell populations in the G0/G1 phase while reduced the cell populations in the S phase (DNA synthesis) as compared with the control group.
Lead-changed cell cycle transition in granulosa cells: flow cytometric analysis of bovine GCs cultured under Pb exposure and their corresponding control. The analyzed cell counts are shown on the Y-axis and the DNA content of cells detected by PI staining is indicated on the X-axis (a–a′′′). a: control group; a′: 1 μg/ml group; a′′: 2 μg/ml group; a′′′: 3 μg/ml group. Quantitative analysis of cell populations at different cell cycle stages (b). Data shown as means ± SEM (n = 3). Lowercase letters a and b indicate statistically significant differences (P < 0.05)
Lead exposure induces the expression of GRP78 and CHOP
To determine the potential effect of Pb in the ER stress response of GCs, the expression of GRP78 and CHOP was investigated. Results showed that Pb triggered both GRP78 and CHOP expression only in the dose of 3 μg/ml (Fig. 7a, b). The protein level of GRP78 also showed upregulation in all Pb-exposed groups as indicated in Fig. 7 (c, d).
Lead-triggered expression of unfolded protein response (UPR) marker genes: mRNA expression level of GRP78 (a), CHOP (b) and protein level of GRP78 (c) in bovine GCs cultured under Pb exposure and their corresponding control. The density of GRP78 protein bands was quantified by ImageJ and normalized against β-actin (d). β-actin and GAPDH were used to normalize the expression of targets genes. The results are expressed as the mean ± SEM (n = 3). Lowercase letters a and b indicate statistically significant differences (P < 0.05)
Discussion
Lead exposure is known to affect female fertility in human and animals (Sharma et al. 2012). It has been suggested that ROS-mediated cellular damage could be one of the major mechanisms involved in Pb pathogenesis (Flora et al. 2012). The integrity of ovarian GCs, being the key marker of oocyte quality and viability, is critical to protect the oocytes from oxidative stress damage (Tripathi et al. 2013). According to Luderer (2014), ROS-mediated ovarian toxicity is induced by different stimuli including environmental toxicants. In this context, our results demonstrated that, Pb exposure induced significant ROS accumulation in bovine GCs, which remain prominent even after 24 h of culturing without Pb content.
In the current work, we attempted to investigate the effect of Pb on fine-tuned events induced by two major redox-sensitive transcription factors, NF-κB and Nrf2, which are crucial to maintain cellular hemostasis. The Nrf2 pathway is a pivotal protective self-defense mechanism against oxidative stress within the cell by activating an array of downstream detoxifying genes (Zhang et al. 2013). Although we detected a significant reduction in the mRNA level of its inhibitor; Keap-1 with Pb challenge, Nrf2 also showed a significant decrease with concomitant downregulation of both SOD and CAT levels. These results comply with the finding of Liu et al. (2017), which revealed that Pb decreased the levels of both Nrf2 and Keap-1 in the rat kidney. They further demonstrated the ability of Pb to activate miR153 that target Nrf2 mRNA suggesting the probable effect of Pb as an epigenetic modifier. However, further investigations are required with different Pb doses and exposure periods.
NF-κB is an inducible, pleiotropic transcription factor, efficiently regulating the expression of different genes involved in many physiological and pathological processes (Bellezza et al. 2010). In the present work, the activity of NF-κB was markedly reduced in Pb-treated groups, without a significant difference in the mRNA level of its regulator; IKK, while the NF-κB protein level, being mostly cytoplasmic, increased only at the dose of 3 μg/ml. Though sequential studies have been conducted to unravel the interplay between Nrf2 and NF-ĸB, discrepant results still remain. It was reported that the absence of Nrf2 can exacerbate NF-κB activity inducing cytokine production (Pan et al. 2012). Usually, phytochemicals like curcumin and quercetin activate Nrf2 by inhibiting NF-κB and its downregulated genes (Liu et al. 2015; Sahin et al. 2016). In contrast, many factors such as ischemia, LPS and cigarette smoke have been found to increase both Nrf2 and NF-κB activity (Wakabayashi et al. 2010; Meng et al. 2017). The significant finding in our work was the downregulation of both transcriptional factors under metal exposure at the transcriptional level. There is growing consensus that the ROS level may define the cellular fate by modulating these redox-sensitive TFs. According to Bolisetty and Jaimes (2013), low ROS levels are neutralized by Nrf2 activation and its downstream signaling, while NF-κB is activated with moderate ROS levels. However, apoptosis is induced with persistent ROS accumulation in the cell. Reports also showed that ROS can mediate either NF-κB activation or repression in many cell types (Nakajima and Kitamura 2013).
Another intriguing point is that many metals are able to affect the activity of NF-κB mediating both activation and inhibition of NF-κB (Chen and Shi 2002). For example, the work of Xie and Shaikh (2006) showed the involvement of ROS in apoptosis induction through the suppression of NF-κB signaling under cadmium challenge. Since NF-κB signaling triggers the expression of a wide array of genes, it is widely accepted that NF-κB is involved in both cellular death and survival (Perkins and Gilmore 2006). Thus, it appears that suppression of NF-κB and its downstream pathway is an underlying mechanism drive to apoptosis by a number of divalent metals, either through the binding of metals to the sulfhydryl groups of target protein molecules or the oxidative stress induced by these metals (Dieguez-Acuna et al. 2004). Similarly, it has been reported that the thiol group in enzymes and proteins is a target for Pb and further its irreversible binding might alter their function, being a major underling cause of Pb-driven oxidative stress (Hasanein et al. 2017).
Proteins can undergo post-translational modifications by a large number of reactions involving ROS. Among these reactions is carbonylation, which can emerge through either oxidative cleavage of proteins or by a secondary reaction of a protein side chain at cysteine, lysine and histidine through lipid peroxidation by-products like malondialdehyde (MDA) (Levine et al. 2000). Indeed, Pb is unable to induce ROS directly; however, it could affect the process of lipid peroxidation by antioxidant exhaustion. This in turn results in loss of plasma membrane structure and function, which are partially responsible for Pb accumulation (Ashafaq et al. 2016). In the present work, the oxyblot results showed a significantly higher protein carbonyl content in Pb-exposed groups than in their control counterpart.
The cell cycle is a fine-regulated process that controls cellular growth and differentiation (Darzynkiewicz et al. 2012). A previous study reported that Pb increased the cell population at G0/G1 yet reduced the cell population at the S phase in human leukemia cells (Yedjou et al. 2015). In the current work, similar results were found with Pb-exposed bovine GCs. Additionally, we investigated two markers of proliferation (PCNA and CCDN2) that showed downregulation under the Pb challenge. Our findings showed that the level of BCL-2 mRNA was significantly downregulated in all Pb-exposed groups, while there was no change in BAX level; however, the expression levels of caspase-3 were significantly upregulated only in 3 μg/ml Pb-exposed groups. This was further confirmed by the results obtained by flow cytometry showing a higher percentage of apoptotic GCs with Pb exposure than the control group. These data were consistent with previous results of Abdel Moneim (2016).
Previous studies have shown that Pb induces protein misfolding and endoplasmic reticulum (ER) stress responses in the liver tissue of rats (Liu et al. 2013). Consistence with these data, our results displayed that Pb induced ER stress in bovine GCs by upregulating ER stress-related genes; GRP78 and CHOP. It is known that, under ER stress and accumulation of unfolded proteins, cells trigger a cascade of protective signaling pathways, named the unfolded protein response (UPR). According to Luo et al. (2006), GRP78 is necessary in early mouse embryo for cell proliferation protecting the inner cell mass against apoptosis. In goat ovarian GCs, ER stress is the main signaling pathway involved in apoptosis and follicular atresia (Lin et al. 2012). The early study of Zhang et al. (2008) suggested that Pb, even at low concentration, could bind firmly to GRP78 to facilitate its removal from the cell or sequester it in a nontoxic site. Accordingly, the upregulation of GRP78 implied a cellular defense mechanism against Pb accumulation. Meanwhile, this upregulation may be an adaptive response to the potential inactivation of GRP78 by its binding to Pb and this may also reveal the paradox of upregulation of CHOP simultaneously with GRP78. The depletion of GRP78 triggers not only the proapoptotic CHOP but also intrinsic apoptosis as discussed by Lee (2014). Moreover, it was reported that, in the absence of Nrf2, UPR is compromised and CHOP was also found to be upregulated (Meakin et al. 2014).
Collectively, the present work has demonstrated that exposure to Pb induces oxidative stress that attenuates bovine GCs proliferation and alters cell cycle progression leading to apoptosis may be through disrupting Nrf2/NF-κB interaction. However, the mechanisms involved in this interaction need to be investigated in other cell types and tissues considering different Pb doses and exposure time. Therefore, further functional investigations are warranted to understand different aspects of Nrf2 and NF-κB interactions and their potential role for development of strategies aiming at modulating the response to environmental toxins.
Abbreviations
- BAX:
-
Bcl-2-associated X protein
- BCL-2:
-
B cell lymphoma 2
- CAT:
-
catalase
- CCND2:
-
cyclin-D2
- CHOP:
-
C/EBP homologous protein
- GRP78:
-
78 kDa glucose-regulated protein
- HO-1:
-
Heme oxygenase-1
- IKK:
-
inhibitor of nuclear factor-kappaB kinase
- Keap-1:
-
kelch-like ECH-associated protein 1
- NF-κB:
-
nuclear factor-kappa-light-chain-enhancer of activated B cells
- Nrf2:
-
nuclear factor (erythroid-derived 2)-like 2
- PCNA:
-
proliferating cell nuclear antigen
- SOD:
-
superoxide dismutase
- TNF-α:
-
tumor necrosis factor alpha
- Thrx:
-
thioredoxin
References
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One 11(7):e0158965. https://doi.org/10.1371/journal.pone.0158965
Ahamed M, Siddiqui MKJ (2007) Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta 383(1–2):57–64
Alemu TW, Pandey HO, Salilew Wondim D, Gebremedhn S, Neuhof C, Tholen E, Holker M, Schellander K, Tesfaye D (2018) Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 110:130–141. https://doi.org/10.1016/j.theriogenology.2017.12.042
Ashafaq M, Tabassum H, Vishnoi S, Salman M, Raisuddin S, Parvez S (2016) Tannic acid alleviates lead acetate-induced neurochemical perturbations in rat brain. Neurosci Lett 617:94–100. https://doi.org/10.1016/j.neulet.2016.02.001
Bayat F, Akbari SAA, Dabirioskoei A, Nasiri M, Mellati A (2016) The relationship between blood lead level and preeclampsia. Electron Physician 8:3450–3455. https://doi.org/10.19082/3450
Bellezza I, Mierla AL, Minelli A (2010) Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2:483–497. https://doi.org/10.3390/cancers2020483
Bires J, Maracek I, Bartko P, Biresova M, Weissova T (1995) Accumulation of trace elements in sheep and the effects upon qualitative and quantitative ovarian changes. Vet Hum Toxicol 37:349–356
Bolisetty S, Jaimes EA (2013) Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 214:6306–6344. https://doi.org/10.3390/ijms14036306
Chen F, Shi X (2002) Signaling from toxic metals to NF-kappaB and beyond: not just a matter of reactive oxygen species. Environ Health Perspect 110:807–811
Chowdhury AR (2009) Recent advances in heavy metals induced effect on male reproductive function-a retrospective. Al Ameen J Med Sci 2:37–42
Darzynkiewicz Z, Zhao H, Halicka HD, Rybak P, Dobrucki J, Wlodkowic D (2012) DNA damage signaling assessed in individual cells in relation to the cell cycle phase and induction of apoptosis. Crit Rev Clin Lab Sci 49:199–217. https://doi.org/10.3109/10408363.2012.738808
Dieguez-Acuna FJ, Polk WW, Ellis ME, Simmonds PL, Kushleika JV, Woods JS (2004) Nuclear factor kappaB activity determines the sensitivity of kidney epithelial cells to apoptosis. Implications for mercury-induced renal failure. Toxicol Sci 82:114–123. https://doi.org/10.1093/toxsci/kfh236
Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, Perrea DN (2009) The effect of lead intoxication on endocrine functions. J Endocrinol Investig 32:175–183. https://doi.org/10.1007/BF03345710
Eum K-D, Weisskopf MG, Nie LH, Hu H, Korrick SA (2014) Cumulative lead exposure and age at menopause in the Nurses’ Health Study cohort. Environ Health Perspect 122:229–234. https://doi.org/10.1289/ehp.1206399
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58. https://doi.org/10.2478/v10102-012-0009-2
Gebremedhn S, Salilew-Wondim D, Ahmad I, Sahadevan S, Hossain MM, Hoelker M, Rings F, Neuhoff C, Tholen E, Looft C, Schellander K, Tesfaye D, Zhang M (2015) MicroRNA expression profile in bovine granulosa cells of preovulatory dominant and subordinate follicles during the late follicular phase of the estrous cycle. PLoS One 10:e0125912. https://doi.org/10.1371/journal.pone.0125912
Hasanein P, Ghafari-Vahed M, Khodadadi I (2017) Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Rep 22:42–50. https://doi.org/10.1080/13510002.2016.1140406
Jahromi BN, Mosallanezhad Z, Matloob N, Davari M, Ghobadifar MA (2015) The potential role of granulosa cells in the maturation rate of immature human oocytes and embryo development: a co-culture study. Clin Exp Reprod Med 42:111–117. https://doi.org/10.5653/cerm.2015.42.3.111
Lee AS (2014) Glucose regulated proteins in cancer. Molecular mechanisms and therapeutic potential. Nat Rev Cancer 14:263–276. https://doi.org/10.1038/nrc3701
Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E (2000) Determination of carbonyl groups in oxidized proteins. Methods Mol Biol 99:15–24. https://doi.org/10.1385/1-59259-054-3:15
Lim J, Ortiz L, Nakamura BN, Hoang YD, Banuelos J, Flores VN, Chan JY, Luderer U (2015) Effects of deletion of the transcription factor Nrf2 and benzo[a]pyrene treatment on ovarian follicles and ovarian surface epithelial cells in mice. Reprod Toxicol 58:24–32. https://doi.org/10.1016/j.reprotox.2015.07.080
Lin P, Yang Y, Li X, Chen F, Cui C, Hu L, Li Q, Liu W, Jin Y (2012) Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol Reprod Dev 79:423–432. https://doi.org/10.1002/mrd.22045
Liu C-M, Zheng GH, Ming QL, Sun JM, Cheng C (2013) Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radic Res 47:192–201. https://doi.org/10.3109/10715762.2012.760198
Liu CM, Ma JQ, Xie WR, Liu SS, Feng ZJ, Zheng GH, Wang AM (2015) Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-κB pathway. Food Chem Toxicol 82:19–26. https://doi.org/10.1016/j.fct.2015.05.001
Liu B, Zhang H, Tan X, Yang D, Lv Z, Jiang H, Lu J, Baiyun R, Zhang Z (2017) GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3beta in rat kidney. Oncotarget 8:42226–42237. https://doi.org/10.18632/oncotarget.15033
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu J, Wang Z, Cao J, Chen Y, Dong Y (2018) A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol 16:80. https://doi.org/10.1186/s12958-018-0391-5
Luderer U (2014) Ovarian toxicity from reactive oxygen species. Vitam Horm 94:99–127. https://doi.org/10.1016/B978-0-12-800095-3.00004-3
Luo S, Mao C, Lee B, Lee AS (2006) GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 26:5688–5697. https://doi.org/10.1128/MCB.00779-06
Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford MLJ (2014) Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol 34:3305–3320. https://doi.org/10.1128/MCB.00677-14
Meng Q-T, Chen R, Chen C, Su K, Li W, Tang L-H, Liu H-M, Xue R, Sun Q, Leng Y, Hou J-B, Wu Y, Xia Z-Y (2017) Transcription factors Nrf2 and NF-κB contribute to inflammation and apoptosis induced by intestinal ischemia-reperfusion in mice. Int J Mol Med 40:1731–1740. https://doi.org/10.3892/ijmm.2017.3170
Nakajima S, Kitamura M (2013) Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med 65:162–174. https://doi.org/10.1016/j.freeradbiomed.2013.06.020
Nandi S, Gupta PS, Selvaraju S, Roy SC, Ravindra JP (2010) Effects of exposure to heavy metals on viability, maturation, fertilization, and embryonic development of buffalo (Bubalus bubalis) oocytes in vitro. Arch Environ Contam Toxicol 58:194–204. https://doi.org/10.1007/s00244-009-9342-7
Paksy K, Gati I, Naray M, Rajczy K (2001) Lead accumulation in human ovarian follicular fluid, and in vitro effect of lead on progesterone production by cultured human ovarian granulosa cells. J Toxicol Environ Health A 62:359–366. https://doi.org/10.1080/152873901300018093
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat Inflamm 2012:217580. https://doi.org/10.1155/2012/217580
Perkins ND, Gilmore TD (2006) Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ 213:759–772. https://doi.org/10.1038/sj.cdd.4401838
Qureshi N, Sharma R, Mogra S, Panwar K (2010) Protective effects of combined treatment of vitamin E and C on lead induced folliculogenesis in swiss mice. J Herbal Med Toxic 4:207–213
Sahin K, Pala R, Tuzcu M, Ozdemir O, Orhan C, Sahin N, Juturu V (2016) Curcumin prevents muscle damage by regulating NF-kappaB and Nrf2 pathways and improves performance. an in vivo model. J Inflamm Res 9:147–154. https://doi.org/10.2147/JIR.S110873
Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24:15–45
Santos RR, Schoevers EJ, Roelen BAJ (2014) Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol 12:117. https://doi.org/10.1186/1477-7827-12-117
Seyom E, Abera M, Tesfaye M, Fentahun N (2015) Maternal and fetal outcome of pregnancy related hypertension in Mettu Karl Referral Hospital, Ethiopia. J Ovarian Res 8:10. https://doi.org/10.1186/s13048-015-0135-5
Shan G, Tang T, Zhang X (2009) The protective effect of ascorbic acid and thiamine supplementation against damage caused by lead in the testes of mice. J Huazhong Univ Sci Technolog Med Sci 29:68–72. https://doi.org/10.1007/s11596-009-0114-4
Sharma R, Qureshi N, Mogra S, Panwar K (2012) Lead induced infertility in Swiss mice and role of antioxidants. Univers J Environ Res Technol 2:75–82
Swarup D, Patra RC, Naresh R, Kumar P, Shekhar P (2005) Blood lead levels in lactating cows reared around polluted localities; transfer of lead into milk. Sci Total Environ 347:106–110. https://doi.org/10.1016/j.scitotenv.2004.12.055
Taupeau C, Poupon J, Nome F, Lefevre B (2001) Lead accumulation in the mouse ovary after treatment-induced follicular atresia. Reprod Toxicol 15:385–391
Taylor CM, Golding J, Hibbeln J, Emond AM (2013) Environmental factors predicting blood lead levels in pregnant women in the UK. PLoS One 8:e72371–e72371. https://doi.org/10.1371/journal.pone.0072371
van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP (1996) A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 24:131–139. https://doi.org/10.1002/(SICI)1097-0320(19960601)24:2<131:AID-CYTO5>3.0.CO;2-M
Vigeh M, Yokoyama K, Seyedaghamiri Z, Shinohara A, Matsukawa T, Chiba M, Yunesian M (2011) Blood lead at currently acceptable levels may cause preterm labour. Occup Environ Med 68:231–234. https://doi.org/10.1136/oem.2009.050419
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13:1649–1663. https://doi.org/10.1089/ars.2010.3216
Wang Y, Chan S, Tsang BK (2002) Involvement of inhibitory nuclear factor-kappaB (NFkappaB)-independent NFkappaB activation in the gonadotropic regulation of X-linked inhibitor of apoptosis expression during ovarian follicular development in vitro. Endocrinology 143:2732–2740. https://doi.org/10.1210/endo.143.7.8902
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43:621–626. https://doi.org/10.1042/BST20150014
Xie J, Shaikh ZA (2006) Cadmium-induced apoptosis in rat kidney epithelial cells involves decrease in nuclear factor-kappa B activity. Toxicol Sci 91:299–308. https://doi.org/10.1093/toxsci/kfj131
Yedjou CG, Tchounwou HM, Tchounwou PB (2015) DNA damage, cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int J Environ Res Public Health 13:ijerph13010056. https://doi.org/10.3390/ijerph13010056
Zhang Y, Sun L-G, Ye L-P, Wang B, Li Y (2008) Lead-induced stress response in endoplasmic reticulum of astrocytes in CNS. Toxicol Mech Methods 18:751–757. https://doi.org/10.1080/15376510802390908
Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F (2013) Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100:30–47. https://doi.org/10.1016/j.pneurobio.2012.09.003
Acknowledgments
We are grateful to members of the Flow Cytometry Core Facility at the Institute of Molecular Medicine, University of Bonn and to the Confocal Microscope Facility in Institut für Nutzpflanzenwissenschaften und Ressourcenschutz (INRES), University of Bonn.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Ethical statement
All authors declare that this research was done by strictly adhering to the rules of good scientific practice and are responsible for its content. All experiments were performed in a manner that maximized rigor and reproducibility and without bias.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aglan, H.S., Gebremedhn, S., Salilew-Wondim, D. et al. Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell Tissue Res 380, 643–655 (2020). https://doi.org/10.1007/s00441-020-03177-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03177-x