Abstract
Fenofibrate has been shown to have therapeutic effects on diabetic retinopathy (DR). Our previous studies demonstrated that the oxidative stress–activated Wnt/β-catenin pathway plays a pathogenic role in diabetic complications. In the present study, we evaluate the effect and mechanism of fenofibrate on regulating the oxidative stress–activated Wnt/β-catenin pathway by using the genetic type 1 diabetes model of C57BL/6J-Ins2Akita mice and high glucose (HG)–treated ARPE-19. Our results demonstrated that retinal phosphorylation of LRP6 and nuclear β-catenin were increased in C57BL/6J-Ins2Akita mice suggesting activation of Wnt/β-catenin signaling. Meanwhile, C57BL/6J-Ins2Akita showed upregulation of oxidant enzyme Nox4 and Nox2 and downregulation of antioxidant enzyme SOD1 and SOD2. All these alterations were reversed in C57BL/6J-Ins2Akita mice with fenofibrate treatment. Moreover, fenofibrate significantly ameliorated diabetes-induced retinal vascular leakage in C57BL/6J-Ins2Akita mice. In cultured ARPE-19, fenofibrate decreased HG-induced Nox2 and Nox4 upregulation, attenuated SOD1 and SOD2 downregulation and inhibited LRP6 phosphorylation. Moreover, activation of Wnt/β-catenin by Wnt3a conditional medium (WCM) reduced SOD1 and SOD2 and did not affect Nox2 and Nox4. Fenofibrate suppressed WCM-induced LRP6 phosphorylation and reversed SOD downregulation. Importantly, Nox4 overexpression directly phosphorylated LPR6 in ARPE19; conversely, Nox4 knockdown suppressed HG-induced LPR6 phosphorylation. Taken together, Nox-mediated oxidative stress contributes to Wnt/β-catenin activation in DR. Fenofibrate ameliorated DR through coordinate attenuation of oxidative stress and blockade of Wnt/β-catenin signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is a common cause of visual impairment and one of the major neurovascular complications of both type 1 and type 2 diabetes mellitus. The overall prevalence of DR has been estimated at approximately 30% of diabetic patients, with the risk factors including the duration of diabetes, increased HbA1c, high blood pressure and hyperlipidemia. Clinical trials have demonstrated that intensive control of hyperglycemia and hypertension reduced the incidence and progression of DR (1998; Diabetes et al. 1993). A commonly used clinical treatment of DR is retinal laser coagulation and vitrectomy. Recently, intravitreal injection of anti-VEGF agent has proven to reduce retinal neovascularization and attenuate diabetic macular edema. However, there are also drawbacks of retinal laser coagulation with the loss of peripheral visual fields and blurred vision at night. Anti-VEGF agent requires multiple injections, which brings a heavy economic burden, especially in developing countries. In addition, repetitive intraocular injections will increase the risk of infection. A systemic drug treatment for DR is desired.
Fenofibrate is a PPAR-α agonist and originally developed to treat dyslipidemia. It is known to effectively reduce both low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL), while elevate high-density lipoprotein (HDL) levels. Intriguingly, FIELD (Fenofibrate Invention and Event Lowering in Diabetes) study and ACCORD (Action to Control Cardiovascular Risk in Diabetes)-Eye Study demonstrated that fenofibrate significantly slowed the progression of DR (Chew et al. 2014; Keech et al. 2007). However, the protecting mechanisms of fenofibrate on DR remain elusive.
The Wnt signaling pathway is involved in regulating retinal vascular development (Drenser 2016). Mutations of Wnt cascade components are associated with inherited retinal vascular dysplasia such as familial exudative vitreoretinopathy and Norrie’s disease (Warden et al. 2007). The Wnt signaling mainly branches into two distinct pathways: canonical and non-canonical. The canonical Wnt pathway or Wnt/β-catenin pathway is initiated when Wnt ligands bind with the seven-transmembrane domain frizzled (Fz) receptor and form a complex with a single-transmembrane domain LDL receptor–related protein 5/6 (LRP5/6). The extracellular signals were transduced into the cytoplasm by translocation of Dishevelled (Dvl) to fizzled co-receptors and subsequent recruitment and degradation of Axin by LRP5/6. The “destruction complex” composed by Axin, adenomatous polyposis coli (APC) and glycogen synthase kinase-3β (GSK-3β) were disassembled. The phosphorylation and degradation of β-catenin are attenuated. Stabilized β-catenin accumulates in the cytoplasma, translocates into the nucleus and associates with transcription factor T cell factor (TCF)/lymphoid enhancer factor (LEF) to activate target gene expression. Emerging evidence indicates that Wnt signaling activity is regulated by multiple post-transcriptional modifications such as phosphorylation, ubiquitination and glycosylation (Gao et al. 2014). Phosphorylation is one of the most efficient and well-characterized modifications in regulating cellular signal transduction. Many components of the Wnt/β-catenin pathway are regulated by phosphorylation including Fz, LRP6, Dvl and “destruction complex” members. Phosphorylation of LRP6 serves as a critical step for initiating Wnt/β-catenin cascade. Our group first reported that activation of Wnt/β-catenin signaling was implicated in and contributed to the development of DR (Chen et al. 2009).
Hyperglycemia-induced oxidative stress contributes to Wnt/β-catenin pathway activation and renal dysfunction in diabetic kidney (Zhou et al. 2012). Oxidative stress is characterized by the excessive generation of reactive oxygen species (ROS). Nicotinamide adenine dinu`cleotide phosphate (NADPH) oxidase or Nox, the major source of ROS production in the retinal microvasculature, neuroglial cells and circulating leukocytes, is closely associated with retinal vascular leakage, neuroinflammation and neovascularization in DR or ischemic retinopathy (Deliyanti and Wilkinson-Berka 2015; Li et al. 2015; Saito et al. 2007; Wilkinson-Berka et al. 2014). The Nox family consists of seven members (Nox1–Nox5, Duox1 and Duox2) that transfer electrons across the biological membrane to generate superoxide. Among multiple isoforms, Nox2 and Nox4 have been shown to play a central role in retinal oxidative damage during the development of DR (Li et al. 2010; Rojas et al. 2013). Superoxide dismutase (SOD) is one of the major antioxidants against ROS. Three distinct isoforms of SOD have been identified (SOD1–SOD3). Loss of SOD activity increased ROS production and exaggerated retinal oxidative damage (Hashizume et al. 2008; Justilien et al. 2007).
Our previous study demonstrated that fenofibrate attenuated retinal inflammation and microvascular dysfunction in genetic diabetes model of C57BL/6J-Ins2Akita mice and streptozotocin-induced diabetic mice (Chen et al. 2013; Ding et al. 2014). In addition, fenofibrate has been shown to ameliorate oxidative stress–mediated renal injuries in diabetic nephropathy (Cheng et al. 2016a; Cheng et al. 2016b). Thus, we speculated that fenofibrate might also exert an anti-oxidative effect on diabetic retinas by regulating expression of oxidant and antioxidant enzymes. Meanwhile, inhibition of Wnt/β-catenin signaling protected retinal cells from diabetes-induced injuries. In the present study, we explore whether fenofibrate directly affects oxidative stress–elicited Wnt/β-catenin activation in retinas of C57BL/6J-Ins2Akita mice.
Materials and methods
Animal models
Genetic type 1 diabetes model of C57BL/6J-Ins2Akita and littermate control mice were purchased from Jackson Laboratory (Bar Harbor, MI). Blood glucose and body weight were monitored. At the age of 12 weeks, C57BL/6J-Ins2Akita and littermate control mice were fed 0.1% fenofibrate (LabDiet, Fort Worth, TX) in their diet for 4 weeks. All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of Animals in Ophthalmic and Vision Research and University of Oklahoma Health Sciences Center (OUHSC) guideline for Animal in research.
Immunohistochemistry
Mice were humanly euthanized. Eye balls were harvested and briefly fixed in 4% paraformaldehyde. After removing cornea and lens, the eyecups were post-fixed in 4% paraformaldehyde for 30 min and immersed in gradient sucrose solution. Eyecups were embed in Optimal Cutting Temperature Compound (OCT; Sakura Finetek, Torrance, CA) and sectioned with Leica Cryostat. Sections were blocked with blocking buffer for 1 h and then incubated with the following primary antibodies for overnight: anti-Nox4 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Nox2 (Santa Cruz), anti-SOD1 (Abcam), anti-SOD2 (Stressgen, Victoria, BC), anti-3-nitrotyrosine (3-NT, Millipore, Billerica, MA), anti-albumin (Bethyl Laboratories, Montgomery, TX) and anti-CD31 (BD Pharmingen, San Diego, CA). After intensive rinsing, sections were incubated with FITC- or Cy3-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 1 h. The slides were coverslipped with VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and examined under fluorescence microscope (Zeiss, Germany). The representative images were taken with the same exposure time.
Retinal nuclear protein fraction
Mouse retinas were harvested and thoroughly homogenized by mortar and pestle in liquid nitrogen. Retinal nuclear extracts were prepared by using Nuclear extraction kit (Active Motif, Carlsbad, CA) following the manufacturer’s instructions.
Retinal oxidative stress detection
Generation of retinal ROS was analyzed by fluorometric assay with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, Invitrogen) probe as described previously (Li et al. 2010). Briefly, eyeballs were embed with OCT. Retinal cryosections were obtained and then incubated with 5 μM H2DCF-DA dissolved in HBSS for 30 min at 37 °C under dark. The retinal sections were washed with PBS and nuclei were counterstained with DAPI. The representative images were quickly captured with fluorescence microscope (Zeiss) to avoid photo-oxidation.
Retinal vascular leakage assay
Retinal vascular permeability was measured by quantifying fluorescein isothiocyanate (FITC)-dextran leakage from the retinal vasculature as described previously (Chen et al. 2012). Deeply anesthetized mice were injected with FITC-dextran (4kD, 50 mg/mL in PBS, 100 μL, Sigma-Aldrich) via the caudal vein. After circulation for 15 min, blood was withdrawn from the right ventricle and serum was collected by centrifugation. PBS was perfused into the left ventricle to thoroughly remove the FITC-dextran from the retinal vessels. The retinas were dissected, weighted and homogenized in equal amount of water. Fluorescence of retinal extracts and plasma was measured using a Spectrofluorometer (Perkin Elmer, Waltham, MA) with excitation wavelength at 485 nm and emission wavelength at 535 nm. Retinal vascular permeability was calculated using the following equation: [retinal FITC − dextran (μg)/retinal weight (g)]/[serum FITC-dextran concentration (μg/μL) × circulating time (h)].
Cell culture
ARPE-19, a cell line derived from human retinal pigment epithelium, was obtained from ATCC (Manassas, VA) and maintained in DMEM with 10% FBS. L-cells and L-cells expressing Wnt3a were obtained from ATCC and maintained in DMEM with 10% FBS and 0.4 mg/ml G418 (Invitrogen). The conditioned medium from L-cells (LCM) and the L-cells expressing Wnt3a (WCM) were collected following ATCC guidelines. Fenofibrate, D-glucose and L-glucose were all purchased from Sigma-Aldrich.
Western blot analysis
Mouse retinas and ARPE-19 cells were lysed in RIPA buffer containing protease inhibitors. Twenty-five micrograms of total protein were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. After blocking with 5% non-fatty milk in TBST, the membranes were incubated overnight with the following antibodies anti-phospho-LRP6 (Cell Signaling, Danvers, MA), anti-LRP6 (generated in Dr. Ma’s lab), anti-β-catenin (Abcam), anti-Nox4 and anti-Nox2 (both from Santa Cruz), SOD1 (Abcam) and SOD2 (Stressgen). After washing with TBST, membranes were incubated with secondary antibodies for 1 h. The same membranes were re-blotted with anti-β-actin antibody (Sigma-Aldrich) or anti-Histone H3 (Cell Signaling) as loading controls.
Real-time RT-PCR
Total RNA was isolated from ARPE-19 cells with the RNeasy mRNA extraction kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. One microgram of total RNA was used for cDNA synthesis with iScript cDNA synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacture’s protocol. Real-time PCR was performed with SYBR Green PCR master (Bio-Rad). Primer sequences for real-time RT-PCR were as follows: Nox2: forward: 5′-CCAGTGAAGATGTGTTCAGCT-3′, reverse: 5′-GCACAGCCAGTAGAAGTAGAT-3′ (Ago et al. 2004); Nox4: forward: 5′-AGTCAAACAGATGGGATA-3′, reverse: 5′-TGTCCCATATGAGTTGTT-3′ (Ago, Kitazono, Ooboshi, Iyama, Han, Takada, Wakisaka, Ibayashi, Utsumi and Iida 2004); SOD1: forward: 5′-AGGGCATCATCAATTTCGAGC-3′, reverse: 5′-GCCCACCGTGTTTTCTGGA-3′; SOD2: forward: 5′-AACCTCAGCCCTAACGGTG-3′, reverse: 5′-AGCAGCAATTTGTAAGTGTCCC-3′.
Detection of intracellular reactive oxygen species
Intracellular reactive oxygen species was measured by H2DCF-DA assay as described previously (Liu et al. 2013). The generated fluorescence intensity was determined by a spectrofluorometer (Perkin Elmer) with the excitation wavelength at 485 nm and emission wavelength at 535 nm.
Adenoviral infection and RNAi transfection
Adenoviral vectors expressing Nox4 (Ad-Nox4) or β-galactosidase (Ad-LacZ) were amplified in 293 cells and tittered with Adeno-X™ rapid titer kit (Clontech Laboratories, Mountain View, CA) as described previously (Cheng et al. 2016a). Knockdown of Nox4 was achieved by transfection with the Nox4 siRNA (Santa Cruz) using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions.
Statistical analysis
Data were shown as mean ± S.D. Statistical analysis was performed with two-tailed Student’s t tests for comparing difference between two groups and one-way analysis of variance (ANOVA) with Bonferroni’s post hoc tests for the comparison of difference among four groups. A p value less than 0.05 was considered as statistical significance.
Results
Activation of Wnt/β-catenin signaling and imbalance between Nox and SOD in retinas of diabetic C57BL/6J-Ins2 Akita mice
First, we evaluated activation of the Wnt/β-catenin pathway in retinas of C57BL/6J-Ins2Akita mice, which is a commonly used genetic model of type 1 diabetes. As shown in Fig. 1(a, a’), C57BL/6J-Ins2Akita mouse retinas displayed increased phosphorylated and total LRP6, when compared with non-diabetic littermate controls. It is known that activation of Wnt/β-catenin receptors resulted in dephosphorylation, stabilization and nuclear distribution of β-catenin. Consistently, increased nuclear β-catenin levels were found in the retinas of C57BL/6J-Ins2Akita mice (Fig. 1b, b’), which further suggested activation of Wnt/β-catenin signaling in diabetic retinas. Our previous studies demonstrated that oxidative stress can trigger activation of Wnt/β-catenin signaling. Nox are enzymes that catalyze the production of reactive oxygen species (ROS). However, SOD are responsible for decomposition of ROS. We next compared expression of Nox and SOD in the retinas of C57BL/6J-Ins2Akita mice and their non-diabetic littermate controls. We found that Nox4 expression, predominantly co-immunostained with retinal endothelial marker CD31, was significantly increased in C57BL/6J-Ins2Akita retinas (Fig. 1c, c’,d, d””’). In addition, Nox2 expression was also increased in retinas of C57BL/6J-Ins2Akita mice as shown by immunofluorescence staining (Fig. 1e–e””’). In contrast, SOD1 and SOD2, two major antioxidant enzymes in the retinas, were significantly reduced in C57BL/6J-Ins2Akita mice (Fig. 1f–f””’,g, g ””’). Retinal ROS production was examined by an ROS-sensitive fluorogenic probe (H2DCF-DA). The result demonstrated that retinal ROS production indicated by DCF fluorescence intensity was markedly increased in C57BL/6J-Ins2Akita mice compared with littermate control mice (Fig. 1h–h””’). Collectively, upregulation of Nox and downregulation of SOD may attribute to increased ROS generation and compromised ROS clearance in diabetic retinas. The imbalance of oxidant enzymes and antioxidant enzymes may synergistically exaggerate retinal oxidative stress.
Activation of the Wnt/β-catenin signaling pathway, upregulation of oxidants and downregulation of antioxidants in retinas of C57BL/6J-Ins2Akita mice. (a–a’) Phosphorylation of LRP-6 (p-LRP6) and total LRP6 were increased in the retinas of C57BL/6J-Ins2Akita mice compared to littermate controls. (b–b’) Retinal nuclear of β-catenin was determined by Western blot analysis. (c–e””’) Nox4 and Nox2 were upregulated in the retinas of C57BL/6J-Ins2Akita mice compared to non-diabetic littermate controls. Expression of Nox4 was determined by Western blot analysis (c–c’). Retinal distribution of Nox4 (green) was examined by immunofluorescence. CD31 (red) were used as an endothelial marker. Note that the major Nox4 immunosignal was prominently colocalized with CD31 (d–d””’). Expression of Nox2 was displayed by immunofluorescence (e–e””’). (f–g””’) Downregulation of SOD1 (f–f””’) and SOD2 (g–g””’) in the retinas of C57BL/6J-Ins2Akita mice compared to littermate controls was indicated by immunofluorescence. (h–h””’) Retinal ROS production was detected with H2DCF-DA fluorogenic probe. n = 6, **p < 0.01 vs Ctrl. Scale bar 50 μm
Fenofibrate attenuated high glucose (HG)–induced activation of Wnt/β-catenin signaling and oxidative stress in ARPE-19 cells
Previous studies from our group reported that fenofibrate attenuated retinal inflammation and vascular leakage in rodent models of type 1 diabetes (Chen et al. 2013). Here, we determined whether fenofibrate exerted its protecting effect on diabetic retinopathy via inhibition of the Wnt/β-catenin pathway. ARPE-19 cells were cultured with 25 mM D-glucose (high glucose, HG) for 48 h and then treated with different concentrations of fenofibrate for another 16 h. Cells maintained in 25 mM L-glucose (LG) were used as an osmotic control. As shown in Fig. 2(a, a’), fenofibrate inhibited HG-induced LRP6 phosphorylation in a concentration-dependent manner. Hyperglycemia could increase retinal ROS generation during diabetes. We next determined fenofibrate’s influence on HG-elicited ROS generation in ARPE-19 cells. ROS was significantly increased by HG, which was dose-dependently suppressed by fenofibrate treatment (Fig. 2b). Together with increased ROS generation, Nox2 and Nox4 expression was markedly upregulated by HG (Fig. 2c, c’). Meanwhile, SOD1 and SOD2 were downregulated by HG (Fig. 2d, d’). Fenofibrate treatment lowered HG-induced upregulation of Nox2 and Nox4 (Fig. 2c, c’). Similarly, HG increased mRNA levels of Nox2 and Nox4 in ARPE-19 cells, which were abolished by fenofibrate (Fig. 2e). In addition, fenofibrate treatment reversed HG-induced downregulation of SOD1 and SOD2 (Fig. 2d, d’). These data suggested that fenofibrate could ameliorate HG-induced ROS production via regulating cellular expression of Nox and SOD.
Fenofibrate inhibited HG-induced Wnt/β-catenin activation and oxidative stress in ARPE-19 cells. ARPE-19 cells were treated with HG (25 mM D-glucose) for 48 h followed by incubation with fenofibrate for 16 h. LG (25 mM L-glucose) was used as osmotic controls. (a–a’) Fenofibrate inhibited HG-induced LRP6 phosphorylation in a dose-dependent manner. (b) Fenofibrate suppressed HG-induced ROS generation in ARPE-19 cells. (c–c’) Fenofibrate downregulated HG-induced Nox2 and Nox4 expression in ARPE-19 cells. (d–d’) Expression of SOD1 and SOD2 was decreased in APRE-19 cells treated with HG, which was reversed by fenofibrate. (e) Fenofibrate inhibited HG-induced Nox2 and Nox4 expression at the transcriptional level. Nox2 and Nox4 mRNAs were measured by real-time RT-PCR in ARPE-19 cells. n = 3, **p < 0.01 vs LG and †p < 0.05 or ‡p < 0.01 vs HG
Fenofibrate suppressed WCM-induced Wnt/β-catenin activation and ROS generation in ARPE-19 cells
To understand how the Wnt/β-catenin signaling pathway was directly involved in retinal oxidative stress, we treated ARPE-19 cells with WCM with different concentrations of fenofibrate. We found that WCM induced robust phosphorylation of LRP6 (Fig.3a, a’). Furthermore, WCM could also promote ROS generation in APRE-19 cells (Fig. 3b). Fenofibrate treatment significantly attenuated LRP6 phosphorylation and reduced ROS generation (Fig. 3a, a’,b, b’). We next determined whether Wnt/β-catenin signaling regulated expression of Nox and SOD. We found that WCM does not alter Nox expression at neither protein nor mRNA levels (Fig. 3c, c’,e). In contrast, WCM dramatically reduced SOD1 and SOD2 expression (Fig. 3d, d’). Fenofibrate reversed WCM-induced downregulation of SOD1 and SOD2 (Fig. 3d, d’). Moreover, WCM significantly decreased mRNA levels of SOD1 and SOD2 (Fig. 3e). Taken together, these results indicated that the Wnt/β-catenin signaling may regulate expression of SOD at the transcriptional level. Nox and their derived ROS may be upstream effectors that activate Wnt/β-catenin signaling during diabetes.
Fenofibrate suppressed WCM-induced Wnt/β-catenin activation and ROS generation in ARPE-19 cells. ARPE-19 cells were treated with WCM for 16 h in the presence or absence of fenofibrate. (a–a’) Fenofibrate inhibited WCM-induced LRP6 phosphorylation. (b) WCM increased ROS generation in APRE-19 cells, which was inhibited by fenofibrate treatment. (c–c’) WCM does not alter Nox2 and Nox4 expression in ARPE-19 cells. (d–d’) WCM decreased SOD1 and SOD2 expression in ARPE-19 cells, which was reversed by fenofibrate. (e) WCM decreased SOD1 and SOD2 at the mRNA level but had no effect on Nox2 and Nox4 mRNA. n = 3, *p < 0.05, **p < 0.01 vs LCM and ‡p < 0.01 vs WCM
Nox4 is essential for activation of Wnt/β-catenin signaling pathway in ARPE-19 cells
Nox4, one of the major NADPH oxidase isoforms, has been shown to be responsible for retinal vascular leakage and neovascularization of diabetic retinopathy (Li et al. 2010, 2015). Here, we evaluated whether Nox4 was required for Wnt/β-catenin pathway activation during diabetic condition. As shown in Fig. 4(a, a’), increase of Nox4 was achieved by infection of ARPE-19 cells with the wild-type Nox4 adenoviral vector (Ad-Nox4). Adenoviral vector with overexpression of β-galactosidase (Ad-LacZ) was used as a control. We found that overexpression of Nox4 significantly increased phosphorylation of LRP6 (Fig. 4b, b’). However, knockdown of Nox4 by specific siRNA significantly inhibited HG-induced LRP6 phosphorylation (Fig. 4c, c’). Nox4 knockdown efficiency was confirmed by real-time RT-PCR (Fig. 4d). Our results indicated that Nox4 played a causal role in activation of the Wnt/β-catenin signaling pathway.
Nox4 is required for activation of the Wnt/β-catenin signaling in ARPE-19 cells. ARPE-19 cells were infected with adenovirus expressing Nox4 (Ad-Nox4) or transfected with Nox4-specific siRNA (Nox4i). (a–b’) Overexpression of Nox4 promoted phosphorylation of LRP6. Expression of Nox4 (a and a’) and p-LRP6 (b and b’) was examined by Western blot analysis. (c–d) Knockdown of Nox4 decreased phosphorylation of LRP6. Expression of p-LRP6 was determined by Western blot analysis (c) and semi-quantified by densitometry (c’). Nox4 knockdown efficiency was evaluated by real-time RT-PCR (d). n = 3, **p < 0.01 vs Ad-LacZ or Ctrli
Fenofibrate attenuated oxidative stress and blocked Wnt/β-catenin signaling activation in retinas of C57BL/6J-Ins2 Akita mice
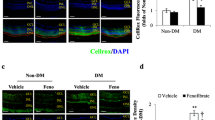
Next, we clarified whether fenofibrate could attenuate oxidative stress and block Wnt/β-catenin signaling in the retinas of C57BL/6J-Ins2Akita mice. C57BL/6J-Ins2Akita mice were fed with chow containing fenofibrate for 4 weeks. Retinal oxidation was determined by H2DCF-DA assay and immunostaining of 3-NT formation, respectively. We found that retinal fluorescence intensity of DCF and immunoreactivity of 3-NT expression were drastically reduced in fenofibrate-treated C57BL/6J-Ins2Akita mice compared with vehicle-treated ones (Fig. 5a–a””’,b, b””’), indicating a significant anti-oxidation effect of fenofibrate on type 1 DR. Furthermore, fenofibrate intervention also suppressed nuclear β-catenin accumulation in the retinas of C57BL/6J-Ins2Akita mice (Fig. 5c, c’), which suggested that fenofibrate attenuated activation of Wnt/β-catenin signaling during DR. In addition, our results clearly demonstrated that retinal Nox4 and Nox2 expression were markedly reduced in C57BL/6J-Ins2Akita mice treated with fenofibrate (Fig. 5d–d”””’,e, e””’). Furthermore, retinal levels of SOD1, the pivotal antioxidant enzyme, were markedly elevated by fenofibrate in C57BL/6J-Ins2Akita mice (Fig. 5f–f””’). These results suggest that fenofibrate could attenuate retinal oxidative stress by reducing ROS-generating enzymes as well as by upregulating the antioxidant systems.
Fenofibrate attenuated oxidative stress and inhibited β-catenin expression in the retinas of C57BL/6J-Ins2Akita mice. C57BL/6J-Ins2Akita mice were fed with fenofibrate. (a–a””’) Retinal ROS production was detected by H2DCF-DA assay. (b–f””’) Expression of 3-NT (b–b””’), β-catenin (c and c’), Nox4 (d-d”””’), Nox2 (e–e””’), and SOD1 (f–f””’) were determined by immunofluorescence staining or Western blot analysis. n = 6, *p < 0.05 vs Akita+Veh. Veh, vehicle; Feno, fenofibrate. Scale bar 50 μm
Fenofibrate reduced vascular leakage in retinas of C57BL/6J-Ins2 Akita mice
Finally, we addressed fenofibrate’s salutary effect on retinal microvasculopathy of diabetic C57BL/6J-Ins2Akita mice. Vascular leakage, a hallmark of diabetic retinopathy, was analyzed by FITC-dextran vascular permeability assay in mouse retinas. Compared with littermate controls, C57BL/6J-Ins2Akita mice showed a dramatic increase of retinal vascular leakage of FITC-dextran (Fig. 6a). However, fenofibrate significantly attenuated retinal hyperpermeability of FITC-dextran in C57BL/6J-Ins2Akita mice. Meanwhile, the protective role of fenofibrate on diabetes-induced vascular leakage was further verified by immunostaining of extravasated albumin in retinas of C57BL/6J-Ins2Akita mice. As shown in Fig. 6(b, b’), albumin was weakly detected within blood vessels in non-diabetic retinas. Extravasated albumin was more diffusely distributed in retinas of diabetic C57BL/6J-Ins2Akita mice (Fig. 6b”, b”’), especially in inner retinal layers, which was markedly ameliorated by fenofibrate treatment (Fig. 6b”””, b”””’). These results suggested that fenofibrate protect against diabetes-caused blood-retinal barrier breakdown.
Fenofibrate prevented retinal vascular leakage in C57BL/6J-Ins2Akita mice. (a–b) Retinal vascular leakage was evaluated by FITC-dextran permeability assay (a) and immunostaining of extravasated albumin (b–b”””’), respectively. n = 6, **p < 0.01 vs Ctrl+Veh, †p < 0.05 vs Akita+Veh. Veh, vehicle; Feno, fenofibrate. Scale bar 50 μm
Discussion
The robust and consistent evidence from the FIELD study and ACCORD-EYE study reported that fenofibrate has therapeutic effects in patients with DR. Here, we utilized C57BL/6J-Ins2Akita mice as a type 1 diabetic animal model and identified a possible mechanism underlying fenofibrate’s protective effect against DR. We found that fenofibrate ameliorated retinal ROS burden by downregulating oxidant enzyme Nox2 and Nox4 expression and upregulating antioxidant enzyme SOD1 and SOD2 expression in diabetic mice; thereby, our study manifested the anti-oxidative property of fenofibrate on DR. Previous studies indicated that activation of Wnt/β-catinin contributes to diabetic complications. In the present study, we showed that Nox4-mediated ROS generation was responsible for activation of the Wnt/β-catenin pathway by inducing phosphorylation of LRP6. Activation of the Wnt/β-catinin pathway resulted in transcriptional downregulation of SOD1 and SOD2, which, in turn, exacerbated retinal oxidative stress and vascular leakage.
Physiological generation of ROS serves as the signaling molecule; however, excessive or sustained ROS generation, especially exceeding the capacity of antioxidant defense systems, increases ROS burden and eventually leads to oxidative damage. The major source of ROS generation in metabolic disorders including diabetes is Nox enzymes, which trigger the transfer of an electron from NADPH substrate to oxygen and catalyze generation of superoxide. Nox family is composed of seven members with distinct cellular distributions and varied activation mechanisms. Notably, Nox1 and Nox2 require the membrane-bound p22phox component for membrane stabilization. Nox1, highly expressed in epithelial cells of the gastrointestinal tract, is preferentially activated by assembly with NADPH oxidase activator 1 (Noxa1), NADPH oxidase organizer 1 (Noxo1) and GTPase Rac1. More recently, Nox1 overexpression has been shown to be responsible for retinal neovascularization and ganglion cell (RGC) death in ischemic retinopathy (Dvoriantchikova et al. 2012; Wilkinson-Berka et al. 2014). Nox2, also known as gp91phox, first identified in phagocytes, required recruitment of cytosolic regulatory subunits p47phox, p40phox and GTPase Rac1, forming the active enzyme complex. Nox2 was shown to be expressed in retinal vessels and circulating leukocytes (Al-Shabrawey et al. 2008; Saito et al. 2007; Wei et al. 2016). Nox2-deficient mice displayed reduced aberrant neovascularization and ganglion cell death in ischemic retinopathy (Chan et al. 2013; Yokota et al. 2011). Similarly, diabetes-induced vascular injuries were also attenuated in mice with Nox2 gene depletion or Nox2 activity inhibition (Al-Shabrawey et al. 2008; Rojas et al. 2013). Our findings indicated that fenofibrate might decrease retinal ROS production partially by reducing hyperglycemia-induced Nox2 expression in type 1 diabetes. Nox4 is constitutively active and its activity is mostly regulated at the transcriptional level. A previous study demonstrated that Nox4 is a major source of vascular ROS generation and abundantly expressed in vascular endothelia, smooth muscle cells and fibroblasts (Craige et al. 2011; Kleinschnitz et al. 2010; Li et al. 2015). Nox4-mediated oxidative stress has been shown to lead to blood-retinal barrier breakdown in DR and retinal neovascularization in ischemic retinopathy (Li et al. 2010, 2015; Wang et al. 2014). Genetic depletion of Nox4 inhibited cerebral ischemia–induced neuronal damage via attenuating breakdown of the blood-brain barrier and neuronal apoptosis (Kleinschnitz et al. 2010). Previous findings indicated that fenofibrate protects retinas from diabetes- or ischemia-induced microvasculopathy by reducing vascular hyperpermeability, pericyte loss, and aberrant neovascularization (Chen et al. 2013; Ding et al. 2014; Moran et al. 2014). In the present study, we provided evidence that fenofibrate may protect the blood-retinal barrier by inhibition of diabetes-induced Nox4 expression and ROS production.
Hyperglycemia or high glucose–triggered ROS generation by Nox has been shown to activate diverse aberrant signaling pathways resulting in vascular injuries in diabetic retinas. Accumulating evidence suggests that Wnt/β-catenin cascade could be activated by redox signaling. A previous study reported that nucleoredoxin (NRX) interacted with Dvl and negatively regulated the Wnt/β-catenin signaling pathway (Funato et al. 2006). A further study demonstrated that Nox1-derived ROS caused oxidative-inactivation of the NRX and dissociation of NRX and Dvl, which accelerated activation of Wnt/β-catenin signaling (Kajla et al. 2012). Upon binding of Wnt ligands to the transmembrane receptor Fz and co-receptor LRP5/6, Dvl was recruited to Fz, which results in the relocation of Axin and associated GSK-3 to the plasma membrane to initiate LRP6 phosphorylation. Phosphorylation of LRP5/6 at ProlineProlineProlineSerine/ThreonineProline (PPPS/TP) motifs by GSK-3 in the intracellular domain provides the docking site for Axin and then attacks more GSK-3 to form a LRP6 signaling complex (LRP6 signalosome) and further enhances LRP6 phosphorylation, which accelerates dissociation of “destruction complex” and propagates stabilization of β-catenin and activation of β-catenin-dependent transcription. In the present study, we found that overexpression of Nox4 can directly increase phosphorylation of LRP6; in contrast, knockdown of Nox4 attenuated HG-induced LRP6 phosphorylation, suggesting a causal role of Nox4-derived ROS in activation of the Wnt/β-catenin signaling pathway. One of the intriguing observations in our study was that fenofibrate suppressed HG- and WCM-induced LRP-6 phosphorylation in retinal pigment epithelial cells. Fenofibrate is a PPAR-α agonist. A previous study reported that loss of PPAR-α augmented Wnt/β-catenin signaling by increasing LRP6 stability in cultured murine tubular cells; however, overexpression of PPAR-α accelerated LRP6 degradation and exerted an inhibitory effect on Wnt/β-catenin signaling activation (Cheng et al. 2016a). All of these findings suggested that fenofibrate could be involved in post-transcriptional regulation of LRP6 in a ROS-related and PPAR-α-dependent manner.
SOD is a ubiquitous family of antioxidant enzymes. Among three distinct isoforms, SOD1 (Cu-Zn-SOD) is mainly distributed in the cytoplasm; SOD2 (Mn-SOD) is compartmentalized in the mitochondrial matrix; SOD3 (EC-SOD) is an extracellular enzyme. SOD catalyzes the dismutase of superoxide into hydrogen peroxide, which could be further converted to water by glutathione peroxidase or catalase, thus serving a pivotal antioxidant role. Retinal SOD expression or activity was reduced by diabetes, which compromised the antioxidant defense capacity and aggravated retinal oxidative injuries. In this study, we demonstrated that both SOD1 and SOD2 were downregulated at the transcriptional level via Wnt/β-catenin signaling cascade, which was consistent with a previous finding that SOD activity was dramatically decreased by Wnt3a (Zhang et al. 2013). Fenofibrate treatment abrogated hyperglycemia- and Wnt/β-catenin-induced SOD downregulation. However, the detailed mechanisms by which Wnt/β-catenin activation negatively regulated SOD transcription remain to be elucidated in the future.
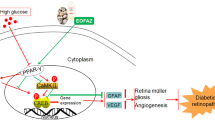
Taken together, hyperglycemia or HG induced Nox2 and Nox4 upregulation and thus increased ROS generation. The overproduction of ROS led to Wnt/β-catenin activation. Activation of Wnt/β-catenin signaling resulted in downregulation of SOD1 and SOD2, which, in turn, further accentuated retinal ROS burden (Fig. 7). Fenofibrate reduced Nox-mediated ROS production and subsequently suppressed Wnt/β-catenin signaling activation. Inhibition of Wnt/β-catenin activation by fenofibrate attenuated SOD downregulation, thereby promoting ROS decomposition in diabetic retinas. Thus, our study indicated that Nox-derived ROS contributed to LRP-6 phosphorylation and subsequent Wnt/β-catenin signaling activation. Activated Wnt/β-catentin led to transcriptional downregulation of SOD to accelerate retinal ROS burden. Fenofibrate ameliorated diabetic vascular leakage likely through coordinate attenuation of oxidative stress and blockade of Wnt/β-catenin signaling.
A schematic diagram summarizing the effect and mechanism for fenofibrate to prevent retinal vascular leakage in diabetic animal models. Hyperglycemia upregulated Nox and contributed to excessive ROS generation, which activated the Wnt/β-catenin signaling pathway. Activation of the Wnt/β-catenin pathway downregulated expression of anti-oxidative enzymes such as SOD1 and SOD2, which further exacerbated cellular oxidative stress. Fenofibrate suppressed hyperglycemia-induced Nox expression, thus attenuated Wnt/β-catenin activation. Inhibition of the Wnt/β-catenin pathway activation may contribute to fenofibrate’s effect on upregulating SOD1 and SOD2
References
(1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj 317:703–713
Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M (2004) Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109:227–233
Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB (2008) Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci 49:3231–3238
Chan EC, van Wijngaarden P, Liu GS, Jiang F, Peshavariya H, Dusting GJ (2013) Involvement of Nox2 NADPH oxidase in retinal neovascularization. Invest Ophthalmol Vis Sci 54:7061–7067
Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX (2013) Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62:261–272
Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, Boulton M, Lyons TJ, Gao G, Ma JX (2009) Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol 175:2676–2685
Chen Y, Wang JJ, Li J, Hosoya KI, Ratan R, Townes T, Zhang SX (2012) Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1. Diabetes Diabetologia 55:2533–2545
Cheng R, Ding L, He X, Takahashi Y, Ma JX (2016a) Interaction of PPARalpha with the canonic Wnt pathway in the regulation of renal fibrosis. Diabetes 65:3730–3743
Cheng Y, Zhang J, Guo W, Li F, Sun W, Chen J, Zhang C, Lu X, Tan Y, Feng W, Fu Y, Liu GC, Xu Z, Cai L (2016b) Up-regulation of Nrf2 is involved in FGF21-mediated fenofibrate protection against type 1 diabetic nephropathy. Free Radic Biol Med 93:94–109
Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT, Action to Control Cardiovascular Risk in Diabetes Eye Study Research G (2014) The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 121:2443–2451
Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF Jr (2011) NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124:731–740
Deliyanti D, Wilkinson-Berka JL (2015) Inhibition of NOX1/4 with GKT137831: a potential novel treatment to attenuate neuroglial cell inflammation in the retina. J Neuroinflammation 12:136
Diabetes C, Complications Trial Research G, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Ding L, Cheng R, Hu Y, Takahashi Y, Jenkins AJ, Keech AC, Humphries KM, Gu X, Elliott MH, Xia X, Ma JX (2014) Peroxisome proliferator-activated receptor alpha protects capillary pericytes in the retina. Am J Pathol 184:2709–2720
Drenser KA (2016) Wnt signaling pathway in retinal vascularization. Eye and Brain 8:141–146
Dvoriantchikova G, Grant J, Santos AR, Hernandez E, Ivanov D (2012) Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Invest Ophthalmol Vis Sci 53:2823–2830
Funato Y, Michiue T, Asashima M, Miki H (2006) The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol 8:501–508
Gao C, Xiao G, Hu J (2014) Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci 4:13
Hashizume K, Hirasawa M, Imamura Y, Noda S, Shimizu T, Shinoda K, Kurihara T, Noda K, Ozawa Y, Ishida S, Miyake Y, Shirasawa T, Tsubota K (2008) Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am J Pathol 172:1325–1331
Justilien V, Pang JJ, Renganathan K, Zhan X, Crabb JW, Kim SR, Sparrow JR, Hauswirth WW, Lewin AS (2007) SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci 48:4407–4420
Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, Kamata T (2012) A crucial role for Nox 1 in redox-dependent regulation of Wnt-beta-catenin signaling. FASEB Journal 26:2049–2059
Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG, Fs i (2007) Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370:1687–1697
Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH (2010) Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8
Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX (2010) Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes 59:1528–1538
Li J, Wang JJ, Zhang SX (2015) NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. J Diabetes Res:963289
Liu Q, Li J, Cheng R, Chen Y, Lee K, Hu Y, Yi J, Liu Z, Ma JX (2013) Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxid Redox Signal 18:1141–1153
Moran E, Ding L, Wang Z, Cheng R, Chen Q, Moore R, Takahashi Y, Ma JX (2014) Protective and antioxidant effects of PPARalpha in the ischemic retina. Invest Ophthalmol Vis Sci 55:4568–4576
Rojas M, Zhang W, Xu Z, Lemtalsi T, Chandler P, Toque HA, Caldwell RW, Caldwell RB (2013) Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. PLoS One 8:e84357
Saito Y, Geisen P, Uppal A, Hartnett ME (2007) Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis 13:840–853
Wang H, Yang Z, Jiang Y, Hartnett ME (2014) Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol Vis 20:231–241
Warden SM, Andreoli CM, Mukai S (2007) The Wnt signaling pathway in familial exudative vitreoretinopathy and Norrie disease. Semin Ophthalmol 22:211–217
Wei Y, Gong J, Xu Z, Duh EJ (2016) Nrf2 promotes reparative angiogenesis through regulation of NADPH oxidase-2 in oxygen-induced retinopathy. Free Radic Biol Med 99:234–243
Wilkinson-Berka JL, Deliyanti D, Rana I, Miller AG, Agrotis A, Armani R, Szyndralewiez C, Wingler K, Touyz RM, Cooper ME, Jandeleit-Dahm KA, Schmidt HH (2014) NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid Redox Signal 20:2726–2740
Yokota H, Narayanan SP, Zhang W, Liu H, Rojas M, Xu Z, Lemtalsi T, Nagaoka T, Yoshida A, Brooks SE, Caldwell RW, Caldwell RB (2011) Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci 52:8123–8131
Zhang DY, Pan Y, Zhang C, Yan BX, Yu SS, Wu DL, Shi MM, Shi K, Cai XX, Zhou SS, Wang JB, Pan JP, Zhang LH (2013) Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem 374:13–20
Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX (2012) Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55:255–266
Funding
This study was financially supported by NSFC Grants 81460163, 81741058, 81400427 and 81300786; Young Talent Scholar Grant from Shaanxi Science and Technology Department 2016KJXX-12; FRFCU Grant xjj2015015; RFDP Grant 20133601120012; Research Grants from Jiangxi Education Department GJJ14094, GJJ13175; Research Grants from Jiangxi Science and Technology Department 20142BDH80005, 20142BAB215029 and 20132BAB205024.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Q., Zhang, X., Cheng, R. et al. Salutary effect of fenofibrate on type 1 diabetic retinopathy via inhibiting oxidative stress–mediated Wnt/β-catenin pathway activation. Cell Tissue Res 376, 165–177 (2019). https://doi.org/10.1007/s00441-018-2974-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2974-z