Abstract
Exposure to nicotine in smoking contributes to most unexplained male infertility but the mechanisms remain to be fully elucidated. Zinc (Zn) is an essential trace element in normal growth, development and reproduction. Zinc oxide nanoparticles (ZnONPs) are well-known antioxidants. Therefore, this work was designed to investigate the potential ability of ZnONPs to protect testis and epididymis in nicotine-treated rats. Forty adult male Wistar albino rats were divided into control group and two experimental groups (treated and supplemented rats). In the treated group, rats received nicotine at a dose of 1 mg/kg/day orally for 30 days. Rats in the supplemented group received ZnONPs (10 mg/kg/day) with nicotine (1 mg/kg/day), orally for the same period. Testicular and epididymal sections were stained with H&E to assess the histological changes. Negrosin-eosin staining of epididymal sperms was performed to assess their viability and morphological changes. Serum testosterone, FSH and LH levels were assessed. Also, oxidative stress parameters and semiquantitative real-time PCR for steroidogenic enzymes were measured. Morphometric studies of both organs were statistically analyzed. Mild to severe testicular and epididymal structural changes together with sperm morphological abnormalities were detected in nicotine-treated rats. Biochemical results also showed a decrease in all measured parameters except for an increased malondialdehyde (MDA) level that meant deterioration of their reproductive function. On the other hand, ZnONP supplementation in the last group showed an obvious improvement in all investigated parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility is a reproductive disease manifested by absence of conception after at least 12 months of regular unprotected intercourse in couples (WHO 2010). It is evident in 15% of the sexually active population. Male infertility contributes between 30 and 50% to this condition. Infertility can cause emotional disorders including stress and social isolation. Several physiological, systemic pathologies, genetic abnormalities, environmental pollution and even oxidative stress in reproductive organs can cause infertility (Sankako et al. 2012).
Cigarette smoking is a common social habit that triggers an economic burden and serious public health problems. According to recent data of the World Health Organization, the number of smokers around the world is estimated at 1 billion people. Smoking is increased in less-developed countries and leads to 6 million deaths per year equivalent to 1 death every 6 s due to smoke exposure (WHO 2010). In the last decades, many available biological, experimental and epidemiological data indicated that cigarette smoking causes up to 13% of human infertility. This lifestyle factor could lead to infertility by declining the concentration and quality of human sperm (Burns 2007).
Cigarette smoke releases a large number of substances of medical importance such as nicotine, carbon monoxide, irritants, carcinogens and mutagens. Some of these substances and/or their metabolites lead to health problems such as heart and vascular diseases, asthma, tuberculosis, emphysema, as well as cancer of the mouth, pharynx, larynx and lung. Also, significant incidence of spontaneous abortion and infertility has been recorded among smokers. Presence of these substances in seminal plasma has been reported, indicating their ability to pass through the blood-testis barrier (Oyeyipo et al. 2010; Ribeiro et al. 2016).
Therefore, smoking can badly affect and damage the sperm function and even its genetic material. Moreover, people exposed passively to cigarette smoke may complain from several reproductive consequences similar to active smokers (PCASRM 2006).
Zinc is an indispensable bio-element for many biochemical processes such as growth and reproduction. It is considered a principle component of different proteins such as copper/zinc superoxide dismutase, so it maintains the structural integrity of DNA and the metabolism of nucleic acids. Additionally, it plays a key role in DNA repair, transcription and translation process (Hirst et al. 2013). Also, zinc is required for the maintenance of germ cells, progression of spermatogenesis and regulation of sperm motility (Zhao et al. 2011). Its deficiency in male reproduction results in gonadal dysfunction, decreased testicular weight and seminiferous tubule shrinkage (Babaei et al. (2010).
Recently, there has been a huge development of nanotechnology in the science and technology field. This nanotechnology is the technology of diagnosing, treating and preventing diseases using nanoscale structured materials. The use of metallic nanoparticles (gold, silver, iron, Zn and metal oxide nanoparticles) has shown great challenges in the field of medicine and its applications (Hirst et al. 2013). Zinc oxide (ZnO) is an environmentally friendly material with minimal hazards according to Wu et al. (2007), so it is used for different bio-applications, such as bio-imaging and cancer detection. Zinc oxide nanoparticles (ZnONPs) have a special importance due to their wide area of applications such as gas sensors, chemical sensors, bio-sensors, cosmetics, optical and electrical devices, solar cells and drug-delivery (Vaseem et al. 2010). ZnONPs are also known to be potent antioxidants and preserve reproductive tissue (Mohammadi et al. 2017; Torabi et al. 2017).
With an increasing rate of cigarette smoking and further nicotine exposure per day, the adverse effects on body tissues and organs become more evident and deleterious in smokers. Although previous studies have investigated the negative effect of nicotine on reproductive and sperm function, few of them studied its histological effects. So, this study was designed to investigate the detrimental effects of cigarette smoke on reproductive male organs and also to evaluate the possible protective role of ZnONPs against nicotine damage on male reproduction. The results open a new era in the clinical applicability of ZnONPs therapy to minimize the significant problem of infertility.
Chemicals
Nicotine preparation
Nicotine hydrogen tartrate with CAS number 65-31-6 and EC Number 200-607-2 (95% nicotine) was purchased from Sigma-Aldrich, 3050 Spruce Street, Saint Louis, MO 63103, USA. The nicotine dosage was prepared in normal saline for each group of animals. It was delivered orally at 1 mg/kg body weight daily. The working solutions were stored in a foil-wrapped glass bottle at 4 °C for no longer than 10 days.
Zinc oxide nanoparticles
In the form of dispersion ˂ 100 nm particle size, ≤ 40 nm average particle size, 20 wt.% in H2O (product number 721077, CAS number 1314-13-2) was purchased from Sigma-Aldrich, 3050 Spruce Street, Saint Louis, MO 63103, USA.
Animals
Forty adult male Wistar albino rats (weighing 180–200 g) were obtained from the Breeding Animal House of the Scientific and Medical Research Center, Faculty of Medicine, Zagazig University, Egypt. The animals were housed in ventilated metal cages. They were given rodent pellet and water ad libitum. The animals were handled and kept in the laboratory under constant conditions of temperature (24 ± 2 °C), (50 ± 5%) humidity and a photoperiod of 12-h dark and light for at least 2 weeks before and throughout the experimental work. All experiments were performed in accordance with the guidelines for animal research issued by the National Institute of Health guide for the care and use of laboratory animals and was approved by the Animal Ethics Committee, Zagazig University, Zagazig, Egypt.
Nanoparticle characterization
The particle size and morphology were detected using transmission electron microscopy in which the aqueous dispersion of the nanoparticles was drop-cast on a carbon-coated copper grid (Hsieh 2007). The grid was air dried at room temperature and visualized using (JEOL JEM -2100) Transmission Electron Microscope (Jeol Ltd., Tokyo, Japan) at the Electron Microscope Research Unit, Faculty of Agriculture, Mansoura University, Mansoura, Egypt (Fig. 1).
Experimental design
After a 2-week acclimation, animals were separated randomly into three groups:
Group I (control)
Animals (20 rats) were further subdivided into two equal subgroups:
-
Subgroup Ia (negative control): Ten rats received no treatment, (only balanced diet and tap water).
-
Subgroup Ib (positive control): Ten rats fed on the standard and given 0.2 ml of normal saline (vehicle), which was used to dissolve the nicotine hydrogen tartrate.
Group II (nicotine-treated group)
Ten rats were treated with nicotine hydrogen tartrate dissolved in normal saline at a dose of 1 mg/kg/day (Oyeyipo et al. 2010).
Group III (ZnONPs-supplemented)
Ten rats received 10 mg/kg/day of ZnONPs (Afifi et al. 2015) with nicotine 1 mg/kg/day (Oyeyipo et al. 2010).
All the previous chemicals were given daily to rats for 30 consecutive days by oral gavage through oral cannula. We did not include a control group receiving only ZnONPs because we aimed to evaluate its role on nicotine-treated rats, not the effect of zinc in normal conditions.
Histological study
At the end of the experiment, body, testis and epididymal weights of all animals were measured. Rats were sacrificed with an intraperitoneal injection of 25 mg/kg sodium thiopental (Kara et al. 2014). For biochemical and molecular analyses (oxidative stress parameters and semiquantitative reverse transcriptase (RT)-PCR of steroidogenic enzymes), the testes and epididymides on the right side of each animal were removed, rinsed with cold normal saline, divided into parts and dried with filter paper. Such parts were quickly frozen in liquid nitrogen then stored at − 80 °C until the preparation of tissue homogenates. For histopathological assessment, the testes and epididymal caput on the left side of the animals were fixed in Bouin’s solution, dehydrated and embedded in paraffin wax. Sections of 5 μm thickness were stained with hematoxylin and eosin (H&E), examined and photographed by the light microscope (Bancroft and Layton 2013).
Hormonal assay
Testosterone was determined using radioimmunoassay kits supplied by Diagnostic Products Corp. (Los Angeles, CA, USA) according to Maruyama et al. (Maruyama et al. 1987). The intra-assay coefficient of variation (CV) was < 9% and the inter-assay CV was < 13% for the testosterone hormone test.
Quantitative estimation of serum follicle-stimulating hormone (FSH) was carried out using enzyme-linked immune sorbent assay (ELISA) according to Knobil (1980). Quantitative estimation of luteinizing hormone (LH) in serum was carried out using ELISA according to Wakabayashi (1977). The kits used for determination of free testosterone, FSH and LH in rat serum were obtained from Glory science. Co., Ltd., USA. The intra-assay CV was < 5 and 4.5% and the inter-assay CV was < 6.5 and 8% for FSH and LH hormones respectively.
Estimation of testicular oxidative stress parameters
Parts of the frozen testicular samples from all groups were washed with phosphate-buffered saline (PBS). Then, they were homogenized in cold phosphate buffer, pH 7.0, containing ethylene diamine tetra acetic acid (EDTA) for measurement of thiobarbituric acid reactive substances (TBARS). The homogenate was centrifuged at 4000 rpm for 15 min at 4 °C and the supernatant was kept at − 80 °C until used for analysis of lipid peroxidation product (MDA), catalase, superoxide dismutase (SOD) and reduced glutathione (GSH), which were measured by using colorimetric kits (Bio-Diagnostics, Dokki, Giza, Egypt) according to the manufacturer’s instructions. TBARS (MDA) levels were expressed as nanomole per milligram protein (Duru et al. 2011).
Sperm quality analysis
To determine these parameters, the epididymal cauda was minced with a scalpel blade in a Petri dish pre-warmed to 37 °C. The fluid of the epididymal cauda was diluted in 2 ml of physiological saline solution (Narayana et al. 2002). One drop of evenly mixed sample was applied to a specific slide and the motility/concentration module of the computer-assisted semen analysis (CASA) system was performed using Mira Lab—Egypt (Mira 9000 sperm Analyzer CASA software). Sperm quality was determined by three parameters: sperm concentration, motility and viability (Emadi et al. 2012).
To assess the sperm viability and morphologic abnormalities, 10 μl of sperm suspension was smeared on a slide, air-dried and stained with 10 μl of eosin-nigrosin dye (1% eosin Y and 5% nigrosin). Examined under the light microscope using × 1000, the live spermatozoa were white and the dead were stained red (Linder et al. 1995; Ezzatabadipour et al. 2012). The sperms were classified into normal and abnormal and the total sperm abnormality was expressed as percentage incidence.
For analysis, a trinocular microscope with a plan objective lens (Olympus) equipped with phase contrast optics and a heated stage (37 °C) was used. Sperm count was expressed as million per milliliter. The number of motile spermatozoa was expressed as percentage of sperm motility.
Semiquantitative reverse transcriptase-PCR of steroidogenic enzymes
After homogenization, total RNA extraction was carried out from rat testis using TriFast TM reagent (PeqLab. Biotechnologie GmbH, Carl-Thiersch St. 2B 91052 Erlongen, Germany, Cat. No. 30-2010). The remaining DNA was removed by digestion with DNase I (Sigma). The RNA purity and concentration were determined spectrophotometrically at A260/A280 nm.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was performed using ready-to-go RT-PCR beads for first cDNA synthesis and PCR reaction provided by Amersham Biosciences, England. Cat. No. 27-9266-01(Berchtold 1989). Gene-specific primers were purchased from Biolegio. BV, PO Box 91, 5600 AB Nijmegen, Netherlands. The primers used for RT-PCR for StAR protein and cytochrome P450scc were gene-specific primers selected according to Akingbemi et al. (Akingebemi et al. 2004).
StAR protein: forward primer, 5′-TTG GGC ATA CTC AAC AAC CA-3′; reverse primer, 5′-ATG ACACCG CTT TGC TCA G-3′.
Cytochrome P450scc: forward primer, 5′-AGG TGT AGC TCA GGA CTT-3′; reverse primer, 5′-AGG AGG CTA TAA AGG ACA CC-3′.
RPS 16 (internal control, housekeeping gene): forward primer, 5′-AAG TCT TCG GAC GCA AGA AA-3′; reverse primer, 5′-TTG CCC AGAAGC AGAACA G-3′.
Thermal cycling reaction was performed with the following program: initial denaturation 94 °C for 5 min, 35 cycles of 94 °C for 0.5 min denaturation, 60 °C for 1 min (StAR protein), 58 °C for 30 s (cytochrome P450scc) and 60 °C for 1 min (RPS 16) as primer annealing, 72 °C for 1 min extension and final extension at 72 °C for 7 min. The products were subjected to agarose gel electrophoresis using 2% agarose stained with ethidium bromide and visualized via light UV trans illuminator (Model TUV-20, OWI Scientific, Inc. 800 242-5560, France) and photographed under fixed conditions (the distance, the light and the zoom).
Histomorphometry
The slides were examined under a microscope and the following measurements were taken; seminiferous tubular diameter, epididymal caput region was analyzed as regards to epididymal tubular diameter, epididymal luminal diameter. Seminiferous and epididymal epithelial height were also measured. For each parameter, ten measurements were made per section. The means of the measurements of the parameter in each section were recorded for each animal. The image analyzer computer system Leica Qwin 500 (Leica Imaging system, Ltd., Cambridge, England) was used to measure the percentage (%) of each parameter in the Pathology Department, Faculty of Dentistry, Cairo University, Cairo, Egypt.
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS, Inc. Chicago, IL, USA). Normally distributed data are expressed as the mean ± standard deviation. p < 0.05 was considered to indicate a statistically significant difference. For analysis of weight gain, hormonal levels and oxidative anti-oxidative parameters and gene expression, one-way ANOVA was done followed by Tukey’s post hoc test.
Results
Histopathological results
Hematoxylin and eosin-stained sections of control rat testis showed normal morphology. It is covered by a thin connective tissue capsule. Well-developed circular or elliptical seminiferous tubules (Sts) were densely packed and lined with multiple layers of spermatogenic cells with little interstitium containing clusters of interstitial cells (Fig. 2a). Nicotine exposure resulted in mild to severe testicular degeneration and distortion accompanied by lumen contraction. Nicotine-treated testis showed thick covering tunica albuginea with dilated and congested blood vessels. Some seminiferous tubules showed separated germinal epithelium from their basement membranes (Fig. 2b). Other sections were severely affected with an irregular separated capsule and wide separation between adjacent Sts that were distorted with an irregular basement membrane; some of them showed detached germinal epithelium in the lumen and a small amount of interstitial tissue could be seen with congested blood vessels (Fig. 2c). Rats receiving nicotine and ZnONPs simultaneously showed a thin connective tissue capsule containing blood vessels and normal shape and arrangement of the seminiferous tubules. Also, the interstitium with normal clusters of interstitial cells could be seen. Acidophilic hyaline material with vacuolation was detected in the interstitium (Fig. 2d).
(A) H&E-stained sections of control rat testis showing a thin connective tissue capsule (curved arrow). The seminiferous tubules (Sts) are densely packed with little interstitium (I) containing clusters of interstitial cells. (B) Nicotine-treated testis show thick covering tunica albuginea (C) with congested blood vessels (B). Some seminiferous tubules (Sts) show separated germinal epithelium from their basement membranes (black arrows). (C) Other sections of the nicotine-treated group show an irregular separated capsule (C) and wide separation between adjacent seminiferous tubules that display an irregular basement membrane (arrowhead); some of them show detached germinal epithelium in the lumen (black arrow) and a small amount of interstitial tissue could be seen (red arrow), notice the congested blood vessel (B). (D) Sections from the ZnONPs-supplemented group showing a thin connective tissue capsule (C) with blood vessels (B) could be seen, normal shape and arrangement of the seminiferous tubules (Sts), the interstitium (I) containing clusters of interstitial cells. Acidophilic hyaline material with vacuolation is detected in the interstitium (black arrow) (A, B, D× 200; C × 100, scale bar 50 μm)
The higher magnification of the previous sections provided us with more details. Control rat testis showed a thin regular basement membrane of Sts with flat myoid cells. The Sts were lined by stratified germinal epithelium and Sertoli cells with large, pale nuclei. Germinal epithelium was formed of spermatogonia, primary spermatocytes and spermatids. Sperms could be seen in the lumen. Leydig cells appeared with acidophilic cytoplasm and rounded vesicular nuclei (Fig. 3a). Nicotine-treated rats showed wide separation between germinal epithelium, with dark-stained nuclei of primary spermatocytes. The interstitial tissue showed Leydig cells with dark-stained nuclei and congested blood vessels in mildly affected sections (Fig. 3b). Some severely affected sections of group II showed distorted Sts with irregular basal lamina and reduction in the thickness of germinal epithelium with a wide separation between adjacent cells due to detachment of some cells in the lumen. They showed irregular outlines and karyolytic nuclei. Primary spermatocytes appeared with dark-stained nuclei. The interstitial tissue showed edema and vacuolation and Leydig cells had small dark-stained nuclei with dark acidophilic cytoplasm (Fig. 3c). ZnONP-supplemented rats showed nearly normal testicular architecture as Sts had a thin regular basement membrane with flat myoid cells and they were lined by stratified germinal epithelium and Sertoli cells with large, pale nuclei. Germinal epithelium was formed of spermatogonia, primary spermatocytes and spermatids. Sperms could be seen in the lumen. Leydig cells appeared with acidophilic cytoplasm and rounded vesicular nuclei (Fig. 3d).
Higher magnification presents (A) the control group showing Sts having a thin regular basement membrane with flat myoid cells (blue arrow). The Sts are lined by stratified germinal epithelium and Sertoli cells with large, pale nuclei (arrowhead). Germinal epithelium is formed of spermatogonia (S), primary spermatocytes (S1) and spermatids (S2). Sperms could be seen in the lumen (S3). Leydig cells (red arrows) appear with acidophilic cytoplasm and rounded vesicular nuclei. (B) Nicotine-treated rats show wide separation between germinal epithelium (black arrow), with darkly stained nuclei of primary spermatocytes (S1). The interstitial tissue show Leydig cells with darkly stained nuclei (red arrows) and congested blood vessels (B). (C) Some sections of nicotine-treated rats display Sts with irregular basal lamina (arrowhead) and reduction in the thickness of germinal epithelium with wide separation between adjacent cells (stars). Some cells appeared detached in the lumen with irregular outlines and karyolytic nuclei (black arrows). Primary spermatocytes (S1) appear with dark-stained nuclei. The interstitial tissue shows edema (E) and vacuolations (V) and Leydig cells exhibit small darkly stained nuclei with dark acidophilic cytoplasm (red arrows). (D) ZnONP-supplemented rats display Sts having a thin regular basal lamina with flat myoid cells (blue arrow). They are lined by Sertoli cells (arrowhead) with large, pale nuclei and stratified germinal epithelium that is formed of spermatogonia (S), primary spermatocytes (S1) and spermatids (S2). Sperms could be seen in the lumen (S3). Leydig cells (red arrows) appear with acidophilic cytoplasm and rounded vesicular nuclei. (H&E × 400, scale bar 30 μm)
The cross-sections of caput epididymis obtained from the control group revealed regular and circular ducts lined with a pseudostratified columnar epithelium that exhibits stereocilia. A dense collection of sperm cells in the lumen was evident. Scanty connective tissue can be seen between the ducts (Fig. 4a). The mildly affected sections of nicotine-treated rats showed epididymal mucosa with dark acidophilic cytoplasm and dark-stained nuclei. Congested blood vessels could be seen in the connective tissue between the ducts. Sperms were noticed in the lumen (Fig. 4b). The highly affected sections of group II showed markedly distorted ducts with vacuolations in the lumen and between the mucosal cells. The connective tissue showed marked extravasations and mononuclear cell infiltration (Fig. 4c). The ZnONP-supplemented group showed ciliated mucosa lining the ducts with some vacuolations. The lumen contained sperms. Congested blood vessels were noticed in between the ducts (Fig. 4d).
H&E-stained sections from rat epididymis display (A) the control group showing the epididymis ducts lined with ciliated mucosa (arrow), the lumen containing sperms (SP). Scanty connective tissue can be seen between the ducts (curved arrow). (B) Nicotine-treated rats exhibit epididymal mucosa with dark acidophilic cytoplasm and dark-stained nuclei (arrow). Congested blood vessels can be seen in the connective tissue between the ducts (B). Notice sperms in the lumen (SP). (C) The highly affected ducts of the nicotine-treated group are markedly distorted (arrow), with vacuolations in the lumen and between the mucosal cells (V). The connective tissue shows marked extravasation (B) and mononuclear cell infiltration (yellow arrow). (D) ZnONPs-supplemented group show ciliated mucosa (arrow) lining the ducts with some vacuolations (V), notice the sperms in the lumen (SP) and congested blood vessels in between the ducts (B). (A and C × 100, B and D × 200, scale bar 50 μm)
At higher magnification, the control rat epididymal caput was lined with principal cells with acidophilic cytoplasm and basal lightly stained nuclei and an apical acidophilic brush border; stereocilia. Basal cells had dark basal nuclei. Smooth muscles of the musculosa layer separated the ducts from the connective tissue that contained blood vessels (Fig. 5a). Nicotine-treated rats showed principal cells with dark acidophilic cytoplasm, dark-stained nuclei and loss of stereocilia. Marked extravasation of blood between the ducts was noticed (Fig. 5b). Rats of group III that received ZnONPs simultaneously with nicotine showed normal epididymis lined with principal cells with an apical acidophilic brush border; stereocilia, basal cells with dark basal nuclei and smooth muscles of the musculosa layer were evident (Fig. 5c).
Higher magnification shows (A) control rat epididymis is lined with principal cells (pr) with acidophilic cytoplasm and basal lightly stained nuclei and apical acidophilic brush border; stereocilia (red arrow). Basal cells have dark basal nuclei (ba). Smooth muscles (sm) of musculosa layer and blood vessels (B) in between the ducts can be seen. (B) Nicotine-treated group shows principal cells (arrows) with dark acidophilic cytoplasm and dark-stained nuclei and loss of stereocilia (red arrows). Marked extravasation of blood (B) between the ducts is noticed. (C) ZnONP-supplemented group shows normal epididymis lined with principal cells (pr) with apical acidophilic brush border; stereocilia (red arrow). Basal cells have dark basal nuclei (ba). Smooth muscles (sm) of musculosa layer can be seen (H&E × 400, scale bar 30 μm)
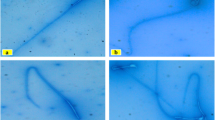
Negrosin-eosin-stained epididymal sperms showed that live sperms were unstained while dead sperms were stained with red color (Fig. 6a, b). Normal shape of sperms was detected in the control group (Fig. 7a) while the nicotine-treated group showed different forms of abnormalities in the form of a twisted body (Fig. 7b); abnormal rounded head (Fig. 7c); abnormal irregular tail (Fig. 7d); and abnormal head and tail (Fig. 7e). The ZnONPs-supplemented group showed normal epididymal sperm morphology (Fig. 7f).
Negrosin-eosin-stained epididymal sperms showing (a) normal shape of sperms in the control group; while the nicotine-treated group shows different forms of abnormalities in the form of (b) a twisted body; (c) abnormal rounded head; (d) abnormal irregular tail; (e) abnormal head (arrowhead) and tail (arrow). (f) The ZnONP-supplemented group shows normal epididymal sperm morphology (negrosin-eosin × 1000, scale bar 10 μm)
Biochemical, statistical and morphometric results
Assessment of body, testicular and epididymal weights and hormonal levels
The positive control group did not show significant differences when compared to the negative control one (p > 0.05). It was found that the nicotine-treated group showed a significant and highly significant difference in this parameter when compared to the control and ZnONP-supplemented groups respectively (p < 0.05, p < 0.001). Furthermore, the ZnONP-supplemented group showed a nonsignificant difference (p > 0.05) when compared to group I (Figs. 8 and 9).
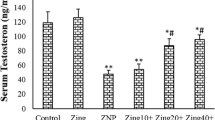
Regarding the level of hormones, the nicotine-treated group showed a significant decrease in all hormones as compared to the control group with a nonsignificant difference between ZnONPs-supplemented groups and control (Fig. 10).
Assessment of lipid peroxidation and oxidative stress in testicular and epididymal tissues
The testicular and epididymal MDA levels were significantly higher in the nicotine-treated group compared to the control one (p < 0.001). However, its level in both tissues of the ZnONP-supplemented group was significantly lower when compared to the nicotine-treated group (p < 0.05) (Fig. 11). Levels of antioxidant enzymes, CAT, SOD, GSH and GPx were highly significantly decreased in the ZnONPs-supplemented group in comparison to the nicotine-treated group (p < 0.05) (Fig. 12).
Assessment of sperm motility, morphology, viability and count
Epididymal sperm concentration in the nicotine-treated group was significantly lower (p < 0.05) than that of the control. The ZnONP-supplemented group revealed an increase in sperm count relative to group II. TSA showed a highly significant increase (p < 0.0001) in the nicotine-treated group in comparison with their respective control ones, also a significant decrease in live sperm and an increase in dead sperm in the nicotine-treated group (nicotine-treated) compared to the control group (Figs. 13 and 14).
Assessment of morphometric results
Morphometric analysis demonstrated a significant decrease (p < 0.05) in the diameter and surface area (SA) of the seminiferous tubule and epididymal tubules in the nicotine-treated group as compared to control groups and ZnONPs-supplemented groups (p < 0.05) (Figs. 15 and 16).
Assessment of semiquantitative reverse transcriptase-PCR of steroidogenic enzymes
Testicular gene expression of StAR and cytochrome P450scc was significantly decreased (p < 0.05) in the nicotine-treated group when compared to the control group. The ZnONP-supplementation group caused a further increase in testicular gene expression of StAR and cytochrome P450scc as compared with the control group (p < 0.05) (Fig. 17).
Discussion
Available data do not conclusively demonstrate that smoking decreases male fertility. However, with much debate on its impact on semen parameters, it is regarded as an infertility risk factor (Mostafa 2010). Zinc is known to be essential for testosterone synthesis and spermatogenesis. Its deficiency leads to atrophy of seminiferous tubules and failure of spermatogenesis. Also, zinc deficiency could impair the steroid receptor function and decrease sex steroid action because the steroid receptors have zinc fingers (Bedwal et al. 2009). Therefore, this study sought to assess the relationship between smoking and male infertility through histological and biochemical impaction on testis and epididymis and the possible ameliorative effect of ZnONPs supplementation.
Regarding body weight, there was a significant decrease in body weight of the nicotine-treated group compared to the control and supplemented groups. Some researchers recorded a significant decrease in BW of cigarette smoke-exposed rats and attributed it to the metabolic effects of nicotine (Sankako et al. 2012; Nerín et al. 2007). Exposure to nicotine may reduce food intake by its effect on feeding behavior (Bellinger et al. 2010). Feeding-related actions of nicotine are correlated to neuropeptides such as neuropeptide Y and peptide hormones, such as leptin (Bishop et al. 2002; Klein et al. 2004). These results disagreed with Oyeyipo et al. (2010) who reported that the mean body weight of nicotine-treated animals showed nonsignificant changes. The weight of testes and epididymes also revealed a significant loss as compared to the control group. These results could be attributed to a decrease in the testosterone level, which is essential for normal growth and development of these organs.
In the present study, shrunken, disorganized seminiferous tubules (Sts) with an irregular basement membrane and wide separation between germinal epithelium with darkly stained nuclei of primary spermatocytes in group II were evident. Germ cells’ detachment and sloughing in the lumen of most Sts may be attributed to nicotine-induced lipid peroxidation and the reduction in testosterone hormone (Oyeyipo et al. 2011) as testosterone is needed for the attachment of different generations of germ cells in Sts (Oyeyemi et al. 2015). Decreased serum testosterone level caused by nicotine may be associated with disruption of testicular cyto-architecture affecting Leydig cell number and function, as well as the fertility rate of the animals (Oyeyipo et al. 2010).
Elshal et al. (2009) reported immature spermatozoa, sperm head defects and disturbances in spermatozoa chromatin and DNA integrities associated with cigarette smoking. It has been suggested that dietary Zn may prevent cellular apoptosis. According to the study of Cao et al. (2015), a positive correlation between Zn levels in testes and sera and levels in food was observed, indicating that dietary Zn accumulates in tissues and serum. Accumulated degenerated germ cells in the lumina of Sts in the present work may be attributed to the failure of Sertoli cells to perform their function in engulfing these bodies (Bin Dohaish et al. 2008). Vacuoles revealed in the present study may be derived from dilatation and vesiculation of the endoplasmic reticulum and mitochondrial swelling (Nolte et al. 1995).
On the other hand, the basal lamina irregularities in the present work might be secondary to tubular shrinkage in degenerated tubules or as a result of contraction of myoid cells (Imran et al. 2003). Acharya et al. (2008) reported that substances having antioxidant activities, such as vitamin C, vitamin E, Zn, selenium and melatonin, reduced and/or prevented both the oxidative stress and damage in the testis caused by cadmium in their study. Cohen (1997) suggested that nicotine activated specific intracellular death-related pathways. In addition, it enhanced the expression of the activated form of caspase-3 and caspase-3 enzyme activity. On the other hand, Mohammadi et al. (2017) proved that ZnONPs prevented both epithelial disorganization of Sts and the reduction of spermatogenic cell counts induced by cyclophosphamide.
Regarding the effect of nicotine on testosterone, LH and FSH hormones in serum showed the significant decrease in group II (nicotine-treated) compared to the control. It was reported that most environmental chemicals and pollutants are hormonally active compounds that target the endocrine system and cause reproductive anomalies. Testosterone levels in plasma are indicators of normal testicular function. It was noted that nicotine could inhibit androgens release, LH, FSH and testosterone, which are necessary for the gonadal development and steroidogenesis in rats (Mathur and D’Cruz 2011). Also, it was documented that nicotine has harmful effects on the hypothalamic/pituitary/testicular axis and a suppressing effect on testicular and androgen production by modifying the function of Leydig cells (Nair and Rajamohan 2014). Reddy et al. (1998) revealed that nicotine is a central nervous system depressor that inhibits the neural stimulus essential for the release of pituitary gonadotropins.
Decreased testosterone level in group II of the present study was emphasized by the manifest decrease in testicular StAR gene expression. Bose et al. (2007) mentioned that environmental factors, such as tobacco smoke that contains nicotine, reduce the interaction of StAR with the outer mitochondrial membrane (OMM), resulting in reduced transport of cholesterol and finally reduced pregnenolone. They added that the effect of cigarette smoke is stronger than that of nicotine alone in decreasing the expression of protein at the OMM. Decline in StAR mRNA transcription and StAR protein expression in nicotine-treated animals was reported in the adult and prenatal period. It was explained by defective cholesterol transfer in the mitochondria or direct suppression of StAR protein expression (Jana et al. 2010; Liu et al. 2016).
Hormonal levels in group III (ZnONP-supplemented) showed an improvement consistent with the results recorded by Al-Ani et al. who reported that testosterone synthesis is dependent on adequate dietary Zn. There is strong evidence that Zn is required for normal function of the hypothalamo-pituitary-gonadal axis. It increases the release of LH and FSH from the pituitary gland, stimulating testosterone production; Zn also inhibits the aromatase enzyme so prevents converting testosterone into excess estrogen (Al-Ani et al. 2015). Also, its deficiency leads to a biochemical lesion in the pathways controlling steroid synthesis, impaired development of the smooth endoplasmic reticulum in the Leydig cells, or leads to the malfunction of the LH receptor mechanism controlling storage and release of testosterone (Bedwal et al. 2009).
The sperm count showed a significant decrease (p < 0.001) in the nicotine-treated group (II) as compared with control ones (Ia, Ib) but showed a significant increase in the supplemented group as compared to group II. The total sperm abnormalities showed a highly significant increase (p < 0.0001) in group II in comparison with their respective control ones. Group III showed a significant increase in the number of normal sperms and decreased incidence of sperm abnormality when compared with the group (II). Also, we observed a significant decrease in live sperm and an increase in dead sperm in group II (nicotine-treated) compared to the control group. These results agree with Nair and Rajamohan (2014) and Jalili et al. (2014) who mentioned that smoking has been associated with overall reduction in semen quality through significantly decreasing the motility, count and normal morphology of sperms in comparison with control groups (p < 0.05).
In smokers, nicotine and its metabolites have been detected in seminal plasma in association with cigarette consumption. Many reports have explained the detrimental effects of cigarette smoking on sperm morphology, density and motility by direct biological and toxic effects on sperm cells (Abdul Ghani Abdul-Ghani 2014). Furthermore, it has been suggested that inhalation of cigarette smoke leads to absorption of nicotine and its metabolites, which reach the reproductive system through the blood-testis barrier causing alterations, including altered antioxidant concentrations and generation of reactive oxygen species (Kapawa et al. 2004). In addition, it has been found that heavy smoking induced DNA damage and is associated with abnormal spermatozoa and male infertility (La Maestra et al. 2015; Dai et al. 2015). In their research, Miranda-Spooner et al. (2016) reported a reduction in sperm motility and mitochondrial activity, also, increased spermatozoa with tail abnormalities in nicotine-exposed rat offspring. They concluded that nicotine is considered a risk factor on male offerings gonads during pregnancy and lactation. This also explains morphological changes in sperms detected in our study by negrosin-eosin staining of epididymal sperms. On the other hand, all sperm parameters improved with ZnONP supplementation that has a positive impact on tissue.
Morphometric analysis of group II confirmed the testicular atrophy with a marked decrease in tubular diameter and seminiferous epithelium height in testis along with a decrease in the same parameters in epididymis. These results agreed with the results of other studies on the reproductive system (Arrighi et al. 2010). The testicular changes indicated decreased spermatogenesis while epididymal changes indicated a lower functional sustainability of this organ, whose activities are towards maturation and conservation of spermatozoa. Group III showed significant improvements of these parameters indicating the protective influence of Zn on reproduction (Al-Ani et al. 2015).
Conclusion and recommendations
Exposure to nicotine was proved to adversely affect the histological and biochemical structure of testis and epididymis of adult rats. Also, sperm parameter results reflected its reproductive drawbacks. ZnONP supplementation to nicotine-exposed rats minimized all these parameters through decreasing oxidative stress and increasing expression of steroidogenic enzymes, indicating the importance of Zn in male reproduction. Further researches are needed to explore its different mechanisms in improving male fertility.
Abbreviations
- Zn:
-
Zinc
- ZnONPs:
-
Zinc oxide nanoparticles
- WHO:
-
World Health Organization
- StAR:
-
Steroidogenic acute regulatory protein
- RT- PCR:
-
Semiquantitative reverse transcriptase-PCR
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- ELISA:
-
Enzyme-linked immune sorbent assay
- CV:
-
Coefficient of variation
- PBS:
-
Phosphate-buffered saline
- EDTA:
-
Ethylenediaminetetraacetic acid
- TBARS:
-
Thiobarbituric acid reactive substances
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- rpm:
-
Round per minute
- TSA:
-
Total sperm abnormalities
- OMM:
-
Outer mitochondrial membrane
- STs:
-
Seminiferous tubules
- NBT:
-
Nitroblue tetrazolium to measure neutrophil oxidative burst
- SH:
-
Thiol
- GSH-Px:
-
Glutathione peroxidase
- BW:
-
Body weight
References
Abdul-Ghani AS (2014) Studies on cigarette smoke induced oxidative DNA damage and reduced spermatogenesis in rats. J Environ Biol 35:943–947
Acharya UR, Mishra M, Patro J, Panda MK (2008) Effect of vitamin C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol 25(1):84–88
Afifi M, Almaghrabi OA, Kadasa NM (2015) Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. BioMed Res Int 19:1–6
Akingebemi BT, Sottas CM, Koulova A, Klinefelter GR, Hardy MP (2004) Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145:592–603
Al-Ani NK, Al-Kawaz U, Saeed BT (2015) Protective influence of zinc on reproductive parameters in male rat treated with cadmium. Am J Med Med Sci 5(2):73–81
Arrighi S, Bosil G, Groppetti D, Cremonesi F (2010) Morpho- and histometric evaluations on the testis and epididymis in buffalo bulls during the different reproductive seasons. Open Anat J 2, 29–33.
Babaei H, Azaril O, Kheirandish R, Abshenas J, Mohammadi N (2010) Zinc therapy improves deleterious effects of experimental unilateral cryptorchidism: histopathological evaluation of testes. IJVS 5(1, 2):77–88
Bancroft J, Layton C (2013) Hematoxylin and eosin. In: Suvarna SK, Layton C, Bancroft JD (eds) Theory and practice of histological techniques, Ch. 10 and 11, 7th edn. Churchill Livingstone of Elsevier, Philadelphia, pp. 172–214.
Bedwal S, Prasad S, Nair N, Saini MR, Bedwal RS (2009) Catalase in testes and epididymidis of Wistar rats fed zinc deficient diet. Indian J Pharmac Sci 71(1):55–58
Bellinger LL, Wellman PJ, Harris R, Kelso EW, Kramer PR (2010) The effects of chronic nicotine on meal patterns, food intake, metabolism and body weight of male rats. Pharmacol Biochem Behav 95:92–99
Berchtold MW (1989) A simple method for direct cloning and sequencing cDNA by the use of a sinle specific oligonucleotide and oligo (dT) in a polymerase chain reaction (PCR). Nucleic Acid Res 17(11):453
Bin Dohaish E, Ali A, Melebary S (2008) Histological changes in the testes of two strains of mice: effect of ecological factors. Saudi J Biol Sci 15(2):279–287
Bishop C, Parker GC, Coscina DV (2002) Nicotine and its withdrawal after feeding induced by paraventricular hypothalamic injections of neuropeptide Y in Sprague- Dawley rats. Psychopharmacology 162(3):265–272
Bose M, Debnath D, Chen Y, Bose HS (2007) Folding, activity and import of steroidogenic acute regulatory protein into mitochondria changed by nicotine exposure. J Mol Endocrinol 39(1):67–79
Burns LH (2007) Psychiatric aspects of infertility and infertility treatments. Psychiatr Clin North Am 30:689–716
Cao Y, Li YS, Li ZJ, Wang F, Li CM (2015) Dietary zinc may attenuate heat-induced testicular oxidative stress in mice via up-regulation of Cu-Zn SOD. Genet Mol Res 14(4):16,616–16,626
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Dai JB, Wang ZX, Qiao ZD (2015) The hazardous effects of tobacco smoking on male fertility. Asian J Androl 17:954–960
Duru FI, Yama OE, Noronha CC, Okanlawon AO (2011) Alterations in morphometry and malondialdehyde levels in adult Sprague-Dawley rat testes in three obstructive vasectomy models: effect of melatonin. Asian J Pharm Clin Res 4(2):27–30
Elshal MF, El-Sayed IH, Elsaied MA, El-Masry SA, Kumosani TA (2009) Sperm head defects and disturbances in spermatozoal chromatin and DNA integrities in idiopathic infertile subjects: association with cigarette smoking. Clin Biochem 42:589–594
Emadi L, Azari O, Gholipour H, Saeed M (2012) Effect of vitamin C on epididymal spermquality in the rat experimentally induced unilateral cryptorchidism. IJVS 7(1, 2):63–74
Ezzatabadipour M, Azizollahi S, Sarvazad A, Mirkahnooj Z, Mahdinia Z, Nematollahi-Mahani SN (2012) Effects of concurrent chronic administration of alcohol and nicotine on rat sperm parameters. Andrologia 44(5):330–336
Hirst SM, Krakatoa A, Singh S et al (2013) Bio-distribution and invivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol 28(2):107–118
Hsieh CH (2007) Spherical zinc oxide nano particles from zinc acetate in the precipitation method. J Chin Chem Soc 54(1):31–34
Imran A, Muhammad S, Khalid FY (2003) Study of the effects of lead poisoning on the testes in albino rats. Pak J Med Res 42(3):97–101
Jalili C, Khani F, Salahshoor MR, Roshankhah S (2014) Protective effect of curcumin against nicotine-induced damage on reproductive parameters in male mice. Int J Morphol 32(3):844–849
Jana K, Samanta PK, De DK (2010) Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci 116:647–659
Kapawa A, Giannakos D, Tsoukanelis K, Kanakas N, Kalogiannis D, Agapitos E, Loutradis D, Miyagawa I, Sofikitis N (2004) Effects of paternal cigarette smoking on testicular function, sperm fertilizing capacity, embryonic development, and blastocyst capacity for implantation in rats. Andrologia 36:57–68
Kara A, Unal D, Simsek N, Yucel A, Yucel N, Selli J (2014) Ultra- structural changes and apoptotic activity in cerebellum of post- menopausal-diabetic rats: a histochemical and ultra-structural study. J Gynecol Endocrinol 30(3):226–231
Klein LC, Corwin EJ, Ceballos RM (2004) Leptin, hunger, and body weight: influence of gender, tobacco smoking, and smoking abstinence. Addic Behav 29:921–927
Knobil E (1980) The neuroendocrine control of the menstrual cycle. Rec Prog Horm Res 36:52–88
La Maestra S, De Flora S, Micale RT (2015) Effect of cigarette smoke on DNA damage, oxidative stress, and morphological alterations in mouse testis and spermatozoa. Int J Hyg Environ Health 218:117–122
Linder RE, Klinefelter GR, Strader LF, Narotsky MG, Suarez JD, Roberts NL, Perreault SD (1995) Dibromoacetic acid affects reproductive competence and sperm quality in the male rat. Fund Appl Toxicol 2825:9–17
Liu L, Wang JF, Fan J, Rao YS, Liu F, Yan YE, Wang H (2016) Nicotine suppressed fetal adrenal star expression via YY1 mediated-histone deacetylation modification mechanism. Int J Mol Sci 17(9):1477
Maruyama Y, Aoki N, Suzuki Y, Ohno Y, Imamura M, Saika T, Sinohara H, Yamamoto T (1987) Sex-steroid-binding plasma protein (SBP), testosterone, oestradiol and dehydroepiandrosterone Reprod, (DHEA) in prepuberty and puberty. Acta Endocrinol. 114:60–67
Mathur PP, D’Cruz SC (2011) The effect of environmental contaminants on testicular function. Asian J Androl 13:585–591 (review)
Miranda-Spooner M, Paccola CC, Neves FM, Oliva SU, Miraglia SM (2016) Late reproductive analysis in rat male offspring exposed to nicotine during pregnancy and lactation. Andrology 4(2):218–231
Mohammadi T, Hoveizi E, Khajehpour L, Jelodar Z (2017) Protective effects of zinc oxide nanoparticles on testis histological structure in cyclophosphamide treated adult mice. J Mazandaran Univ Med Sci 26(144):19–27
Mostafa T (2010) Cigarette smoking and male infertility. Rev J Adv Res 1:179–186
Nair SV, Rajamohan T (2014) The role of coconut water on nicotine-induced reproductive dysfunction in experimental male rat model. Food Nutr Sci 5:1121–1130
Narayana K, D’Souza UJ, Rao KP (2002) Ribavirin induced sperm shape abnormalities in Wistar rat. Mut Res 513:193–196
Nerín I, Beamonte A, Gargallo P, Jiménez-Muro A, Marqueta A (2007) Weight gain and anxiety levels in recent ex-smokers. Arch Bronconeumol 43:9–15
Nolte T, Harleman JH, Jahn W (1995) Histopathology of chemically induced testicular atrophy in rats. Exp Toxic Pathol 47:267–286
Oyeyemi WA, Shittu ST, Kolawole TA, Ubanecheand P, Akinola AO (2015) Protective effect of vitamin E on nicotine induced reproductive toxicity in male rats. Nigerian J Basic Appl Sci 23(1):7–13
Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF (2010) Effects of oral administration of nicotine on organ weight, serum testosterone level and testicular histology in adult male rats. Nig J Physiol Sci 25:81–86
Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF (2011) Effects of nicotine on sperm characteristics and fertility profile in adult male rats: a possible role of cessation. J Reproductive Infertility 12:201–207.
Practice Committee of the American Society for Reproductive Medicine [PCASRM] (2006) Smoking and infertility. Fertil Steril 86:172–177
Reddy S, Londonkar R, Reddy S, Patil SB (1998) Testicular changes due to graded doses of nicotine in albino mice. Indian J Physiol Pharmacol 42:276–280
Ribeiro AG, Ribeiro-paes GT, Stessuk T, Kozma RL, Isabel Camargo IC, Biosci J (2016) Histomorphometrical evaluation in testis and epididymis of rats with pulmonary emphysema experimentally induced by prolonged exposure to cigarette smoke. Biosci J Uberlandia 32(4):1092–1102
Sankako MK, Garcia PC, Piffer RC, Dallaqua B, Damasceno DC, Oduvaldo CM (2012) Pereira1 Possible mechanism by which zinc protects the testicular function of rats exposed to cigarette smoke. Pharmacol Rep 64:1537–1546
Torabi F, Shafaroudi MM, Rezaei N (2017) Combined protective effect of zinc oxide nanoparticles and melatonin on cyclophosphamide-induced toxicity in testicular histology and sperm parameters in adult Wistar rats. Int J Reprod BioMed 15(7):403–412
Vaseem M, Umar A, Hahn YB (2010) ZnO nanoparticles: growth, properties, and applications of copper oxide and nickel oxide/hydroxide nanostructures. Chapter 2 in book: Metal Oxide Nanostructures and Their Applications. American Scientific Publishers, pp 1–39
Wakabayashi K (1977) Heterogeneity of rat luteinizing hormone revealed by radioimmunoassy and electrofocusing studies. Endocrinol Japon 24:473–485
WHO (2010) laboratory manual for the examination and processing of human semen. 5th edn. Sexual and reproductive health (Updated July 2013). http://www.who.int/mediacentre/factsheets/fs339/en/
Wu YL, Tok AI, Boey FY, Zeng XT, Zhang XH (2007) Surface modification of ZnO nanocrystals. Appl Surf Sci 253:5473–5479
Zhao Y, Tan Y, Dai J, Li B, Guo L, Cui J, Wang G, Shi X, Zhang X, Mellen N, Li W (2011) Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett 200(1):100–106
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohamed, D.A., Abdelrahman, S.A. The possible protective role of zinc oxide nanoparticles (ZnONPs) on testicular and epididymal structure and sperm parameters in nicotine-treated adult rats (a histological and biochemical study). Cell Tissue Res 375, 543–558 (2019). https://doi.org/10.1007/s00441-018-2909-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2909-8