Abstract
Epilepsy is a disorder of the central nervous system characterized by spontaneous recurrent seizures. Although current therapies exist to control the number and severity of clinical seizures, there are no pharmacological cures or disease-modifying treatments available. Use of transgenic mouse models has allowed an understanding of neural stem cells in their relation to epileptogenesis in mesial temporal lobe epilepsy. Further, with the significant discovery of factors necessary to reprogram adult somatic cell types into pluripotent stem cells, it has become possible to study monogenic epilepsy-in-a-dish using patient-derived neurons. This discovery along with some of the newest technological advances in recapitulating brain development in a dish has brought us closer than ever to a platform in which to study and understand the mechanisms of this disease. These technologies will be critical in understanding the mechanism of epileptogenesis and ultimately lead to improved therapies and precision medicine for patients with epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
“People think that epilepsy is divine simply because they don’t have any idea what causes epilepsy. But I believe that someday we will understand what causes epilepsy, and at that moment, we will cease to believe that it’s divine.”
Hippocrates
Introduction
Epilepsy can be traced as far back as early 2nd millennia BC Mesopotamia. At that time, the disease was referred to as the “falling disease” (Engel 2013). Later, it would be called the “sacred disease” due to the overwhelming belief that seizures were a result of possession by gods or evil spirits (Engel 2013). Modern medicine and neuroscience has come quite a distance since then and we now know that epilepsy is a disorder of the central nervous system (CNS) characterized by spontaneous recurring seizures. Risk factors for epilepsy include traumatic brain injury (TBI), stroke, cancer, CNS infection and genetic factors affecting brain structure and development (Engel 2013). One of the most common forms of epilepsy in adults is mesial temporal lobe epilepsy (mTLE). Due to the strong clinical features, proclivity to affect the hippocampus, a region of the brain involved in adult neurogenesis and amenability to epilepsy surgery, models of mTLE have advanced our understanding of the pathophysiology of epilepsy. While anti-seizure medications (ASMs) may reduce seizure frequency, they do not modify the disease to prevent epilepsy occurrence (Engel 2013). However, regardless of the suspected cause, nearly 1/3 of patients do not respond to available therapy. In addition, ASMs have significant side effects and require prescribed daily medication regimens with noncompliance rates ranging from 25 to 75% (Conrad 1985). Epilepsy is a complex disease that not only involves seizures but also causes a significant psychological, social and economic impact on the patients, families and communities that it affects (Jacobs and Jensen 2012).
In the following sections, we will review some of the histological and pathological findings in epilepsy. First, we will explore the role of neural stem cells and adult neurogenesis in rodent mTLE models. Next, we will discuss some of the newest technological advances to model genetic causes of epilepsy, and go into detail about how the use of human-induced pluripotent stem cells (iPSCs) has the potential to dissect mechanisms of epilepsy and screen therapeutics.
Aberrant neurogenesis in the epileptic brain
The generation of new neurons, or neurogenesis, is a process that occurs in two areas of the adult brain, the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampus (Gage 2002; Gould et al. 1999; Gross 2000). Within these regions, multipotent neural stem cells can be found that undergo proliferation and give rise to newborn neurons throughout the life of mammals (Gage 2002; Gross 2000). However, in the case of prolonged seizures, the number of proliferating neural stem cells drastically increases in rodents (Parent et al. 1997; Scott et al. 1998, 2000). Many of these newborn cells will go on to migrate ectopically into the hilar region of the dentate where they form abnormal connections with resident neurons (Fig. 1). Additionally, a portion of these newborn neurons will develop aberrant properties such as hilar basal dendrites, mossy fiber sprouting, and increased dendritic arborization (Jessberger et al. 2007; Parent et al. 1997). Because the hippocampus is the main region of the brain implicated in mTLE, it has been hypothesized that the aberrant neurogenesis seen in this region after seizure may contribute to the development of recurrent seizures in patients with epilepsy (Parent 2002; Parent and Lowenstein 2002). Further, the hippocampus plays a significant role in learning and memory, mood regulation and resistance to depression. A dysfunctional hippocampus may lead to the associated behavioral comorbidities of mTLE, including depression, anxiety and memory deficits (Danzer 2012; Deng et al. 2010; Eisch and Petrik 2012; Zhao et al. 2008). In the next section, we will further discuss how acute seizures affect new, developing, and mature neurons and what effect each may have on the development of recurring seizures.
Neural stem cells or mature neurons: who poisoned the well?
Adult neurogenesis is a transient process by which neural stem cells are “born”, differentiate and eventually integrate into the existing circuitry of the dentate gyrus. There is evidence to suggest a significant role for the newborn stem cells in the development of epilepsy. Animals in which neurogenesis is reduced or ablated in the kindling model of seizure induction using ionizing radiation targeted to the hippocampus have a lower seizure threshold, suggesting that the surviving neurons in this region are more hyperexcitable and contribute to the development of epilepsy (Althaus et al. 2015; Jenrow et al. 2001; Raedt et al. 2007). Further, cells born up to 1 week before the initial seizures can develop aberrant dendrites into the hilus when exposed to kainic acid, a potent neurotoxic glutamic acid analogue used to induce acute seizures in rodents (Jessberger et al. 2007). Many of the changes that occur to newborn neurons in the hippocampus after seizure—ectopic migration, hilar basal dendrite formation, mossy fiber sprouting and increased excitability—have been hypothesized to create a recurrent excitatory loop in the dentate leading to impaired gating function of information entering the hippocampal circuit from the cortex resulting in spontaneous seizures (Jessberger and Parent 2015). Earlier studies using non-specific approaches to ablate dividing cells prior to induction of status epilepticus in the dentate led to an attenuation in the number of seizures (Jung et al. 2004, 2006). More recently, using a transgenic mouse model, selective ablation of neurogenesis prior to induction of acute seizures using the pilocarpine mouse model of chronic mTLE, led to a 40–50% reduction in the total number of seizures (Cho et al. 2015; Hosford et al. 2016). In this work, ablation of neurogenesis prior to acute seizures reduced the total number of aberrant and ectopically migrating new neurons, providing evidence that there is a pool of susceptible newborn cells at the time of the initial insult that go on to mature and drive epilepsy progression (Cho et al. 2015; Hosford et al. 2016).
The factors driving epileptogenesis are not completely accounted for by aberrant neurogenesis, as near-complete ablation prior to acute seizures does not prevent the development of spontaneous recurrent seizure activity. Retroviral labeling reveals significant mossy fiber plasticity amongst the mature neuron population in the hippocampus in the pilocarpine model of epilepsy (Althaus et al. 2016). In addition, many studies demonstrate a significant loss of mature GABAergic neurons from the dentate circuitry in various models of rodent epilepsy. Loss of inhibitory GABAergic neurons is hypothesized to make the hippocampus more permissible to excitatory input (Ekdahl et al. 2003; Houser 2014; Pollard et al. 1994). Further, increased innervation of the CA2 region of the hippocampus after seizure bypasses inhibitory neurons that help suppress excess activity flowing into the dentate. Together, these findings suggest an important role for not only newborn neurons of the hippocampus but also mature neurons.
Monogenetic epilepsy models
In 6 out of 10 cases of epilepsy, the cause is unknown, suggesting that genetics may play an underlying role in epileptogenesis. Epidemiological studies have long confirmed the heritable nature of epilepsy, specifically the idiopathic generalized epilepsies (Peljto et al. 2014). Since the completion of the human genome project in 2001 (Lander et al. 2001; Venter et al. 2001), there has been a rapid discovery of monogenic causes of epilepsy. Monogenic epilepsy now likely represents approximately 10–20% of medically refractory epilepsies in childhood and this will expand with the discovery of new genetic variants and the increasing availability and affordability of genetic sequencing (Noebels 2015; Wang et al. 2014). Indeed, our discovery of these monogenic causes of epilepsy has outpaced our scientific resources to study how these genetic mutations cause disease and our clinical capacity to provide specific therapeutics. Genetics is the new frontier in understanding mechanisms of epileptogenesis and developing precision medicine in epilepsy.
The most common monogenic epilepsies occur in children, often starting within the first year of life. These children are more likely to exhibit additional CNS problems such as neurodevelopmental delays, autism spectrum disorders, and intellectual disability. There are many challenges in treating children with monogenic epilepsy, as seizures in this group are commonly resistant to ASMs and, in some cases, treatment may have neurodevelopmental side effects and contribute to behavioral issues. However, in spite of a desperate need for improved therapies, as of 2015, less than half of all known genetic epilepsies have a validated in vivo or in vitro model (EpiPM Consortium 2015). One example of a monogenetic epilepsy is children with KCNQ2 gene mutations, which codes for a voltage-gated potassium channel (Singh et al. 1998). Mutations in KCNQ2 have been associated with epileptic encephalopathy with intractable neonatal seizures and Ohtahara syndrome (Weckhuysen et al. 2012). In vitro models of KCNQ2 mutations using hippocampal pyramidal cells have shown increased cell firing frequency and hyperexcitability (Miceli et al. 2013). Retigabine is an ASM that targets the voltage-gated potassium channel Kv7.2/Kv7.3, encoded in part by KCNQ2. In rodent models, administration of retigabine decreased kainic acid-induced seizure activity in Kcnq2 knock-in mice when compared to phenobarbital (Ihara et al. 2016). However, while this is promising pre-clinical data, translation to humans has been challenging. While Retigabine (also known as ezogabine in the U.S.) is approved for use in adults with focal seizures, there are limited clinical data on its use in children. Small open label trials have shown some benefit; however, larger blinded prospective studies are needed (Millichap et al. 2016). Retigabine will no longer be available clinically after June 30, 2017; however, related compounds are in early development. In addition, developing human-specific pre-clinical models may lead to better screening of compounds directed to the Kv7.2/Kv7.3 receptor, possibly advancing the path to precision medicine in patients affected by KCNQ2 mutations.
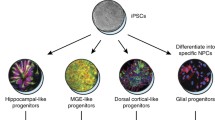
Awarded the 2012 Nobel Prize, the discovery that somatic cells can be reprogrammed into iPSCs has profoundly altered the landscape of human specific models for studying disease (Takahashi and Yamanaka 2006) (Fig. 2). It is important to emphasize, however, that iPSC science is still a very young field, with many unanswered questions regarding the biology and function of these special cells. Recent work with iPSCs has led to new in vitro models for many neurological diseases. There are investigations using human-derived iPSCs to model anorexia nervosa, Alzheimer’s disease and neurodevelopmental diseases (Jones et al. 2017; Negraes et al. 2017; Tidball and Parent 2016). Experiments by Parent and colleagues successfully modeled Dravet syndrome using neurons in two-dimensional cell culture and postulated a novel mechanism of SCN1A mediated epilepsy (Tidball and Parent 2016). Dravet syndrome is most commonly associated with a loss of function mutation in SCN1A, encoding for the sodium channel, Nav1.1. The predominating hypothesis of SCN1A mutation pathogenesis is the “interneuron hypothesis” where epileptogenesis is due to decreased sodium current and loss of excitatory drive within GABAergic interneurons leading to generalized hyperexcitability (Escayg and Goldin 2010). In vitro iPSC models have challenged this hypothesis, showing that pyramidal neurons and bipolar interneurons are both unexpectedly hyperexcitable. These neurons show an increased sodium current and measureable repetitive spontaneous firing and bursting activity, indicating an epileptic phenotype in a dish (Liu et al. 2013). Another study, by Dolmetsch and colleagues, supported the interneuron hypothesis of Dravet syndrome. In this work, iPSCs were used to study Dravet syndrome and led to the discovery of deficits in sodium currents and action potential firing only in inhibitory neurons while excitatory neurons were functionally normal (Sun et al. 2016). Elucidating the mechanisms of childhood epilepsy will bring useful tools in analyzing epileptogenesis and may lead to precision medicine for genetic epilepsy syndromes (Du and Parent 2015). Understanding the fundamental changes perturbed by these monogenic epilepsies may in turn allow for the development of small molecule targets against epileptogenesis and other therapeutic or diagnostic possibilities.
Schematic illustrating utility of human induced pluripotent stem cells (iPSCs) in driving precision medicine for epilepsy: (1) patient recruitment, (2) reprogramming of human iPSCs from patient blood or fibroblasts, (3) CRISPR/Cas9 gene editing to obtain isogenic controls, (4) in vitro culture of human iPSC-derived neurons or organoids and (5) drug screening or diagnostic testing using epilepsy-in-a-dish model
Organoids and CRISPR/Cas9 to study genetic epilepsy syndromes
With approaches in human embryonic stem cells such as homologous recombination and gene-editing technologies such as TALENs and CRISPR/Cas9, it is now possible to investigate monogenetic human diseases with relative ease. Südhof and colleagues successfully studied neurodevelopmental diseases related to SHANK3 haplo-insufficiency using homologous recombination and CRISPR/Cas9 to mutagenize the SHANK3 gene in human H1 embryonic stem cells (Yi et al. 2016). In these experiments, conditional knock-out neurons with matching controls from the same ES cell clones reduced subclone-to-subclone variability. In their study, combined electrophysiology and immunostaining experiments demonstrated that SHANK3 haplo-insufficiency impairs intrinsic neuronal properties and HCN channel function, which is commonly associated with epilepsy and intellectual disability. Other studies using iPSCs from patients with amyotrophic lateral sclerosis and Huntington’s disease have been conducted using isogenic controls through targeted CRISPR/Cas9-mediated gene correction (Wang et al. 2017; Xu et al. 2017). Further studies utilizing patient-derived iPSCs with isogenic controls created by CRISPR/Cas9 will allow for elucidation of the neurobiology of many monogenic diseases for which there is no validated experimental model. In addition, there are efforts to use CRISPR/Cas9 therapeutically in patients to correct causative gene defects; however, delivering this technology across the blood–brain barrier and directing it to specific target cell types are areas of ongoing research (Keener 2015).
Modeling of CNS disease is also possible in three-dimensional cultures called cerebral “organoids” (Fig. 3). Several groups have created cerebral organoids, which retain structural and cellular features reminiscent of cerebral regions, including the neocortex and hippocampus (Lancaster and Knoblich 2014; Lancaster et al. 2013). Previous work in the field has validated the use of organoids in studying normal human brain development, as well as structural defects like lissencephaly and the pathogenesis of congenital Zika syndrome (Bershteyn et al. 2017; Garcez et al. 2016; Matsui et al. 2017). Exciting new work from Paşca and colleagues shows that organoids can be used to evaluate the developmental characteristics of autism and other neurodevelopmental disorders such as epilepsy (Birey et al. 2017). In this study, using patient-derived iPSCs with Timothy syndrome, a rare monogenetic cause of autism, cortical spheroids (a specific type of cerebral organoid) enriched for glutamatergic and GABAergic neural progenitors and neurons were fused, revealing an in vitro phenotype of abnormal interneuron migration. Using advanced techniques, such as single cell RNA sequencing, live cell imaging and pharmacologic rescue, this phenotype was determined to be cell-autonomous to the cultured GABAergic neurons. Additionally, organoids are amenable to electrophysiological experiments by means of slice physiology seen in organotypic culture models or using multielectrode arrays (MEAs) to record network activity of the organoid. Looking to the future, it may one day be possible to screen drugs and other small molecules by utilizing patient cells grown in cerebral organoid culture. While in their relative infancy, these powerful new techniques hold great promise in the evaluation of human disease and will pave the way for precision therapy for epilepsy.
Future outlook
Use of in vivo rodent models in tandem with in vitro human-derived iPSC models represents the future of epilepsy research. Rodent models have elucidated many of the aspects of the environment of the epileptic brain. Due to the widespread availability of modified transgenic rodents, a variety of approaches exist to ask fundamental questions about the underlying pathophysiology of the epileptic brain. Rodent models have also been very useful in providing a better understanding of how various therapeutic interventions may affect behavioral co-morbidities associated with epilepsy, such as anxiety, depression and memory loss. Recently, with the development of several key technological advances, we are now also able to model epilepsy-in-a-dish. By directly harvesting human cells and using CRISPR/Cas9 technology to edit the genetic code, we are now entering a new era of personalized medicine where we are able to study the electrophysiological and histological changes that occur in patients with epilepsy without requiring human neural tissue, which is now only available from surgical specimens and autopsies. Looking to the future, both in vivo rodent models and in vitro patient-derived iPSC models will be critical to our understanding of different aspects of epilepsy and other neurodevelopmental disorders. Each of these models is likely to continue to contribute significantly to our understanding of epilepsy and will be complimentary in reaching our ultimate goal, a cure for epilepsy.
Conclusions
Epilepsy is a complex neurobiological condition characterized by recurrent seizures and significant co-morbidities. The role of neural stem cells in epilepsy is well described in mTLE models, but the exact contribution of aberrant neurogenesis to epileptogenesis is evolving. Moreover, there has been a dearth of translational therapies developed based on our current understandings of epilepsy. IPSC models of monogenic epilepsy provide a human-specific model, which has great potential to elucidate the biology of epilepsy and affect the development of new therapies and strategies to impact the lives of patients with epilepsy.
References
Althaus A, Sagher O, Parent J, Murphy G (2015) Instrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model. J Neurophysiol 113(4):1184–1194. doi:10.1152/jn.00835.2014
Althaus AL, Zhang H, Parent JM (2016) Axonal plasticity of age-defined dentate granule cells in a rat model of mesial temporal lobe epilepsy. Neurobiol Dis 86:187–196. doi:10.1016/j.nbd.2015.11.024
Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, Kriegstein AR (2017) Human iPSC-derived cerebral Organoids model cellular features of Lissencephaly and reveal prolonged mitosis of outer radial Glia. Cell Stem Cell. doi:10.1016/j.stem.2016.12.007
Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O’Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Pasca SP (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545(7652):54–59. doi:10.1038/nature22330
Cho K, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J (2015). Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun, 26(6):6606. doi: 10.1016/j.expneurol.2017.04.005
Conrad P (1985) The meaning of medications: another look at compliance. Soc Sci Med 20(1):29–37
Danzer SC (2012) Depression, stress, epilepsy and adult neurogenesis. Exp Neurol 233(1):22–32. doi:10.1016/j.expneurol.2011.05.023
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogensis affect learning and memory? Nat Rev Neurosci 11:339–350. doi:10.1038/nrn2822
Du X, Parent JM (2015) Using patient-derived induced Pluripotent stem cells to model and treat epilepsies. Curr Neurol Neurosci Rep 15(10):71. doi:10.1007/s11910-015-0588-3
Eisch A, Petrik D (2012) Depression and Hippocampal Neurogenesis: a road to remission? Science 338(6103):72–75. doi:10.1126/science.1222941
Ekdahl CT, Zhu C, Bonde S, Bahr B, Blomgren K, Lindvall O (2003) Death mechanisms in status epilepticus-generated neurons and effects of additional seizures on their survival. Neurobiol Dis 14(3):513–523
EpiPM Consortium (2015) A roadmap for precision medicine in the epilepsies. Lancet Neurol 14(12):1219–1228. doi:10.1016/S1474-4422(15)00199-4
Escayg A, Goldin AL (2010) Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51(9):1650–1658. doi:10.1111/j.1528-1167.2010.02640.x
Gage FH (2002) Neurogenesis in the adult brain. J Neurosci 22(3):612–613
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352(6287):816–818. doi:10.1126/science.aaf6116
Gould E, Reeves AJ, Graziano MSA, Gross CG (1999) Neurogenesis in the neocortex of adult primates. Science 286(5439):548–552
Gross C (2000) Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci 1:67–73. doi:10.1038/35036235
Hosford BE, LIska JP, Danzer SC (2016) Ablation of newly generated hippocampal granule cells has disease modifying effects in epilepsy. J Neurosci 36(43):11013–11023. doi:10.1523/JNEUROSCI.1371-16.2016
Houser CR (2014) Do structural changes in GABA neurons give rise to the epileptic state. Adv Exp Med Biol 813:151–160. doi:10.1007/978-94-017-8914-1_12
Ihara Y, Tomonoh Y, Deshimaru M, Zhang B, Uchida T, Ishii A, Hirose S (2016) Retigabine, a Kv7.2/Kv7.3-channel opener, attenuates drug-induced seizures in knock-in mice harboring Kcnq2 mutations. PLoS ONE 11(2):e0150095. doi:10.1371/journal.pone.0150095
Jacobs M, Jensen FE (2012) Introduction to institute of medicine report: epilepsy across the spectrum: promoting health and understanding. Epilepsy Curr 12(6):243–244. doi:10.5698/1535-7511-12.6.243
Jenrow KA, Ratkewicz AE, Elisevich KV (2001) Ehanced excitability induced by ionizing radiation in the kindled rat. Exp Neurol 169(1):96–104. doi:10.1006/exnr.2000.7616
Engel J (2013) Seizures and Epilepsy 2nd edition. Oxford University Press, New York
Jessberger S, Parent JM (2015) Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol 7:1–10. doi:10.1101/cshperspect.a020677
Jessberger S, Zhao C, Toni N, Clemenson GD, Li Y, Gage FH (2007) Seizure-associated, aberrant Neurogenesis in adult rat Characterizedd with retrovirus-mediated cell labeling. J Neurosci 27(35):9400–9407. doi:10.1523/JNEUROSCI.2002-07.2007
Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L (2017) Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis 8(3):e2696. doi:10.1038/cddis.2017.89
Jung KH, Chu K, Kim J, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK (2004) Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenutation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci 19(12):3219–3226. doi:10.1111/j.0953-816X.2004.03412.x
Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK (2006) Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spotaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 23(2):237–246. doi:10.1016/j.nbd.2006.02.016
Keener AB (2015) Delivering the goods: scientists seek a way to make CRISPR-Cas gene editing more targeted. Nat Med 21(11):1239–1241. doi:10.1038/nm1115-1239
Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9(10):2329–2340. doi:10.1038/nprot.2014.158
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501(7467):373–379. doi:10.1038/nature12517
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, Szustakowki J, International Human Genome Sequencing Consortium (2001) Initial sequencing and analysis of the human genome. Nature 409(6822):860–921. doi:10.1038/35057062
Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O’Malley HA, Patino GA, O’Brien JE, Rusconi R, Gupta A, Thompson RC, Natowicz MR, Meisler MH, Isom LL, Parent JM (2013) Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol 74(1):128–139. doi:10.1002/ana.23897
Matsui T, Nieto-Estevez V, Kyrychenko S, Schneider JW, Hsieh J (2017) Retinoblastoma protein controls growth, survival and neuronal migration in human cerebral organoids. Development 144(6):1025–1034. doi:10.1242/dev.143636
Miceli F, Soldovieri MV, Ambrosino P, Barrese V, Migliore M, Cilio MR, Taglialatela M (2013) Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits. Proc Natl Acad Sci U S A 110(11):4386–4391. doi:10.1073/pnas.1216867110
Millichap JJ, Park KL, Tsuchida T, Ben-Zeev B, Carmant L, Flamini R, Joshi N, Levisohn PM, Marsh E, Nangia S, Narayanan V, Ortiz-Gonzalez XR, Patterson MC, Pearl PL, Porter B, Ramsey K, McGinnis EL, Taglialatela M, Tracy M, Tran B, Venkatesan C, Weckhuysen S, Cooper EC (2016) KCNQ2 encephalopathy: features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol Genet 2(5):e96. doi:10.1212/NXG.0000000000000096
Negraes PD, Cugola FR, Herai RH, Trujillo CA, Cristino AS, Chailangkarn T, Muotri AR, Duvvuri V (2017) Modeling anorexia nervosa: transcriptional insights from human iPSC-derived neurons. Transl Psychiatry 7(3):e1060. doi:10.1038/tp.2017.37
Noebels J (2015) Pathway-driven discovery of epilepsy genes. Nat Neurosci 18(3):344–350. doi:10.1038/nn.3933
Parent JM (2002) The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res 50(1–2):179–189
Parent JM, Lowenstein DH (2002) Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res 135:121–131. doi:10.1016/S0079-6123(02)35012-X
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell Neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17(10):3727–3738
Peljto AL, Barker-Cummings C, Vasoli VM, Leibson CL, Hauser WA, Buchhalter JR, Ottman R (2014) Familial risk of epilepsy: a population-based study. Brain 137(Pt 3):795–805. doi:10.1093/brain/awt368
Pollard H, Charriaut-Marlangue C, Cantagrel S, Represa A, Robain O, Moreau J, Ben-Ari Y (1994) Kainate-induced apoptotic cell death in the hippocampal neurons. Neuroscience 63(1):7–18
Raedt R, Boon P, Persson A, Alborn A-M, Boterberg T, Van Dycke A, Linder B, De Smedt T, Wadman WJ, Ben-Menachem E, Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (2007) Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia 48(10):1952–1963. doi:10.1111/j.1528-1167.2007.01146.x
Scott BW, Wang S, Burnham WM, De Boni U, Wojtowicz JM (1998) Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett 248(2):73–76
Scott BW, Wojtowicz JM, Burnham WM (2000) Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol 165(2):231–236. doi:10.1006/exnr.2000.7458
Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M (1998) A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet 18(1):25–29. doi:10.1038/ng0198-25
Sun Y, Pasca SP, Portmann T, Goold C, Worringer KA, Guan W, Chan KC, Gai H, Vogt D, Chen Y-JJ, Mao R, Chan K, Rubenstein JL, Madison DV, Hallmayer J, Froehlich-Santino WM, Bernstein JA, Dolmetsch RE (2016). A deleterious Nav1.1 mutation selectively impairs telencephalic inhibitory neurons derived from Dravet Syndrome patients. eLife 5. doi: 10.7554/eLife.13073
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024
Tidball AM, Parent JM (2016) Concise review: exciting cells: modeling genetic epilepsies with patient-derived induced Pluripotent stem cells. Stem Cells 34(1):27–33. doi:10.1002/stem.2203
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X (2001) The sequence of the human genome. Science 291(5507):1304–1351. doi:10.1126/science.1058040
Wang J, Gotway G, Pascual JM, Park JY (2014) Diagnostic yield of clinical next-generation sequencing panels for epilepsy. JAMA Neurol 71(5):650–651. doi:10.1001/jamaneurol.2014.405
Wang L, Yi F, Fu L, Yang J, Wang S, Wang Z, Suzuki K, Sun L, Xu X, Yu Y, Qiao J, Belmonte JCI, Yang Z, Yuan Y, Qu J, Liu GH (2017) CRISPR/Cas9-mediated targeted gene correction in amyotrophic lateral sclerosis patient iPSCs. Protein Cell 8(5):365–378. doi:10.1007/s13238-017-0397-3
Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, Berkovic SF, Scheffer IE, de Jonghe P (2012) KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol 71(1):15–25. doi:10.1002/ana.22644
Xu X, Tay Y, Sim B, Yoon SI, Huang Y, Ooi J, Utami KH, Ziaei A, Ng B, Radulescu C, Low D, Ng AY, Loh M, Venkatesh B, Ginhoux F, Augustine GJ, Pouladi MA (2017) Reversal of phenotypic abnormalities by CRISPR/Cas9-mediated gene correction in Huntington disease patient-derived induced Pluripotent stem cells. Stem Cell Rep 8(3):619–633. doi:10.1016/j.stemcr.2017.01.022
Yi F, Danko T, Botelho SC, Patzke C, Pak C, Wernig M, Sudhof TC (2016) Autism-associated SHANK3 haploinsufficiency causes Ih channelopathy in human neurons. Science 352(6286):aaf2669. doi:10.1126/science.aaf2669
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult Neurogenesis. Cell 132(4):645–660. doi:10.1016/j.cell.2008.01.033
Acknowledgements
We thank Jose Cabrera for help with the figures. This work was supported by grants from the National Institute of Health (NIH) R01NS081203, R01NS089770, R01NS093992 and K02AG041815 to J.H., Department of Defense W81XWH-15-1 to J.H., American Heart Association 15GRNT25750034 to J.H., a grant from the Texas Institute for Brain Injury and Repair to J.H. and an award from the National Center for Advancing Translational Sciences (NIH) UL1TR001105 to D.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thodeson, D.M., Brulet, R. & Hsieh, J. Neural stem cells and epilepsy: functional roles and disease-in-a-dish models. Cell Tissue Res 371, 47–54 (2018). https://doi.org/10.1007/s00441-017-2675-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2675-z