Abstract
Porcine models are useful for investigating therapeutic approaches to short bowel syndrome and potentially to intestinal stem cell (ISC) transplantation. Whereas techniques for the culture and genetic manipulation of ISCs from mice and humans are well established, similar methods for porcine stem cells have not been reported. Jejunal crypts were isolated from murine, human, and juvenile and adult porcine small intestine, suspended in Matrigel, and co-cultured with syngeneic intestinal subepithelial myofibroblasts (ISEMFs) or cultured without feeder cells in various culture media. Media containing epidermal growth factor, noggin, and R-spondin 1 (ENR medium) were supplemented with various combinations of Wnt3a- or ISEMF-conditioned medium (CM) and with glycogen synthase kinase 3 inhibitor (GSK3i), and their effects were studied on cultured crypts. Cell lineage differentiation was assessed by immunohistochemistry and quantitative polymerase chain reaction. Cultured porcine cells were serially passaged and transduced with a lentiviral vector. Whereas ENR medium supported murine enteroid growth, it did not sustain porcine crypts beyond 5 days. Supplementation of Wnt3a-CM and GSK3i resulted in the formation of complex porcine enteroids with budding extensions. These enteroids contained a mixture of stem and differentiated cells and were successfully passaged in the presence of GSK3i. Crypts grown in media supplemented with porcine ISEMF-CM formed spheroids that were less well differentiated than enteroids. Enteroids and spheroids were transfected with a lentivirus with high efficiency. Thus, our method maintains juvenile and adult porcine crypt cells long-term in culture. Porcine enteroids and spheroids can be successfully passaged and transduced by using lentiviral vectors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent discoveries of mechanisms that regulate intestinal epithelial stem cell (ISC) growth and differentiation have made possible the long-term in vitro culture and expansion of mouse and human intestinal epithelium (Sato et al. 2009, 2011a; Yin et al. 2014). Essential signaling pathways for the maintenance and differentiation of cultured intestinal epithelial stem cells have been described, and the importance of the stem cell niche is well recognized (Sato et al. 2011b). In particular, intestinal subepithelial myofibroblasts (ISEMFs) have been shown to be critical players in the dynamic interplay between the epithelium and mesenchyme (Lahar et al. 2011; Yeung et al. 2011; Lei et al. 2014). However, although our in vitro armamentarium for interrogating ISC physiology is highly developed, preclinical utilization of intestinal tissue culture techniques in large animals has encountered many challenges and most studies published to date remain centered around rodent models (Yui et al. 2012; Watson et al. 2014).
In order to improve the translational use of ISCs, data are needed from in vivo experiments performed in large animal models, which better predict the clinical response in human patients (Harding et al. 2013). This requires the in-depth characterization of large animal models, including stem cell physiology, cell lineage identification, and development of a robust culture system that might differ from the well-studied rodent systems. Pigs have been widely used for preclinical studies focused on intestinal physiology (Zhang et al. 2013) and intestinal pathology (Stoltz et al. 2013; Linard et al. 2013). Porcine treatment models for short bowel syndrome have been established, and these models might also prove useful as large animal systems to investigate and develop approaches to intestinal tissue engineering (Agopian et al. 2009; Sala et al. 2009). A recent study in neonatal piglets characterized the intestine of newborn animals and demonstrated successful intestinal culture (Gonzalez et al. 2013). However, studies in our laboratory have shown that the culture conditions described for neonatal piglet intestinal crypts are not suitable to maintain juvenile or adult porcine crypts. Here, we report investigations that make it possible to culture adult porcine crypt cells long-term and to transduce these cells successfully with lentiviral vectors.
Materials and methods
Porcine crypt isolation and culture
Two-cm segments of mid-jejunum were isolated from 10- to 14-week-old Yorkshire pigs (n = 19) and 4- to 8-week-old mini-Yucatan pigs (n = 7). Each segment was placed in ice-cold phosphate-buffered saline (PBS). The muscle layers were peeled away with forceps and the segment was cut open to expose the lumen. The mucosal surface was scraped with a glass coverslip to remove the villi. The segment was cut into 5 × 5 mm pieces and repeatedly washed in PBS until the supernatant was clear. Epithelial isolation was performed by incubating the tissue in 10 mM ethylenediaminetetraacetic acid (EDTA; Sigma, St. Louis, Mo., USA) and 1 mM dithiothreitol (DTT, Sigma) in PBS for 30 min at 4 °C on a rocker at 50 rpm. The supernatant containing villi and debris was decanted and discarded, fresh PBS was added, the tube was vortexed thrice for 10 s and the supernatant containing crypts was collected. This was repeated until six fractions were collected. The fractions were centrifuged at 200g for 2 min at 4 °C and then resuspended in PBS with 10 % fetal bovine serum (FBS; Invitrogen, Carlsbad, Calif., USA). The fractions were combined and filtered through a 100-μm strainer, centrifuged at 200g for 2 min at 4 °C and suspended in basic medium consisting of 1× antibiotic-antimycotic solution (ABAM), 2 mM glutaMAX and 10 mM HEPES buffer in 5 ml advanced DMEM/F-12 (all from Invitrogen).
For crypt culture, crypts were pelleted by three quick spins, resuspended in growth-factor-reduced Matrigel (BD Biosciences, San Jose, Calif., USA) and plated in 0.95 cm2 culture plate wells (Thermo Scientific, Waltham, Mass., USA). After a 15-min incubation period at 37 °C, we added 250 μl culture medium, which consisted of basic medium (see above) with the addition of 1 mM N-acetylcysteine (Sigma), 1× N2 supplement (Invitrogen), 1× B27 supplement (Invitrogen), with or without 50 ng/ml recombinant murine epidermal growth factor (EGF; Peprotech, Rocky Hill, N.J., USA), 100 ng/ml recombinant murine noggin (Peprotech), and 500 ng/ml recombinant human R-spondin 1 (R&D Systems, Minneapolis, Minn., USA). This mixture was termed “ENR medium.” In addition, we tested the effects of supplementation with ISEMF- or Wnt3a-conditioned medium (CM), 100 ng/ml recombinant human fibroblast growth factor 10 (FGF10; Peprotech), 1 mM valproic acid (VPA, Sigma), 10 mM nicotinamide (Sigma), 2.5 μM glycogen synthase kinase 3 inhibitor (GSK3i, CHIR99021; Stemgent, Cambridge, Mass., USA), 10 μM p160ROCK inhibitor (Y27632; Stemgent), 10 μM p38 mitogen-activated protein (MAP) kinase inhibitor (SB202190; Sigma) and 500 nM transforming growth factor β (TGFβ) receptor inhibitor (LY2157299; Selleck, Houston, Tex., USA). Colony-forming efficiency (CFE) was determined by the number of live structures in each well on culture day 7 versus day 1.
ISEMF culture and CM collection
Pericryptal ISEMFs were isolated from porcine jejunum (n = 10) as previously described for other mammalian species (Lahar et al. 2011). ISEMFs were cultured in low glucose DMEM with glutaMAX (Invitrogen), with 10 % FBS, 1× penicillin/streptomycin, 20 ng/ml EGF, 0.25 U/ml insulin and 10 mg/ml transferrin in 6-well plates (Corning, Tewksbury, Mass., USA). Once confluent, they were detached with 0.25 % trypsin and 1 mM EDTA, transferred, and cultured in T25 or T75 tissue culture flasks (Corning). For co-culture experiments, ISEMFs were split and plated 2 days prior to co-culture at a seeding density of 37,500 myofibroblasts per 0.95 cm2 well. For CM collection, ISEMF culture medium was collected after a 7-day incubation on confluent ISEMFs and mixed 1:1 with ENR medium, nicotinamide, Y27632, SB202190 and LY2157299. This was termed “spheroid medium.”
Subculture technique
To passage enteroids, Matrigel was digested with 0.6 mg/ml dispase (Invitrogen) in PBS at 37 °C for 10 min with gentle inversion every 2 min. After 3 quick spins, the supernatant was removed and the pellet was resuspended in PBS, passed thrice through a 25-gauge needle (BD Biosciences) and spun down. The pellet was then resuspended in Matrigel with or without 1 μM prostaglandin E2 (PGE2; Sigma) or 1 μM Jagged-1 (Jag1; AnaSpec, Fremont, Calif., USA) and plated. Culture medium after passage consisted of ENR medium with the addition of 25 % Wnt3a-CM, nicotinamide, CHIR99021, Y27632, SB202190 and LY2157299 (“enteroid medium”) and was used with or without the addition of VPA.
Murine and human cultures
In reference studies, murine intestinal crypt three-dimensional culture was carried out as described previously by using 8- to 12-week-old male and female C57BL/6 J mice (n = 9; Jabaji et al. 2013; Lei et al. 2014). Human crypts were isolated from adult duodenal samples (n = 9) as previously described (Lahar et al. 2011). Mouse and human crypts were either co-cultured on confluent mouse and human ISEMFs, respectively, or cultured without feeder cells. Co-cultures were supplemented with ENR medium. Without feeder cells, crypts were grown either in ENR medium alone or with the addition of Wnt3a-CM (1:1) or 1 μM PGE2.
Culture temperatures
Murine, human and porcine crypts were cultured in a 5 % CO2 incubator at 37 °C. To mimic the physiologic temperature of weaned pigs (39.3 ± 0.3 °C; Dewey and Straw 2006), cultures were also carried out at 39 °C. Enteroids were grown in standard enteroid medium and assessed on culture day 6 for morphology and gene expression. The ability to subculture enteroids was assessed at both temperatures.
Cryo-preservation of isolated crypts
To assess freezing as an option for long-term storage, freshly isolated porcine jejunal crypts were suspended in 1 ml freeze-preservation medium consisting of 40 % FBS and 10 % dimethylsulfoxide in 1× culture medium with no growth factors and were snap-frozen. The cryovials were stored for 2 weeks at −80 °C. For culture, crypts were rapidly thawed at 37 °C (n = 5), mixed with 10 ml cold PBS in a 15-ml conical tube and spun down at 100g and 4 °C for 10 min. The pellet was resuspended in Matrigel and plated as described above.
Transduction of porcine cells
Spheroids and enteroids were transduced with a non-concentrated pRRL-CMV-GFP lentivirus based on a modification of a previously described technique (Koo et al. 2012). In brief, spheroids and enteroids were subcultured as above and suspended in 50 μl culture medium with 8 μg/ml hexadimethrine bromide (Sigma). The lentivirus suspension was added to the dissociated structures in a 1:3 ratio by volume and spun down at 600g and 34 °C for 1 h. Following a 4-h incubation at 37 °C, the cells were resuspended in Matrigel and plated. At 24 h later, structures were split into single cells with dispase as above, followed by TrypLE and then pipetted 20 times. Transduction efficiency was quantified with a flow cytometer (LSR II, BD Biosciences) and compared with untransduced controls.
Histology and immunohistochemistry
Intestinal tissue was fixed overnight in 10 % neutral buffered formalin and stored in 70 % ethanol until further histologic processing. Intestinal cultures were fixed in 10 % buffered formalin for 5 min, then resuspended in 30 μl preheated Histogel (American MasterTech) and stored in ethanol as above. Specimens were embedded in paraffin and sectioned at 3 μm thickness. Hematoxylin and eosin staining and immunohistochemistry (IHC) were performed per standard protocol (Lahar et al. 2011; Jabaji et al. 2013). Staining was carried out with antibodies against CD10 (56C6, Dako, Carpinteria, Calif., USA), lysozyme (ab74666, Abcam, Cambridge, Mass., USA), chromogranin A (20086, Immunostar, Hudson, Wis, USA; Gonzalez et al. 2013), villin (1D2C3, Dako), E-cadherin (NCH-38, Dako), β-catenin (β-Catenin-1, Dako), p120-catenin (98, Ventana, Tucson, Ariz., USA) and cytokeratin 8 and 18 (EP17/EP30, Dako). Goblet cells were identified with the periodic acid-Schiff stain (Brown et al. 1988). Porcine ISEMFs were plated in 0.95 cm2 culture wells, fixed as above and stained with antibodies against α-smooth muscle actin (α-SMA, M0851, Dako), desmin (M0760, Dako) and vimentin (ab92547, Abcam). For immunofluorescent stains, conjugated goat secondary antibody (Invitrogen) was added at a 1:200 dilution and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen).
Quantitative polymerase chain reaction
RNA was extracted with the RNeasy mini-kit (Qiagen) and cDNA was constructed with SuperScript III reverse transcriptase (Invitrogen). The polymerase chain reaction (PCR) was performed by using the SYBR green RT-PCR reagent kit (Invitrogen) and a real-time PCR thermal cycler (Applied Biosystems, Carlsbad, Calif., USA). PCR product quality was assessed with DNA electrophoresis. Primer sequences are shown in Table 1. Cycle numbers were normalized to beta-glucuronidase (Gusb) and to whole porcine jejunum.
Statistical analysis
Statistical analysis was performed by using the one-tailed Student’s t-test assuming equal variance. P < 0.05 was regarded as significant.
Ethics statement
The animal research committee at UCLA approved the use of murine and porcine tissue in this study (Protocol nos. 2005-169 and 2013–007, respectively). The UCLA Institutional Review Board (IRB) approved procurement of de-identified human duodenal surgical specimens from the UCLA Translational Pathology Core Laboratory (Protocol no. 11-002778); the IRB waived the need for consent and approved the entire study.
Results
Comparison of murine, human, and porcine intestinal cultures
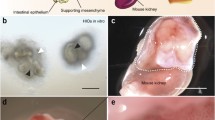
In co-culture with ISEMFs, the growth of murine, human and juvenile or adult porcine crypts was supported by ENR medium; cultured crypts in all systems took on a combination of enteroidal, cystic and flat morphologies (Fig. 1a-c). In the absence of ISEMFs, ENR medium was sufficient for budding enteroids to form from murine crypts (Sato et al. 2009) but not from human or juvenile and adult porcine crypts (Fig. 1d-f). The addition of Wnt3a-CM to ENR resulted in the formation of spheroids from murine but not human or porcine crypts (Fig. 1g-i). The addition of PGE2 to ENR supported murine and human crypt cultures, producing a mixture of spheroids and small enteroids but was insufficient for the growth of porcine crypts (Fig. 1j-l). The addition to ENR medium of FGF10 or nicotinamide and the small molecule inhibitors of p160ROCK (Y27632), p38 MAP kinase (SB202190) and TGFβ receptor (LY2157299; collectively “ENRnYSL medium”) resulted in CFE <10 % in the juvenile and adult porcine system (Fig. 1m). The addition of CHIR99021, a selective GSK3i, to ENR medium dramatically improved CFE to 62.5 % ± 33.5 % (P = 0.039 vs. ENRnYSL medium). This improvement in CFE was more consistent when CHIR99021 was added to ENRnYSL medium with the 1:1 addition of Wnt3a-CM (“enteroid medium”), resulting in 100 % CFE. The addition of ISEMF-CM to ENRnYSL resulted in CFE of 65.8 % ± 16.9 %.
Comparison of murine, human, and porcine intestinal cultures. a–c Intestinal crypts from all three species produce enteroids, spheroids, and flat structures when co-cultured with intestinal subepithelial myofibroblasts (ISEMFs) and grown in ENR medium (containing epidermal growth factor, noggin, and R-spondin 1). d–f Intestinal crypt culture without ISEMFs. Only murine crypts survived in ENR medium (NS non-supportive). g–i Addition of Wnt3aconditioned medium (Wnt3a-CM) produced spheroids in the murine culture system and short-lived enteroids in the human system but did not support adult porcine crypt growth. j–l Addition of prostaglandin E2 (PGE2 ) to ENR medium resulted in spheroid formation in murine and human culture systems but was not supportive of adult porcine crypt culture. Bars 200 μm. m Comparison of colonyforming efficiency (CFE) on day 7 among various culture media combinations demonstrating a notable survival benefit through addition of either CHIR99021 (glycogen synthase kinase 3 inhibitor) or ISEMF-CM (FGF10 fibroblast growth factor 10, Y27632 p160ROCK inhibitor, SB202190 p38 mitogen-activated protein kinase inhibitor, LY2157299 transforming growth factor β receptor inhibitor)
Characterization of cultured porcine epithelium
The culture of porcine intestinal crypts in the enteroid medium resulted in a budding enteroid morphology (Fig. 2a) consisting of columnar epithelium (Fig. 2d) comparable with crypt cells in vivo (Fig. 2c). In contrast, the addition of spheroid medium resulted in a spheroidal phenotype (Fig. 2b) with simple to cuboidal epithelium (Fig. 2e). Immunohistochemical analysis showed the presence of goblet, enteroendocrine, and rare Paneth cell lineages in enteroids consistent with the staining pattern of jejunal crypts, in contrast to spheroids, which lacked these differentiation markers (Fig. 2f-n). The enterocyte brush border marker CD10 was present in the core villus domain of enteroids and native intestinal villi but was absent in spheroids (Fig. 2o-q). The presence of Lgr5-positive stem cells and of goblet, enteroendocrine and Paneth cell lineages in enteroids and spheroids was also assessed by the RNA expression profiles of Lgr5, Muc2, Chga and Lyz, respectively and compared with those of intact adult porcine crypts (Fig. 2r). Enteroids had reduced expression of Paneth and goblet cell markers when compared with whole jejunum or intact crypts by reverse transcription plus PCR. Whereas the enteroendocrine marker chromogranin A was highly expressed in enteroids and spheroids, its expression was an order of magnitude lower in spheroids.
Characterization of cultured porcine enteroids (a-q, middle panels) and spheroids (a-q, right panels) compared to porcine jejunum (c-q, left panels): (a-b) Brightfield, (c-e) H&E, (f-h) PAS, (i-k) chromogranin A, (l-n) lysozyme, (o-q) CD10. (r) Gene expression of porcine crypts, enteroids and spheroids normalized by housekeeping gene Gusb and whole porcine jejunum gene expression. In (l-q), nuclei are counter-stained with DAPI. Scale bar, 200 μm (a-b) and 50 μm (c-q)
Using immunohistochemistry, we investigated the cell polarity of the cultured porcine structures. Both enteroids and spheroids showed evidence of cell surface polarity as evidenced by the basally located nuclei (Fig. 2d, e) and the cell membrane localization of E-cadherin, cytokeratin 8 and 18, villin, p120-catenin, and β-catenin (Fig. 3).
Effect of temperature on porcine crypt culture
Enteroids grown at 37 or 39 °C appeared to have similar budding structures on day 6 of primary culture (Fig. 4a, b). Enteroids from both temperature conditions were successfully subcultured, producing typical budding structures (Fig. 4c, d). The expression of Muc2, Chga and Lyz in enteroids grown at the two temperatures was similar (P > 0.05; Fig. 4e). The expression of Lgr5 was higher in enteroids grown at 37 °C (P = 0.003).
Effect of culture temperature on enteroid growth. Enteroids on culture day 6 cultured at 39 °C (a) and 37 °C (c). Subcultured enteroids 3 days after initial subculture at 39 (b) and 37 °C (d). (e) Gene expression of enteroids grown at the two culture temperatures for 7 days. (f) Thawed crypts produced enteroids; culture day 7. Scale bar, 500 μm (a-b) and 200 μm (c-d, f)
Cryo-preservation of porcine crypts
Porcine crypts were frozen at −80 °C for 2 weeks then thawed and cultured as above in enteroid medium. Enterosphere formation was delayed after a freeze-thaw cycle compared with untreated control crypt cultures but large budding structures were observed by culture day 7 (Fig. 4f).
ISEMF-CM produces intestinal spheroids
Porcine ISEMFs showed markers analogous to those of ISEMFs isolated from murine and human intestines, namely α smooth muscle actin and vimentin (Electronic Supplementary Material, Fig. S1a, b). In a few isolations, we noticed an admixture of smooth muscle cells as demonstrated by positive desmin immunostaining (Electronic Supplementary Material, Fig. S1c). However, this did not result in differences in biological activity. ISEMF-CM mixed 1:1 with ENRnYSL medium produced spheroids (Fig. 2b, e, h, k, n, q).
Subculture
Long-term culture of porcine intestinal crypts was attempted with and without the addition of Wnt3a-CM, CHIR99021, PGE2, Jag1 and ISEMF-CM. ENR medium supplemented with Wnt3a-CM alone did not support transfer and survival beyond four passages. Using enteroid medium, we were able to passage porcine crypts up to 10 times (mean 9.3 ± 0.6 passages with an expansion of up to 640-fold in cell mass (Fig. 5a, b). After 10 passages, we observed arrest of growth and no further expansion. Jag1 had no discernible effect on enteroid passage, even when added in the presence of Wnt3a-CM and CHIR99021 or PGE2 (data not shown). Addition of VPA to the enteroid medium resulted in the formation of budding enteroids in primary culture but once subcultured, small simple enteroids formed without buds (Fig. 5c) and these could only be passaged 2–3 times with limited expansion ability (up to six-fold). This was in contrast to cultures grown in enteroid medium, which maintained budding structures after subculture (Fig. 5d). The combination of Wnt3a-CM and PGE2 (but not PGE2 alone, Fig. 1l) produced spheroids and permitted up to 10 passages (9.5 ± 0.7 passages; P = 0.39 vs. enteroid medium, Fig. 5a) and expansion up to 66-fold. Spheroids cultured in ISEMF-CM were subcultured up to 8 times (6.3 ± 1.5 passages, P = 0.02 vs. enteroid medium; Fig. 5a); addition of PGE2 did not improve the capacity of spheroids to undergo passage. The expansion capacity of spheroids in ISEMF-CM was limited to 10- to 12-fold over 6 weeks and 10 passages.
Subculture of porcine intestinal epithelium. (a) Maximum number of passages for various culture conditions. (b) Fold-expansion of porcine enteroids grown in enteroid medium. (c) Addition of VPA resulted in small enteroids without buds and limited expansion ability. (d) Budding was preserved in enteroids subcultured in enteroid medium without VPA. Scale bar, 500 μm
Transduction
The transduction efficiency of enteroids and spheroids was 24.2 % ± 0.21 % and 65.3 % ± 4.95 %, respectively (n = 2; Fig. 6e–j). Nearly all enteroids and spheroids contained green fluorescent protein (GFP)-positive cells but GFP expression within each structure was inhomogeneous (Fig. 6a-d).
Transduction of porcine intestinal enteroids and spheroids. a-d Brightfield and fluorescence microscopy of transduced enteroids (a,b) and spheroids (c,d), respectively. Bars 100 μm (a, b), 200 μm (c, d). e Transduction efficiency of enteroids and spheroids compared with non-transduced controls. Scatter plot of cultured porcine cells. g–j Flow cytometric quantification of GFP-positive cells among (g) untransduced enteroids, (h) untransduced spheroids, (i) transduced enteroids, and (j) transduced spheroids
Discussion
Clinical utilization of intestinal stem cells requires preclinical assessment in animal models. In the area of intestinal mucosal stem cell transplantation, in vivo experience has thus far been concentrated on rodents. Orthotopic transplantation of intestinal crypt organoid units has been previously demonstrated in mice and rats (Chen et al. 2006; Avansino et al. 2006). Furthermore, transplantation of mouse colonic stem cells has been demonstrated in a murine colitis model (Yui et al. 2012). Long-term engraftment of murine and human ISCs co-cultured with ISEMFs has been shown in the subcutaneous position in mice (Lahar et al. 2011; Lei et al. 2014). These models demonstrate the feasibility of functional engraftment of cultured ISCs in vivo. However, these studies are limited by the inherent differences of rodents from humans in terms of intestinal physiology (Cao et al. 2006; Gibbons and Spencer 2011), although transplantation of intestinal crypts has been reported in one publication each for dogs and pigs, respectively (Agopian et al. 2009; Sala et al. 2009). Porcine models are well-established as large animal models for preclinical assays because of both the anatomic and phylogenetic similarities of pigs and humans (Yandza et al. 2012; Swartz and Andreadis 2013). Thus, we pursued the establishment of culture methods for ISCs from juvenile and adult pigs by building on lessons that have been learned from murine, human and neonatal porcine ISC cultures (Sato et al. 2009; Jung et al. 2011; Gonzalez et al. 2013). Fetal porcine crypts were readily grown in our laboratory under the culture conditions established by Gonzalez et al. (2013) for neonatal pigs (data not shown). However, initial attempts to use this method for juvenile or adult porcine intestinal crypts met with failure, leading us to consider that donor age plays an important role in intestinal culture of porcine cells, as has recently been shown in mice (Fordham et al. 2013).
As Sato et al. (2009) have shown previously, the addition of various growth factors (EGF, Noggin, and Rspondin1) is necessary and sufficient for the long-term culture of mouse intestinal crypts in the absence of mesenchymal cells. Building on this knowledge and our experience with murine, human and neonatal porcine cultures, we developed a culture protocol for juvenile and adult porcine small bowel crypts (Jung et al. 2011; Sato et al. 2011a). We show that ENR medium, which is sufficient for murine crypt culture, does not support porcine crypt growth. Whereas the addition of PGE2 allows us to culture human crypts, this culture condition is non-supportive of juvenile and adult porcine cultures. In the case of the pig, we found that the addition of CHIR99021, a specific GSK3 inhibitor, dramatically improves the short-term survival of primary cultures. This is probably a result of the expansion of the stem cell population (Yin et al. 2014). These differences highlight the importance of optimizing culture conditions for intestinal epithelium from various species.
Similarly, although the neonatal culture medium (Gonzalez et al. 2013) supports the growth of fetal and neonatal porcine intestinal crypts in primary culture, in our experience, this medium does not permit the culture of juvenile or adult porcine crypts for more than 4–5 days. Our data support the notion that, in comparison with their fetal and neonatal counterparts, adult pig intestinal crypts in culture are more Wnt-dependent as evidenced by the robust effects of GSK3 inhibition on enteroid survival in primary culture and following subculture. Canonical Wnt signaling is needed for the adult porcine jejunal crypts to survive in the absence of ISEMFs. Comparable differences have also been observed between fetal and adult intestinal crypts from mice (Mustata et al. 2013).
Passage of juvenile and adult porcine intestinal cultures poses another special challenge. In murine cultures, simple mechanical passage of enteroids in ENR medium, with or without the chemical detachment of cells, is feasible (Sato et al. 2009; Fuller et al. 2012). In contrast, human colonic enteroids require the addition of recombinant human Wnt3a, nicotinamide, the TGFβ inhibitor A83-01 and the p38 MAP kinase inhibitor SB202190 to ENR medium for successful transfer and subculture (Jung et al. 2011). However, we found these procedures unsuited for the splitting and passaging of adult porcine intestinal enteroid cultures and developed a novel enteroid subculture technique. Once budding enteroids were produced in primary culture, these were split mechanically in order to break off the buds. We then evaluated the effect of various additives on subculture survival. Jag1, a notch activator important for stem cell regeneration (VanDussen et al. 2012) and VPA, a histone deacetylase inhibitor (Yin et al. 2014), had no discernible effect when added after passage. In contrast, addition of CHIR99021 (a GSK3i), which we had found to be essential for short-term enteroid survival, made all the difference, allowing up to 10 successful enteroid passages. In subsequent studies, we assessed the effects of PGE2 because of its role in the inhibition of anoikis, mitogenic signaling (Jung et al. 2011), and its effect on the upregulation or stabilization of Lgr5 (Al-Kharusi et al. 2013). Whereas the addition of PGE2 alone to ENR medium did not support growth after subculture, simultaneous addition of ENRnYSL medium, Wnt3a-CM and PGE2 resulted in a spheroidal morphology and the lack of budding extensions and this mixture permitted up to 10 passages in culture.
Unlike mice and humans, pigs have a relatively high resting physiologic body temperature (39.3 ± 0.3 °C; Dewey and Straw 2006). However, porcine tissue culture has traditionally been performed at 37 °C (Mueller et al. 2013; Wang et al. 2011; Gonzalez et al. 2013), despite the paucity of literature to document the physiologic basis of this culture temperature. To assess the effect of culture temperature on porcine intestinal cultures, we isolated porcine jejunal crypts and cultured them in enteroid medium at either 37 °C or 39 °C. No differences were seen in the expression of differentiation markers at either temperature; the exception was a higher expression of Lgr5 expression at 37 °C, although enteroids at both temperatures were subcultured equally successfully. The normal growth of porcine intestinal cultures at 37 °C obviates the need for maintaining incubators at different temperatures, a practical consideration for laboratories in which cells from various species are maintained in the same incubator.
We demonstrated successful cryo-preservation of adult porcine intestinal crypts with the ability of the thawed crypts to form enteroids. Similar data have been demonstrated by using murine enteroids (Yui et al. 2012; Fuller et al. 2012). The ability to cryo-preserve isolated intestinal crypts has important implications both for research and therapeutic purposes and provides the ability to share intestinal tissue among collaborating institutions.
Although we have now shown that porcine crypts can be successfully cultured and transduced in enteroid and spheroid media, a method for long-term culture of juvenile and adult porcine crypts beyond several months remains elusive. The practical utility of long-term culture revolves around the use of porcine models in tissue engineering experiments; long-term culture is required for ISC expansion in intestinal tissue regeneration (Bitar and Raghavan 2012; Orlando et al. 2013). To this end, a more formal mechanistic approach will probably be needed to uncover important pathways in adult porcine intestinal cultures. The comparison of intestinal epithelial culture in the adult porcine model with those in the murine and human systems, as we performed here, is likely to be the most expedient route to pursue this mechanistic approach thanks to the large volume of preexisting data on murine and human intestinal cultures.
In summary, we describe a novel method for the maintenance of juvenile and adult porcine intestinal crypts in culture over several weeks and show improved short-term plating efficiency in the setting of continuous canonical Wnt stimulation by the inhibition of GSK3. We demonstrate that porcine crypts can be induced by specific supplements to form spheroids or budding enteroids that can be transduced with lentiviruses.
References
Agopian VG, Chen DC, Avansino JR, Stelzner M (2009) Intestinal stem cell organoid transplantation generates neomucosa in dogs. J Gastrointest Surg 13:971–982
Al-Kharusi MRA, Smartt HJM, Greenhough A, Collard TJ, Emery ED, Williams AC, Paraskeva C (2013) LGR5 promotes survival in human colorectal adenoma cells and is upregulated by PGE2: implications for targeting adenoma stem cells with NSAIDs. Carcinogenesis 34:1150–1157
Avansino JR, Chen DC, Hoagland VD, Woolman JD, Stelzner M (2006) Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery 140:423–434
Bitar KN, Raghavan S (2012) Intestinal tissue engineering: current concepts and future vision of regenerative medicine in the gut. Neurogastroenterol Motil 24:7–19
Brown PJ, Miller BG, Stokes CR, Blazquez NB, Bourne FJ (1988) Histochemistry of mucins of pig intestinal secretory epithelial cells before and after weaning. J Comp Pathol 98:313–323
Cao X, Gibbs ST, Fang L, Miller HA, Landowski CP, Shin HC, Lennernas H, Zhong Y, Amidon GL, Yu LX, Sun D (2006) Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharm Res 23:1675–1686
Chen DC, Agopian VG, Avansino JR, Lee JK, Farley SM, Stelzner M (2006) Optical tissue window: a novel model for optimizing engraftment of intestinal stem cell organoids. J Surg Res 134:52–60
Dewey CE, Straw BE (2006) Herd examination. In: Straw BE, Zimmerman JJ, D’Alliare S, Taylor DJ (eds) Diseases of swine, 9th edn. Blackwell, Ames, pp 13–14
Fordham RP, Yui S, Hannan NRF, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T, Watanabe M, Jensen KB (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13:734–744
Fuller MK, Faulk DM, Sundaram N, Shroyer NF, Henning SJ, Helmrath MA (2012) Intestinal crypts reproducibly expand in culture. J Surg Res 178:48–54. doi:10.1016/j.jss.2012.03.037
Gibbons DL, Spencer J (2011) Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol 4:148–157
Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, Magness ST (2013) Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One 8:e66465
Harding J, Roberts RM, Mirochnitchenko O (2013) Large animal models for stem cell therapy. Stem Cell Res Ther 4:23
Jabaji Z, Sears CM, Brinkley GJ, Lei NY, Joshi VS, Wang J, Lewis M, Stelzner M, Martín MG, Dunn JC (2013) Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Methods 19:961–969
Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, Clevers H, Batlle E (2011) Isolation and in vitro expansion of human colonic stem cells. Nat Med 17:1225–1227
Koo BK, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, Es JH van, Clevers H (2012) Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods 9:81–83
Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martín MG, Dunn JC (2011) Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One 6:e26898
Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, Wang F, Jaroszewicz A, Pellegrini M, Li L, Lewis M, Stelzner M, Dunn JC, Martín MG (2014) Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One 9:e84651
Linard C, Busson E, Holler V, Strup-Perrot C, Lacave-Lapalun JV, Lhomme B, Prat M, Devauchelle P, Sabourin JC, Simon JM, Bonneau M, Lataillade JJ, Benderitter M (2013) Repeated autologous bone marrow-derived mesenchymal stem cell injections improve radiation-induced proctitis in pigs. Stem Cells Transl Med 2:916–927
Mueller KR, Martins KV, Murtaugh MP, Schuurman HJ, Papas KK (2013) Manufacturing porcine islets: culture at 22 °C has no advantage above culture at 37 °C: a gene expression evaluation. Xenotransplantation 20:418–428
Mustata RC, Vasile G, Fernandez-Vallone V, Strollo S, Lefort A, Libert F, Monteyne D, Pérez-Morga D, Vassart G, Garcia MI (2013) Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep 5:421–432
Orlando G, Domínguez-Bendala J, Shupe T, Bergman C, Bitar KN, Booth C, Carbone M, Koch KL, Lerut JP, Neuberger JM, Petersen B, Ricordi C, Atala A, Stratta RJ, Soker S (2013) Cell and organ bioengineering technology as applied to gastrointestinal diseases. Gut 62:774–786
Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC (2009) Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res 156:205–212
Sato T, Vries RG, Snippert HJ, Wetering M van de, Barker N, Stange DE, Es JH van, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265
Sato T, Stange DE, Ferrante M, Vries RG, Es JH van, Brink S van den, Houdt WJ van, Pronk A, Gorp J van, Siersema PD, Clevers H (2011a) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, Born M van den, Barker N, Shroyer NF, Wetering M van de, Clevers H (2011b) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418
Stoltz DA, Rokhlina T, Ernst SE, Pezzulo AA, Ostedgaard LS, Karp PH, Samuel MS, Reznikov LR, Rector MV, Gansemer ND, Bouzek DC, Alaiwa MH, Hoegger MJ, Ludwig PS, Taft PJ, Wallen TJ, Wohlford-Lenane C, McMenimen JD, Chen JH, Bogan KL, Adam RJ, Hornick EE, Nelson GA 4th, Hoffman EA, Chang EH, Zabner J, McCray PB Jr, Prather RS, Meyerholz DK, Welsh MJ (2013) Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest 123:2685–2693
Swartz DD, Andreadis ST (2013) Animal models for vascular tissue-engineering. Curr Opin Biotechnol 24:916–925
VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC (2012) Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139:488–497
Wang J, Kolomeyer AM, Zarbin MA, Townes-Anderson E (2011) Organotypic culture of full-thickness adult porcine retina. J Vis Exp 2011:2655. 10.3791/2655doi: 10.3791/2655
Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, Helmrath MA (2014) An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20:1310–1314
Yandza T, Tauc M, Saint-Paul MC, Ouaissi M, Gugenheim J, Hébuterne X (2012) The pig as a preclinical model for intestinal ischemia-reperfusion and transplantation studies. J Surg Res 178:807–819
Yeung TM, Chia LA, Kosinski CM, Kuo CJ (2011) Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci 68:2513–2523
Yin X, Farin H, van Es J, Clevers H (2014) Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11:106–112
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18:618–623
Zhang Q, Widmer G, Tzipori S (2013) A pig model of the human gastrointestinal tract. Gut Microbes 4:193–200
Acknowledgments
We sincerely thank Dr. Randal Buddington (University of Memphis) for providing fetal porcine intestinal samples; Drs. JanLee Jensen and Sandra Duarte-Vogel, Sonia Watt, Guillermo Moreno, and Eileen So (University of California, Los Angeles) for providing porcine tissue; Liara Gonzalez (North Carolina State University) for sharing expertise with neonatal piglet culture; Scott Magness (University of North Carolina) for helpful discussions; and Emmanuelle Faure (University of California, Los Angeles Vectorcore) for help and guidance with viral transduction.
Author information
Authors and Affiliations
Corresponding author
Additional information
The UCLA Vectorcore is supported by CURE/P30 DK041301. This research was performed as a project of the Intestinal Stem Cell Consortium, a collaborative research project funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infections Diseases (DK085535-01 and DK085535-02S2) and was supported in part by the California Institute of Regenerative Medicine (RT2-01985).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Characterization of porcine ISEMFs by immunostaining (green) for (a) α smooth muscle actin, (b) vimentin, and (c) desmin. Nuclei are counter-stained with DAPI (blue). Bar 100 μm.(GIF 137 kb)
Rights and permissions
About this article
Cite this article
Khalil, H.A., Lei, N.Y., Brinkley, G. et al. A novel culture system for adult porcine intestinal crypts. Cell Tissue Res 365, 123–134 (2016). https://doi.org/10.1007/s00441-016-2367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2367-0