Abstract
Wound healing is a highly orchestrated physiological process consisting in a complex interaction of cellular and biochemical events. Human amniotic epithelial stem cells (HAESCs) have been shown to be an attractive resource for wound healing because they are primitive stem cells. However, the exact effects of amnion-derived stem cells on the migration or proliferation of keratinocytes and their potential mechanism are not fully understood. We have found that HAESCs accelerate the migration of keratinocytes and induce a remarkable increase in the activity of phospho-ERK, phospho-JNK, and phospho-AKT, the blockade of which by their specific inhibitors significantly inhibits migration induced by HAESC-conditioned medium (CM). Furthermore, the co-culture of keratinocytes with HAESCs up-regulates the expression levels of cell proliferation proteins Cyclin D1, Cyclin D3 and Mdm2. In vivo animal experiments have shown that HAESC-CM improves wound healing, whereas blockade with ERK, JNK and AKT inhibitors significantly impairs wound healing. Taken together, these results reveal, for the first time, that HAESCs promote wound healing by facilitating the migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways and might be a potential therapy in skin wound healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wound healing process relies on a multitude of cells that execute and regulate a complex signaling network altering the growth, migration and metabolism of fibroblasts, keratinocytes and stem cells (Gurtner et al. 2008). Keratinocytes, a major cellular component of the epidermis, are responsible for restoring the integrity of the injured epidermis injury through a process termed epithelialization (Pastar et al. 2014). A better understanding of the epithelialization process might provide insights for new therapeutic approaches to accelerate wound healing. Evidence indicates that stem cells mediate regeneration and repair through migration to the wound sites, where they secrete various growth factors, which then stimulate the migration and proliferation of epithelial cells or fibroblasts (Blanpain 2010).
Human amnion is derived from the epiblast as early as 8 days after fertilization and before gastrulation (Parolini et al. 2008). Human amniotic epithelial stem cells (HAESCs) have several advantages that make them an excellent alternative for embryonic stem cells (Wolfrum et al. 2010). HAESCs can directly differentiate into the three embryonic lineages (Lazzarini et al. 2014). Moreover, HAESCs express low levels of major histocompatibility complex (MHC) class I surface antigens, express no MHC class II antigens and do not present disadvantages such as ethical controversies and the risk of teratoma formation (Niknejad et al. 2008; Wolbank et al. 2007). Growing evidence has shown that amniotic membrane-derived cells secrete a number of proteins and cytokines, including epidermal growth factor (EGF), keratinocyte growth factor (KGF), human growth factor and basic fibroblast growth factor (bFGF), which potentially affect cellular growth and proliferation (Diaz-Prado et al. 2011; Yamahara et al. 2014). In addition, a broad spectrum of immunomodulatory factors, including interleukin-1 receptor antagonist, interleukin-6, interleukin-10 and interferon-γ have been found in the amnion, most of which are potentially involved in epithelial stromal interactions at the ocular surface and therefore play a crucial role in the process of corneal wound healing, such as epithelial wound closure (Ravishanker et al. 2003). Researchers have shown that fresh amniotic fluid significantly improves the rate of wound closure and the outcome of the repair process in various adult tissues, such as tendon, nerve and retina (Grueterich et al. 2003; Manuelpillai et al. 2012; Mariotti et al. 2015). However, the therapeutic properties of HAESCs in skin wounds need to be fully elucidated and the transduction mechanism is also not clear.
We focus, in this work, on the pivotal role of HAESCs on keratinocytes during wound healing. Scratch wound assay and cell cycle analysis revealed that HAESCs significantly accelerate the proliferation and migration of keratinocytes. Furthermore, we observed that the mitogenic effect is accompanied by the up-regulation of proteins related to cell proliferation (such as Cyclin D1, Cyclin D3, Mdm2) and is mediated through the activation of the AKT (also called Protein kinase B) signaling pathway. In addition to the proliferation of keratinocytes, the conditioned medium (CM) accelerates the migration of keratinocytes and the migration effect is mediated through the activation of the extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) signaling pathways. In vivo wound assays have also shown that HAESC-CM significantly accelerates wound closure. These data indicate that HAESCs represent a possible therapeutic strategy in skin wound healing.
Materials and methods
Ethics statement
All experimental procedures including the use of human and animal samples were conducted under the protocol reviewed and approved by the Xijing Hospital Ethics Committee and the Institutional Ethical Committee of the Fourth Military Medical University.
Human amnion samples were obtained from 5 women (age range: 25–30 years) with normal singleton pregnancies between January 2013 and January 2014. Patients received no treatment before the procedure and the surgeries were performed in the Department of Obstetrics, Xijing Hospital, Xi’an, China. Written informed consent was obtained from all patients or their legal guardians.
In vivo wound healing model
Male 6-week-old C57BL/6 mice that weighed 20–25 g (purchased from The Center of Experimental Animal, The Fourth Military Medical University) were housed under standard laboratory conditions of 12 h light/12 h dark at 25 °C with food and water supplied daily. The mice were randomly divided into five groups (n = 5): phosphate-buffered saline (PBS) control group; HAESC-CM treatment group; CM+ERK inhibitor (Sigma, USA); CM+JNK inhibitor (Sigma); CM+AKT inhibitor (Sigma). Mice were first anesthetized by intraperitoneal (i.p.) injection of pentobarbital sodium (20 mg/kg b.w.). The hair on the back and flank was clipped and a 1 cm × 1 cm full-thickness wound was generated on each mouse under sodium pentobarbital anesthesia as previously described (Galiano et al. 2004). For mice treated with CM or CM+inhibitors, 100 μl CM or 100 μl CM+inhibitors (10 μM) was injected into the surrounding tissues of the wound at four sites on days 1 and 3. For control mice group, an equal amount of PBS was injected. The wound size was measured and the wound closure rate was assessed in a timely manner. At day 14, mice were anesthetized by i.p. injection of pentobarbital sodium (20 mg/kg b.w.) and then killed by CO2 asphyxiation. All efforts were made to minimize animal suffering. The skin tissue samples were collected for further histological analysis and the wound area was calculated with Image-Pro Plus 6.0 software (Medical Cybernetics, USA) according to the protocol described previously (Zhang et al. 2011). In brief, the wound was imaged by the aid of a transparent sheet. The image was then scanned, imported to a computer and analyzed by Image-Pro Plus 6.0 software. The rate of wound closure was calculated as wound closure rate (%) = [(original wound area − open area on final day)/ original wound area] × 100 %.
Histological examination
For histological observation, wounded skin tissues at day 14 were collected and dehydrated in a series of graded ethanol baths and then in a xylene bath before being immersed in paraffin wax. The samples were then embedded in paraffin molds and sliced into 4-μm-thick sections that were mounted on glass slides and then stained with hematoxylin-eosin (H&E) and Masson. The stained sections were imaged by using a Olympus FSX100 microscope (Olympus, Japan).
Isolation and culture of cells
Human amnion were obtained from women with normal singleton pregnancies after the exclusion of hepatitis B virus, hepatitis C virus, human immunodeficiency virus, infectious syphilis and other related indicators. For cell culture, the amnion layer was mechanically peeled off from the placenta and rinsed with sterilized PBS. It was then cut into 1 cm × 1 cm pieces with scissors as in the previous methods with modifications (Akle et al. 1981). The amnion pieces were subjected to 0.05 % trypsin containing 0.2 g/l ethylene diamine tetraacetic acid (EDTA) and incubated at 37 °C with constant agitation. The trypsin was inactivated by the addition of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % heat-inactivated fetal bovine serum (FBS). The suspension was collected and cultured in DMEM supplemented with 10 % FBS, 10 ng/ml human leukemia inhibitory factor (PeproTech, USA), 0.2 mmol/l mercaptoethanol, 1 % antibiotic-antimycotic and 1 % nonessential amino acids in a humidified incubator at 37 °C with 5 % CO2. All reagents were purchased from Invitrogen unless otherwise stated. The proliferative ability of HAESCs was measured by using MTT assay (Liu et al. 2012). To assess the dependency of HAESC proliferation on EGF treatment, 10 ng/ml EGF was added to some wells. During exponential growth, approximately 1 × 104 cells were seeded into 96-well microtiter plates and absorbance was determined in a timely manner every day. Keratinocytes (HaCaT) were obtained from ATCC and grown in DMEM containing 10 % FBS and 1 % antibiotic-antimycotic.
Analysis by fluorescence-activated cell sorting
The expression of the stem cell markers by the cells isolated from human amnion was examined with flow cytometry. Briefly, HAESCs were harvested by treatment with 0.125 % trypsin-EDTA and stained with fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated or allophycocyanin (APC)-conjugated or peridinin chlorophylla protein cyanine-5.5 complex (PerCP-Cy5.5)-conjugated antibodies against CD29, CD31, CD34, CD90, CD105, SSEA-3, SSEA-4, Sox2, OCT4, HLA-ABC and HLA-DR (all BD pharmingen, USA). For controls, proper isotype antibodies were used. Fluorescence-activated cell sorting (FACS) was carried out on a FACSAria III (Becton Dickinson, USA) instrument for analysis of surface markers.
Differentiation potential of HAESCs
Adipogenic differentiation medium A (Cyagen, China) was prepared as follows: 10 mM ascorbate, 50 mM IBMX, 0.5 mM dexamethasome, 4 mg/ml insulin, 10 % FBS and rosiglitazone was transferred into DMEM in a laminar flow hood. The fully supplemented medium was gently swirled to ensure a homogeneous mixture; DMEM supplemented with 4 mg/ml insulin and 10 % FBS served as adipogenic differentiation medium B (Cyagen, China). After five cycles of culture in medium A/medium B (3 day:1 day), cultured cells were incubated in adipogenic differentiation medium B for additional 7 days. The cells were then fixed with 4 % formaldehyde, rinsed with PBS and stained with Oil Red O working solution. For osteogenic differentiation, cells were cultured in osteogenic differentiation medium (Cyagen, China) containing 50 μg/ml ascorbate, 0.5 mM dexamethasone and 10 % FBS. After 2 weeks, the cells were fixed with 4 % formaldehyde and stained with Alizarin Red working solution.
CM preparation
CM was collected as described (Yang et al. 2014). Briefly, the HAESCs were seeded into T75 culture flasks (Corning Costar, USA). On reaching approximately 60–70 % confluence, the cells were washed with PBS. The medium was then replaced by serum-free medium for further culture until 100 % confluence. Culture medium from each sample was collected and used as CM in this study.
Labeling of keratinocytes with green fluorescence protein
Keratinocytes (HaCaT) were infected with a lentiviral vector for green fluorescence protein (GFP) by standard procedures (Bai et al. 2007). Briefly, keratinocytes were seeded in 60-mm culture dishes and infected with unconcentrated lentiviral supernatant (Clontec Laboratories, Calif., USA) after 24 h at a multiplicity of infection of 5–10. At 6 h after transduction, the virus-containing medium was removed and fresh culture medium supplemented with 10 % FBS was added. GFP expression was visualized under a fluorescence microscope (Olympus IX71, Japan) and GFP-positive cells were further analyzed by FACSAria III flow cytometry (BD, USA) 3 days after transduction (Fig. S1).
Scratch wound assay and time-lapse video
For wound healing assays, Ibidi Culture-Inserts were used to analyze cell migration (Navone et al. 2014). After the cells reached 100 % confluence, the Culture-Inserts were removed by using sterile tweezers, creating a cell-free gap of 500 μm. We placed the Culture-Inserts into a laser confocal Petri dish (NEST Biotech, China) under sterile conditions. Fluo View FV 10i Laser Scanning Confocal Microscopy (Olympus Corp, Japan) was used to take real-time dynamic images with a computer. The migration area of keratinocytes at each time point was measured by using Image Pro Plus 6.0 software and were normalized by the scratch area.
Cell cycle analysis
For cell cycle analysis (Sabapathy et al. 2012), about 1 × 106 keratinocytes were collected and fixed with 75 % ice-cold ethanol for at least 12 h at 4 °C. Before analysis by flow cytometry, the cells were treated with 10 mg/ml RNase A (Sigma) and 50 mg/ml propidium iodide (Sigma). The samples were then analyzed by FACSAria III flow cytometry (BD, USA).
Transwell co-culture assay
Keratinocytes were co-cultured with HAESCs in transwell chambers (Corning Costar, USA) with 3-μm pore filters for 4 days. HAESCs were seeded in 6-well plates and keratinocytes were seeded onto the surface of a porous polycarbonate transwell membrane. Once the cells had adhered onto the membrane, each transwell membrane was inserted into a well of a 6-well culture plate. The cells were then co-cultured for 4 days.
Western blot analysis
Proteins (40 μg) were loaded onto a 5–10 % polyacrylamide gel, separated by electrophoresis and transferred to a polyvinylidene difluoride membrane (PVDF). After being blocked with 5 % non-fat milk, the PVDF membrane was incubated with specific primary antibodies: rabbit anti-human Cyclin D1, Cyclin D3, or Mdm2 (Santa Cruz, Calif., USA) and rabbit anti-human AKT, phospho-AKT, ERK, phospho-ERK, JNK, phospho-JNK, p38, phospho-p38, or β-actin (Cell signaling, USA). Horseradish-peroxidase-conjugated goat anti-rabbit (Boster, China) antibody was used as a secondary antibody. Proteins were visualized by an enhanced chemiluminescence system by using FluorChem FC (Alpha Innotech, USA).
Real-time reverse transcription plus polymerase chain reaction
The Trizol Reagent kit (Invitrogen, USA) was used for RNA extraction. The isolated RNA was reverse-transcribed into complementary DNA by using the PrimeScript RT Kit (TaKaRa, China). Primers were obtained from Integrated DNA Technologies. Real-time polymerase chain reaction (PCR) was performed in a Bio-Rad IQ5 Real-Time System (Bio-Rad, USA) by using the SYBR1 Premix Ex Taq II kit (TaKaRa, China) in a 20-ml volume of the PCR solution. The sequences for primers are listed in Table 1. The results were normalized against mean Ct-values of D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by using the ΔCt-method: ΔCt = Ct gene of interest - Ct mean (GAPDH). The fold increase was calculated as 2-(ΔΔCt).
Statistical analysis
Statistical analyses were performed by using SPSS13.0 software (SPSS, USA). Data are presented as the means ± standard error of three independent experiments. Independent samples were tested by the t-test for comparison between two groups or by one-way analysis of variance among the groups, with *P < 0.05 being considered statistically significant.
Results
Isolation and characterization of HAESCs
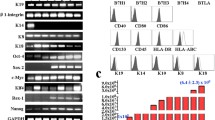
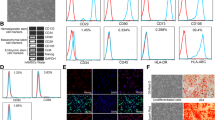
As depicted in Fig. 1a, the amniotic membrane was composed of a single layer of flattened cuboidal cells resting on a basal lamina, beneath which lay a stromal layer composed of amniotic mesenchymal cells. HAESCs showed a typical cobblestone-like morphology (Fig. 1b) and could be maintained in DMEM for at least 12 passages (data not shown). The growth curve of HAESCs was measured by using the MTT assay, which showed that passage 2 HAESCs reached a logarithmic growth phase on day 4 (Fig. 1c). Passage 2 HAESCs were also indentified by incubating the cells in differentiation medium to promote differentiation into osteogenic and adipogenic lineage. The differentiation of HAESCs into osteoblasts was detected by using Alizarin Red (Fig. 1d) and the differentiation into adipocytes was assessed by using Oil Red O stain (Fig. 1e). Cells isolated from human amnion were subjected to flow cytometry to examine the expression of stem cell markers. HAESCs were positive for embryonic stem cell markers SSEA-3 (55.4 % ± 6.7 %, Fig. 1l), SSEA-4 (74.1 % ± 3.2 %, Fig. 1m); mesenchymal stem cell markers CD29 (99.1 % ± 2.4 %, Fig. 1g), CD90 (94.0 ± 1.6 %, Fig. 1j), CD105 (8.0 % ± 2.3 %, Fig. 1k); pluripotent transcription factors Sox2 (15.4 % ± 5.7 %, Fig. 1n), OCT4 (9.9 % ± 3.8 %, Fig. 1o); and immunological marker HLA-ABC (91.1 % ± 1.4 %, Fig. 1p); they were negative for hematopoietic marker CD34 (0.0 % ± 0 %, Fig. 1i); endothelial marker CD31 (0.0 % ± 0 %, Fig. 1h); and immunological marker HLA-DR (0.0 % ± 0 %, Fig. 1q). Proper isotype antibodies were used as controls (Fig. 1f).
Characterization of HAESCs. a Structure of the human amniotic membrane (HAM). HAM was stained with hematoxylin and eosin. Human amniotic epithelial stem cells (HAESCs) are a single layer of flattened cuboidal cells resting on a basal lamina. Bar 50 μm. b Morphological assessment of freshly isolated HAESCs. HAESCs show a typical cobblestone-like morphology. Bar 50 μm. c Growth rate graphics of HAESCs (Abs.570 absorbance at 570 nm). HAESCs (passage 2) have a doubling time of about 4 days without epidermal growth factor (EGF) treatment, whereas the EGF treatment group a faster growth rate. d, e Osteogenic and adipogenic differentiation of HAESCs. Detection of cells differentiated into osteoblasts and adipocyte by Alizarin Red and Oil Red O. Bar 50 μm. f–q Flow cytometry of HAESC surface markers (FITC fluorescein-isothiocyanate-conjugated antibody, PE phycoerythrin-conjugated antibody, APC allophycocyanin-conjugated antibody, PerCP-Cy5.5 peridinin chlorophylla protein cyanine-5.5 complex-conjugated antibody). HAESCs were positive for CD29 (g), CD90 (j), CD105 (k), SSEA-3 (l), SSEA-4 (m), Sox2 (n), OCT4 (o) and HLA-ABC (p) and negative for CD31 (h), CD34 (i) and HLA-DR (q). Proper isotype antibodies were used as controls (f)
HAESCs promoted migration of keratinocytes
To investigate the migration of keratinocytes treated with HAESC-CM, keratinocytes were divided into four groups. Cells in group a were treated with serum-free DMEM only; cells in group b were treated with CM; cells in group c were pre-incubated with mitomycin C for 1 h and then treated with CM; and cells in group d were also pre-incubated with mitomycin C for 1 h and treated with serum-free DMEM. Representative images of the scratch wound assay at various time points (0, 6, 12, 18 and 24 h) are shown (Fig. 2a). Quantified data (Fig. 2b) calculated by Image-Pro Plus 6.0 software revealed that, at 6 h, a significant difference in the migration area was observed between group a and group b and between group b and group c, both sets of which demonstrated that CM promoted the migration ability of keratinocytes (16.4 % ± 2.9 % for group a, 35.9 % ± 3.5 % for group b; 15.5 % ± 2.1 % for group c; 3.5 % ± 1.2 % for group d, *P < 0.05). Moreover, a significant difference was seen in the migration between group a and d and between group c and group d at 6 h, both sets of which indicated that CM not only promoted the migration ability of keratinocytes but also accelerated the proliferation of keratinocytes. At 18 h, the scratch area treated with CM only had completely healed and the migration area was significantly larger compared with other groups (54.6 % ± 2.3 % for group a; 94.1 % ± 4.5 % for group b; 32.9 % ± 4.5 % for group c; 10.3 % ± 2.3 % for group d, *P < 0.05).
HAESCs promote migration of keratinocytes. a Representative images of the scratch wound assay at various time points: group a scratch wound assay of keratinocyte migration treated with conditioned medium (CM), group b scratch wound assay of keratinocyte migration exposed to serum-free DMEM only as a control group. To prevent cell proliferation, group c and group d were pre-incubated with mitomycin C and treated with CM and serum-free DMEM, respectively. Bar 500 μm. b Histogram showing the quantified cell migration area in each group at various time points (*P < 0.05)
ERK and JNK were involved in CM-promoted keratinocyte migration
To clarify which signaling pathways are involved in the regulation of wound healing promoted by HAESCs, we investigated the specific role of the mitogen-activated protein kinase (MAPK) and AKT pathways during this process through Western blotting. The levels of ERK, JNK and AKT phosphorylation significantly increased at 15 min after treatment with CM and peaked at 60 min (Fig. 3a). Densitometric analysis of ERK, JNK and AKT phosphorylation are also presented below the blots, respectively (Fig. 3b, b’, b’’). Subsequently, we studied the effect of inhibitors against the activation of ERK, JNK, and AKT (Fig. 3c). The ERK inhibitor PD98059 reduced the CM-induced phosphorylation of ERK from its peak at 72.5 % to 48.3 % (*P < 0.05), the JNK inhibitor SP600125 reduced the CM-induced phosphorylation of JNK from 122 % to 58 % (*P < 0.05), and the AKT inhibitor LY294002 reduced the CM-induced phosphorylation of AKT from 100 % to 70.5 %. Densitometric analysis of the inhibition of ERK, JNK and AKT phosphorylation is also presented below the blots, respectively (Fig. 3d, d’, d’’). In contrast, p38 phosphorylation showed no significant changes after CM treatment (Fig. S2).
MAPK (mitogen-activated protein kinase) and AKT (also called Protein kinase B) phosphorylation in human keratinocytes after conditioned-medium (CM) treatment. a Western blot analyses of CM-induced MAPK and AKT phosphorylation in keratinocytes. Densitometric analyses of phospho-ERK (b), phospho-JNK (b’) and phospho-AKT (b’’) are presented below the blots (KD kiloDalton). c Inhibition of CM-induced MAPK and AKT phosphorylation in human keratinocytes treated with combined CM and PD98059 (10 μM) or SP600125 (10 μM) or LY294002 (10 μM) for 60 min. Densitometric analysis of ERK (d), JNK (d’) and AKT (d’’) is also shown. A representative of three similar experiments is depicted and the mean values (standard deviation) of the three experiments are presented below the blots (*P < 0.05)
In light of the critical role that MAPKs and AKT play in wound healing, we focused on the effect of inhibitors of MAPKs and AKT on keratinocytes migration. Keratinocytes migration was markedly blocked by treatment with ERK inhibitor PD98059 and JNK inhibitor SP600125; in order to avoid effects attributable to cell proliferation, we further pre-incubated cells with mitomycin C and ERK inhibitor PD98059 or JNK inhibitor SP600125 for 1 h and treated them with CM to evaluate the migration of keratinocytes. Keratinocyte migration was completely blocked when cells were pre-treated with mitomycin C and ERK inhibitor PD98059 or JNK inhibitor SP600125, whereas when pre-incubated with mitomycin C and AKT inhibitor LY294002, cell migration was not completely inhibited. These results suggested that the phosphorylation of ERK and of JNK was activated in CM-promoted keratinocytes migration and that the AKT pathway was responsible for the mitogenic action of CM (Fig. 4a). Qualitative data regarding migration evaluation are also shown in Fig. 4b obtained by using Image-Pro Plus 6.0 software.
Effect on keratinocytes migration of blocking JNK, ERK, or AKT pathways with specific inhibitors. a Migration of human keratinocytes induced by CM was significantly impaired after pre-incubation with inhibitors alone or combined inhibitors and mitomycin C. Bar 500 μm. (*p < 0.05). b Histogram quantifyiing the cell migration area in each group. See also Fig. S7
HAESCs promoted proliferation of keratinocytes and AKT was involved in CM-promoted keratinocyte proliferation
Because cultured HAESCs reached the logarithmic growth phase on day 4, keratinocytes co-cultured with HAESCs for 4 days were harvested for further analysis. The effects of HAESCs on the proliferation of keratinocytes (Fig. 5a–c) were examined by cell cycle analysis, which showed that the fraction of S phase in the co-culture group (total S-phase: 43.9 % ± 2.5 %, Fig. 5b) was higher than that in the control group (total S-phase: 24.4 % ± 3.6 %, Fig. 5a, *p < 0.05). The co-culture of keratinocytes with HAESCs in transwells caused the dramatic promotion of keratinocyte proliferation (Fig. 5e, *P < 0.05). These observations were further confirmed by Western blotting, which showed that the expression of Cyclin D1 (Fig. 5f, *P < 0.05) and Cyclin D3 (Fig. 5g, *P < 0.05), which are related to cell survival and proliferation, markedly increased. Co-cultured keratinocytes with HAESCs have a higher expression level of Mdm2 compared with the control group (Fig. 5h, *p < 0.05). The densitometric analysis of Cyclin D1, Cyclin D3 and Mdm2 is shown in Fig. 5i–k, respectively.
Analysis of cell-proliferation-associated molecules co-cultured with HAESCs. a–d Analysis of the cell cycle in the control and the co-cultured groups. e Flow cytometry analyses of cell numbers in the control and the co-cultured groups. f–k Western blots of cell-proliferation-regulatory molecules Cyclin D1, Cyclin D3 and Mdm2 and their densitometric analysis (*P < 0.05)
Next, we investigated the AKT signaling pathway in the regulation of keratinocyte proliferation promoted by CM. Scratch wound healing assays (see Movies. S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11) showed that the specific inhibitor against the AKT pathway, LY294002, or pre-treatment with mitomycin C and LY294002 significantly reduced the proliferation of keratinocytes (Fig. 4a). Moreover, FACS analysis revealed that the AKT inhibitor LY294002 reduced the CM-mediated stimulation of DNA synthesis (S-phase) in keratinocytes from 43.9 % to 28.9 % (Fig. 5c, *P < 0.05). Collectively, the data presented above suggested that the AKT signaling pathway was responsible for the mitogenic effect of CM on keratinocytes.
HAESCs promoted wound healing in an animal model
The wound closure process treated with HAESC-CM in the presence or absence of specific inhibitors was determined by observation and histological examination for 2 weeks. None of the animals became sick or died during the treatment. From macro-perspectives, wounds treated with CM exhibited a similar size to that of the control group on day 1 after treatment (data not show). However, the lesion area of the CM group at day 3 significantly decreased compared with control lesions (Fig. 6a). In particular, the wound closure rate at postoperative day 3 was 75.3 % ± 2.9 % in the control group, 70.1 % ± 4.5 % in the CM group, 92.3 % ± 3.1 % in the CM + PD98059 group, 85.9 % ± 2.9 % in the CM + SP600125 group and 87.4 % ± 3.6 % in the CM+LY294002 group. Almost complete epithelialization of the wound was reached at day 7 in mice treated with CM, whereas another few days were necessary for completion in control mice. Evaluations of the wound area at postoperative day 14 were 3.8 % ± 1.1 % in the control group, 0.0 % ± 0 % in the CM-treated group, 8.3 % ± 3.2 % in the CM+PD98059 group, 5.9 % ± 4.3 % in the CM+SP600125 group and 5.4 % ± 1.8 % in the CM+LY294002 group (*p < 0.05). The area of the wound was measured by using a metric ruler that was placed adjacent to the wound. The photographs indicate the kinetics of wound healing in all groups (Fig. 6b). Histological examination showed that the wound healing in CM-treated mice (Fig. 7c) was faster compared with control mice (Fig. 7b) and that treatment with ERK inhibitors+CM (Fig. 7d), JNK inhibitors+CM (Fig. 7e) and AKT inhibitors+CM (Fig. 7f) significantly impaired wound healing; normal skin (Fig. 7a) served as another control. Indeed, after 2 weeks, those mice treated with CM (Fig. 7i) showed a fast degree of tissue reorganization with skin appendages resembling those of normal skin (Fig. 7g) in comparison with the control (Fig. 7h) and treatment with ERK inhibitors+CM (Fig. 7j), JNK inhibitors+CM (Fig. 7k) and AKT inhibitors+CM (Fig. 7l) significantly impaired the organization of collagen fibers.
Wound closure after CM treatment. a Representative photographs of full-thickness excisional wounds treated with phosphate-buffered saline (PBS), with CM, or with CM+inhibitors. HAESC-derived conditioned medium (CM) improved skin wound healing, whereas blockade with ERK, JNK and AKT inhibitors significantly impaired wound healing. b Wound closure rate of healing after treatment with PBS, with CM, or with CM+inhibitors (*P < 0.05)
Histological structure of wounded skin sections. a–f Hematoxylin and eosin staining of wounded skin sections in the various groups at day 14. g–l Masson staining of wounded skin at day 14. Mice treated with CM (c, i) showed a fast degree of tissue reorganization and replacement of skin appendages compared with control mice (b, h); treatment with ERK inhibitors+CM (d, j), JNK inhibitors+CM (e, k) and AKT inhibitors+CM (f, l) significantly impaired wound healing; normal skin (a, g) acted as another control. The area between the two vertical black lines indicates unhealed skin. Bar 500 μm
Growth factors implicated in proliferative and migration activity of HAESCs
To clarify that the activities of the AKT and MAPK pathways and the promotion of growth and migration were not attributable to other cell lines, serum, or growth factors, we investigated the intracellular signaling pathways with adult fibroblasts, platelet-derived growth factor (PDGF) and 10 % FBS as shown in Fig. S3a, b. The results revealed that, compared with CM from fibroblasts, ERK phosphorylation was activated in the treatment with PDGF for 1 h (*P < 0.05); ERK, JNK and AKT phosphorylation were activated after the treatment with HAESC-CM (*P < 0.05), whereas ERK, JNK, p38 and AKT phosphorylation were all activated after treatment with 10 % serum (*P < 0.05).
We further conducted an online search of PubMed and critically analyzed the literature regarding the role of growth factors and cytokines in the management of wound healing. Here, we discuss six growth factors and cytokines currently being used for the healing of wounds. These include: epithelialization growth factors, namely EGF, KGF and PDGF and chemotactic factors, namely transforming growth factor-beta 1 (TGF-β1), stromal cell-derived factors (SDF-1) and CXC chemokine ligand-5 (CXCL-5; Hofmann and Abramowicz 1990; Lang and Searle 1994; Lee et al. 2010; Mamede et al. 2012; Wang and Cheng 2009). In our efforts to clarify which of the above factors was responsible for the mitogenic action of HAESCs, we explored the growth factors in CM from human dermal fibroblasts, human amnion mesenchymal cells, or human amniotic epithelial cells by real-time reverse transcription. Our data (Fig. S3c-h) showed that, compared with CM from fibroblasts, treatment with HAESC-CM gave high expression of the genes EGF, KGF, PDGF, CXCL-5 and SDF-1 and low expression of the genes TGF-β1; compared with CM from amniotic mesenchymal stem cells, treatment with HAESC-CM gave high expression of the genes EGF, KGF and CXCL-5 and low expression of the genes PDGF, TGF-β1 and SDF-1. The morphology of the human amniotic mesenchymal stem cells and HAESCs is shown in Fig. S4.
Discussion
Wound healing requires the successful completion of orchestrated biochemical and cellular events. Overlapping phases of inflammation, proliferation and migration are common to wounds of all etiologies (Fong et al. 2010). Stem cells are known to manipulate their environment and to regulate wound healing by secreting bioactive factors (Blanpain 2010). The human amniotic membrane contains amnion epithelial cells and amnion mesenchymal stromal cells. Human amniotic epithelial cells are sources of diverse progenitor cell populations and possibly more primitive stem cells (Park et al. 2012). Growing evidence has shown that HAESCs secrete a number of proteins and cytokines (Miki et al. 2005) that potentially affect cellular growth and proliferation and that play indispensable roles in the wound healing process (Montesano and Orci 1988). Further studies have shown that human amniotic fluid stimulates the proliferation of human fetal and adult skin fibroblasts during wound healing. These effects might be attributed to the presence of growth factors in the amniotic fluid, especially bFGF and PDGF (Chrissouli et al. 2010). Interestingly, our results suggest that HAESC-CM significantly promotes the proliferation of keratinocytes and that this mitogenic effect of wound healing is attributable to the bioactive factors secreted by HAESCs.
Cyclin D is a known member of the cyclin protein family, which is involved in regulating cell cycle progression. The synthesis of Cyclin D is initiated during G1 and drives the G1/S phase transition. Therefore, Cyclin D is an important regulator of cell cycle progression and can function as a transcriptional co-regulator in various cell types and particularly in scratch areas in wound healing (Bramanti et al. 2010). In the present study, we observed that, in the co-culture group, more keratinocytes are present in S phase than those in the control group. The co-cultured keratinocytes also exhibit markedly increased expression of the cell-proliferation-related proteins Cyclin D1 and Cyclin D3. Our findings are in agreement with the data reported by others who have shown that fresh amniotic fluid accelerates the repair process in various adult tissues and improves the quality of repair by activating mitosis (Bazrafshan et al. 2014). Mdm2, an intracellular molecule with multiple biological functions, is an E3 ubiquitin ligase that negatively regulates p53 mainly by ubiquitin-mediated degradation and as such, Mdm2 suppresses coordinated cell cycle arrest or apoptosis and promotes cell survival and growth (Vazquez et al. 2008). Evidence is growing that Mdm2 has a number of p53-independent functions in cell cycle regulation, differentiation, transcription, or DNA synthesis (Marine and Lozano 2010). The results of our study revealed that HAESC-CM might promote the proliferation of keratinocytes through the up-regulation of Mdm2.
Previous studies have shown that amnion-derived cellular cytokine solution promotes the migration of fibroblasts during the process of wound healing (Uberti et al. 2010). In our present study, we made a full-thickness excisional skin wound model in mice and topically applied HAESC-CM to the wound. Our results demonstrate that the topical application of CM also significantly accelerates the rate of wound healing in mice and the formation of skin appendages such as hair follicle and sebaceous gland. Despite the understanding of HAESCs function in physiological processes, the mechanisms by which the these stem cells regulate wound healing are still poorly understood. As several studies have indicated, the MAPK pathways (including JNK, ERK and p38) and the AKT pathway play a crucial role in skin wound healing (Barrientos et al. 2014, 2008; Chrissouli et al. 2010; Lima et al. 2012). To clarify which signaling pathways are involved in the regulation of keratinocytes migration by HAESC-CM, we investigated the specific role of MAPKs and AKT signaling pathways in CM-accelerated keratinocyte migration. In our study, the cultured keratinocytes show an obvious increase in ERK, JNK and AKT activity after treatment with CM. The inhibition of ERK, JNK and AKT phosphorylation significantly inhibits the migration of keratinocytes promoted by CM (see also Figs. S5, S6). This implies that HAESCs facilitate the wound healing process by the ERK, JNK and AKT signaling pathways but not by the p38 signaling pathways. Accumulating evidence has suggested that ERK plays a crucial role in the regulation of cell motility and migration (Kanaji et al. 2013; Zhang et al. 2014). The inhibition of ERK activation results in markedly reduced movement of endothelial cells (Matsubayashi et al. 2004). Further study has indicated that the spreading and migrating features of leading-edge cells in embryonic dorsal closure and wound healing are controlled by genes of the JNK signaling pathway (Bosch et al. 2005). p38-MAPK is reportedly essential for the migration of cultured keratinocytes (Klekotka et al. 2001). However, our results suggest no significant phospho-p38 activity occurs after treatment with HAESC-CM.
Previous investigations have demonstrated the presence of various known growth factors, such as EGF, KGF, PDGF, TGF-β1, CXCL-5 and SDF-1, in amniotic fluid. In our efforts to clarify which of the above factors is responsible for the mitogenic action of amnion, we explored the growth factors in CM from human dermal fibroblasts, human amnion mesenchymal cell, or human amniotic epithelial cells by real-time reverse transcription and our data show that the factors mainly responsible for this stimulatory action are EGF and KGF. Furthermore, the observed data reveal that HAESCs probably secrete a number of chemotactic proteins or chemotactic cytokines, including CXCL-5 and SDF-1, which potentially affect cell migration. This is in agreement with results showing that CXCL-5 and SDF-1 are potent stimulators of keratinocyte migration (Mishra et al. 2012).
In summary, we provided direct evidence, for the first time, that HAESCs promote skin wound healing by accelerating keratinocyte proliferation and migration via the ERK, JNK and AKT signal pathways. Results from our group indicate that HAESCs is a promising therapeutic strategy for the management of skin wounds. Notably, HAESC-CM might regulate additional genes that participate in the ERK, JNK and AKT pathways in skin wound healing. Thus, the detailed signal transduction cascade underlying HAESC-CM-induced wound healing needs to be further studied.
Abbreviations
- HAESCs:
-
Human amniotic epithelial stem cells
- EGF:
-
Epidermal growth factor
- CM:
-
Conditioned medium
- GFP:
-
Green fluorescence protein
- Mdm2:
-
Murine double minute-2
References
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I (1981) Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2:1003–1005
Bai X, Pinkernell K, Song YH, Nabzdyk C, Reiser J, Alt E (2007) Genetically selected stem cells from human adipose tissue express cardiac markers. Biochem Biophys Res Commun 353:665–671
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601
Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M (2014) Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 22:569–578
Bazrafshan A, Owji M, Yazdani M, Varedi M (2014) Activation of mitosis and angiogenesis in diabetes-impaired wound healing by processed human amniotic fluid. J Surg Res 188:545–552
Blanpain C (2010) Stem cells: skin regeneration and repair. Nature 464:686–687
Bosch M, Serras F, Martin-Blanco E, Baguna J (2005) JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol 280:73–86
Bramanti V, Tomassoni D, Bronzi D, Grasso S, Curro M, Avitabile M, Li Volsi G, Renis M, Ientile R, Amenta F, Avola R (2010) Alpha-lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase expression in astroglial cell cultures. Neurochem Res 35:2070–2077
Chrissouli S, Pratsinis H, Velissariou V, Anastasiou A, Kletsas D (2010) Human amniotic fluid stimulates the proliferation of human fetal and adult skin fibroblasts: the roles of bFGF and PDGF and of the ERK and AKT signaling pathways. Wound Repair Regen 18:643–654
Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T, Cicione C, Rendal-Vazquez ME, Fuentes-Boquete I, Toro FJ de, Blanco FJ (2011) Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation 81:162–171
Fong E, Tzlil S, Tirrell DA (2010) Boundary crossing in epithelial wound healing. Proc Natl Acad Sci U S A 107:19302–19307
Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC (2004) Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12:485–492
Grueterich M, Espana EM, Tseng SC (2003) Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol 48:631–646
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Hofmann GE, Abramowicz JS (1990) Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta Obstet Gynecol Scand 69:217–221
Kanaji N, Nelson A, Wang X, Sato T, Nakanishi M, Gunji Y, Basma H, Michalski J, Farid M, Rennard SI, Liu X (2013) Differential roles of JNK, ERK1/2, and p38 mitogen-activated protein kinases on endothelial cell tissue repair functions in response to tumor necrosis factor-alpha. J Vasc Res 50:145–156
Klekotka PA, Santoro SA, Zutter MM (2001) Alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J Biol Chem 276:9503–9511
Lang AK, Searle RF (1994) The immunomodulatory activity of human amniotic fluid can be correlated with transforming growth factor-beta 1 (TGF-beta 1) and beta 2 activity. Clin Exp Immunol 97:158–163
Lazzarini R, Olivieri F, Ferretti C, Mattioli-Belmonte M, Di Primio R, Orciani M (2014) mRNAs and miRNAs profiling of mesenchymal stem cells derived from amniotic fluid and skin: the double face of the coin. Cell Tissue Res 355:121–130
Lee DC, Romero R, Kim CJ, Chaiworapongsa T, Tarca AL, Lee J, Suh YL, Mazaki-Tovi S, Vaisbuch E, Mittal P, Draghici S, Erez O, Kusanovic JP, Hassan SS, Kim JS (2010) Surfactant protein-A as an anti-inflammatory component in the amnion: implications for human pregnancy. J Immunol 184:6479–6491
Lima MH, Caricilli AM, Abreu LL de, Araujo EP, Pelegrinelli FF, Thirone AC, Tsukumo DM, Pessoa AF, Santos MF dos, Moraes MA de, Carvalheira JB, Velloso LA, Saad MJ (2012) Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One 7:e36974
Liu J, Pang Y, Chen J, Huang P, Huang W, Zhu X, Yan D (2012) Hyperbranched polydiselenide as a self assembling broad spectrum anticancer agent. Biomaterials 33:7765–7774
Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349:447–458
Manuelpillai U, Lourensz D, Vaghjiani V, Tchongue J, Lacey D, Tee JY, Murthi P, Chan J, Hodge A, Sievert W (2012) Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One 7:e38631
Marine JC, Lozano G (2010) Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ 17:93–102
Mariotti C, Lazzarini R, Nicolai M, Saitta A, Orsini E, Orciani M, Di Primio R (2015) Comparative study between amniotic-fluid mesenchymal stem cells and retinal pigmented epithelium (RPE) stem cells ability to differentiate towards RPE cells. Cell Tissue Res 362:21–31
Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E (2004) ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr Biol 14:731–735
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC (2005) Stem cell characteristics of amniotic epithelial cells. Stem Cells 23:1549–1559
Mishra PJ, Mishra PJ, Banerjee D (2012) Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells 4:35-43
Montesano R, Orci L (1988) Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A 85:4894–4897
Navone SE, Pascucci L, Dossena M, Ferri A, Invernici G, Acerbi F, Cristini S, Bedini G, Tosetti V, Ceserani V, Bonomi A, Pessina A, Freddi G, Alessandrino A, Ceccarelli P, Campanella R, Marfia G, Alessandri G, Parati EA (2014) Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Curr Stem Cell Res Ther 5:7
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 15:88–99
Park SB, Seo MS, Kim HS, Kang KS (2012) Isolation and characterization of canine amniotic membrane-derived multipotent stem cells. PLoS One 7:e44693
Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first International Workshop on Placenta Derived Stem Cells. Stem Cells 26:300–311
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care 3:445–464
Ravishanker R, Bath AS, Roy R (2003) “Amnion Bank”—the use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns 29:369–374
Sabapathy V, Ravi S, Srivastava V, Srivastava A, Kumar S (2012) Long-term cultured human term placenta-derived mesenchymal stem cells of maternal origin displays plasticity. Stem Cells Int 2012:174328
Uberti MG, Pierpont YN, Ko F, Wright TE, Smith CA, Cruse CW, Robson MC, Payne WG (2010) Amnion-derived cellular cytokine solution (ACCS) promotes migration of keratinocytes and fibroblasts. Ann Plast Surg 64:632–635
Vazquez A, Bond EE, Levine AJ, Bond GL (2008) The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov 7:979–987
Wang PH, Cheng MH (2009) Amniotic fluid cytokines predict pregnancy outcome: myth or reality? J Chin Med Assoc 72:617–618
Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, Griensven M van, Stadler G, Redl H, Gabriel C (2007) Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 13:1173–1183
Wolfrum K, Wang Y, Prigione A, Sperling K, Lehrach H, Adjaye J (2010) The LARGE principle of cellular reprogramming: lost, acquired and retained gene expression in foreskin and amniotic fluid-derived human iPS cells. PLoS One 5:e13703
Yamahara K, Harada K, Ohshima M, Ishikane S, Ohnishi S, Tsuda H, Otani K, Taguchi A, Soma T, Ogawa H, Katsuragi S, Yoshimatsu J, Harada-Shiba M, Kangawa K, Ikeda T (2014) Comparison of angiogenic, cytoprotective, and immunosuppressive properties of human amnion- and chorion-derived mesenchymal stem cells. PLoS One 9:e88319
Yang C, Lei D, Ouyang W, Ren J, Li H, Hu J, Huang S (2014) Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int 2014:109389
Zhang M, Liu NY, Wang XE, Chen YH, Li QL, Lu KR, Sun L, Jia Q, Zhang L, Zhang L (2011) Activin B promotes epithelial wound healing in vivo through RhoA-JNK signaling pathway. PLoS One 6:e25143
Zhang M, Sun L, Wang X, Chen S, Kong Y, Liu N, Chen Y, Jia Q, Zhang L, Zhang L (2014) Activin B promotes BMSC-mediated cutaneous wound healing by regulating cell migration via the JNK-ERK signaling pathway. Cell Transplant 23:1061–1073
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Bin Zhao, Jia-Qi Liu and Zhao Zheng contributed equally to this work.
This work was supported by the National Health and Family Planning Commission of China (2015SQ00060) and the National Natural Science Foundation of China (81201470).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Labeling of keratinocytes with green fluorescent protein (GFP). Flow cytometry analysis of GFP-keratinocytes. Results show that the transfection efficiency was as high as 98.3 % (n=3). (GIF 36 kb)
Fig. S2
Western blot analyses of the effect of CM on the p38 pathway. No significant phospho-p38 activity is seen after treatment with HAESC-CM. (GIF 13 kb)
Fig. S3
a, b Western blot analyses of effect of adult fibroblasts, PDGF, and 10 % fetal bovine serum on MAPK and AKT pathways and related densitometric analysis. Compared with conditioned medium (CM) from fibroblasts, ERK phosphorylation was activated by treatment with PDGF for 1 h (*p<0.05); ERK, JNK and AKT phosphorylation was activated after the treatment with HAESC-CM (*p<0.05), whereas ERK, JNK, p38 and AKT phosphorylation was activated after treatment with 10 % serum (*p<0.05). c–h Growth factor and cytokines expression. Six growth factors and cytokines EGF, KGF, PDGF, TGF-β1, SDF-1 and CXCL-5 were quantified by real-time polymerase chain reaction (PCR). Changes in the expression of each target gene were measured relative to the mean critical threshold (CT) values of the GAPDH housekeeping gene by the ΔCt method. Compared with CM from fibroblasts, treatment with HAESC-CM showed high expression of the genes EGF, KGF, PDGF, CXCL-5 and SDF-1 and low expression of the TGF-β1 gene. Compared with CM from amniotic mesenchymal stem cells, HAESC-CM showed high expression of the genes EGF, KGF and CXCL-5 and low expression of the genes PDGF, TGF-β1 and SDF-1. (GIF 22 kb)
Fig. S4
a, b Morphology of human amniotic mesenchymal stem cells (HAMCs) and human amniotic epithelial stem cells (HAESCs). HAMCs (a) show a typical fibroblast-like morphology, whereas HAESCs (b) have a cobblestone-like morphology. c Self-renewal ability of HAMCs and HAESCs. From the curve, HAMCs reach logarithmic growth phase on day 3 and HAESCs reach logarithmic growth phase on day 4. (GIF 42 kb)
Fig. S5
Effects of inhibitors on the cell cycle of keratinocytes. a–d Analysis of the cell cycle in control and inhibitor groups. Keratinocytes were treated with ERK inhibitor PD98059 (10 μM) JNK inhibitor SP600125 (10 μM) and AKT inhibitor LY294002 (10 μM), and DNA synthesis was analyzed by FACSAria III flow cytometry. (GIF 55 kb)
Fig. S6
Effects of inhibitors on the cell apoptosis of keratinocytes. a–d Analysis of cell apoptosis in control and inhibitor groups by using the FITC Annexin V apoptosis detection kit. A significant increase in apoptosis and dead cells was seen in keratinocytes treated with AKT inhibitor LY294002 (10 μM), whereas no obvious differences were detected after treatment with 10 μM ERK inhibitor PD98059 and JNK inhibitor SP600125. (GIF 45 kb)
Fig. S7
Scratch wound assay. a Keratinocytes grown in serum-free medium (control). b Keratinocytes grown in HAESC-CM. c Keratinocytes pre-incubated with mitomycin C and treated with HAESC-CM. d Keratinocytes grown in HAESC-CM and ERK inhibitor PD98059 (the treatment medium was changed every 3 h). e To prevent cell proliferation, group c was pre-incubated with mitomycin C for 1 h. f Keratinocytes grown in HAESC-CM and JNK inhibitor SP600125 (the treatment medium was changed every 3 h). g To prevent cell proliferation, group g was pre-incubated with mitomycin C for 1 h. h Keratinocytes grown in HAESC-CM and AKT inhibitor LY294002 (the treatment medium was changed every 3 h). i To prevent cell proliferation, group h was pre-incubated with mitomycin C for 1 h. j Histogram quantifying the cell migration area in each group at the various time points. (GIF 112 kb)
Movie. S1–S11 Real-time dynamic laser scanning confocal microscopy images of scratch wound assay.
Movie. S1
Scratch wound assay of keratinocytes migration treated with CM (group a, 0–24 h). (AVI 1557 kb)
Movie. S2
Scratch wound assay of keratinocytes migration exposed to serum free DMEM only as control group (group b, 0–24 h). (AVI 1575 kb)
Movie. S3
To prevent cell proliferation, group c was pre-incubated with mitomycin C and treated with CM (0–24 h). (AVI 1548 kb)
Movie. S4
To prevent cell proliferation, group d was pre-incubated with mitomycin C and treated with serum free DMEM (0–24 h). (AVI 1537 kb)
Movie. S5
Scratch wound assay of keratinocyte migration treated with CM (0–24 h). (AVI 1601 kb)
Movie. S6
Scratch wound assay of migration of keratinocytes pre-incubated with ERK inhibitor PD98059 and treated with CM (0–24 h). (AVI 1147 kb)
Movie. S7
Scratch wound assay of migration of keratinocytes pre-incubated with mitomycin C and ERK inhibitor PD98059 and treated with CM (0–24 h). (AVI 1474 kb)
Movie. S8
Scratch wound assay of migration of keratinocytes pre-incubated with JNK inhibitor SP600125 and treated with CM (0–24 h). (AVI 1546 kb)
Movie. S9
Scratch wound assay of migration of keratinocytes pre-incubated with mitomycin C and JNK inhibitor SP600125 and treated with CM (0–24 h). (AVI 1303 kb)
Movie. S10
Scratch wound assay of migration of keratinocytes pre-incubated with AKT inhibitor LY294002 and treated with CM (0–24 h). (AVI 1141 kb)
Movie. S11
Scratch wound assay of migration of keratinocytes pre-incubated with mitomycin C and AKT inhibitor LY294002 and treated with CM (0–24 h). (AVI 1545 kb)
Time lapse images of cell growth and motility were continuously acquired every 30 min by FV10i confocal microscopy. Data were analyzed with FV10-ASW 3.0 software. Images were captured at high resolution (1024×1024) with a 10× objective and speed (1×) scans. Dynamic video was recorded in the AVI format (frame rate = 600 ms/frame).
Rights and permissions
About this article
Cite this article
Zhao, B., Liu, JQ., Zheng, Z. et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res 365, 85–99 (2016). https://doi.org/10.1007/s00441-016-2366-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2366-1