Abstract
Uncultured bone marrow mononuclear cells (BMMC) were recently used to successfully repair damaged cartilage. However, the effect of BMMCs on the proliferation and differentiation of chondrocytes that are critical to cartilage repair is unclear. Here, we investigate the influence of BMMCs on chondrocyte dedifferentiation in pellet culture. We isolated and mixed BMMCs and chondrocytes in a 1:1 (BMMC/C) ratio and cultured in pellets (1.6 × 106 cells per pellet) for 2, 4, or 8 weeks. Chondrocyte differentiation was evaluated using macrography, histological examination, immunohistochemistry and gene expression analysis. While a transparent and smooth surface was observed in both BMMC/C and chondrocyte cultures over time, the former was smaller in size after 2 and 4 weeks of culture. Interestingly, after 8 weeks, BMMC/C cultures became significantly larger than chondrocyte cultures (P = 0.003). The distribution of a cartilage-specific extracellular matrix (ECM), that includes components like glycosaminoglycan (GAG) and type II collagen, was gradually reduced in chondrocyte cultures. On the other hand, while we found no obvious differences in the ECM in BMMC/C cultures between 2 and 4 weeks in vitro, after 8 weeks the concentration of ECM components decreased significantly. Further, we detected an upregulation of cartilage-specific genes in BMMC/C cultures, when compared with chondrocytes. Altogether, we demonstrate that co-culture with BMMCs delays the dedifferentiation of chondrocytes in pellet cultures in vitro. This suggests that uncultured BMMC, which can be quickly and safely obtained, could serve as a potential alternative cell source for engineering of cartilage tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restoring the structure and function of articular cartilage following an injury is a crucial problem in orthopedic therapy (Schindler 2011). Current strategies to repair damaged cartilage include bone marrow stimulation (Mithoefer et al. 2005; Steadman et al. 2003), autologous matrix-induced chondrogenesis (AMIC) (Benthien and Behrens 2011), autologous osteochondral grafting (Hangody and Füles 2003), autologous chondrocyte implantation (ACI) (Peterson et al. 2010) and knee arthroplasty (Carr et al. 2012). Amongst these, ACI has the most curative potential to repair cartilage lesions, as noted during long-term follow-up (Beris et al. 2012; Brittberg 2008; Peterson et al. 2010). For example, at 12 months of follow-up, knee function and the quality of life were markedly improved by this method and this recovery was sustained up to 24 months following treatment (Minas 1998). Another study reported significant graft survival and functional outcome in patients at 36 months of follow-up after ACI (Micheli et al. 2001). Other reports noted good clinical outcome in patients diagnosed with various degrees of cartilage defects and treated with ACI at 2–9 years of follow-up. Finally, the best outcomes were obtained in patients diagnosed with an isolated defect in the femoral condyle (Peterson et al. 2000).
Despite promising outcomes, ACI has several disadvantages for use in clinical therapy. First, ACI is a two-step procedure, increasing the chances of damage to the donor site from where the cartilage samples are collected. Second, the limited availability of donor cartilage tissue is a problem because the curative effect of ACI can be reduced by an inadequate number of chondrocytes obtained from in vitro culture of the donor cartilage. Third, the implanted chondrocytes are often unevenly distributed and leak easily from the site of injury (Sohn et al. 2002). Fourth, the repaired tissue consists in a combination fibrocartilage and hyaline cartilage (McNickle et al. 2009). Finally, multiple passaging may alter the properties of chondrocytes, specifically, downregulation of the cartilage-specific extracellular matrix (ECM) and upregulation of fibrotic genes (Benya et al. 1978; Lin et al. 2008; von der Mark et al. 1977). Many researchers, therefore, have focused on using mesenchymal stromal (stem) cells (MSCs) derived from various sources (bone marrow, umbilical cord blood, adipose tissue, synovial tissue) to treat damaged cartilage (Koga et al. 2009). Amongst these, bone marrow mesenchymal stem cells (BMSCs) have been widely studied due to their abundant availability and reduced donor site morbidity (Beane and Darling 2012; Inui et al. 2012; Matsumoto et al. 2010). However, in vitro BMSC culture can be challenging because of the need for prolonged culture leading to elevated risk of contamination and high cost (Wise et al. 2014). Moreover, complete purification of MSCs is difficult, their differentiation cannot be controlled precisely and the mechanism of migration of MSCs remains unclear (Chamberlain et al. 2007).
Recently, several groups have reported isolating uncultured bone marrow mononuclear cells (BMMC) for cartilage tissue engineering (Chang et al. 2008; Wise et al. 2014; Zhang et al. 2012). The mononuclear fraction of bone marrow is a primary source of cytokines and growth factors and can be obtained easily without the requirement of long-term culture expansion (Balakumaran et al. 2010). Meanwhile, other studies have found paracrine interactions between BMSCs and other cells derived from the bone marrow mononuclear fraction, including hematopoietic stem cells and endothelial progenitor cells (Wise et al. 2014). Others have shown that a trophic and stable microenvironment can be provided to induce proliferation and differentiation of BMMCs, which could in turn assist the repair of damaged cartilage (Chang et al. 2008; Zhang et al. 2012). Importantly, a recent study showed that a combination of uncultured BMMCs and isolated chondrocytes could improve cartilage regeneration in a microfracture (Bekkers et al. 2013). However, there are no reports on the effect of BMMCs on the proliferation and differentiation of chondrocytes in pellet culture. This is important for chondrocyte-based cartilage repair. In the present study, we successfully test the hypothesis that uncultured BMMCs could delay or inhibit dedifferentiation of unexpanded chondrocytes in pellet culture and promote the expression of cartilage-specific ECM.
Materials and methods
Isolation of BMMCs and chondrocytes
All animal experiments were approved by the Institutional Review Board and the Animal Research Committee of Nanjing Medical University. BMMCs were obtained according to our previously published method (Wei et al. 2014). Briefly, bone marrow was aspirated from the posterior superior iliac spine of 24 female New Zealand white rabbits (5–6 months old), mixed with saline and added to a 15-ml conical tube containing Percoll (Pharmacia, USA) at a 1:1:1 ratio of bone marrow:saline:Percoll. BMMCs were isolated form this mixture by density gradient centrifugation at 500g for 5 min.
To isolate cartilage tissue, a medial parapatellar incision was made to expose the femur condyle. A biopsy punch (6 mm in diameter; Corning, USA) was used to obtain the samples, which were then digested using a rapid digestion procedure described previously (Bekkers et al. 2013). Briefly, samples were cut into pieces and digested in 2 % type II collagenase (Gibco, USA) at 37 °C for 45 min with continuous shaking. The resulting cell suspension was centrifuged at 600g for 10 min and the pellet of chondrocytes was washed three times with phosphate-buffered saline (PBS).
Preparation of BMMC/C and chondrocyte pellets and macroscopic examination
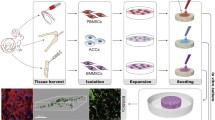
Following sample collection, all animals were euthanized using an overdose of sodium pentobarbital. Uncultured BMMCs were mixed with digested chondrocytes in a 1:1 ratio and centrifuged at 500g for 10 min in order to prepare the pellets (BMMC/C; 1.6 × 106 cells per pellet). In parallel, a suspension of chondrocytes was centrifuged at 500g for 10 min to create chondrocyte pellets (C; 1.6 × 106 cells per pellet) (Fig. 1). All pellets were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10 % fetal bovine serum (FBS; Gibco) and 1 % antibiotics-antimycotics and incubated at 37 °C.
The preparation of BMMC/C and chondrocyte pellets. Uncultured BMMCs were mixed with digested chondrocyte cell suspension at a 1:1 ratio and centrifuged to prepare BMMC/C pellets (1.6 × 106 cells per pellet). Unexpanded chondrocytes were centrifuged separately to create chondrocyte pellets for culture (1.6 × 106 cells per pellet)
The gross morphology of BMMC/C and chondrocyte pellets was examined after 2, 4 and 8 weeks of culture in vitro. The size of the pellets was accurately measured using Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA, USA).
Histology and immunohistochemistry
Harvested cultured pellets were fixed in 10 % neutral formalin, dehydrated through an ethanol gradient series, cleared in xylene and embedded in paraffin. Paraffin sections, 5 μm in thickness, were stained with Safranin-O/Fast-Green (Safranin-O) to detect the distribution of glycosaminoglycan (GAG).
For immunohistochemical staining, 5 μm thick paraffin sections were incubated with proteinase K for 30 min, 3 % H2O2–methanol for 10 min and 10 % normal goat serum for 30 min. Next, slides were incubated with the following primary antibodies for 90 min: mouse anti-type I collagen (Sigma, St. Louis, MO, USA), mouse anti-type II collagen (Novus Biologicals, Littleton, CO, USA) and rabbit anti-type X collagen (Abcam, Shatin, N.T., Hong Kong). The sections were then sequentially treated with goat anti-mouse/rabbit secondary antibody (Nanjing KeyGen Biotech, Jiangsu, China) for 30 min, followed by DAB for 3–5 min and Mayer’s hematoxylin (Sigma) for 8 min. They were finally mounted with neutral balsam for microscopic examination (Ci-L; Nikon, Tokyo, Japan).
Real-time polymerase chain reaction (RT-PCR) analysis
Harvested pellets were washed three times with PBS and total RNA was isolated by treating the samples with Trizol (Invitrogen, CA, USA) for 10 min. Next, they were reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA). RT-PCR was performed on an ABI Prism 7,500 sequence detection system (Applied Biosystems) and used to detect the expression of the following genes: aggrecan (Acan), type I collagen (Col1a1), type II collagen (Col2a1) and type X collagen (Col10a1). The housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The following primer sequences were used for RT-PCR: Acan forward: ATGGCTTCCACCAGTGCG, reverse: CGGATGCCGTAGGTTCTCA; Col1a1 forward: GCGGTGGTTACGACTTTGGTT, reverse: AGTGAGGAGGGT CTCAATCTG; Col2a1 forward: CAGGCAGAGGCAGGAAACTAAC, reverse: CAGAGGTGTTTGACACGGAGTAG; Col10a1 forward: ATCAGCCACTGGGAA GCC, reverse: TTCGGTCCACTTGGTCCTC; GAPDH forward: CGTCTGCCCT ATCAACTTTCG, reverse: CGTTTCTCAGGCTCCCTCT (Wang et al. 2010). The cDNA samples were amplified for 40 cycles.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) post hoc test were performed to compare differences in size and gene expression between different durations of culture within the BMMC/C and chondrocyte groups. Moreover, the unpaired Student’s t test was used to compare differences in size and gene expression between the two experimental groups at different durations of culture. P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0 statistical software (SPSS, Chicago, IL, USA).
Results
Gross morphology and size of BMMC/C and chondrocyte pellets
We performed macroscopic examination of BMMC/C and chondrocyte pellet cultures and found that both have a smooth and transparent surface that started to resemble native cartilage with increasing time in culture (Fig. 2a–f). The size of chondrocyte pellet cultures decreased gradually with increasing duration of culture. The size of the BMMC/C pellets at 4 weeks was less than that at 2 and 8 weeks, respectively (P < 0.001); however, there was no significant difference in size between 2 and 8 weeks of culture (P = 0.343). Importantly, the size of the hondrocyte pellet cultures was significantly greater than the BMMC/C pellets after 2 and 4 weeks in culture (P < 0.001). However, after 8 weeks in culture, we found the MMC/C cultures to be significantly larger than the chondrocyte cultures (P = 0.003) (Fig. 2g).
The size and morphology of BMMC/C and chondrocyte pellets. a–f Both BMMC/C and chondrocyte pellets develop a transparent and smooth surface over time, resembling native cartilage. g Chondrocyte pellet size decreased gradually with increasing duration of culture. BMMC/C pellets are smaller than chondrocyte pellets at 2 and 4 weeks (P < 0.001) but larger at 8 weeks of culture (P = 0.003). **P < 0.01, ***P < 0.001
Histological evaluation of BMMC/C and chondrocyte pellets
We next analyzed the cellular characteristics of both types of pellet cultures by first labeling GAG using Safranin-O staining. We found that the distribution of GAG in the chondrocyte pellets decreased gradually from the periphery towards the center with increasing duration of culture (Fig. 3d–f). At 2 weeks of culture, GAG concentration in the BMMC/C was slightly reduced around the periphery of the pellets (Fig. 3a). After 4 weeks of culture, no obvious loss in the accumulation of GAG was detected in the BMMC/C (Fig. 3b). However, after 8 weeks of culture, staining for GAG was reduced and unevenly distributed in the BMMC/C pellets (Fig. 3c). Moreover, in comparison to the chondrocyte cultures, cells in the BMMC/C pellets proliferated and aggregated gradually with time (Fig. 3).
Distribution of GAG in BMMC/C and chondrocyte pellets. a Slightly reduced accumulation of GAG around the periphery of BMMC/C pellets at 2 weeks. b No obvious loss of GAG distribution in BMMC/C pellets at 4 weeks. c Uneven and reduced distribution of GAG in BMMC/C pellets after 8 weeks of culture. d–f Gradual decrease in GAG distribution from the periphery towards the center of chondrocyte pellets over time. Original magnification ×40, detailed magnification ×100
Immunohistochemical evaluation of BMMC/C and chondrocyte pellet cultures
Similar to Safranin-O staining, type II collagen levels in chondrocyte cultures were also reduced gradually with increasing time in culture (Fig. 4d–f). We detected no obvious decrease in the distribution of type II collagen after both 2 as well as 4 weeks of culture (Fig. 4a, b). However, after 8 weeks in vitro, we observed a reduced and uneven distribution of type II collagen (Fig. 4c). Type I and type X collagen were mostly distributed in the outermost layer of chondrocyte pellet cultures at 2 and 4 weeks (Figs. 5d, e, 6d, e). While type I collagen levels increased slightly after 8 weeks (Fig. 5f), we found no obvious difference in the staining for type X collagen (Fig. 6f). In the BMMC/C pellet cultures, we detected no expression of either type I or type X collagen at 2 and 4 weeks of culture (Figs. 5a, b, 6a, b). However, after 8 weeks in vitro, both were found to be distributed around the periphery of the BMMC/C cultures (Figs. 5c, 6c).
Concentration of type II collagen in BMMC/C and chondrocyte pellets. a, b No obvious loss of type II collagen in the BMMC/C pellets at 2 and 4 weeks. c Uneven and reduced distribution of type II collagen in the BMMC/C pellets after 8 weeks of culture. d–f Reduction in the accumulation of type II collagen from the periphery towards the center of the chondrocyte pellets over time. Original magnification ×40, detailed magnification ×100
Concentration of type I collagen in BMMC/C and chondrocyte pellets. a, b No obvious changes in the distribution of type I collagen in the BMMC/C pellets at 2 and 4 weeks. c Distribution of type I collagen in the periphery of the BMMC/C pellets at 8 weeks of culture. d, e Distribution of type I collagen in the outermost layer of the chondrocyte pellets at 2 and 4 weeks. f Slightly increased accumulation of type I collagen in the chondrocyte pellets at 8 weeks of culture. Original magnification ×40, detailed magnification ×100
Concentration of type X collagen in the BMMC/C and chondrocyte pellets. a, b No obvious changes in the distribution of type X collagen in the BMMC/C pellets at 2 and 4 weeks. c Distribution of type X collagen in the periphery of BMMC/C pellets at 8 weeks. d, e Distribution of type X collagen in the outermost layer of the chondrocyte pellets at 2 and 4 weeks. f Absence of type X collagen in the chondrocyte pellets after 8 weeks of culture. Original magnification ×40, detailed magnification ×100
Gene expression analysis of BMMC/C and C pellets
We next performed RT-PCR analysis to quantify the expression level of chondrocyte-specific genes. While Acan expression decreased gradually in the chondrocyte cultures, in the BMMC/C pellets, its expression first underwent an increase, then decreased significantly (P = 0.026). At different time points during culture, the level of Acan expression in the BMMC/C pellets was significantly higher than in the chondrocyte pellets (Fig. 7a). The expression of Col2a1 was similar to that of Acan in both the BMMC/C and chondrocyte cultures at all time points in vitro. Importantly, while we found a significantly higher expression of Col2a1 in the BMMC/C pellets in comparison to the chondrocyte pellets at 4 and 8 weeks of culture, no difference was detected at 2 weeks (P = 0.319) (Fig. 7b). The expression of Col1a1 showed no obvious difference in the BMMC/C pellets with varying periods in culture (P > 0.05). In contrast, in the hondrocyte pellets, Col1a1 levels were significantly higher at 8 weeks than at 4 weeks of culture (P = 0.023). In addition, between the two experimental groups, we found significantly higher Col1a1 expression in the chondrocytes pellets after 8 weeks in vitro (P = 0.034) (Fig. 7c). Finally,we found no difference between the BMMC/C and chondrocyte pellets, in its expression at different time points during culture (P > 0.05) (Fig. 7d).
Gene expression analysis of the BMMC/C and chondrocyte pellets. a Initial insignificant increase in Acan expression is followed by a significant decrease in the BMMC/C pellets (P = 0.026); gradual decrease in expression in the chondrocyte pellets; significantly higher Acan expression in the BMMC/C pellets compared to the chondrocyte pellets at different time points during culture. b Significantly higher expression of Col2a1 in the BMMC/C than the chondrocyte pellets at 4 and 8 weeks. c Significant upregulation of Col1a1 in the chondrocyte pellets at 8 weeks than at 4 weeks (P = 0.023). Significantly higher expression of Col1a1 in the chondrocyte pellets compared to the BMMC/C pellets after 8 weeks of culture (P = 0.034). d No significant difference in Col10a1 expression between the BMMC/C and chondrocyte pellets at different durations of culture (P > 0.05). *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
In the present study, we investigated the effect of uncultured BMMCs on the in vitro culture of unexpanded chondrocytes, specifically with regard to their differentiation and secretion of ECM proteins. Our results show that BMMCs can delay the dedifferentiation of chondrocytes in pellet culture. Cartilage-specific ECM is known to be composed mainly of GAG and type II collagen. Previous studies have shown that repeated passaging of chondrocytes in monolayer cultures leads to alterations in the chondrogenic phenotype, through a process called dedifferentiation. The latter is characterized by decreased expression of cartilage-specific ECM proteins (Dessau et al. 1981; Diaz-Romero et al. 2005). Therefore, various protocols have been developed to induce chondrocytes to differentiate into specific desired lineages. For example, co-culture of primary and passaged chondrocytes on filter inserts has been shown to trigger the secretion and accumulation of ECM proteins proteoglycan and collagen, as well as expression of aggrecan and type II collagen (Gan and Kandel 2007). In other studies, redifferentiated passaged chondrocytes were shown to have similar properties as primary chondrocytes (Ahmed et al. 2009). Interestingly, dedifferentiated chondrocytes (monolayer passages P1–P4) can induce their own redifferentiation when cultured in the form of pellets, suggesting that an in vitro pellet culture system could serve as a platform to efficiently and robustly differentiate chondrocytes into desired cell lineages (Schulze-Tanzil et al. 2002). In the current study, we isolated the chondrocytes, immediately fabricated their pellets and cultured them over various time periods in vitro. Our data show that the concentration of GAG and type II collagen undergoes a gradual reduction from the periphery towards the center of the chondrocyte pellets, while the expression of cartilage-specific genes decreases with increasing duration of culture. This indicates that unexpanded chondrocytes might undergo dedifferentiation in long-term pellet cultures. These results are also consistent with our observation that the chondrocyte pellets gradually decrease in size during culture.
In order to resolve the problem of chondrocyte dedifferentiation, we devised a strategy where we mixed uncultured BMMCs with digested chondrocytes to prepare the BMMC/C pellets. This is because BMMCs can be acquired easily and rapidly, they are trophic and a low-cost source of cells (Chang et al. 2008; Wise et al. 2014; Zhang et al. 2012). Researchers have shown that the increased chondrocyte proliferation and ECM deposition seen in pellet co-cultures of BMSCs and chondrocytes depended on the trophic function of the BMSCs (Wu et al. 2011). Furthermore, the effect of BMSCs on chondrocyte proliferation and cartilage formation in pellet co-culture systems was independent of co-culture media conditions (chondrogenic-specific vs. non-specific differentiation medium) or the origin of the primary chondrocytes (bone marrow vs. adipose tissue vs. synovial membrane) (Wu et al. 2012). One interesting feature of this approach is that BMSCs are a critical component of the bone marrow mononuclear fraction and are known to secrete various growth factors and cytokines (Balakumaran et al. 2010) that can have a paracrine effect on other cells of the same fraction, such as hematopoietic stem cells and endothelial progenitor cells (Wise et al. 2014). Therefore, BMMCs are a promising cell source that could potentially delay or even prevent the dedifferentiation of chondrocytes in pellet co-cultures. In the present study, we allowed cells in the BMMC/C pellet co-cultures to proliferate, expand and aggregate in vitro, which led to an increase in pellet size over time and to a high concentration of cartilage-specific matrix proteins at 2 and 4 weeks. Importantly, the expression of Acan and Col2a1 in the BMMC/C cultures showed no significant changes after 2 and 4 weeks in culture, indicating that the BMMCs cause a delay in the reduction of expression of cartilage-specific matrix proteins in chondrocyte pellet cultures. However, after 8 weeks, we did observe a significant decrease in ECM proteins. Additionally, the Col1a1 expression in the BMMC/C was significantly lower than in the chondrocyte cultures after 8 week. Altogether, these findings suggest that uncultured BMMCs can delay the dedifferentiation of unexpanded chondrocytes in a pellet co-culture paradigm.
However, this study has certain limitations. First, we did not investigate the underlying mechanism of cellular interaction behind the findings of this study. Further research is thus needed to track different cell types, detect changes in ratios of different cell types with culture time and investigate the underlying mechanisms associated with changes in chondrocyte properties. In addition, we mixed uncultured BMMCs with the digested chondrocyte cell suspension in a 1:1 ratio; no other ratios were tested. Further studies are needed to identify the most appropriate relative proportion of BMMCs and chondrocytes that can best prevent dedifferentiation of the latter. Moreover, the difference between the effect of BMMCs versus BMSCs on the dedifferentiation of chondrocytes in pellet co-cultures was also not investigated in this study. Previous studies have shown that growth factors can play a key role in the chondrogenesis of MSCs (Danišovič et al. 2012) and can enhance the proliferation and redifferentiation of chondrocytes in vitro (Jakob et al. 2001). Therefore, in future studies, we might test the application of various growth factors to the pellet co-culture system in order to improve cartilage formation in vitro. Finally, a pellet co-culture system may alter the cellular interactions and thus limit the eventual clinical applicability; however, a porous scaffold can act as a cell carrier and facilitate cell adhesion, proliferation and chondrogenesis (Nuernberger et al. 2011; Wei et al. 2014; Zhang et al. 2014). Therefore, the presence of a cell carrier is important and will be investigated in future studies on cartilage regeneration using BMMCs.
Taken together, our results show that uncultured BMMCs can delay dedifferentiation of unexpanded chondrocytes in pellet culture. To the best of our knowledge, no studies have reported the effect of BMMCs on the proliferation and differentiation of chondrocytes in pellet cultures, which is crucial for chondrocyte-based cartilage repair. Our findings contribute significantly to this field, since uncultured BMMCs can be obtained rapidly and safely and are an easily available alternative cell source. In addition, they offer enormous potential as a source of trophic support for cartilage tissue engineering.
References
Ahmed N, Gan L, Nagy A, Zheng J, Wang C, Kandel RA (2009) Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng Part A 15:665–673
Balakumaran A, Robey PG, Fedarko N, Landgren O (2010) Bone marrow microenvironment in myelomagenesis: its potential role in early diagnosis. Expert Rev Mol Diagn 10:465–480
Beane OS, Darling EM (2012) Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann Biomed Eng 40:2079–2097
Bekkers JE, Creemers LB, Tsuchida AI, van Rijen MH, Custers RJ, Dhert WJ, Saris DB (2013) One-stage focal cartilage defect treatment with bone marrow mononuclear cells and chondrocytes leads to better macroscopic cartilage regeneration compared to microfracture in goats. Osteoarthritis Cartilage 21:950–956
Benthien JP, Behrens P (2011) The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc 19:1316–1319
Benya PD, Padilla SR, Nimni ME (1978) Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 15:1313–1321
Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN (2012) Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med 40:562–567
Brittberg M (2008) Autologous chondrocyte implantation–technique and long-term follow-up. Injury 39(Suppl 1):S40–S49
Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, Beard DJ (2012) Knee replacement. Lancet 379:1331–1340
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749
Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, Ogawa T, Ochiai N (2008) Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res 26:18–26
Danišovič L, Varga I, Polák S (2012) Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell 44:69–73
Dessau W, Vertel BM, von der Mark H, von der Mark K (1981) Extracellular matrix formation by chondrocytes in monolayer culture. J Cell Biol 90:78–83
Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P (2005) Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol 202:731–742
Gan L, Kandel RA (2007) In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng 13:831–842
Hangody L, Füles P (2003) Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 85-A(Suppl 2):25–32
Inui A, Iwakura T, Reddi AH (2012) Human stem cells and articular cartilage regeneration. Cells 1:994–1009
Jakob M, Démarteau O, Schäfer D, Hintermann B, Dick W, Heberer M, Martin I (2001) Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem 81:368–377
Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I (2009) Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc 17:1289–1297
Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, Zheng MH (2008) Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res 26:1230–1237
Matsumoto T, Okabe T, Ikawa T, Iida T, Yasuda H, Nakamura H, Wakitani S (2010) Articular cartilage repair with autologous bone marrow mesenchymal cells. J Cell Physiol 225:291–295
McNickle AG, L’Heureux DR, Yanke AB, Cole BJ (2009) Outcomes of autologous chondrocyte implantation in a diverse patient population. Am J Sports Med 37:1344–1350
Micheli LJ, Browne JE, Erggelet C, Fu F, Mandelbaum B, Moseley JB, Zurakowski D (2001) Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med 11:223–228
Minas T (1998) Chondrocyte implantation in the repair of chondral lesions of the knee: economics and quality of life. Am J Orthop 27:739–744
Mithoefer K, Williams RJ 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG (2005) The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 87:1911–1920
Nuernberger S, Cyran N, Albrecht C, Redl H, Vecsei V, Marlovits S (2011) The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 32:1032–1040
Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 374:212–234
Peterson L, Vasiliadis HS, Brittberg M, Lindahl A (2010) Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med 38:1117–1124
Schindler OS (2011) Current concepts of articular cartilage repair. Acta Orthop Belg 77:709–726
Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, Shakibaei M (2002) Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res 308:371–379
Sohn DH, Lottman LM, Lum LY, Kim SG, Pedowitz RA, Coutts RD, Sah RL (2002) Effect of gravity on localization of chondrocytes implanted in cartilage defects. Clin Orthop Relat Res 394:254–262
Steadman JR, Rodkey WG, Briggs KK (2003) Microfracture chondroplasty: indications, techniques, and outcomes. Sports Med Arthrosc 11:236–244
von der Mark K, Gauss V, von der Mark H, Müller P (1977) Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267:531–532
Wang W, Li B, Yang J, Xin L, Li Y, Yin H, Qi Y, Jiang Y, Ouyang H, Gao C (2010) The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials 31:8964–8973
Wei B, Jin C, Xu Y, Du X, Yan C, Tang C, Ansari M, Wang L (2014) Chondrogenic differentiation of marrow clots after microfracture with BMSC-derived ECM scaffold in vitro. Tissue Eng Part A 20:2646–2655
Wise JK, Alford AI, Goldstein SA, Stegemann JP (2014) Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopedic tissue engineering. Tissue Eng Part A 20:210–224
Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M (2011) Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 17:1425–1436
Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M (2012) Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A 18:1542–1551
Zhang Y, Wang F, Chen J, Ning Z, Yang L (2012) Bone marrow-derived mesenchymal stem cells versus bone marrow nucleated cells in the treatment of chondral defects. Int Orthop 36:1079–1086
Zhang Q, Lu H, Kawazoe N, Chen G (2014) Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater 10:2005–2013
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81171745), the Science and Technology Planning Project of Xuzhou City, China (No. KC14SH010) and the Orthopedic Clinical Medical Center of Nanjing City, China.
Conflict of interest
All authors declare no conflict of interest regarding the design and outcomes of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao Ouyang and Bo Wei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ouyang, X., Wei, B., Mao, F. et al. Uncultured bone marrow mononuclear cells delay the dedifferentiation of unexpanded chondrocytes in pellet culture. Cell Tissue Res 361, 811–821 (2015). https://doi.org/10.1007/s00441-015-2156-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2156-1