Abstract
Desmosomes are cell-cell adhesive organelles with a well-known role in forming strong intercellular adhesion during embryogenesis and in adult tissues subject to mechanical stress, such as the heart and skin. More recently, desmosome components have also emerged as cell signaling regulators. Loss of expression or interference with the function of desmosome molecules results in diseases of the heart and skin and contributes to cancer progression. However, the underlying molecular mechanisms that result in inherited and acquired disorders remain poorly understood. To address this question, researchers are directing their studies towards determining the functions that occur inside and outside of the junctions and the extent to which functions are adhesion-dependent or independent. This review focuses on recent discoveries that provide insights into the role of desmosomes and desmosome components in cell signaling and disease; wherever possible, we address molecular functions within and outside of the adhesive structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Desmosomes are intercellular junctional complexes that confer strong cell-cell adhesion in tissues that undergo large amounts of mechanical strain, such as in the heart and skin (Green and Simpson 2007). They act as anchors linking the intermediate filament (IF) cytoskeletons of neighboring cells thus forming an integrated, mechanically resistant unit throughout a tissue. In addition to mediating adhesion, desmosomes have been recognized as signaling scaffolds that regulate pathways involved in normal physiological processes such as proliferation and differentiation (Johnson et al. 2014).
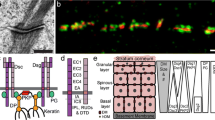
Desmosomes are calcium-dependent junctions. The core desmosomal components comprise three main protein families (Fig. 1): transmembrane cadherins (desmogleins and desmocollins), armadillo proteins (plakophilins and plakoglobin) and plakin proteins (mainly desmoplakin). The desmosomal cadherins of adjacent cells form trans-interactions coupling the two cells together (Garrod and Chidgey 2008). Intracellularly, desmosomal cadherins interact with the linker armadillo proteins, which are in turn linked to desmoplakin. They are similar in molecular organization to the adherens junction (AJ), which is a cell-cell adhesion complex that links instead to the actin cytoskeleton through different adaptor molecules. Components of the AJ have widely been studied both in and out of the context of junctions (Niessen and Gottardi 2008; Kim et al. 2013). On the other hand, desmosome functions outside of adhesion are not as well understood.
Desmosomes are compromised in human diseases, including genetic disorders leading to blistering diseases of the skin, in cardiomyopathies and in some cancers. This review focuses on recent reports of desmosomal constituents in human disease and associated signaling mechanisms (Tables 1 and 2).
Desmosomal cadherins
Desmosomal cadherins couple adjoining cells together through homo- and heterophilic interactions, although the specifics of these molecular interactions are not well understood. In humans, four desmogleins (Dsg1-4) and three desmocollins (Dsc1-3) have been detected, which are expressed in a tissue- and differentiation-dependent manner (Kowalczyk and Green 2013). Dsg2 and Dsc2 are the primary isoforms in simple epithelia and are present at low levels in the basal layer of stratified epithelia, such as the epidermis (Garrod and Chidgey 2008). Dsg1/3 and Dsc1/3 are present in stratified epithelia and Dsg4 is found in stratified epithelia and hair (Garrod and Chidgey 2008; Brooke et al. 2012; Johnson et al. 2014). Desmosomal cadherins are important in regulating normal physiological processes, such as epithelial morphogenesis and differentiation. Moreover, their misregulation is associated with diseases of the skin, hair, heart and digestive tract and with cancer (Thomason et al. 2010).
In skin epithelium, Dsg1 expression increases in the suprabasal layers in which it plays a role in both normal epidermal differentiation and in skin diseases such as pemphigus foliaceus, bullous impetigo, staphylococcal scalded skin syndrome and striate palmoplantar keratoderma (Amagai and Stanley 2012). Dsg1 can support keratinocyte differentiation through the suppression of the mitogen-activated protein kinase (MAPK) pathway via epidermal growth factor receptor (EGFR) signaling and modulating the interaction of Erbin, SHOC2 and Ras (Getsios et al. 2009; Harmon et al. 2013). These functions do not require the extracellular regions of Dsg1 that are needed for adhesion. In addition, the receptor tyrosine kinase EphA2, in a ligand-dependent manner, promotes entry of keratinocytes into a terminal differentiation pathway through a mechanism reliant on Dsg1 (Lin et al. 2010). Finally, the RhoA GEF breakpoint cluster region (Bcr) has been shown to promote keratinocyte differentiation through the regulation of MAL/SRF signaling, again in a manner that is dependent on Dsg1 (Dubash et al. 2013).
Although progress has been made delineating the signaling pathways by which Dsg1 regulates physiological processes such as differentiation, much less is known about the mechanism by which perturbation of Dsg1 leads to disease. Recent familial studies have identified two homozygous mutations in Dsg1 that lead to severe skin dermatitis, multiple allergies and metabolic wasting (SAM) syndrome (Samuelov et al. 2013; Has et al. 2015). One mutation led to a loss of Dsg1 expression and was associated with an apparently more severe phenotype. The other mutation occurred within the Dsg1 signal peptide and resulted in cytoplasmic mislocalization of the protein. Differences in the observed phenotypic severity raise the possibility that this non-junctional Dsg1 could still be partially functional, conceivably through signaling outside of the adhesive plaque. This condition is also associated with an increase in cytokine expression and points to a role for Dsg1 in regulating skin allergies in addition to its other dermatological effects (Samuelov et al. 2013).
Dsgs contain a Dsg unique region (DUR) that lies within their C-terminal tail and whose function remains poorly understood. Removal of this region in Dsg1 abrogates its interaction with Erbin and reduces the effects of Dsg1 on dampening MAPK signaling to promote differentiation (Harmon et al. 2013). In addition, the DUR in Dsg2 has been shown to promote strong adhesion by increasing surface retention of the cadherin via promotion of dimerization and inhibition of internalization (Chen et al. 2012). A mutation in the DUR of Dsg2 that is associated with cardiomyopathy, V977fsX1006, promotes the rapid internalization of Dsg2 and leads to the loss of dimerization in cardiac cells (Chen et al. 2012). Other mutations related to heart disease in Dsg2 have been suggested to alter post-translational modifications of Dsg2 itself (Gehmlich et al. 2010) and phosphorylation of connexin 43 (Gehmlich et al. 2012).
Recently, Dsg2 has been identified as the primary high-affinity receptor used by a group of species B adenoviruses: Ad3, Ad7, Ad11 and Ad14 (Wang et al. 2011a). The binding of adenovirus to Dsg2 leads to a transient opening of intercellular junctions, uncovering receptors that are normally inaccessible, such as CAR and Her2 (Wang et al. 2011a, 2011b); a recombinant protein called junctional opener (JO) has been generated, which contains the Ad3 domain responsible for Dsg2 interaction. Multiple versions of junctional opening proteins have been used to improve the efficacy, safety and penetration of anti-cancer drugs in mice (Beyer et al. 2011, 2012; Wang et al. 2013).
Autoantibodies against Dsg1 and Dsg3 lead to the autoimmune skin disorders pemphigus foliaceus (PF) and pemphigus vulgaris (PV), respectively. In the epidermis, Dsg1 and Dsg3 have inverse distribution patterns. High levels of Dsg3 occur in the basal layer with little Dsg1, whereas the upper layers have high levels of Dsg1 and little Dsg3. PF (targets Dsg1) induces blister formation only in the most superficial layers of the skin, whereas PV (targets Dsg3) causes blisters in the basal layer of the skin (Kitajima 2013). In the intermediate layers, a mixture of Dsg1/3 is expressed and blisters do not typically form in these layers. This is attributable to the apparent ability of these two desmogleins to compensate for the loss of each other in the so-called compensation hypothesis (Shirakata et al. 1998). At a cellular level, IgG against Dsg1 affects the size of desmosomes but this does not occur with Dsg3 IgG (van der Wier et al. 2014). The exposure of keratinocytes to PV IgG induces Dsg3 redistribution, endocytosis and subsequent protein degradation (Jolly et al. 2010).
The relative contribution of PV IgG-dependent steric hindrance of cadherin interactions and induced cellular signaling changes to loss of cell-cell adhesion remains to be determined. A recent study provided evidence that non-desmosomal Dsg3 regulates Src activity, whereby PV IgG reduces the expression of Dsg3 and is associated with a decrease in active Src (Tsang et al. 2012). However, another study found that, under some conditions, Src activity promotes PV acantholysis (Cirillo et al. 2014). Here, PKP3 is phosphorylated in response to PV IgG leading to dissociation from Dsg3 (Cirillo et al. 2014). This results in the destabilization of adhesion and can be attenuated by using a Src inhibitor (Cirillo et al. 2014). Possible explanations for these disparities in signaling include the different model systems utilized and the type of antibodies employed to study the effects of IgG on PV. A recent study examined the differences between monoclonal and polyclonal versions of PV IgG. Polyclonal PV patient IgG leads to aberrant clustering of Dsg3 on the cell surface followed by endocytosis, both of which are dependent on p38 MAPK (Saito et al. 2012). However, pathogenic monoclonal IgG targeting Dsg3 leads to the loss of adhesion independently of p38 MAPK, possibly functioning through the steric hindrance of cadherin oligomerization (Saito et al. 2012).
Several reports have identified alterations in desmosomal cadherin expression in cancer; nevertheless, these results are somewhat contradictory. For example, the expression of desmosome components, including Dsg2 and Dsg3, are elevated in cancers of the lung, prostate, head and neck and skin in which they are associated with disease progression (Kurzen et al. 2003; Furukawa et al. 2005; Chen et al. 2007; Brennan and Mahoney 2009; Breuninger et al. 2010). However, Dsg1-3 and Dsc2/3 have also been reported to be downregulated in gastric, colorectal, prostate, bladder, breast, skin and head and neck cancers (Dusek and Attardi 2011). These differences might be attributable to specific tissue contexts and/or to the finding that some desmosomal cadherins signal differently from others. Below, we will discuss recent discoveries in signaling associated with desmosomal cadherins and cancer.
The molecular mechanisms linking the desmosomal cadherins to cancer remain poorly understood but some recent progress has been made. For instance, in intestinal cancer cells, knockdown of Dsg2 leads to decreased EGFR and downstream Erk1/2 signaling and suppresses cell proliferation (Kamekura et al. 2014). In some head and neck cancers, Dsg3 functions as an oncogene, promoting cancer cell growth and invasion through a mechanism mediated by plakoglobin (PG) signaling (Chen et al. 2013). This occurs through the modulation of c-myc, cyclin D1 and MMP-7 (Chen et al. 2013). Moreover, overexpression of Dsg3 in various epithelial cell lines leads to the downregulation of E-cadherin with an increase in migratory capacity and filopodial formation, which is attributed to downstream regulation of Src kinase activity, whereas knockdown of Dsg3 has the opposite effects (Tsang et al. 2010). On the other hand, loss of Dsc2 has been reported in colorectal cancer, facilitating cell proliferation and growth through the activation of Akt/beta-catenin signaling (Kolegraff et al. 2011). Furthermore, in esophageal squamous cell carcinoma (SCC), overexpression of Dsc2 leads to an inhibition of beta-catenin-mediated transcription by altering its localization in an E-cadherin-dependent manner (Fang et al. 2013). The same study identified Dsc2 as a downstream target of miR-25, which reduces Dsc2 expression and promotes cancer aggressiveness (Fang et al. 2013). Clearly, signaling downstream of the desmosomal cadherins regulates multiple processes underlying cancer progression, including migratory and growth capacity. However, mechanistic differences exerted by specific cadherin isoforms in each tissue context are not well understood.
We are beginning to understand the functions of individual desmosomal cadherins in both normal tissue and in disease. However, they coexist in the context of a tissue-type and differentiation-dependent milieu. An understanding of the way that the array of desmosomal cadherins is coregulated and works in collaboration to regulate important signaling pathways might give us insights into their complex biological and pathogenic roles.
Desmoplakin
Desmoplakin (DP) is a member of the plakin family of cytolinker proteins and is an essential desmosomal component that links the desmosomal core proteins to the IF cytoskeleton. Global knockout of DP in mouse leads to a dramatic decrease in the number of desmosomes within null embryos and results in lethality at embryonic day 6.5 (Gallicano et al. 1998). Tissue-specific mouse knockout studies indicate critical roles for DP in the skin and heart (Vasioukhin et al. 2001; Yang et al. 2006). Indeed, human mutations have been identified that result in diseases of the skin and heart (Thomason et al. 2010) and some recent progress is discussed below.
DP assembles into junctions in three temporally overlapping phases that are differentially regulated by the IF and actin cytoskeletons (Godsel et al. 2005). Two major isoforms of DP, namely DPI and DPII, are known and they have recently been discovered not to be equally capable of promoting adhesive strength. Work in keratinocytes indicates that the loss of DPII leads to a more severe adhesion defect in response to mechanical stress (Cabral et al. 2012). In addition, DP-mediated desmosome adhesive strengthening is regulated by post-translational modification of the DP c-terminal tail region in which a non-phosphorylatable mutant of a protein kinase C (PKC) consensus site promotes hyperadhesion (Hobbs and Green 2012). In an epidermal-derived system, sarcoendoplasmic reticulum Ca2+-ATPase isoform 2 (SERCA2), which is often mutated in Darier’s disease, has been shown to regulate DP translocation to sites of cell-cell adhesion in a PKC-dependent manner (Hobbs et al. 2011). An understanding of the way that DP is post-translationally modified might help to elucidate the complex nature of its incorporation into junctions.
Mutations in DP that result either in the loss of the IF-binding c-terminus or in complete protein loss have been identified in cases of lethal acantholytic epidermolysis bullosa, an inherited blistering disease attributed to the loss of cell-cell adhesion, with and without apparent associated cardiomyopathy (Asimaki et al. 2009; Hobbs et al. 2010). DP missense mutations can lead to Carvajal/Naxos syndrome, which is characterized by cardiomyopathy, palmoplantar keratoderma and wooly hair and is occasionally coupled with dental phenotypes (Chalabreysse et al. 2011; Keller et al. 2012). Recent genetic familial studies of arrhythmogenic cardiomyopathies have identified additional DP nonsense mutations that result in right- or left-dominant cardiomyopathies (Campuzano et al. 2013; Lopez-Ayala et al. 2014). Finally, a “hotspot” for arrhythmogenic cardiomyopathy mutations was identified within the N terminus of DP spanning residues 250–604 (Kapplinger et al. 2011). A wide range of DP mutations have been identified in cardiocutaneous disease but the basis for onset of skin vs. heart vs. mixed disease phenotypes remains poorly understood.
Although detailed molecular mechanisms that cause cardio/cutaneous disease remain poorly understood, several reports indicate that desmosome mutations can lead to aberrant gap junctions, which could detrimentally impact both heart and epidermal functions. In the skin, gap junctions play an important role in normal barrier homeostasis, wound closure and skin disease pathogenesis (Scott et al. 2012). In the heart, cardiomyocytes are electrically coupled through gap junctions allowing action potentials to propagate throughout the cardiac tissue. Abnormal electrical signaling can lead to defects in cardiac rhythm and contraction (van der Velden and Jongsma 2002). Recent evidence highlights the potential for desmosome-associated cardiomyopathies being linked to improper electrical coupling. For example, mutations in DP that lead to cardiomyopathies, with or without described associated skin abnormalities, induce the loss of the gap junction protein connexin 43 at cell-cell junctions (Asimaki et al. 2009; Gomes et al. 2012). Some of these changes can be detected earlier than is possible by using conventional cardiac imaging and this could have important implications for the earlier diagnosis of cardiomyopathies.
Recent work has revealed that the desmosome-dependent regulation of connexins can occur through its ability to modulate microtubule dynamics. In the heart, desmosome and gap junction remodeling occurs concurrently with the relocalization of the microtubule-associated proteins end-binding 1 (EB1) and Kif5b (Chkourko et al. 2012) and EB1 is important in mediating the proper trafficking of connexin 43 to gap junctions (Shaw et al. 2007). Recently, DP has been shown to interact with EB1 and a subset of previously identified DP cardio/cutaneous disease mutations inhibits DP interaction with EB1, alters microtubule dynamics and hinders proper connexin 43 localization and gap junction function in both heart and skin cells (Patel et al. 2014). A point mutant associated with skin fragility and wooly hair syndrome induces aberrant DP localization and loss of connexin 43 localization at the cell membrane (Patel et al. 2014). Two of the identified arrhythmogenic cardiomyopathy mutations disrupt DP binding to EB1 but are still incorporated into desmosomes to which they confer adhesive strength (Patel et al. 2014). Again, these mutations lead to a loss of connexin 43 localization at the cell membrane. The proposed model suggests that DP captures EB1-associated microtubule “plus ends” at sites of cell-cell contact. This stabilizes the route for targeted trafficking of connexin 43 to gap junctions. This mechanism might also be relevant beyond arrhythmogenic cardiomyopathy to heart failure in general, as decreased levels of connexin 43 at cardiomyocyte borders are associated with both ischemic and non-ischemic cardiomyopathies (Shaw 2014).
Unsurprisingly, because of the important role of DP in regulating cell-cell adhesion, the loss of DP has been associated with some cancers. Deletion of DP in a mouse model of pancreatic neuroendocrine carcinogenesis promotes local tumor invasion, without affecting widespread invasion or metastasis (Chun and Hanahan 2010). Interestingly, this study provides evidence for the independent roles of desmosomes and AJs in cancer progression, as no effects have been seen on the AJ component E-cadherin (Chun and Hanahan 2010). It also provides support for a two-step cancer progression model, in which local invasion is promoted by the loss of desmosomal function and further loss of AJs promotes full disease progression (Dusek and Attardi 2011). In humans, DP is often downregulated in non-small cell lung cancer (NSCLC) and ectopic DP expression leads to an inhibition of cell proliferation, migration and invasion (Yang et al. 2012). In addition to its role in adhesion, DP expression also increases the levels of PG with the concurrent downregulation of beta-catenin (Yang et al. 2012) suggesting that DP has a tumor suppressor function in NSCLC via the inhibition of Wnt/beta-catenin signaling.
The connection between DP mutation and phenotypic outcome remains poorly understood. However, advances in the elucidation of the molecular mechanisms underlying DP-related diseases, including the ability of DP to regulate junctions other than desmosomes and critical signaling pathways such as Wnt/beta-catenin, might clarify the relationship of DP genotype to phenotype.
Plakophilins
Desmosomal armadillo protein constituents include the plakophilins PKP1, 2 and 3 and have diverse biological and pathological roles (for a review, see Hatzfeld 2007). Like desmosomal cadherins, PKP1-3 exhibit tissue- and differentiation-dependent expression patterns (Hatzfeld 2007). PKP1 expression is high in the suprabasal layers of complex and stratified epithelia. PKP2 is more widely expressed and is present in simple, complex and stratified epithelia and in other cell types such as cardiomyocytes. PKP3 is present more uniformly in most simple and stratified epithelia, with the exception of hepatocytes. The PKPs act as scaffolds, interacting with both desmosomal cadherins and desmoplakin and, to variable degrees, promote desmosome assembly, maturation and linkage to the cytoskeleton (Hatzfeld 2007). PKP1/2 are also present in the nucleus, although their nuclear functions remain poorly understood.
All three PKPs have been implicated in human genetic disorders and/or cancer. For example, the first identified desmosome-associated disorder involved mutations in PKP1 leading to a skin disease called ectodermal dysplasia-skin fragility syndrome (McGrath et al. 1997). These loss-of-function PKP1 mutations result in defects in skin integrity, hair development, sweating and inflammation (McGrath et al. 1997; Lai-Cheong et al. 2007; Tanaka et al. 2009). In the autoimmune blistering disease PV, keratinocyte adhesion is compromised by loss of Dsg3 from cell-cell junctions. However, overexpression of PKP1 can rescue this loss of Dsg3 and other desmosome proteins at the cell borders and also induces formation of calcium-independent desmosomes (Tucker et al. 2014).
In addition to its role in skin diseases, PKP1 is elevated in some tumors such as head and neck carcinomas and Ewing sarcoma but the molecular mechanisms involved are poorly understood. PKP1 controls cell proliferation and size by regulating protein synthesis via eIF4A1 in an adhesion-independent manner (Wolf et al. 2010). PKP1 recruits eIF4A1 to the initiation complex and directly regulates its activity (Wolf et al. 2010). Moreover, insulin signaling via Akt2 can switch the function of PKP1 from stabilizing cell adhesion, via recruitment of desmosomal components to cell borders, to promoting hyperproliferation (Wolf et al. 2013). These studies support a role for PKP1 in tumor formation by regulating cell proliferation and growth.
PKP2 is the only PKP in cardiomyocytes and heterozygous mutations in PKP2 are the most common genetic source of arrhythmogenic right ventricular cardiomyopathy (ARVC; Bass-Zubek et al. 2009). ARVC-related missense mutations in PKP2 can cause instability of the protein via calpain-mediated proteolysis (Kirchner et al. 2012). Moreover, truncating PKP2 mutations are associated with decreased PKP2 protein expression in both myocardial and epidermal tissues (Rasmussen et al. 2014). As with other forms of ARVC, heterozygous mutations in PKP2 lead to abnormal gap junction connexin 43 localization and expression at the intercalated disk (Fidler et al. 2009). This loss strengthens the concept that defects in electrical communication are a major component underlying arrhythmogenic disorders such as ARVC. Supporting this notion, a recent study demonstrated a molecular interaction of PKP2 with connexin 43 and Ankyrin-G, a cytoskeletal adaptor protein that regulates the voltage-gated sodium channel complex in the heart (Sato et al. 2011). Moreover, missense mutations in PKP2 have been identified in patients with Brugada syndrome and lead to defects in sodium channel function through the loss of INa and NaV1.5 at sites of cell contact, although without signs of ARVC (Cerrone et al. 2014). PKP2 is required for the recruitment of DP to desmosomes (Bass-Zubek et al. 2008); therefore, a connection between the loss of PKP2 and the loss of DP at desmosomes might exist in regulating these diseases as they exhibit similar changes in gap junctions.
In addition to modulating intercellular adhesion, PKP2 has also been implicated in the regulation of actin cytoskeletal dynamics, cell migration and tumor development. Knockdown of PKP2 leads to a defect in the remodeling of the cortical actin network after cadherin engagement, without affecting the AJ component p120 catenin (Godsel et al. 2010). This is presumably attributable to a lack of active RhoA at sites of cell-cell contact. PKP2 can affect cell migration via the regulation of focal adhesion dynamics, a process that is also regulated by RhoA signaling (Koetsier et al. 2014). Finally, PKP2 is a novel positive regulator of EGFR activation and knockdown of PKP2 attenuates EGFR-mediated signaling, decreasing cancer cell migration, proliferation and tumor development (Arimoto et al. 2014).
Although no human genetic diseases have been found to be associated with PKP3, mice lacking this PKP exhibit morphological abnormalities in hair follicles and often exhibit cutaneous inflammation (Sklyarova et al. 2008). In addition, a role for this protein is emerging in cancer. For example, in gastrointestinal cancer, relative PKP3 mRNA expression has been found to be significantly higher in cancer patients compared with controls (Valladares-Ayerbes et al. 2010). A higher blood level of PKP3 is also associated with advanced-stage tumors and metastases (Valladares-Ayerbes et al. 2010). Moreover, PKP3 protein expression is increased in breast and pancreatic cancers (Breuninger et al. 2010; Demirag et al. 2012). However, the mechanistic basis of these connections has not been determined. In contrast, loss of PKP3 in HCT116 colon cancer cells leads to an increase in cancer progression and metastasis through changes in keratin 8 phosphorylation (Khapare et al. 2012). Furthermore, a recent study in the SCC line, SCC9, demonstrated that PKP3 mediates both desmosome assembly and E-cadherin-based junctional maturation via Rap1 GTPase (Todorović et al. 2014), thereby strengthening cell-cell adhesion. Further studies will be needed in order to understand this apparent contradiction in the role of PKP3 in cancer.
PKPs are clearly more than just linker proteins that strengthen intercellular adhesion. They are emerging as critical regulators of diverse signaling programs controlling cellular processes ranging from protein synthesis, growth, proliferation, migration, to invasion and tumor development. Future work is warranted that examines the adhesion-dependent and -independent roles of the PKPs and their as yet elusive nuclear functions.
Plakoglobin
PG is another member of the armadillo family of proteins that is present in desmosomes in which it promotes adhesion through interactions with desmosomal cadherins, PKPs and DP. PG, like the PKPs, has both a cytoplasmic and nuclear pool; however, it has some known nuclear functions, such as the regulation of transcription and the inhibition of Wnt/beta-catenin signaling (Chidgey and Dawson 2007; Aktary and Pasdar 2012).
Mice lacking PG usually die between embryonic day 10.5 and birth because of severe heart and skin defects (Bierkamp et al. 1996). In humans, alterations in PG have been associated with various diseases of the skin, heart and some types of cancer. The first reported desmosome-associated cardiocutaneous syndromes stemmed from PG mutations (McKoy et al. 2000; Asimaki et al. 2007). Recently, a study reported the discovery of two human mutations that resulted in skin disease without associated cardiomyopathy (Cabral et al. 2010). The two homozygous mutations led to a marked decrease in the protein expression of PG, skin fragility, diffuse palmoplantar keratoderma and woolly hair (Cabral et al. 2010). Another study identified a lethal nonsense mutation in PG that resulted in undetectable PG protein expression in the skin; the patient displayed severe congenital skin fragility, generalized epidermolysis and considerable transcutaneous fluid loss, with no apparent cardiac dysfunction (Pigors et al. 2011). The more severe skin phenotype in this study might be attributable to a dramatic decrease in both PG protein and mRNA, compared instead with just the expression of a mutant version of PG. Since PG is critically important to both skin and heart, it remains a mystery as to why defects in this protein can lead to such severe phenotypes in one tissue without apparent consequences in the other.
PG is important in normal skin physiology, as knockout of PG in mouse epidermal keratinocytes leads to an increase in cornification, epidermal thickening, ulceration and inflammation (Li et al. 2012). The assembly of both desmosomes and AJs is disturbed and a compensational effect also occurs in beta-catenin expression without apparent change in its signaling activity (Li et al. 2012). Knockdown of PG in human keratinocytes causes activation of p38 MAPK-dependent loss of cell adhesion and keratin network collapse, which can be rescued by extranuclear PG expression (Spindler et al. 2014). This suggests that the adhesive and signaling functions of the cytoplasmic pool of PG are more important in triggering associated skin diseases than in the transcriptional function of nuclear PG.
PG has important roles in normal heart development and in multiple reported cardiomyopathies. Two recent PG conditional loss-of-function studies in mouse cardiac tissue have been shown to recapitulate the clinical phenotypes of human ARVC, including progressive loss of cardiac myocytes, inflammation, fibrosis and arrhythmias (Li et al. 2011a, b). An increase in the expression of beta-catenin was reported in both studies; however, in one case, signaling downstream of beta-catenin is described as being activated (Li et al. 2011b), whereas in the other, no changes are apparent (Li et al. 2011a). This discrepancy can be explained by the level and timing of genetic PG ablation. Simultaneous knockout of both PG and beta-catenin in the mouse heart results in the disruption of the intercalated disk, loss of connexin 43 from cell borders and sudden cardiac death, thus demonstrating their requirement for maintaining normal mechanoelectrical properties in the heart (Swope et al. 2012). ARVC is also characterized by fibrofatty deposits and nuclear PG has been shown to be necessary for the differentiation of cardiac progenitor cells into adipocytes in ARVC (Lombardi et al. 2011). In addition, PG forms a complex with beta-catenin and Yes-associated protein (YAP) to regulate adipogenesis through the Hippo signaling pathway (Chen et al. 2014). These results point to a clear role for nuclear PG in regulating cardiac function, both in normal and diseased states, although they do not exclude the involvement of cytoplasmic PG.
Unlike the well-defined oncogenic activity of beta-catenin, signaling downstream of PG typically has a tumor suppressor function. Although the molecular mechanisms for this are not well understood, recent progress has been made in identifying signaling events downstream of PG that regulate tumor biology. PG has been shown to promote the expression and stability of the metastasis suppressor Nm23 (Aktary et al. 2010) and increases p53-mediated expression of the tumor suppressor 14-3-3sigma (Aktary et al. 2013). Moreover, PG functions to decrease the expression of SATB1, which leads to decreased proliferation, migration and invasion (Aktary and Pasdar 2013). PG has also been reported to be downregulated in esophageal cancer in which it is associated with a decrease in the Triton-X 100 insoluble pool of E-cadherin and desmocollin-2 and decreased E-cadherin at the cell membrane, suggesting decreased cell-cell adhesion (Fang et al. 2014). The nucleolar phosphoprotein nucleophosmin interacts directly with PG in MDA-MB-231 carcinoma cells and PG expression inhibits nucleophosmin-mediated cell growth and invasion, potentially through the regulation of its subcellular localization (Lam et al. 2012).
A mechanism by which PG might inhibit cancer progression is through its role in impeding cell migration (Yin et al. 2005). PG inhibits cell motility in both primary keratinocytes and prostate cancer cell lines at least in part by modulating cell-substrate adhesion dynamics in a Src-dependent and extracellular-matrix-dependent (fibronectin and vitronectin) manner (Todorović et al. 2010; Franzen et al. 2012). In contrast, in acute myeloid leukemia, PG has been reported to be upregulated (Morgan et al. 2013). Here, PG expression promotes the stabilization beta-catenin and its nuclear translocation and enhances TCF-dependent transcription (Morgan et al. 2013), all of which are associated with enhanced cell motility and tumorigenesis (Muller et al. 2002).
PG has also been shown to promote cancer progression. A recent study highlighted the role of PG in circulating tumor cell clusters. These clusters have a much higher metastatic potential than single circulating tumor cells and are dependent on PG as the knockdown of PG abrogates both cluster formation and metastasis in mouse models (Aceto et al. 2014).
Some of the discrepancies mentioned above can be explained by the site in the cell in which the changes in PG functions are occurring. For instance, loss-of-function of PG at junctions might decrease intercellular adhesion and promote cancer progression, whereas the same loss-of-function of PG in the nucleus might promote proliferation, migration and invasion. A similar issue might underlie differences seen in cardiomyopathies. Therefore, we need to determine the location in the cell at which PG is acting and the way in which the balance of junctional and non-junctional (cytoplasmic and nuclear) protein affects these processes.
Concluding remarks
Desmosomes play a clear role in the normal physiology of tissues that experience frequent mechanical insult, such as the heart and skin. Emerging evidence points to their misregulation occurring in these tissues in several disease states, including mechanically sensitive diseases such as cancer. In cancer, some uncertainty exists as to the specific contribution of individual desmosomal components to disease progression; this can potentially be explained by their complex expression pattern in epithelia and variable downstream signaling events. Because of their heavy involvement in tissues and diseases that either bear or are regulated by mechanical forces, the role of desmosomes in mechanotransduction (the transformation of mechanical stimulation into biochemical signals) has surprisingly not been a major focus of investigation. Recent work has indicated that AJs are responsible for the establishment of tension in epithelial monolayers and that interference with desmosome function does not have an effect on this early event (Harris et al. 2014). However, a gap in our knowledge remains at the level of complex tissue morphogenesis and homeostasis.
Recently, we have begun to appreciate the non-adhesive and non-junctional functions of desmosomal components in signaling. As progress is made, it will be important to identify the signaling networks regulated downstream of the desmosome constituents and also distinguish their dependence on functioning within the adhesive plaque. This might be accomplished through the use of specific mutants functioning only in particular pools coupled with super-resolution imaging to improve the definition of junctional and non-junctional pools. An understanding of the disease contributions of desmosomal constituents and the underlying molecular mechanisms both within and outside of the adhesive plaque should ultimately enhance our ability to treat desmosome-associated diseases.
References
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158:1110–1122
Aktary Z, Pasdar M (2012) Plakoglobin: role in tumorigenesis and metastasis. Int J Cell Biol 2012:189521
Aktary Z, Pasdar M (2013) Plakoglobin represses SATB1 expression and decreases in vitro proliferation, migration and invasion. PLoS One 8:e78388
Aktary Z, Chapman K, Lam L, Lo A, Ji C, Graham K, Cook L, Li L, Mackey JR, Pasdar M (2010) Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene 29:2118–2129
Aktary Z, Kulak S, Mackey J, Jahroudi N, Pasdar M (2013) Plakoglobin interacts with the transcription factor p53 and regulates the expression of 14-3-3sigma. J Cell Sci 126:3031–3042
Amagai M, Stanley JR (2012) Desmoglein as a target in skin disease and beyond. J Invest Dermatol 132:776–784
Arimoto K, Burkart C, Yan M, Ran D, Weng S, Zhang DE (2014) Plakophilin-2 promotes tumor development by enhancing ligand-dependent and -independent epidermal growth factor receptor dimerization and activation. Mol Cell Biol 34:3843–3854
Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ (2007) A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 81:964–973
Asimaki A, Syrris P, Ward D, Guereta LG, Saffitz JE, McKenna WJ (2009) Unique epidermolytic bullous dermatosis with associated lethal cardiomyopathy related to novel desmoplakin mutations. J Cutan Pathol 36:553–559
Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, Wahl JK 3rd, Denning MF, Green KJ (2008) Plakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assembly. J Cell Biol 181:605–613
Bass-Zubek AE, Godsel LM, Delmar M, Green KJ (2009) Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol 21:708–716
Beyer I, van Rensburg R, Strauss R, Li Z, Wang H, Persson J, Yumul R, Feng Q, Song H, Bartek J, Fender P, Lieber A (2011) Epithelial junction opener JO-1 improves monoclonal antibody therapy of cancer. Cancer Res 71:7080–7090
Beyer I, Cao H, Persson J, Song H, Richter M, Feng Q, Yumul R, van Rensburg R, Li Z, Berenson R, Carter D, Roffler S, Drescher C, Lieber A (2012) Coadministration of epithelial junction opener JO-1 improves the efficacy and safety of chemotherapeutic drugs. Clin Cancer Res 18:3340–3351
Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R (1996) Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol 180:780–785
Brennan D, Mahoney MG (2009) Increased expression of Dsg2 in malignant skin carcinomas: a tissue-microarray based study. Cell Adh Migr 3:148–154
Breuninger S, Reidenbach S, Sauer CG, Ströbel P, Pfitzenmaier J, Trojan L, Hofmann I (2010) Desmosomal plakophilins in the prostate and prostatic adenocarcinomas: implications for diagnosis and tumor progression. Am J Pathol 176:2509–2519
Brooke MA, Nitoiu D, Kelsell DP (2012) Cell-cell connectivity: desmosomes and disease. J Pathol 226:158–171
Cabral RM, Liu L, Hogan C, Dopping-Hepenstal PJ, Winik BC, Asial RA, Dobson R, Mein CA, Baselaga PA, Mellerio JE, Nanda A, Boente Mdel C, Kelsell DP, McGrath JA, South AP (2010) Homozygous mutations in the 5′ region of the JUP gene result in cutaneous disease but normal heart development in children. J Invest Dermatol 130:1543–1550
Cabral RM, Tattersall D, Patel V, McPhail GD, Hatzimasoura E, Abrams DJ, South AP, Kelsell DP (2012) The DSPII splice variant is crucial for desmosome-mediated adhesion in HaCaT keratinocytes. J Cell Sci 125:2853–2861
Campuzano O, Alcalde M, Berne P, Zorio E, Iglesias A, Navarro-Manchón J, Brugada J, Brugada R (2013) Role of novel DSP_p.Q986X genetic variation in arrhythmogenic right ventricular cardiomyopathy. Eur J Med Genet 56:541–545
Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko Gusky H, Novelli V, Kim C, Tirasawadichai T, Judge DP, Rothenberg E, Chen HS, Napolitano C, Priori SG, Delmar M (2014) Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 129:1092–1103
Chalabreysse L, Senni F, Bruyère P, Aime B, Ollagnier C, Bozio A, Bouvagnet P (2011) A new hypo/oligodontia syndrome: Carvajal/Naxos syndrome secondary to desmoplakin-dominant mutations. J Dent Res 90:58–64
Chen YJ, Chang JT, Lee L, Wang HM, Liao CT, Chiu CC, Chen PJ, Cheng AJ (2007) DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene 26:467–476
Chen J, Nekrasova OE, Patel DM, Klessner JL, Godsel LM, Koetsier JL, Amargo EV, Desai BV, Green KJ (2012) The C-terminal unique region of desmoglein 2 inhibits its internalization via tail-tail interactions. J Cell Biol 199:699–711
Chen YJ, Lee LY, Chao YK, Chang JT, Lu YC, Li HF, Chiu CC, Li YC, Li YL, Chiou JF, Cheng AJ (2013) DSG3 facilitates cancer cell growth and invasion through the DSG3-plakoglobin-TCF/LEF-Myc/cyclin D1/MMP signaling pathway. PLoS One 8:e64088
Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ (2014) The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res 114:454–468
Chidgey M, Dawson C (2007) Desmosomes: a role in cancer? Br J Cancer 96:1783–1787
Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, Martens JR, Rothenberg E, Musa H, Delmar M (2012) Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm 9:1133–1140
Chun MG, Hanahan D (2010) Genetic deletion of the desmosomal component desmoplakin promotes tumor microinvasion in a mouse model of pancreatic neuroendocrine carcinogenesis. PLoS Genet 6:e1001120
Cirillo N, AlShwaimi E, McCullough M, Prime SS (2014) Pemphigus vulgaris autoimmune globulin induces Src-dependent tyrosine-phosphorylation of plakophilin 3 and its detachment from desmoglein 3. Autoimmunity 47:134–140
Demirag GG, Sullu Y, Yucel I (2012) Expression of Plakophilins (PKP1, PKP2, and PKP3) in breast cancers. Med Oncol 29:1518–1522
Dubash AD, Koetsier JL, Amargo EV, Najor NA, Harmon RM, Green KJ (2013) The GEF Bcr activates RhoA/MAL signaling to promote keratinocyte differentiation via desmoglein-1. J Cell Biol 202:653–666
Dusek RL, Attardi LD (2011) Desmosomes: new perpetrators in tumour suppression. Nat Rev Cancer 11:317–323
Fang WK, Liao LD, Li LY, Xie YM, Xu XE, Zhao WJ, Wu JY, Zhu MX, Wu ZY, Du ZP, Wu BL, Xie D, Guo MZ, Xu LY, Li EM (2013) Down-regulated desmocollin-2 promotes cell aggressiveness through redistributing adherens junctions and activating beta-catenin signalling in oesophageal squamous cell carcinoma. J Pathol 231:257–270
Fang WK, Liao LD, Gu W, Chen B, Wu ZY, Wu JY, Shen J, Xu LY, Li EM (2014) Down-regulated gamma-catenin expression is associated with tumor aggressiveness in esophageal cancer. World J Gastroenterol 20:5839–5848
Fidler LM, Wilson GJ, Liu F, Cui X, Scherer SW, Taylor GP, Hamilton RM (2009) Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med 13:4219–4228
Franzen CA, Todorović V, Desai BV, Mirzoeva S, Yang XJ, Green KJ, Pelling JC (2012) The desmosomal armadillo protein plakoglobin regulates prostate cancer cell adhesion and motility through vitronectin-dependent Src signaling. PLoS One 7:e42132
Furukawa C, Daigo Y, Ishikawa N, Kato T, Ito T, Tsuchiya E, Sone S, Nakamura Y (2005) Plakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancer. Cancer Res 65):7102–7110
Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E (1998) Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol 143:2009–2022
Garrod D, Chidgey M (2008) Desmosome structure, composition and function. Biochim Biophys Acta 1778:572–587
Gehmlich K, Asimaki A, Cahill TJ, Ehler E, Syrris P, Zachara E, Re F, Avella A, Monserrat L, Saffitz JE, McKenna WJ (2010) Novel missense mutations in exon 15 of desmoglein-2: role of the intracellular cadherin segment in arrhythmogenic right ventricular cardiomyopathy? Heart Rhythm 7:1446–1453
Gehmlich K, Syrris P, Reimann M, Asimaki A, Ehler E, Evans A, Quarta G, Pantazis A, Saffitz JE, McKenna WJ (2012) Molecular changes in the heart of a severe case of arrhythmogenic right ventricular cardiomyopathy caused by a desmoglein-2 null allele. Cardiovasc Pathol 21:275–282
Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ (2009) Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol 185:1243–1258
Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LT, Huen AC, Thorne ME, Gaudry CA, Park JK, Myung K, Goldman RD, Chew TL, Green KJ (2005) Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J Cell Biol 171:1045–1059
Godsel LM, Dubash AD, Bass-Zubek AE, Amargo EV, Klessner JL, Hobbs RP, Chen X, Green KJ (2010) Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol Biol Cell 21:2844–2859
Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JE, Quarta G, Nobles M, Syrris P, Chaubey S, McKenna WJ, Tinker A, Lambiase PD (2012) Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J 33:1942–1953
Green KJ, Simpson CL (2007) Desmosomes: new perspectives on a classic. J Invest Dermatol 127:2499–2515
Harmon RM, Simpson CL, Johnson JL, Koetsier JL, Dubash AD, Najor NA, Sarig O, Sprecher E, Green KJ (2013) Desmoglein-1/Erbin interaction suppresses ERK activation to support epidermal differentiation. J Clin Invest 123:1556–1570
Harris AR, Daeden A, Charras GT (2014) Formation of adherens junctions leads to the emergence of a tissue-level tension in epithelial monolayers. J Cell Sci 127:2507–2517
Has C, Jakob T, He Y, Kiritsi D, Hausser I, Bruckner-Tuderman L (2015) Loss of desmoglein 1 associated with palmoplantar keratoderma, dermatitis and multiple allergies. Br J Dermatol 172:257–261
Hatzfeld M (2007) Plakophilins: multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta 1773:69–77
Hobbs RP, Green KJ (2012) Desmoplakin regulates desmosome hyperadhesion. J Invest Dermatol 132:482–485
Hobbs RP, Han SY, van der Zwaag PA, Bolling MC, Jongbloed JD, Jonkman MF, Getsios S, Paller AS, Green KJ (2010) Insights from a desmoplakin mutation identified in lethal acantholytic epidermolysis bullosa. J Invest Dermatol 130:2680–2683
Hobbs RP, Amargo EV, Somasundaram A, Simpson CL, Prakriya M, Denning MF, Green KJ (2011) The calcium ATPase SERCA2 regulates desmoplakin dynamics and intercellular adhesive strength through modulation of PKCα signaling. FASEB J 25:990–1001
Johnson JL, Najor NA, Green KJ (2014) Desmosomes: regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harb Perspect Med 4:a015297
Jolly PS, Berkowitz P, Bektas M, Lee HE, Chua M, Diaz LA, Rubenstein DS (2010) p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. J Biol Chem 285:8936–8941
Kamekura R, Kolegraff KN, Nava P, Hilgarth RS, Feng M, Parkos CA, Nusrat A (2014) Loss of the desmosomal cadherin desmoglein-2 suppresses colon cancer cell proliferation through EGFR signaling. Oncogene 33:4531–4536
Kapplinger JD, Landstrom AP, Salisbury BA, Callis TE, Pollevick GD, Tester DJ, Cox MG, Bhuiyan Z, Bikker H, Wiesfeld AC, Hauer RN, van Tintelen JP, Jongbloed JD, Calkins H, Judge DP, Wilde AA, Ackerman MJ (2011) Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J Am Coll Cardiol 57:2317–2327
Keller DI, Stepowski D, Balmer C, Simon F, Guenthard J, Bauer F, Itin P, David N, Drouin-Garraud V, Fressart V (2012) De novo heterozygous desmoplakin mutations leading to Naxos-Carvajal disease. Swiss Med Wkly 142:w13670
Khapare N, Kundu ST, Sehgal L, Sawant M, Priya R, Gosavi P, Gupta N, Alam H, Karkhanis M, Naik N, Vaidya MM, Dalal SN (2012) Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis. PLoS One 7:e38561
Kim W, Kim M, Jho EH (2013) Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J 450:9–21
Kirchner F, Schuetz A, Boldt LH, Martens K, Dittmar G, Haverkamp W, Thierfelder L, Heinemann U, Gerull B (2012) Molecular insights into arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 missense mutations. Circ Cardiovasc Genet 5:400–411
Kitajima Y (2013) New insights into desmosome regulation and pemphigus blistering as a desmosome-remodeling disease. Kaohsiung J Med Sci 29:1–13
Koetsier JL, Amargo EV, Todorović V, Green KJ, Godsel LM (2014) Plakophilin 2 affects cell migration by modulating focal adhesion dynamics and integrin protein expression. J Invest Dermatol 134:112–122
Kolegraff K, Nava P, Helms MN, Parkos CA, Nusrat A (2011) Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/beta-catenin signaling. Mol Biol Cell 22:1121–1134
Kowalczyk AP, Green KJ (2013) Structure, function, and regulation of desmosomes. Prog Mol Biol Transl Sci 116:95–118
Kurzen H, Munzing I, Hartschuh W (2003) Expression of desmosomal proteins in squamous cell carcinomas of the skin. J Cutan Pathol 30:621–630
Lai-Cheong JE, Arita K, McGrath JA (2007) Genetic diseases of junctions. J Invest Dermatol 127:2713–2725
Lam L, Aktary Z, Bishay M, Werkman C, Kuo CY, Heacock M, Srivastava N, Mackey JR, Pasdar M (2012) Regulation of subcellular distribution and oncogenic potential of nucleophosmin by plakoglobin. Oncogenesis 1:e4
Li D, Liu Y, Maruyama M, Zhu W, Chen H, Zhang W, Reuter S, Lin SF, Haneline LS, Field LJ, Chen PS, Shou W (2011a) Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum Mol Genet 20:4582–4596
Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL (2011b) Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol 31:1134–1144
Li D, Zhang W, Liu Y, Haneline LS, Shou W (2012) Lack of plakoglobin in epidermis leads to keratoderma. J Biol Chem 287:10435–10443
Lin S, Gordon K, Kaplan N, Getsios S (2010) Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol Biol Cell 21:3902–3914
Lombardi R, da Graca Cabreira-Hansen M, Bell A, Fromm RR, Willerson JT, Marian AJ (2011) Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res 109:1342–1353
Lopez-Ayala JM, Gomez-Milanes I, Sánchez Muñoz JJ, Ruiz-Espejo F, Ortíz M, González-Carrillo J, López-Cuenca D, Oliva-Sandoval MJ, Monserrat L, Valdés M, Gimeno JR (2014) Desmoplakin truncations and arrhythmogenic left ventricular cardiomyopathy: characterizing a phenotype. Europace 16:1838–1846
McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RA (1997) Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet 17:240–244
McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ (2000) Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355:2119–2124
Morgan RG, Pearn L, Liddiard K, Pumford SL, Burnett AK, Tonks A, Darley RL (2013) Gamma-catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of betacatenin. Leukemia 27:336–343
Muller T, Bain G, Wang X, Papkoff J (2002) Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp Cell Res 280:119–133
Niessen CM, Gottardi CJ (2008) Molecular components of the adherens junction. Biochim Biophys Acta 1778:562–571
Patel DM, Dubash AD, Kreitzer G, Green KJ (2014) Disease mutations in desmoplakin inhibit Cx43 membrane targeting mediated by desmoplakin-EB1 interactions. J Cell Biol 206:779–797
Pigors M, Kiritsi D, Krümpelmann S, Wagner N, He Y, Podda M, Kohlhase J, Hausser I, Bruckner-Tuderman L, Has C (2011) Lack of plakoglobin leads to lethal congenital epidermolysis bullosa: a novel clinico-genetic entity. Hum Mol Genet 20:1811–1819
Rasmussen TB, Nissen PH, Palmfeldt J, Gehmlich K, Dalager S, Jensen UB, Kim WY, Heickendorff L, Mølgaard H, Jensen HK, Baandrup UT, Bross P, Mogensen J (2014) Truncating plakophilin-2 mutations in arrhythmogenic cardiomyopathy are associated with protein haploinsufficiency in both myocardium and epidermis. Circ Cardiovasc Genet 7:230–240
Saito M, Stahley SN, Caughman CY, Mao X, Tucker DK, Payne AS, Amagai M, Kowalczyk AP (2012) Signaling dependent and independent mechanisms in pemphigus vulgaris blister formation. PLoS One 7:e50696
Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, Spiegel R, Eytan O, Geller S, Peleg S, Shomron N, Goh CS, Wilson NJ, Smith FJ, Pohler E, Simpson MA, McLean WH, Irvine AD, Horowitz M, McGrath JA, Green KJ, Sprecher E (2013) Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 45:1244–1248
Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M (2011) Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ Res 109:193–201
Scott CA, Tattersall D, O’Toole EA, Kelsell DP (2012) Connexins in epidermal homeostasis and skin disease. Biochim Biophys Acta 1818:1952–1961
Shaw RM (2014) Desmosomal hotspots, microtubule delivery, and cardiac arrhythmogenesis. Dev Cell 31:139–140
Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY (2007) Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128:547–560
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K (1998) Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol 110:76–78
Sklyarova T, Bonne S, D’Hooge P, Denecker G, Goossens S, De Rycke R, Borgonie G, Bösl M, van Roy F, van Hengel J (2008) Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J Invest Dermatol 128:1375–1385
Spindler V, Dehner C, Hübner S, Waschke J (2014) Plakoglobin but not desmoplakin regulates keratinocyte cohesion via modulation of p38MAPK signaling. J Invest Dermatol 134:1655–1664
Swope D, Cheng L, Gao E, Li J, Radice GL (2012) Loss of cadherin-binding proteins beta-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis. Mol Cell Biol 32:1056–1067
Tanaka A, Lai-Cheong JE, Café ME, Gontijo B, Salomão PR, Pereira L, McGrath JA (2009) Novel truncating mutations in PKP1 and DSP cause similar skin phenotypes in two Brazilian families. Br J Dermatol 160:692–697
Thomason HA, Scothern A, McHarg S, Garrod DR (2010) Desmosomes: adhesive strength and signalling in health and disease. Biochem J 429:419–433
Todorović V, Desai BV, Patterson MJ, Amargo EV, Dubash AD, Yin T, Jones JC, Green KJ (2010) Plakoglobin regulates cell motility through Rho- and fibronectin-dependent Src signaling. J Cell Sci 123:3576–3586
Todorović V, Koetsier JL, Godsel LM, Green KJ (2014) Plakophilin 3 mediates Rap1-dependent desmosome assembly and adherens junction maturation. Mol Biol Cell 25:3749–3764
Tsang SM, Liu L, Teh MT, Wheeler A, Grose R, Hart IR, Garrod DR, Fortune F, Wan H (2010) Desmoglein 3, via an interaction with E-cadherin, is associated with activation of Src. PLoS One 5:e14211
Tsang SM, Brown L, Lin K, Liu L, Piper K, O’Toole EA, Grose R, Hart IR, Garrod DR, Fortune F, Wan H (2012) Non-junctional human desmoglein 3 acts as an upstream regulator of Src in E-cadherin adhesion, a pathway possibly involved in the pathogenesis of pemphigus vulgaris. J Pathol 227:81–93
Tucker DK, Stahley SN, Kowalczyk AP (2014) Plakophilin-1 protects keratinocytes from pemphigus vulgaris IgG by forming calcium-independent desmosomes. J Invest Dermatol 134:1033–1043
Valladares-Ayerbes M, Diaz-Prado S, Reboredo M, Medina V, Lorenzo-Patiño MJ, Iglesias-Díaz P, Haz M, Pértega S, Santamarina I, Blanco M, Quindós-Varela M, Figueroa A, Antón-Aparicio LM (2010) Evaluation of plakophilin-3 mRNA as a biomarker for detection of circulating tumor cells in gastrointestinal cancer patients. Cancer Epidemiol Biomarkers Prev 19:1432–1440
van der Velden HM, Jongsma HJ (2002) Cardiac gap junctions and connexins: their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc Res 54:270–279
van der Wier G, Pas HH, Kramer D, Diercks GF, Jonkman MF (2014) Smaller desmosomes are seen in the skin of pemphigus patients with anti-desmoglein 1 antibodies but not in patients with anti-desmoglein 3 antibodies. J Invest Dermatol 134:2287–2290
Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E (2001) Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol 3:1076–1085
Wang H, Li Z, Yumul R, Lara S, Hemminki A, Fender P, Lieber A (2011a) Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions. J Virol 85:6390–6402
Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, Wahl JK 3rd, Urban N, Drescher C, Hemminki A, Fender P, Lieber A (2011b) Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17:96–104
Wang H, Yumul R, Cao H, Ran L, Fan X, Richter M, Epstein F, Gralow J, Zubieta C, Fender P, Lieber A (2013) Structural and functional studies on the interaction of adenovirus fiber knobs and desmoglein 2. J Virol 87:11346–11362
Wolf A, Krause-Gruszczynska M, Birkenmeier O, Ostareck-Lederer A, Hüttelmaier S, Hatzfeld M (2010) Plakophilin 1 stimulates translation by promoting eIF4A1 activity. J Cell Biol 188:463–471
Wolf A, Rietscher K, Glaß M, Hüttelmaier S, Schutkowski M, Ihling C, Sinz A, Wingenfeld A, Mun A, Hatzfeld M (2013) Insulin signaling via Akt2 switches plakophilin 1 function from stabilizing cell adhesion to promoting cell proliferation. J Cell Sci 126:1832–1844
Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI, Godsel LM, Green KJ, Saffitz JE, Li H, Danieli GA, Calkins H, Marcus F, Towbin JA (2006) Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res 99:646–655
Yang L, Chen Y, Cui T, Knösel T, Zhang Q, Albring KF, Huber O, Petersen I (2012) Desmoplakin acts as a tumor suppressor by inhibition of the Wnt/beta-catenin signaling pathway in human lung cancer. Carcinogenesis 33:1863–1870
Yin T, Getsios S, Caldelari R, Kowalczyk AP, Müller EJ, Jones JC, Green KJ (2005) Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci U S A 102:5420–5425
Acknowledgements
The Getsios lab is supported by the National Institutes of Health (NIH) grants R01-AR062110 and a Skin Disease Research Center Grant P30-AR057216. The Green lab is supported by NIH grants R01-AR041836, R37-AR043380, and R01-CA122151, by a grant from the Leducq Foundation, and by the Joseph L. Mayberry Senior Endowment. J.A.B. is supported by a Ruth L. Kirschstein “Post Graduate Program in Cutaneous Biology” training grant T32-AR060710.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Broussard, J.A., Getsios, S. & Green, K.J. Desmosome regulation and signaling in disease. Cell Tissue Res 360, 501–512 (2015). https://doi.org/10.1007/s00441-015-2136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2136-5