Abstract
Desmin is a muscle-specific type III intermediate filament essential for proper muscular structure and function. In human, mutations affecting desmin expression or promoting its aggregation lead to skeletal (desmin-related myopathies), or cardiac (desmin-related cardiomyopathy) phenotypes, or both. Patient muscles display intracellular accumulations of misfolded proteins and desmin-positive insoluble granulofilamentous aggregates, leading to a large spectrum of molecular alterations. Increasing evidence shows that desmin function is not limited to the structural and mechanical integrity of cells. This novel perception is strongly supported by the finding that diseases featuring desmin aggregates cannot be easily associated with mechanical defects, but rather involve desmin filaments in a broader spectrum of functions, such as in organelle positioning and integrity and in signaling. Here, we review desmin functions and related diseases affecting striated muscles. We detail emergent cellular functions of desmin based on reported phenotypes in patients and animal models. We discuss known desmin protein partners and propose an overview of the way that this molecular network could serve as a signal transduction platform necessary for proper muscle function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intermediate filaments
Classification, structure and biochemical properties
The intermediate filament (IF) protein family encompasses 73 members encoded by a large group of genes that have been commonly implicated in human disease accounting for at least 94 different disease entities (Eriksson et al. 2009). IF family members have been subdivided into five distinct types on the basis of their primary structure, their properties of assembly and their developmentally regulated tissue-specific expression pattern (Fig. 1a). IF types I and II, which are composed of keratins, form obligatory heteropolymers in epithelial cells. By contrast, type III IF proteins, which include desmin, syncoilin, vimentin, peripherin and GFAP (glial fibrillary acidic protein), form homopolymers. The type IV group comprises three neurofilament (NF) subunits (NF-L [light], NF-M [middle] and NF-H [heavy]), α-internexin, nestin and synemin α and β. Group V is composed of the nuclear IF proteins (lamin A, B1, B2, C1 and C2), the oldest type of filaments in terms of evolution. The last group (group VI) includes the two eye lens IFs or “beaded filament” proteins, namely CP49 (phakinin) and filensin (CP115; Coulombe and Wong 2004). Cytoplasmic IF proteins have a similar secondary structure, which consists in a central α-helical rod domain flanked by non-helical domains of variable size. The rod domain forms the core structure of 10-nm filaments. Two segments of the rod domain, namely the 1A subdomain located in the amino-terminal region and the 2B subdomain in the carboxy-terminal end, are the most conserved regions among the various types of IF. The non-helical domains share little homology among the various types of IF but are similar within a given type. The amino terminal domains are essential for IF assembly, whereas the carboxy-terminal domain might be involved in lateral interaction and the organization of the IF network (Goldman et al. 2012). These non-helical domains have numerous phosphorylation sites involved in the regulation of IF properties and in their assembly/disassembly and subcellular organization (Eriksson et al. 2004). IF assembly can be performed by “autarky” (self-assembly of IF leading to functional filaments starting from purified protein) and takes place in a three-step process. First, eight tetramers rapidly associate laterally to form unit-length filaments (ULFs; Fig. 1b). These ULFs are approximately 16 nm in diameter and 60 nm in length. The ULFs then anneal end-to-end to yield loosely packed filaments that are several hundred nanometers long. These filaments start to compact for further elongation by stochastically reducing their diameter; the loosely packed filaments undergo an internal reorganization or compaction that propagates throughout the filament to yield mature IFs (Herrmann et al. 2009; Schopferer et al. 2009).
Structural organization of intermediate filament (IF) proteins and their classification. a Tripartite structure of IFs, consisting in a highly conserved α-helical central rod domain flanked by non-α-helical head and tail domains. The rod domain consists in heptad repeats that are the signature of α-helical proteins. The heptads are interrupted by short linker sequences (L1, L12, L2), which result in four α-helical segments: 1A, 1B, 2A and 2B. The central rod domain is highly conserved in vertebrate IF proteins, with the exception of the nuclear lamins, which contain six extra heptads in the 1B segment. The variability of IF proteins lies in the length and sequence of the head and tail domains (e.g., nestin has a longer tail domain, whereas lamins have longer tail and head domains). IFs have been classified into five distinct types on the basis of their sequence identity and tissue distribution. The type I and type II sequence-homology groups contain the keratins of epithelial cells. Heterodimers are formed with one keratin of each type (one acidic and one basic). Type III IFs include vimentin, desmin, glial fibrillary acidic protein (GFAP), peripherin and syncoilin. The neurofilament (NF) triplet proteins (NF-L, NF-M, NF-H), α-internexin, synemins (α and β) and nestin comprise the type IV intermediate filaments. Nuclear lamin A and its splice variant, lamin C, together with lamin B1 and B2 constitute the type V IFs. The type VI IFs encompass the cytoplasmic lens IFs CP49/phakinin and filensin/CP115. b General model of IF assembly and polymer formation. Two dimers associate in an anti-parallel half-staggered manner occurring after the overlapping of two coiled-coil (Coil 1A and Coil 1B) domains (red). Upon initiation of the assembly condition, the tetramers associate laterally into a multi-length filament (ULF unit-length filament). Individual ULFs anneal longitudinally with other ULF (1) or two annealed ULFs (2), which in turn can anneal with three annealed ULFs to form longer filaments. These longer filaments will spontaneously reduce their diameter by radial compaction to form mature cytoplasmic IFs
Cellular functions
IFs are highly dynamic structures and increasing evidence shows that IF functions are not restricted to maintaining the structural and mechanical integrity of cells (Buehler 2013). This recent perception is strongly supported by the finding that many of the diseases associated with mutations in the genes encoding IF proteins cannot easily be related to structural defects but rather involve a broader spectrum of functions, such as organelle positioning and function, signaling and/or the regulation of transcription (Chang et al. 2009; Chang and Goldman 2004; Schofield and Bernard 2013). Indeed, subsequent studies have highlighted the involvement of IFs in adhesion, migration and signal transduction (Chung et al. 2013; Windoffer et al. 2011). Numerous pieces of evidence have demonstrated that IFs act as a scaffold for and as a functional determinant of signaling molecules such as kinases, phosphatases, or ion channels (Dingli et al. 2012; Schofield and Bernard 2013). In many cases, post-translational modifications by phosphorylation, glycosylation, or other methods regulate the IF-related signaling functions, in particular their interactions with individual signaling proteins and their dynamic properties (Snider and Omary 2014). An example of an IF-kinase complex is the interaction of vimentin with Rho-binding kinases (ROKs) in the RAF-1/RhoA signaling pathway to mediate actin dynamics and focal adhesion formation. Activated ROKα phosphorylates vimentin, which leads to filament collapse and the consequent release of ROKα from the vimentin IFs and its translocation to the periphery of the cell. IFs can also influence cell fate by directly controlling death receptor complexes. In particular, K8/K18 filaments protect liver hepatocytes during apoptosis-promoting stress conditions, either by regulating the targeting of death receptors at the cell surface or by controlling the formation of the death-inducing signaling complex through protein sequestration and thereby controlling downstream signaling (Gilbert et al. 2008).
Desmin: the muscle IF-specific protein
In contrast to smooth muscle, cardiac and skeletal muscles are both striated, resulting from the overlapping patterns of actin and myosin filaments. Fibers from skeletal muscle are multinucleated and result from the fusion of many mononucleated myoblasts, whereas cardiac muscle is composed of individual cardiomyocytes. Cardiomyocyte plasma membranes (sarcolemma) are connected mechanically and electrically through highly specialized cell–cell junctions, intercalated disks, composed of three types of cell-cell contacts: adherent junctions, desmosomes and gap junctions. Skeletal muscles have two specialized cell–cell junctions: the neuromuscular junction where they are innervated by motor neurons and the myotendinous junction where they attach to the tendons. IFs associate with the sarcolemma of both cardiac and skeletal muscle at structures termed “costameres”, present at the membrane overlying Z-disks and M-bands. The IF cytoskeleton of mature muscle is composed predominantly of type III IF proteins, especially desmin (Lazarides and Hubbard, 1976). The non-muscle-specific IF proteins, synemin and paranemin, are co-expressed and form copolymers with desmin (Breckler and Lazarides 1982; Granger and Lazarides 1980). In addition, the atypical type III IF syncoilin can bind desmin and α-dystrobrevin, a component of the dystrophin-associated protein complex (DAPC; Newey et al. 2001). The majority of these IFs are typically localized around the α-actinin-rich Z-disks and are also found at the costameres. Nestin, originally identified as a brain-stem-cell-specific IF protein, can also polymerize with type III IF proteins (Steinert et al. 1999). It is present in early cardiac and skeletal muscles but is only maintained at significant levels in adult skeletal muscle at the neuromuscular and myotendinous junctions (Kachinsky et al. 1994). Adult striated muscle also contains IFs of the cytokeratin family, specifically keratins 8 (K8) and 19 (K19), which form heteropolymers in vitro (Stone et al. 2005). Like desmin, K8 and K19 associate with the periphery of Z-disks and are present at the sarcolemma (Stone et al. 2005; Ursitti et al. 2004).
Desmin in myogenesis and developing muscle
The predominant type III protein in myoblasts is vimentin, which is known to be downregulated with development (Farrell et al. 1990). In cultured muscle cells, during myoblast elongation and fusion, desmin becomes integrated with the pre-existing vimentin filaments and forms longitudinal strands. Upon the maturation of myotubes, these strands are transformed to transversely organized filaments and are localized between the myofibrils at the level of the Z-disks (Barbet et al. 1991). Previous studies have demonstrated that desmin deficiency blocks myoblast fusion and myotube formation, both in differentiating C2C12 myoblasts and in embryoid bodies (Schultheiss et al. 1991). Myogenesis can be divided into several phases including cell commitment, the exit from the cell cycle, the initiation of differentiation, cell contact and alignment and cell adhesion and fusion (Bentzinger et al. 2012). Desmin might actively participate in one or more of these processes and be directly involved in myoblast fusion. Furthermore, the ectopic expression of desmin in the lenses of mice causes partial plasma membrane fusion (Dunia et al. 1990). Fusion between two cells can take place only between desmin-positive cells; however, additional reports in which bone marrow stem cells have been used to generate myogenic cells suggest that, at least in this case, only one of the cells requires desmin for fusion (Camargo et al. 2003). Intriguing observations from desmin knockout mice showing normal muscle development (Li et al. 1997) indicate the compensation of desmin expression by other IFs such as cytokeratins; the latter might orchestrate myoblast fusion and differentiation or be involved later during myofiber structural organization. Like several IFs proteins (such as synemin, vimentin), desmin is well expressed in developing muscle (Kuisk et al. 1996). It is one of the earliest known myogenic markers, both in heart and in somites (Herrmann et al. 1989). In contrast to most muscle-specific genes, desmin is also expressed, albeit at low levels, in satellite cells and replicating myoblasts (Allen et al. 1991). During development, desmin expression precedes the other muscle-specific structural genes and the bHLH transcription factors myoD, myogenin and MRF4, with the exception of Myf-5 (Li and Capetanaki 1993), suggesting that desmin has a modulating role in myogenic commitment and differentiation (Lazarides 1982). This is also supported by the observation of a total inhibition of skeletal and smooth muscle development in cultured desmin null mutant embryoid cells (Weitzer et al. 1995). However, the results obtained in mice lacking the desmin gene, which develop normal muscles, show that desmin is not essential, either for the proliferation and differentiation of myotubes or for the subsequent myofibrillar organization (Capetanaki et al. 1997). Initial studies of a desmin knockout model have reported no overexpression of vimentin (Li et al. 1997). Further investigations are needed to determine whether another IF can compensate for the lack of desmin during skeletal and cardiac muscle development.
Desmin in adult skeletal muscle
In mature striated muscle, desmin IFs form three-dimensional scaffolds that extend across the entire diameter of the myofibril. Desmin IFs surround the Z-disks, interconnecting them to each other and to the sarcolemma, at the level of costameres in skeletal muscle and at intercalated disks in the case of cardiac muscle (Capetanaki et al. 2007; Fig. 2a). Costameres in rodent fast-twitch skeletal muscle consist in three distinct structures: Z-domains, which are linked to the Z-disks of nearby myofibrils; M-domains, which align with M-lines in nearby sarcomeres; and longitudinally oriented or l-domains, which lie parallel to the long axis of the myofibers and have no known correlate in the fiber interior (Ervasti 2003). Three types of cytoplasmic filaments link costameres to the underlying contractile apparatus. γ-Actin, which comprises the best characterized of these filaments, associates with the sarcolemma (at least in part) through its ability to bind to dystrophin (Rybakova et al. 2000). The two other structures, both composed of IFs, also link the contractile apparatus to the sarcolemma in striated muscle. One contains desmin, whereas the other is composed of the two keratins K19 and K8. Desmin is the most abundant IF protein in mature striated muscle in which it surrounds each Z-disk and extends from the Z-disks of superficial myofibrils to the Z-domains of costameres at the sarcolemma. Desmin filaments are linked to costameres through plectin (Konieczny et al. 2008) and through the binding of synemin to dystrophin (Bhosle et al. 2006), the dystrophin-binding protein α-dystrobrevin (Mizuno et al. 2001) and other costameric components (Bellin et al. 2001). Desmin knockout in mice leads to costameres disruption, especially at Z-domains (O'Neill et al. 2002) but it has otherwise surprisingly mild effects on striated muscle function (Sam et al. 2000). K8 and K19 are also expressed in mature striated muscle (Ursitti et al. 2004). Although they are more difficult to detect and are present in smaller amounts than desmin, K8 and K19 are found at both the Z-disks and M-lines of myofibrils near the sarcolemma (Ursitti et al. 2004), where they are appropriately localized for linking myofibrils to the M- and Z-domains of costameres. Deeper in the myofibers, they, like desmin, concentrate primarily around Z-disks (Ursitti et al. 2004). At the sarcolemma, they are also enriched at the l-domains and are thus the most likely cytoplasmic structures to stabilize the l- and M-domains of costameres. When overexpressed in skeletal myofibers, K8 accumulates in the middle of sarcomeres (Stone et al. 2005), suggesting an affinity for the proteins of M-bands, as both K8 and K19 co-purify with the dystrophin-dystroglycan complex (Ursitti et al. 2004). The absence of K19 in fast-twitch skeletal muscle is accompanied by a mild myopathy associated with disruption of costameres and mitochondria. These mice exhibit normal localization but a higher level of desmin in the tibialis muscle (but not in the gastrocnemius) suggesting that K19 absence is compensated by desmin in fast-twitch skeletal muscle (Stone et al. 2007). More recently, Lovering et al. (2011) generated desmin/K19 double-knockout mice on an FVB (Friend leukemia virus B strain) genetic background and showed that the elimination of both K19 and desmin in fast-twitch skeletal muscle disrupts the organization of muscle fibers and compromises contractile force and protection from injury. They further suggested that desmin and K19 play distinct, complementary, or, in some cases, antagonistic roles in muscle structure and function (Lovering et al. 2011).
Desmin IF as a cellular organizer. a Representation of muscle fiber organization showing compacted myofibrils surrounded by the sarcoplasmic reticulum (SR) network. Intracellular sarcolemma (plasma membrane) invaginations called the T-tubules connect the SR to form a triad (two SR saccules connected to one T-tubule). Boxes 1–3 indicate the various regions of muscle fibers in which desmin IFs act as a scaffold for membranous compartments and organelles. a’ Desmin IFs associated with costameres via dystrobrevin and syncoilin, two proteins from the dystrophin-associated protein complex (DAPC). The DAPC bridges the extracellular matrix to the contractile apparatus and is composed of dystrophin and its associated transmembrane glycosylated proteins (dystroglycans and sarcoglycans). Plectin 1f is a cytolinker bridging desmin IF to dystrophin and its associated proteins dystrobrevin and β-dystroglycan. a’’ Desmin lattice connecting the nucleus (via the cytolinker plectin 1) and other organelles such as mitochondria (via the cytolinker plectin 1b) or lysosome (via myospryn). Desmin filaments also connect the sarcomere via titin and myomesin at the M-line and plectin 1d at the Z-disk. a’’’ Possible link of desmin to the SR via the phosphoinositide phosphatase myotubularin (MTM1), whereas the link between desmin and mitochondria via the plectin 1b is established. b Physical interaction map based on published data and interaction databases (BioGrid, MINT, STRING) displaying desmin interactors such as cytolinkers and chaperones and proteins involved in signaling or sarcomere and cytoskeleton regulation
Desmin filaments also link the entire contractile apparatus to various membranous compartments and organelles, including the nucleus, mitochondria, lysosomes (Capetanaki et al. 2007) and potentially the sarcoplasmic reticulum (SR; Amoasii et al. 2013; Hnia et al. 2011). Connection to many organelles is mainly through a physical link with plectin isoforms (Castanon et al. 2013). Such interactions control mitochondrial dynamics and nuclear positioning (Winter and Wiche 2013; Fig. 2a). Consequently, nuclear shape and positioning and the link between the myofibrils and the sarcomere are altered in desmin-null mice (Capetanaki et al. 1997). Whether the movement of the nuclei to the periphery and the assembly of the myofibril network are both functionally linked to desmin transversal organization and/or whether these processes are interdependent is unclear. Noteworthy, the coincident nature of these events and the prevalence of aberrant nuclear positioning and desmin networks in individuals with several muscle diseases support the idea that adequate distribution and function of desmin IF are required for normal nuclear positioning. The absence of desmin also results in a delayed and modified regeneration with accumulation of adipocytes when desmin knockout muscle is damaged following notexin treatment, as reported by Meyer and Lieber (2012). These authors observed the persistence of small diameter muscle fibers containing NCAM (neural cell adhesion molecule) and developmental myosin isoforms, markedly disorganized neuromuscular junctions and the absence of post-junctional folds in some cases. These data suggest that desmin is essential for terminal muscle regeneration, the maturation of muscle fibers and the maintenance of the folded structure of the postsynaptic apparatus at the neuromuscular junction (Meyer and Lieber 2012).
Desmin partners and ensuing functions
Clues about desmin functions have arisen recently from the identification of proteins able to bind to desmin dynamically and from the assessment of these interactions in the context of animal models. Based on reported physical interactions in the literature and protein-protein interaction databases (BioGRID, MINT, GeneMANIA; Mostafavi et al. 2008; Stark et al. 2006), we established an interaction network for desmin (Fig. 2b). Desmin interactors include other members of the IF family, cytolinkers bridging organelles and cytoskeleton, chaperones and adaptor proteins and proteins that have been implicated in proteolysis, posttranslational modifications and signaling important for proper skeletal or cardiac muscle functions (Capetanaki et al. 2007; Costa et al. 2004). The architectural role of desmin filaments has been enriched by these new findings suggesting that desmin serves as a platform for signaling events perturbed upon desmin misfolding/aggregation under pathological conditions. Here, we detail two categories of desmin protein partners, namely the IF proteins and the signaling proteins. The other categories have been described and discussed in previous reviews (Clemen et al. 2013; Costa et al. 2004; Li et al. 1997; Winter et al. 2014).
Intermediate filaments
Desmin might bind or co-polymerize other type III IFs such as vimentin, syncoilin, or GFAP (Schultheiss et al. 1991; Guma et al. 2001; Newey et al. 2001), type IV IFs, namely synemin and nestin (Granger and Lazarides 1980; Kachinsky et al. 1994) and the type V IFs, namely the lamins (Cartaud et al. 1995; Fig. 2b). Co-polymerization of desmin with type III IFs might co-exist in some cell types or during development. GFAP can polymerize with desmin in vitro but only a few cell types co-express both proteins at the same time, such as myofibroblast (with additional expression of vimentin; Guma et al. 2001). Nestin is typically expressed in stem cells from the central nervous system but is also present with desmin in muscle ligaments and at the myotendinous junction (Kachinsky et al. 1994). Synemin, initially purified as a desmin-associated molecule (Granger and Lazarides 1980), is present at a higher level in striated muscle and might bridge desmin to both myofibrillar Z-lines via its binding site to α-actinin and to costameres via its interaction with the vinculin/α7β-integrin complex (Sun et al. 2008). Like desmin, syncoilin is highly expressed in skeletal and cardiac muscle. Syncoilin is found throughout the sarcolemma but is enriched at the neuromuscular and myotendinous junctions and around the nucleus (Moorwood 2008). Syncoilin binds α-dystrobrevin, a component of the DAPC, thus linking desmin to the sarcolemma (Blake and Martin-Rendon 2002; Poon et al. 2002). Interestingly, in mice devoid of desmin, syncoilin is redistributed to the soluble fraction of the cell, indicating that it is dependent upon desmin for its normal location and association with the cytoskeleton (Howman et al. 2003). The association of syncoilin with both the DAPC and the desmin IF network suggests that it plays a role in the transduction of signals between these two entities. For example, IF networks are known to be dynamic and to be modulated in response to cell signaling, particularly with respect to mechanical and non-mechanical stress (Mohamed and Boriek 2012). Some evidence has been presented that DAPC is involved in mechanotransduction (Kumar et al. 2004). Therefore, syncoilin might help to convey mechanical stress signaling from the DAPC to the desmin IF network, allowing the latter to respond accordingly. In addition, α-dystrobrevin might bind signaling proteins such as the syntrophins/neural nitric oxide synthetase further suggesting that the desmin/syncoilin/α-dystrobrevin complex serves as a platform for localized signal transduction through the sarcolemma (Constantin 2014; Mizuno et al. 2001).
Signaling proteins
-
CMYA5/myospryn: the cardiomyopathy associated protein 5 (CMYA5), also called myospryn or TRIM76, is a protein member of the tripartite motif (TRIM) superfamily mainly expressed in cardiac and skeletal muscle (Benson et al. 2004; Tsoupri and Capetanaki 2013). CMYA5 has been found to bind desmin by yeast two-hybrid screening (Y2H), as confirmed by biochemical analysis (Kouloumenta et al. 2007). The desmin N-terminus binds CMYA5 via its SPRY domain and both proteins co-localize at the periphery of the nucleus in mice cardiomyocytes. In the absence of desmin, myospryn loses its sharp perinuclear localization (Kouloumenta et al. 2007). Myospryn also co-localizes with markers for the endoplasmic reticulum (ER) and Golgi sorting machineries (KDEL receptor and TGN38, respectively) suggesting that it resides close to membrane structures of the ER and, potentially, the ER-Golgi intermediate compartment (Kouloumenta et al. 2007). In adult muscle, the two proteins co-localize predominantly at intercalated disks in cardiac muscle and at the Z-line connecting the sarcolemma and costameres (Kouloumenta et al. 2007). In addition, CMYA5 binds the dystrobrevin-binding protein dysbindin, a component of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Interestingly, desmin can immunoprecipitate the BLOC-1 subunits dysbindin and pallidin, potentially through myospryn (Benson et al. 2004; Kouloumenta et al. 2007). These findings suggest a role of desmin IFs in vesicle trafficking and organelle biogenesis and/or positioning. A plausible speculation is that desmin/CMYA5 interaction with the lysosomal machinery requires local signal transduction. Indeed, an in vitro study has suggested that CMYA5 is phosphorylated by protein kinase A (PKA; Reynolds et al. 2007). As such, CMYA5 is proposed as an A-kinase anchoring protein. The PKA signaling pathway is a universal mechanism of signal transduction and of particular importance for muscle function (Reynolds et al. 2008). On the other hand, CMYA5 can also interact with the calcium- and calmodulin (CaM)-regulated protein phosphatase calcineurin at the Z-disk/coastamere region (Kielbasa et al. 2011). Because of its important and diverse roles in muscle physiology, calcineurin is subjected to stringent regulation. Overall, the desmin/CMYA5 interaction is likely to coordinate the activity of specific kinases and phosphatases, particularly in response to changes in muscle activity or damage.
-
S100A1: S100A1 is a member of the calcium-binding S100 protein family and represents the most abundant S100 isoform in cardiac muscle (Prosser et al. 2011; Volkers et al. 2010). Early studies have revealed distinct expression patterns of S100A1 in healthy and diseased cardiac tissues from animal models and humans (Most et al. 2007). Further elaborate investigations have uncovered S100A1 protein as one of the main regulators of calcium-handling in skeletal muscle (Volkers et al. 2010). S100A1 binds desmin and regulates the formation of desmin polymers in vitro in the presence of micromolar levels of Ca2+ (Garbuglia et al. 1999). S100A1 also co-localizes with RyR (ryanodine receptor) in both heart and skeletal muscles (RyR1 and RyR2, respectively) and decreases RyR calcium transients via a calcium-dependent S100A1-RyR interaction (Prosser et al. 2008). Importantly, S100A1 competes with CaM for the same binding site on RyR1 and RyR2 (Prosser et al. 2008). Because calcium-loaded CaM-binding to RyR inhibits RyR-induced calcium release in both skeletal and cardiac myocytes, the simplest model for the S100A1-dependent activation of RyR involves competing away a well-established RyR inhibitor (i.e., CaM). Alternatively, we cannot rule out that the S100A1-RyR interaction stabilizes active RyR and that the CaM-dependent inhibition of RyR occurs by CaM displacing S100A1. In addition, S100A1 might also bind the SR Ca2+ ATPase SERCA (Remppis et al. 2002). Overall, a tempting speculation is that the interaction of desmin with S100A1 indirectly regulates Ca2+ channel activity (RyR, SERCA). Indeed, abnormal expression levels of SERCA2, phospholamban, ryanodine receptor 2 and calsequestrin 2 are significantly decreased in the 28-week-old αB-Cry R120G transgenic mouse (Jiao et al. 2014). Desmin might sequester S100A1 protein at the sarcomere, favoring the CaM inhibition of RyR. However, further investigations are needed to decipher the exact mechanism by which desmin can influence Ca2+ homeostasis in adult and developmental muscles.
-
PLEKHA5: the pleckstrin homology domain-containing protein family A member 5 (PLEKHA5), also named phosphatidylinositol 3-phosphate-binding PH domain protein-2 (PEPP2), is a protein that binds to membrane phosphoinositides (PtdIns3P, PtdIns4P, PtdIns5P and PtdIns3,5P 2) with different specificities (Yamada et al. 2012). Initially identified in brain, PLEKHA5 was proposed to play an important role in brain development through a potential role in vesicle trafficking (Zou and Zhong 2012). Bandyopadhyay et al. (2010) applied a systematic experimental (Y2H) and computational approach (interaction databases) to map 2269 interactions between human mitogen-activated protein kinase (MAPK)-related proteins and other cellular machinery. PLEKHA5 was found to interact with desmin with high confidence. Interestingly, the same screen identified PLEKHA5 interaction with plectin 1, a desmin-binding protein belonging to the plectin cytolinker family (Bandyopadhyay et al. 2010). Plectin anchors desmin to several organelles and cell compartments suggesting that the PLEKHA5-desmin interaction is involved in organelle dynamics. Given the binding capacity of PLEKHA5 toward specific phosphoinositides that are mostly enriched on the endosomal machinery, the PLEKHA5-desmin-plectin 1 complex might control the sorting or motility of such organelles.
-
MTM1: myotubularin, the protein mutated in the X-linked form of centronuclear myopathy (XLCNM, also called myotubular myopathy), has been found to bind desmin in vitro and in vivo in skeletal muscle; this interaction is not conserved in cardiac muscle suggesting that the MTM1-desmin complex has a specific functional significance in skeletal muscle (Hnia et al. 2011). MTM1 is a phosphoinositide 3-phosphatase that metabolizes PtdIns3P and PtdIns(3,5)P 2 into PtdIns and PtdIns5P, respectively. MTM1 shares the C(X)5R signature motif with the protein tyrosine phosphatase/dual specificity phosphatase family (PTP/DSP) and was initially suggested to be involved in membrane trafficking (Taylor et al. 2000). In skeletal muscle, MTM1 co-localizes partially with desmin IFs at the periphery of the Z-line (within the I band; Hnia et al. 2011). In addition, MTM1 co-localizes with the junctional SR markers RyR1 and triadin (Amoasii et al. 2013) and defects in calcium homeostasis and related excitation-contraction-coupling machinery have been observed in MTM1-deficient animal models (Al-Qusairi et al. 2009; Dowling et al. 2009). Interestingly, MTM1 can co-fractionate with desmin in the mitochondrial fraction from skeletal muscle homogenates, suggesting that the MTM1-desmin interaction also plays a role in mitochondrial dynamics or localization (Hnia et al. 2011). Indeed, XLCNM patient and Mtm1 knockdown mouse muscle cells have decreased mitochondrial dynamics and motility and ER/SR shape defects suggesting a potential role of the MTM1-desmin complex in mitochondrial-SR/ER contacts (Amoasii et al. 2013; Fig. 2a).
-
ITSN1: intersectin 1 belongs to the intersectin family, which are scaffold proteins linking endocytosis and signal transduction pathways. Members of this protein family contain multiple SH3 protein interaction domains, each capable of binding various ligands as signaling proteins or components of the endocytic machinery (Tsyba et al. 2011). Growing evidence supports a model in which ITSNs regulate various biochemical pathways at specific sites such as actin polymerization or receptor tyrosine kinase ubiquitination (Humphries et al. 2014; Okur et al. 2014). Desmin and syncoilin have been found by Y2H with ITSN1 as bait (Wong et al. 2012). In addition, other protein partners of ITSN1 involved in endosome trafficking and the regulation of the Rab and Arf GTPase pathways have been identified (Keating et al. 2006). ITSN1 might thus link desmin to the endocytic machinery or recruit signaling proteins such as Rab GTPases to specific compartments enriched in desmin.
-
MLH1: MutL Homolog 1 is a component of the post-replicative DNA mismatch repair system (MMR) found to interact with desmin by utilising a bacterial two-hybrid system (Brieger et al. 2010). MLH1 exhibits relationships to three interacting pairs of proteins involved in cytoskeletal and filament organization: thymosin beta 4 and actin gamma, cathepsin B and annexin A2 and spectrin alpha and desmin (Brieger et al. 2010). Co-immunoprecipitation and co-localization experiments have validated the interaction of MLH1 with desmin and the other cytoskeletal complexes (Brieger et al. 2010). In the same study, the authors confirmed that short interfering RNA knockdown of MLH1 shows a functional impact on the MLH1-actin interaction in filament organization but they did not access desmin filaments experimentally . They proposed that the dysregulation of MLH1 plays an essential role in cytoskeleton dynamics (Brieger et al. 2010). Further experimental data are needed to address the functional impacts of the MHL1-desmin interaction under physiological conditions. Of interest, like other IFs, desmin might specifically bind single-stranded DNA and RNA in vitro via the non-α-helical head domain, which contains 12 arginine residues (positively charged; Tolstonog et al. 2002). In addition, desmin has been shown to interact with MyoD and to regulate its transcriptional activity (Li et al. 1994). Overall, this suggests a role of desmin IFs in the regulation of gene expression (Cartaud et al. 1995). Further research will be required to confirm these findings and to fully elucidate the mechanisms.
Desmin and its associated proteins form a cellular scaffold that could fine-tune mechanochemical signaling and trafficking processes, thus regulating cell homeostasis and survival. Nevertheless, the exact molecular mechanisms underlying the majority of these associations remain unknown.
Desminopathies: genetics and physiopathology
Genetics and etiology
The lack of detailed epidemiological studies precludes the assessment of desminopathy incidence and prevalence; it is however considered as a rare disease with less than 5 affected in 10,000 individuals (Clemen et al. 2013). Dominant inheritance is the most frequent in familial desminopathies and mutations lead to the formation of desmin aggregates (Goldfarb et al. 2008). Autosomal recessive inheritance has recently been observed in five families (Munoz-Marmol et al. 1998; Clemen et al. 2013). In two cases, the mutations result in the absence of desmin. Moreover, a few cases with de novo mutations have been reported (Clemen et al. 2013). The majority of mutations found in the desmin gene (2q35) are missense mutations (van Spaendonck-Zwarts et al. 2011). Few splice site mutations leading to the loss of in-frame exon 3 have been reported (p. Asp214_Glu245del; Munoz-Marmol et al. 1998). Small in-frame deletion has been described in two families leading to p. Arg.173_Glu197del (Munoz-Marmol et al. 1998; Piñol-Ripoll et al. 2009) and the deletion of 22 bases in exon 6 leading to a premature stop codon has been identified in one family (Clemen et al. 2013). Mutations are spread over the entire desmin gene and have been found in both the head and tail domains and in the four domains composing the central conserved α-helical region. However, a cluster of mutations could be observed in exon 6 corresponding to the end of the 2B coil domain (Clemen et al. 2013). The updated list (up until June 2014) of mutations reported in the literature is summarized in Fig. 3a. DES mutations are subdivided into three groups depending on the related phenotype: specific for skeletal muscle, specific for a cardiac phenotype and both skeletal muscle and cardiac. Phenotypes are highly variable and include skeletal muscle weakness, cardiomyopathy, cardiac conduction disease, respiratory insufficiency and smooth muscle defects (Fig. 3b). The age of onset is highly variable, varying from birth to the late 80s but generally occurs during the 30s.
Desmin mutations and pathophysiology of desminopathies. a Desmin mutations are found over the entire gene but with a majority in the α-helical-rod 2B and in the tail domains. Mutations are subdivided into three groups depending on their related phenotype: skeletal muscle (blue), cardiac muscle (red) and both skeletal and cardiac muscle (purple). b The major phenotype is muscular with distal muscle impairment that generally precedes proximal muscle impairment. Desmin mutations also lead to desmin-related limb girdle muscular dystrophy and scapuloperoneal syndrome type Kaeser distal myopathy. Cardiomyopathy is common in desminopathies, being present in up to 74 % of patients. Additional phenotypes such as cataract or gastric/intestinal problems have been noted. c Meta-analysis of 159 patients (van Spaendonck-Zwarts et al. 2011). Both distal and proximal skeletal (SK) muscle phenotypes are present in the majority of patients; 49 % of patients with desminopathies develop both cardiac and skeletal muscle phenotypes. Within patients with the cardiac phenotype, 17 % have dilated cardiomyopathy (DCM); however, no cardiomyopathy (No CM) was detected in 51 %. Hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM), or arrhythmogenic right ventricular cardiomyopathy (ARVC) are also noted but to a lesser extent
Skeletal muscle involvement
Desmin-related myopathy is one of the myofibrillar myopathies and is associated with highly variable progressive skeletal myopathy. A meta-analysis based on the interpretation of published data from 159 patients carrying 40 different heterozygous desmin mutations revealed that 67 % of patients have combined distal and proximal muscular weakness. True distal weakness was found in 27 % and true proximal in 6 % (van Spaendonck-Zwarts et al. 2011). The distal muscle impairment generally precedes the proximal muscle impairment with muscle weakness in lower and then upper limbs (Fig. 3b). Muscle weakness can later spread to truncal, neck flexor, facial and bulbar muscles. Desmin mutations also lead to desmin-related limb girdle muscular dystrophy and scapuloperoneal distal myopathy (Walter et al. 2007). A recognizable imaging pattern of muscle involvement has been reported for desminopathy diagnostics, with sensitivity to detecting desminopathy of 100 % and a specificity of 95 % in the studied cohort (Fischer et al. 2008). Muscles such as the peroneal, gluteus maximus, sartorius and gracilis and semitendinosus are globally the most seriously and often the earliest affected. The tibialis anterior, soleus and gastrocnemius muscles are generally affected later (Fischer et al. 2008). The adductor magnus, biceps femoris and semimembranosus muscles are the last to be involved and to a lesser extent (Fischer et al. 2008). Respiratory muscle weakness, present in 26 % of patients, is a common feature of advanced desminopathies and often leads to the patient's death. Desmin is thought to be necessary for diaphragm longitudinal and transversal force transmission (Boriek et al. 2001). Patient biopsies have shown muscle fibers with irregular shape and intracellular amorphous deposits or inclusion bodies. Abnormal mitochondrial enzyme staining is a hallmark for desminopathies (Reimann et al. 2003). Classical myopathy features are observed including a variation of fiber size atrophic fibers, and internal nuclei (Olivé et al. 2004). Electron microscopy has shown the accumulation of dense granulofilamentous material located in the subsarcolemmal, interfibrillar, or perinuclear regions, corresponding to desmin aggregates (Claeys et al. 2008). Z-disk alteration, increased autophagic structures and the focal grouping of mitochondria are frequently also present (Claeys et al. 2008).
Cardiac involvement
Among the mutations in the desmin gene, few mutations (red in Fig. 3a) have been identified to affect only heart function. In particular, two mutations in the tail domain (p. Ile451Met and p. Val459Ile) and a mutation in the 2B domain (p. Asp312Asn) specifically lead to cardiomyopathy without any skeletal muscle phenotype (Li et al. 1999; Taylor et al. 2007). Moreover, several reported cases of desmin-related myopathies could evolve to include cardiac involvement (Olivé et al. 2004). Generally, 74 % of all desminopathy patients present a cardiomyopathy including dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, or arrhythmogenic right ventricular cardiomyopathy in, respectively, 17, 12, 6 and 1 % of patients depending on the site and nature of the mutation (Fig. 3c). Cardiac conduction defects have been observed in 62 % of patients, two thirds of them having cardiomyopathy (van Spaendonck-Zwarts et al. 2011). The most frequent manifestation of cardiac conduction defects is the atrioventricular block (47 % of cases) and requires the urgent implantation of a pacemaker. Similar to skeletal muscle, cardiac muscle shows desmin-positive aggregates and granulofilamentous and electron-dense amorphous material in intermyofibrillar, subsarcolemmal and perinuclear regions (Claeys et al. 2008). Cardiomyocyte hypertrophy and disarray and misshaped nuclei and degenerating mitochondria are frequent pathological signs (Goldfarb and Dalakas 2009).
Other pathological involvement
Smooth muscle involvement is not a major feature of desminopathies. Nevertheless, some manifestations have been reported such as intestinal involvement with chronic diarrhea or constipation (Bar et al. 2007) or intestinal pseudo-obstruction (Ariza et al. 1995), swallowing difficulties (Goldfarb et al. 1998), and cataracts (Olivé et al. 2004; Fig. 3b). Patients with a desmin mutation are at risk of developing respiratory problems. Indeed, a meta-analysis of desminopathies showed that 26 % of carriers have respiratory insufficiency (van Spaendonck-Zwarts et al. 2011).
Animal models and pathological mechanisms for desmin-related diseases
Desmin knockout mice
Two desmin knockout mice models from two different groups were described in 1996–1997 (Li et al. 1997; Milner et al. 1996; Fig. 4a). Desmin knockout mice develop and reproduce normally and display no obvious anatomical defects showing that desmin is not essential for myofibrillogenesis. No evidence of compensation by other IF proteins such as vimentin or nestin has been found by immunofluorescence. After birth, defects appear in skeletal, smooth and cardiac muscles and have been shown to be more severe in weight-bearing muscles such as the soleus or in continually used muscles such as the diaphragm, heart and aortic vessel wall. Excessive mitochondria clumping in skeletal and cardiac muscles and extensive mitochondria proliferation in the myocardium, often accompanied by swollen and disintegrating mitochondria, have been observed at the very early stage of the disease, before other structural effects become obvious (Milner et al. 2000). In situ mitochondrial respiration is significantly altered in the cardiac and soleus muscles of desmin null mice (Milner et al. 2000), despite increased creatine kinase activity and energy production and a lower amount of cytochrome c in heart mitochondria (Linden et al. 2001; Milner et al. 2000). The heart mitochondrial proteome of Des−/− mice showed differences in most metabolic pathways and, in particular, in ketone body and acetate metabolism, NADH shuttle components, amino-acid metabolism and respiratory enzymes. Levels of proteins that have been implicated in apoptosis, calcium homeostasis and fibrosis pathways have also been found to be perturbed (Fountoulakis et al. 2005). These perturbations in mitochondria localization and function can be explained by the lack of the desmin-mediated association of the mitochondria with the microtubule-associated plus-end-directed motor kinesin, which has been shown to be mislocalized (Linden et al. 2001) and by the reduced capacity of mitochondria to resist calcium exposure in the absence of desmin (Weisleder et al. 2004a).
Desmin animal models and associated phenotypes. a Desmin knockout mice (Des−/−) present affected cardiac and skeletal muscles. The skeletal muscle phenotype is exacerbated upon regeneration or aging (NMJ neuromuscular junction). b Desmin-related disease models displaying skeletal or cardiac phenotypes with common histological (myofibril disorganization and misshaped mitochondria) and physiological (decreased muscle function) signs (SR sarcoplasmic reticulum, WT wild-type, AAV adeno-associated virus)
In skeletal muscles, sarcomeres aligned normally until 2 months of age and presented a normal distribution of sarcomeric proteins (such as α-actinin, tropomyosin; Milner et al. 1996; Fig. 4a). Progressively, skeletal muscles showed Z-disk streaming and the loss of tension and misalignment of myofibrils, which become fragile and prone to breakage upon mechanical stress. A loss of association of desmin interactors synemin and paranemin with the Z-disk but not with the myotendinous and neuromuscular junctions, was observed (Carlsson et al. 2000). After degeneration of the myofibers, a cycle of regeneration was seen but was often aberrant with the disorganization of muscle fibers and subsarcolemmal accumulation of mitochondria and a relative increase of slow compared with fast myosin heavy chains, revealing a role for desmin in the maintenance of the structural integrity of solicited skeletal muscles (Li et al. 1997). Upon regeneration or aging, neuromuscular junctions appeared to be disorganized and elongated, with diffuse acetylcholinesterase staining and reduced overall activity in muscle cells (Agbulut et al. 2001). Consequently, Des−/− mice were weaker and fatigued more easily, showing an impaired performance on endurance running tests (Haubold et al. 2003). Moreover, morphological abnormalities were observed in the diaphragm, which appeared thinner and showed a misalignment of myofibrils and abnormal sarcomeres with no clear demarcation between the A and I bands (Li et al. 1997).
In cardiac muscle, myofiber loss of tension and disorganization were visible from the third week postnatally and gradually increased with age (Milner et al. 1996). Heart tissue from Des−/− mice contains degenerating cardiomyofibers from 5 days post-partum. From 10 days post-partum onward, an accumulation of macrophages, fibrosis and calcification is observed preferentially in the inter-ventricular septum and the free wall of the right ventricle. Perturbation of the intercalated disks, disruption of the sarcolemma and overcontraction of myofibrils have also been described (Thornell et al. 1997; Fig. 4). Des−/− mice develop concentric cardiomyocyte hypertrophy accompanied later by ventricular dilation and perturbed systolic function. The molecular hallmark of pressure-overload hypertrophy is observed with the overexpression of biomarkers such as the α-skeletal actin and the reduction of SERCA2A (Li et al. 1999). Magnetic resonance imaging of heart from Des−/− mice shows reduced left and right ventricular ejection fractions and cardiac output. Left ventricle weight is significantly increased, and the mice exhibit segmental wall thinning and akinesia suggesting myocardial necrosis (Sprinkart et al. 2012). In electrocardiography studies, Des−/− mice present a reduced atrial but prolonged ventricular refractory period and an enhanced inducibility of atrial fibrillation but lower susceptibility to ventricular arrhythmias. Ventricular conduction is slower than that in control animals (Sprinkart et al. 2012).
Loose organization of smooth muscle cells in the aortic vessel has also been observed in Des−/− mice (Li et al. 1997; Milner et al. 1996). Finally, the re-localization of BCL2 from the inner to the outer membrane of mitochondria and to the cytoplasm has been seen indicating apoptotic conditions (Linden et al. 2001). Importantly, the overexpression of BCL2 in Des−/− heart rescues most of the previously described phenotypes, in particular mitochondrial defects and myocardial lesions and fibrosis (Weisleder et al. 2004b).
Zebrafish knockdown models
In zebrafish, the function of desmin is not well characterized. In situ hybridization and immunofluorescence studies have shown the expression of desmin in skeletal muscles and myocardium with enrichment at Z-bands and intercalated disks at embryonic (Costa et al. 2008) and adult (Camara-Pereira et al. 2009) stages. Two versions of the desmin gene are present in zebrafish: desmina (desma; Loh et al. 2000) and desminb (desmb). Desma and Desmb share, respectively, 68.7 % and 72.0 % similarity with the human desmin protein. Desma knockdown embryos were generated by using morpholino antisense oligonucleotide injection and exhibited strong phenotypes including massive cardiac oedema, tail deformity and dramatic effects on heart rate (Vogel et al. 2009). A recent study reported defects in interfilament spacing by using X-ray diffraction in 50 % desma and desmb knockdowns in larvae at 4–6 days postfertilization. Mechanical properties of skeletal muscles, including active force and responses to stretch during active contraction, were lower in the context of desma knockdown (Li et al. 2013). The phenotype and contraction of the heart have not been assessed in this zebrafish model so far. Moreover, currently, no fish model is available that is a phenocopy of major desmin-related diseases, with the accumulation of protein aggregates, thus precluding the investigation of any pathological mechanisms.
Desmin-related myopathy models
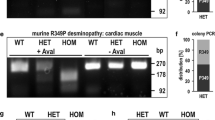
The first desminopathy model was generated in 1996 through the expression of the hamster desmin with its last 129 amino acids being substituted by the last 13 amino acids of hamster vimentin under the control of the hamster desmin promoter (Raats et al. 1996). This Delta129Des-Vim mouse model exhibited only weak expression of the transgene in all muscle types. Strong diffuse desmin staining was observed in skeletal muscles and only occasionally in the heart. Desmin-positive dots were present only in a few skeletal muscle cells. The expression of the transgene leads to a dominant-negative effect on the desmin network resulting in the disorganization of the transverse and longitudinal sarcoplasmic tubular system including fragmentation and swelling but without noticeable alteration of the myofibrillar and sarcomeric organization (Raats et al. 1996; Fig. 4). Kostareva et al. (2008) reported a desmin-related myopathy mouse model with overexpression of the HA-tagged mutated mouse desmin with the p. Leu345Pro missense mutation found in 28 patients. The transgene was expressed at a low level, corresponding to 5 % of wild-type desmin in the insoluble fraction and 17.4 % in the soluble fraction, with no difference in the pattern or intensity of desmin staining and no desmin aggregates being observed (Kostareva et al. 2008). No sign of myofibrillar or sarcomeric disturbance or misalignment was found but mitochondria presented a mitochondrial calcium increase and a reduced cristae density with circular membranes structures attributable to vacuolization of their matrix. This led to a lower relative force during recovery from fatigue, reduced muscle strength in the soleus muscle, a thickening of the left ventricular wall and increased fibrosis in the heart (Kostareva et al. 2008). Recently, two transitory models of desminopathy were generated by the injection of adeno-associated viruses carrying p. Arg406Trp or p. Glu413Lys mutated versions of murine desmin (Joanne et al. 2013). Ectopic expression of p. Arg406Trp desmin led to its accumulation in the perinuclear region, whereas the p. Glu413Lys mutant accumulated in the subsarcolemmal compartment. Muscle fibers had a perturbed architecture with central nuclei, increased muscle regeneration and mislocalized mitochondria. Perturbation of Z-disks alignment was observed at the sites of desmin accumulation. The affected muscles showed impaired maximal force generation capacity in both models (Joanne et al. 2013).
Desmin-related cardiomyopathy models
The DesD7 transgenic mouse strain expressing a desmin mutation and leading specifically to desmin-related cardiomyopathy was bred in 2001 (Wang et al. 2001). This model expresses, under the control of the myocardial-specific α-myosin heavy chain promoter, a mouse desmin transgene with a 7-amino-acid deletion (p. Arg173_Glu179del) corresponding to a known mutation in patients with desmin-related cardiomyopathy. Heterozygous DesD7 mice displayed characteristic electron-dense granular and filamentous desmin aggregates in the cardiomyocyte perinuclear region and intermyofibrillar space. Additional analyses revealed myofibrillar disarray and cardiac hypertrophy with compensation in the adult (Wang et al. 2001; Fig. 4). Crossbreeding with ubiquitin-proteasome system (UPS) reporter mice (GFPdgn) showed that the UPS proteolytic function was impaired, probably at the level of the entry of ubiquitinated proteins into the 20S proteasome (Liu et al. 2006). Crossbreeding with autophagy reporter mice (GFP-LC3) showed that the autophagy flux was increased and accompanied by the upregulation of p62 in DesD7 cardiomyocytes (Zheng et al. 2011). A second desmin-related cardiomyopathy model expressing the p. Ile451Met desmin mutant under the control of the cardiac α-MHC promoter was reported in 2008 (Mavroidis et al. 2008). In offspring of this mouse with Des−/− mice (Mavroidis et al. 2008), no desmin aggregates were detected; however, desmin lost its Z-disk localization but could still associate with intercalated disks. The latter had an altered architecture, resembling structural defects noted in dilated cardiomyopathy (Mavroidis et al. 2008).
Concluding remarks and outlook
Desmin obviously plays a central role in integrating the regulation of structure and function in striated muscles. Two decades of research have led to a number of important insights into the mechanisms by which desmin regulates several cellular processes such as organelle positioning, the organization and stability of the sarcolemma and the integration of mechanotransduction signals. In addition, desmin might serve as a signaling platform for the integration of signals from the outside to the inside of organelles such as mitochondria or the nucleus. The increasing number of desmin partners leads us to emphasize additional roles for IFs in the regulation of muscle function. Some of these partners are involved in processes such as lipid signaling, DNA repair, endocytosis and calcium homeostasis. The rationale for desmin IFs playing such a broad role in the physiology of striated muscles is intriguing and challenging for biologists, with potentially significant implications for the understanding of myopathies, dilated cardiomyopathies and heart failure. One of the critical steps for a future therapeutic approach is to characterize faithful animal models (e.g, mice, zebrafish) that phenocopy desmin aggregation in patients. These models could help us to decipher the way that desmin aggregation or misfolding can have an impact on the cellular process in vivo; live imaging by tracking the evolution of muscle function could be used to follow disease progression. Finally, more work should be directed at screening drugs or molecules that hinder the aggregation of desmin filaments or that prevent granulofilamentous deposits congregating within cells. For example, the unravelling of the molecular mechanisms by which desmin interactors impact on IF aggregation will pave the way for therapeutic approaches that could target desmin partners. Similarly, the modulation of the expression or activity of specific chaperones or proteins that affect desmin filaments could be a promising strategy for targeting desmin aggregation in vivo.
References
Agbulut O, Li Z, Perie S, Ludosky MA, Paulin D, Cartaud J, Butler-Browne G (2001) Lack of desmin results in abortive muscle regeneration and modifications in synaptic structure. Cell Motil Cytoskeleton 49:51–66
Allen RE, Rankin LL, Greene EA, Boxhorn LK, Johnson SE, Taylor RG, Pierce PR (1991) Desmin is present in proliferating rat muscle satellite cells but not in bovine muscle satellite cells. J Cell Physiol 149:525–535
Al-Qusairi L, Weiss N, Toussaint A, Berbey C, Messaddeq N, Kretz C, Sanoudou D, Beggs AH, Allard B, Mandel JL, Laporte J, Jacquemond V, Buj-Bello A (2009) T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci U S A 106:18763–18768
Amoasii L, Hnia K, Chicanne G, Brech A, Cowling BS, Muller MM, Schwab Y, Koebel P, Ferry A, Payrastre B, Laporte J (2013) Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle in vivo. J Cell Sci 126:1806–1819
Ariza A, Coll J, Fernandez-Figueras MT, Lopez MD, Mate JL, Garcia O, Fernandez-Vasalo A, Navas-Palacios JJ (1995) Desmin myopathy: a multisystem disorder involving skeletal, cardiac, and smooth muscle. Hum Pathol 26:1032–1037
Bandyopadhyay S, Chiang CY, Srivastava J, Gersten M, White S, Bell R, Kurschner C, Martin C, Smoot M, Sahasrabudhe S, Barber DL, Chanda SK, Ideker T (2010) A human MAP kinase interactome. Nat Methods 7:801–805
Bar H, Mucke N, Katus HA, Aebi U, Herrmann H (2007) Assembly defects of desmin disease mutants carrying deletions in the alpha-helical rod domain are rescued by wild type protein. J Struct Biol 158:107–115
Barbet JP, Thornell LE, Butler-Browne GS (1991) Immunocytochemical characterisation of two generations of fibers during the development of the human quadriceps muscle. Mech Dev 35:3–11
Bellin RM, Huiatt TW, Critchley DR, Robson RM (2001) Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J Biol Chem 276:32330–32337
Benson MA, Tinsley CL, Blake DJ (2004) Myospryn is a novel binding partner for dysbindin in muscle. J Biol Chem 279:10450–10458
Bentzinger CF, Wang YX, Rudnicki MA (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4:a008342
Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM (2006) Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem Biophys Res Commun 346:768–777
Blake DJ, Martin-Rendon E (2002) Intermediate filaments and the function of the dystrophin-protein complex. Trends Cardiovasc Med 12:224–228
Boriek AM, Capetanaki Y, Hwang W, Officer T, Badshah M, Rodarte J, Tidball JG (2001) Desmin integrates the three-dimensional mechanical properties of muscles. Am J Physiol Cell Physiol 280:C46–C52
Breckler J, Lazarides E (1982) Isolation of a new high molecular weight protein associated with desmin and vimentin filaments from avian embryonic skeletal muscle. J Cell Biol 92:795–806
Brieger A, Adryan B, Wolpert F, Passmann S, Zeuzem S, Trojan J (2010) Cytoskeletal scaffolding proteins interact with Lynch-Syndrome associated mismatch repair protein MLH1. Proteomics 10:3343–3355
Buehler MJ (2013) Mechanical players—the role of intermediate filaments in cell mechanics and organization. Biophys J 105:1733–1734
Camara-Pereira ES, Campos LM, Vannier-Santos MA, Mermelstein CS, Costa ML (2009) Distribution of cytoskeletal and adhesion proteins in adult zebrafish skeletal muscle. Histol Histopathol 24:187–196
Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA (2003) Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 9:1520–1527
Capetanaki Y, Milner DJ, Weitzer G (1997) Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct Funct 22:103–116
Capetanaki Y, Bloch RJ, Kouloumenta A, Mavroidis M, Psarras S (2007) Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res 313:2063–2076
Carlsson L, Li ZL, Paulin D, Price MG, Breckler J, Robson RM, Wiche G, Thornell LE (2000) Differences in the distribution of synemin, paranemin, and plectin in skeletal muscles of wild-type and desmin knock-out mice. Histochem Cell Biol 114:39–47
Cartaud A, Jasmin BJ, Changeux JP, Cartaud J (1995) Direct involvement of a lamin-B-related (54 kDa) protein in the association of intermediate filaments with the postsynaptic membrane of the Torpedo marmorata electrocyte. J Cell Sci 108:153–160
Castanon MJ, Walko G, Winter L, Wiche G (2013) Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem Cell Biol 140:33–53
Chang L, Goldman RD (2004) Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5:601–613
Chang L, Barlan K, Chou YH, Grin B, Lakonishok M, Serpinskaya AS, Shumaker DK, Herrmann H, Gelfand VI, Goldman RD (2009) The dynamic properties of intermediate filaments during organelle transport. J Cell Sci 122:2914–2923
Chung BM, Rotty JD, Coulombe PA (2013) Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol 25:600–612
Claeys KG, Fardeau M, Schroder R, Suominen T, Tolksdorf K, Behin A, Dubourg O, Eymard B, Maisonobe T, Stojkovic T, Faulkner G, Richard P, Vicart P, Udd B, Voit T, Stoltenburg G (2008) Electron microscopy in myofibrillar myopathies reveals clues to the mutated gene. Neuromuscul Disord 18:656–666
Clemen CS, Herrmann H, Strelkov SV, Schroder R (2013) Desminopathies: pathology and mechanisms. Acta Neuropathol 125:47–75
Constantin B (2014) Dystrophin complex functions as a scaffold for signalling proteins. Biochim Biophys Acta 1838:635–642
Costa ML, Escaleira R, Cataldo A, Oliveira F, Mermelstein CS (2004) Desmin: molecular interactions and putative functions of the muscle intermediate filament protein. Braz J Med Biol Res 37:1819–1830
Costa ML, Escaleira RC, Jazenko F, Mermelstein CS (2008) Cell adhesion in zebrafish myogenesis: distribution of intermediate filaments, microfilaments, intracellular adhesion structures and extracellular matrix. Cell Motil Cytoskeleton 65:801–815
Coulombe PA, Wong P (2004) Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 6:699–706
Dingli F, Parys JB, Loew D, Saule S, Mery L (2012) Vimentin and the K-Ras-induced actin-binding protein control inositol-(1,4,5)-trisphosphate receptor redistribution during MDCK cell differentiation. J Cell Sci 125:5428–5440
Dowling JJ, Vreede AP, Low SE, Gibbs EM, Kuwada JY, Bonnemann CG, Feldman EL (2009) Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5:e1000372
Dunia I, Pieper F, Manenti S, Kemp A van de, Devilliers G, Benedetti EL, Bloemendal H (1990) Plasma membrane-cytoskeleton damage in eye lenses of transgenic mice expressing desmin. Eur J Cell Biol 53:59–74
Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD (2004) Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci 117:919–932
Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD (2009) Introducing intermediate filaments: from discovery to disease. J Clin Invest 119:1763–1771
Ervasti JM (2003) Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278:13591–13594
Farrell FX, Sax CM, Zehner ZE (1990) A negative element involved in vimentin gene expression. Mol Cell Biol 10:2349–2358
Fischer D, Kley RA, Strach K, Meyer C, Sommer T, Eger K, Rolfs A, Meyer W, Pou A, Pradas J, Heyer CM, Grossmann A, Huebner A, Kress W, Reimann J, Schröder R, Eymard B, Fardeau M, Udd B, Goldfarb L, Vorgerd M, Olivé M (2008) Distinct muscle imaging patterns in myofibrillar myopathies. Neurology 71:758–765
Fountoulakis M, Soumaka E, Rapti K, Mavroidis M, Tsangaris G, Maris A, Weisleder N, Capetanaki Y (2005) Alterations in the heart mitochondrial proteome in a desmin null heart failure model. J Mol Cell Cardiol 38:461–474
Garbuglia M, Verzini M, Sorci G, Bianchi R, Giambanco I, Agneletti AL, Donato R (1999) The calcium-modulated proteins, S100A1 and S100B, as potential regulators of the dynamics of type III intermediate filaments. Braz J Med Biol Res 32:1177–1185
Gilbert S, Ruel A, Loranger A, Marceau N (2008) Switch in Fas-activated death signaling pathway as result of keratin 8/18-intermediate filament loss. Apoptosis 13:1479–1493
Goldfarb LG, Dalakas MC (2009) Tragedy in a heartbeat: malfunctioning desmin causes skeletal and cardiac muscle disease. J Clin Invest 119:1806–1813
Goldfarb LG, Park KY, Cervenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle JW, Semino-Mora C, Sivakumar K, Dalakas MC (1998) Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet 19:402–403
Goldfarb LG, Olivé M, Vicart P, Goebel HH (2008) Intermediate filament diseases: desminopathy. Adv Exp Med Biol 642:131–164
Goldman RD, Cleland MM, Murthy SN, Mahammad S, Kuczmarski ER (2012) Inroads into the structure and function of intermediate filament networks. J Struct Biol 177:14–23
Granger BL, Lazarides E (1980) Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell 22:727–738
Guma FCR, Mello TG, Mermelstein CS, Fortuna VA, Wofchuk ST, Gottfried C, Guaragna RM, Costa ML, Borojevic R (2001) Intermediate filaments modulation in an in vitro model of the hepatic stellate cell activation or conversion into the lipocyte phenotype. Biochem Cell Biol 79:409–417
Haubold KW, Allen DL, Capetanaki Y, Leinwand LA (2003) Loss of desmin leads to impaired voluntary wheel running and treadmill exercise performance. J Appl Physiol 95:1617–1622
Herrmann H, Fouquet B, Franke WW (1989) Expression of intermediate filament proteins during development of Xenopus laevis. I. cDNA clones encoding different forms of vimentin. Development 105:279–298
Herrmann H, Strelkov SV, Burkhard P, Aebi U (2009) Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest 119:1772–1783
Hnia K, Tronchere H, Tomczak KK, Amoasii L, Schultz P, Beggs AH, Payrastre B, Mandel JL, Laporte J (2011) Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J Clin Invest 121:70–85
Howman EV, Sullivan N, Poon EP, Britton JE, Hilton-Jones D, Davies KE (2003) Syncoilin accumulation in two patients with desmin-related myopathy. Neuromuscul Disord 13:42–48
Humphries AC, Donnelly SK, Way M (2014) Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation. J Cell Sci 127:673–685
Jiao Q, Sanbe A, Zhang X, Liu JP, Minamisawa S (2014) alphaB-Crystallin R120G variant causes cardiac arrhythmias and alterations in the expression of Ca handling proteins and ER stress in mice. Clin Exp Pharmacol Physiol 41:589–599
Joanne P, Chourbagi O, Hourde C, Ferry A, Butler-Browne G, Vicart P, Dumonceaux J, Agbulut O (2013) Viral-mediated expression of desmin mutants to create mouse models of myofibrillar myopathy. Skelet Muscle 3:4
Kachinsky AM, Dominov JA, Miller JB (1994) Myogenesis and the intermediate filament protein, nestin. Dev Biol 165:216–228
Keating DJ, Chen C, Pritchard MA (2006) Alzheimer's disease and endocytic dysfunction: clues from the Down syndrome-related proteins, DSCR1 and ITSN1. Ageing Res Rev 5:388–401
Kielbasa OM, Reynolds JG, Wu CL, Snyder CM, Cho MY, Weiler H, Kandarian S, Naya FJ (2011) Myospryn is a calcineurin-interacting protein that negatively modulates slow-fiber-type transformation and skeletal muscle regeneration. FASEB J 25:2276–2286
Konieczny P, Fuchs P, Reipert S, Kunz WS, Zeold A, Fischer I, Paulin D, Schroder R, Wiche G (2008) Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J Cell Biol 181:667–681
Kostareva A, Sjoberg G, Bruton J, Zhang SJ, Balogh J, Gudkova A, Hedberg B, Edstrom L, Westerblad H, Sejersen T (2008) Mice expressing L345P mutant desmin exhibit morphological and functional changes of skeletal and cardiac mitochondria. J Muscle Res Cell Motil 29:25–36
Kouloumenta A, Mavroidis M, Capetanaki Y (2007) Proper perinuclear localization of the TRIM-like protein myospryn requires its binding partner desmin. J Biol Chem 282:35211–35221
Kuisk IR, Li H, Tran D, Capetanaki Y (1996) A single MEF2 site governs desmin transcription in both heart and skeletal muscle during mouse embryogenesis. Dev Biol 174:1–13
Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM (2004) Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J 18:102–113
Lazarides E (1982) Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem 51:219–250
Lazarides E, Hubbard BD (1976) Immunological characterization of the subunit of the 100 Å filaments from muscle cells. Proc Natl Acad Sci U S A 73:4344–4348
Li D, Tapscoft T, Gonzalez O, Burch PE, Quinones MA, Zoghbi WA, Hill R, Bachinski LL, Mann DL, Roberts R (1999) Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation 100:461–464
Li H, Capetanaki Y (1993) Regulation of the mouse desmin gene: transactivated by MyoD, myogenin, MRF4 and Myf5. Nucleic Acids Res 21:335–343
Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y (1994) Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol 124:827–841
Li M, Andersson-Lendahl M, Sejersen T, Arner A (2013) Knockdown of desmin in zebrafish larvae affects interfilament spacing and mechanical properties of skeletal muscle. J Gen Physiol 141:335–345
Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D (1997) Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 139:129–144
Linden M, Li Z, Paulin D, Gotow T, Leterrier JF (2001) Effects of desmin gene knockout on mice heart mitochondria. J Bioenerg Biomembr 33:333–341
Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X (2006) Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J 20:362–364
Loh SH, Chan WT, Gong Z, Lim TM, Chua KL (2000) Characterization of a zebrafish (Danio rerio) desmin cDNA: an early molecular marker of myogenesis. Differentiation 65:247–254
Lovering RM, O'Neill A, Muriel JM, Prosser BL, Strong J, Bloch RJ (2011) Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am J Physiol Cell Physiol 300:C803–C813
Mavroidis M, Panagopoulou P, Kostavasili I, Weisleder N, Capetanaki Y (2008) A missense mutation in desmin tail domain linked to human dilated cardiomyopathy promotes cleavage of the head domain and abolishes its Z-disc localization. FASEB J 22:3318–3327
Meyer GA, Lieber RL (2012) Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol 302:C1609–C1620
Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y (1996) Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol 134:1255–1270
Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y (2000) Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J Cell Biol 150:1283–1298
Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM (2001) Desmuslin, an intermediate filament protein that interacts with alpha-dystrobrevin and desmin. Proc Natl Acad Sci U S A 98:6156–6161
Mohamed JS, Boriek AM (2012) Loss of desmin triggers mechanosensitivity and up-regulation of Ankrd1 expression through Akt-NF-kappaB signaling pathway in smooth muscle cells. FASEB J 26:757–765
Moorwood C (2008) Syncoilin, an intermediate filament-like protein linked to the dystrophin associated protein complex in skeletal muscle. Cell Mol Life Sci 65:2957–2963
Most P, Remppis A, Pleger ST, Katus HA, Koch WJ (2007) S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Physiol Regul Integr Comp Physiol 293:R568–R577
Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q (2008) GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome Biol 9 (Suppl 1):S4
Munoz-Marmol AM, Strasser G, Isamat M, Coulombe PA, Yang Y, Roca X, Vela E, Mate JL, Coll J, Fernandez-Figueras MT, Navas-Palacios JJ, Ariza A, Fuchs E (1998) A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc Natl Acad Sci U S A 95:11312–11317
Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ (2001) Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem 276:6645–6655
Okur MN, Russo A, O'Bryan JP (2014) Receptor tyrosine kinase ubiquitylation involves the dynamic regulation of Cbl-Spry2 by intersectin 1 and the Shp2 tyrosine phosphatase. Mol Cell Biol 34:271–279
Olivé M, Goldfarb L, Moreno D, Laforet E, Dagvadorj A, Sambuughin N, Martínez-Matos JA, Martínez F, Alió J, Farrero E, Vicart P, Ferrer I (2004) Desmin-related myopathy: clinical, electrophysiological, radiological, neuropathological and genetic studies. J Neurol Sci 219:125–137
O'Neill A, Williams MW, Resneck WG, Milner DJ, Capetanaki Y, Bloch RJ (2002) Sarcolemmal organization in skeletal muscle lacking desmin: evidence for cytokeratins associated with the membrane skeleton at costameres. Mol Biol Cell 13:2347–2359
Piñol-Ripoll G, Shatunov A, Cabello A, Larrode P, Puerta I de la, Pelegrín J, Ramos FJ, Olivé M, Goldfarb LG (2009) Severe infantile-onset cardiomyopathy associated with a homozygous deletion in desmin. Neuromuscul Disord 19:418–422
Poon E, Howman EV, Newey SE, Davies KE (2002) Association of syncoilin and desmin: linking intermediate filament proteins to the dystrophin-associated protein complex. J Biol Chem 277:3433–3439
Prosser BL, Wright NT, Hernandez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF (2008) S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem 283:5046–5057
Prosser BL, Hernandez-Ochoa EO, Schneider MF (2011) S100A1 and calmodulin regulation of ryanodine receptor in striated muscle. Cell Calcium 50:323–331
Raats JM, Schaart G, Henderik JB, Kemp A van der, Dunia I, Benedetti EL, Pieper FR, Ramaekers FC, Bloemendal H (1996) Muscle-specific expression of a dominant negative desmin mutant in transgenic mice. Eur J Cell Biol 71:221–236
Reimann J, Kunz WS, Vielhaber S, Kappes-Horn K, Schroder R (2003) Mitochondrial dysfunction in myofibrillar myopathy. Neuropathol Appl Neurobiol 29:45–51
Remppis A, Most P, Loffler E, Ehlermann P, Bernotat J, Pleger S, Borries M, Reppel M, Fischer J, Koch WJ, Smith G, Katus HA (2002) The small EF-hand Ca2+ binding protein S100A1 increases contractility and Ca2+ cycling in rat cardiac myocytes. Basic Res Cardiol 97 (Suppl 1):I56–I62
Reynolds JG, McCalmon SA, Tomczyk T, Naya FJ (2007) Identification and mapping of protein kinase A binding sites in the costameric protein myospryn. Biochim Biophys Acta 1773:891–902
Reynolds JG, McCalmon SA, Donaghey JA, Naya FJ (2008) Deregulated protein kinase A signaling and myospryn expression in muscular dystrophy. J Biol Chem 283:8070–8074
Rybakova IN, Patel JR, Ervasti JM (2000) The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol 150:1209–1214
Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL (2000) Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 279:C1116–C1122
Schofield AV, Bernard O (2013) Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol 48:301–316
Schopferer M, Bar H, Hochstein B, Sharma S, Mucke N, Herrmann H, Willenbacher N (2009) Desmin and vimentin intermediate filament networks: their viscoelastic properties investigated by mechanical rheometry. J Mol Biol 388:133–143
Schultheiss T, Lin ZX, Ishikawa H, Zamir I, Stoeckert CJ, Holtzer H (1991) Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol 114:953–966
Snider NT, Omary MB (2014) Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 15:163–177
Spaendonck-Zwarts KY van, Hessem L van, Jongbloed JD, Walle HE de, Capetanaki Y, Kooi AJ van der, Langen IM van, Berg MP van den, Tintelen JP van (2011) Desmin-related myopathy. Clin Genet 80:354–366
Sprinkart AM, Block W, Träber F, Meyer R, Paulin D, Clemen CS, Schröder R, Gieseke J, Schild H, Thomas D (2012) Characterization of the failing murine heart in a desmin knock-out model using a clinical 3 T MRI scanner. Int J Cardiovasc Imaging 28:1699–1705
Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M (2006) BioGRID: a general repository for interaction datasets. Nucleic Acids Res 34:D535–D539
Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD (1999) A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem 274:9881–9890
Stone MR, O'Neill A, Catino D, Bloch RJ (2005) Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol Biol Cell 16:4280–4293
Stone MR, O'Neill A, Lovering RM, Strong J, Resneck WG, Reed PW, Toivola DM, Ursitti JA, Omary MB, Bloch RJ (2007) Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci 120:3999–4008
Sun N, Critchley DR, Paulin D, Li Z, Robson RM (2008) Human alpha-synemin interacts directly with vinculin and metavinculin. Biochem J 409:657–667
Taylor GS, Maehama T, Dixon JE (2000) Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci U S A 97:8910–8915
Taylor MR, Slavov D, Ku L, Di Lenarda A, Sinagra G, Carniel E, Haubold K, Boucek MM, Ferguson D, Graw SL, Zhu X, Cavanaugh J, Sucharov CC, Long CS, Bristow MR, Lavori P, Mestroni L, Familial Cardiomyopathy Registry, BEST (Beta-Blocker Evaluation of Survival Trial) DNA Bank (2007) Prevalence of desmin mutations in dilated cardiomyopathy. Circulation 115:1244–1251
Thornell L, Carlsson L, Li Z, Mericskay M, Paulin D (1997) Null mutation in the desmin gene gives rise to a cardiomyopathy. J Mol Cell Cardiol 29:2107–2124
Tolstonog GV, Sabasch M, Traub P (2002) Cytoplasmic intermediate filaments are stably associated with nuclear matrices and potentially modulate their DNA-binding function. DNA Cell Biol 21:213–239
Tsoupri E, Capetanaki Y (2013) Muyospryn: a multifunctional desmin-associated protein. Histochem Cell Biol 140:55–63
Tsyba L, Nikolaienko O, Dergai O, Dergai M, Novokhatska O, Skrypkina I, Rynditch A (2011) Intersectin multidomain adaptor proteins: regulation of functional diversity. Gene 473:67–75
Ursitti JA, Lee PC, Resneck WG, McNally MM, Bowman AL, O'Neill A, Stone MR, Bloch RJ (2004) Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J Biol Chem 279:41830–41838
Vogel B, Meder B, Just S, Laufer C, Berger I, Weber S, Katus HA, Rottbauer W (2009) In-vivo characterization of human dilated cardiomyopathy genes in zebrafish. Biochem Biophys Res Commun 390:516–522
Volkers M, Rohde D, Goodman C, Most P (2010) S100A1: a regulator of striated muscle sarcoplasmic reticulum Ca2+ handling, sarcomeric, and mitochondrial function. J Biomed Biotechnol 2010:178614
Walter MC, Reilich P, Huebner A, Fischer D, Schroder R, Vorgerd M, Kress W, Born C, Schoser BG, Krause KH, Klutzny U, Bulst S, Frey JR, Lochmüller H (2007) Scapuloperoneal syndrome type Kaeser and a wide phenotypic spectrum of adult-onset, dominant myopathies are associated with the desmin mutation R350P. Brain 130:1485–1496
Wang X, Osinska H, Dorn GW 2nd, Nieman M, Lorenz JN, Gerdes AM, Witt S, Kimball T, Gulick J, Robbins J (2001) Mouse model of desmin-related cardiomyopathy. Circulation 103:2402–2407
Weisleder N, Soumaka E, Abbasi S, Taegtmeyer H, Capetanaki Y (2004a) Cardiomyocyte-specific desmin rescue of desmin null cardiomyopathy excludes vascular involvement. J Mol Cell Cardiol 36:121–128
Weisleder N, Taffet GE, Capetanaki Y (2004b) Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci U S A 101:769–774
Weitzer G, Milner DJ, Kim JU, Bradley A, Capetanaki Y (1995) Cytoskeletal control of myogenesis: a desmin null mutation blocks the myogenic pathway during embryonic stem cell differentiation. Dev Biol 172:422–439
Windoffer R, Beil M, Magin TM, Leube RE (2011) Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol 194:669–678
Winter DL, Paulin D, Mericskay M, Li Z (2014) Posttranslational modifications of desmin and their implication in biological processes and pathologies. Histochem Cell Biol 141:1–16
Winter L, Wiche G (2013) The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol 125:77–93
Wong KA, Wilson J, Russo A, Wang L, Okur MN, Wang X, Martin NP, Scappini E, Carnegie GK, O'Bryan JP (2012) Intersectin (ITSN) family of scaffolds function as molecular hubs in protein interaction networks. PLoS One 7:e36023
Yamada K, Nomura N, Yamano A, Yamada Y, Wakamatsu N (2012) Identification and characterization of splicing variants of PLEKHA5 (Plekha5) during brain development. Gene 492:270–275
Zheng Q, Su H, Ranek MJ, Wang X (2011) Autophagy and p62 in cardiac proteinopathy. Circ Res 109:296–308
Zou Y, Zhong W (2012) A likely role for a novel PH-domain containing protein, PEPP2, in connecting membrane and cytoskeleton. Biocell 36:127–132
Acknowledgment
We thank Pr. Denise Paulin for her helpful suggestions and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Karim Hnia and Caroline Ramspacher contributed equally to this work.
This work is supported by INSERM, CNRS, UDS (University of Strasbourg) and AFM (Association Française Contre la Myopathy).
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hnia, K., Ramspacher, C., Vermot, J. et al. Desmin in muscle and associated diseases: beyond the structural function. Cell Tissue Res 360, 591–608 (2015). https://doi.org/10.1007/s00441-014-2016-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-2016-4