Abstract
In addition to their differentiation potential, self-renewal capability is an important characteristic of stem cells. The limited self-renewal activity of mesenchymal stem cells is the greatest obstacle to the application of stem cell therapy in regenerative medicine. The human TERT gene enhances the self-renewal of MSCs, but the mechanism of self-renewal and the interactions among TERT-gene-related molecules remain unknown. The objectives of this study were to generate immortalized MSCs derived from MSCs isolated from placenta (naive) by human TERT gene transfection with the AMAXA gene delivery system, to compare their characteristics, and to investigate whether increased TERT expression affected the pituitary tumor transforming gene (PTTG1; also known as securin), which is involved in chromosome segregation during mitosis. TERT-immortalized cells (TERT+) with a prolonged life span displayed high PTTG1 expression. TERT+ cells also retained the stemness capacity and multipotency of naive cells and displayed high PTTG1 expression. However, down-regulation of PTTG1 by treatment with short interfering RNA induced cell senescence and decreased telomerase activity. Moreover, TERT bound to PTTG1 formed complexes with chaperones such as Ku70 and heat shock protein 90. Thus, placental MSCs immortalized by TERT gene transfection display differentiation potential and exhibit enhanced self-renewal through a balanced interaction of PTTG1 and chaperones. The interaction between TERT and PTTG1 by association of Ku70 might be important for the enhancement of the limited self-renewal activity of MSCs and for understanding the regulatory mechanisms of self-renewal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human mesenchymal stem cells (hMSCs), which can be isolated from a variety of organs, are attractive for use in regenerative medicine because they have the potential for self-renewal and can differentiate into multiple lineages under specific culture conditions (Pittenger et al. 1999). Although MSCs have the capacity for self-renewal, they generally have limited self-renewal activity and exhibit an average life span of less than 20 population doublings making the cells difficult to study for potential clinical use. Normal human cells undergo limited cell division when cultured before entering a non-dividing state known as “senescence” (Campisi et al. 1996). Replicative senescence, originally described by Hayflick (1965), is a state of arrested growth and altered function that occurs in normal somatic cells after a finite number of cell divisions.
Recently, human placenta-derived MSCs (PDSCs) have been identified as an alternative to traditional MSCs, including bone-marrow-derived MSCs (BM-MSCs). PDSCs have the potential for self-renewal activity and differentiation into multiple lineages and advantages such as easy acquisition, ethical acceptability, and strong immunomodulatory effects (Igura et al. 2004). We have previously characterized several types of PDSCs found in normal-term placentas based on placental anatomy (Kim et al. 2011). The self-renewal activity of chorionic plate-derived MSCs (CP-MSCs) and Whaton’s Jelly-derived MSCs (WJ-MSCs) is higher than that of other types of PDSCs. However, even though self-renewal activity is higher than that of traditional MSCs, the average life span of CP-MSC and WJ-MSC is 21 population doublings, limiting the ability to obtain sufficient cells for use in clinical applications.
Senescent cells are arrested in the G1 phase of the cell cycle and display a large flattened morphology. Although senescent cells are metabolically active, they are unable to synthesize DNA, even in response to stimulation with growth factors (Fridman et al. 2006). This is because these cells have one or more short dysfunctional telomeres and lack telomerase expression as a result of continuous sub-culturing (Harley et al. 1990). Cell-cycle-related factors have been reported to be closely involved in the self-renewal activity of MSCs (Napolitano et al. 2007). Therefore, many researchers have attempted to transfect the human telomerase reverse transcriptase (TERT) gene into MSCs to overcome their senescence (Tsai et al. 2010). The human TERT gene might enhance the proliferation of MSCs when ectopically expressed (Batista et al. 2011; Bodnar et al. 1998; Tsai et al. 2010). However, gene delivery systems involving viral vectors are limited by recent findings that these cells undergo neoplastic transformation. In addition, a strong link between telomerase expression and many types of cancer has been observed (Miura et al. 2006). Primary cells can be induced to grow indefinitely in vitro by genetic manipulation through a process known as ex vivo immortalization (Okamoto et al. 2002). However, limitations still hamper this technique, including difficulties in expanding adult stem cells to obtain large numbers of cells and the safety of this technique for clinical application.
Pituitary tumor-transforming gene-1 (PTTG1), also known as securin, was isolated from rat pituitary tumor cells in 1997 (Pei and Melmed 1997). It regulates sister-chromatid separation during mitosis (Zou et al. 1999). PTTG1 is overexpressed in rapidly proliferating cells and various primary tumors and tumor cell lines and functions in cell replication, cell cycle-progression, appropriate cell division (Heaney et al. 2000; Saez et al. 1999; Tong et al. 2007; Wang et al. 2001), chromosomal stability (Jallepalli et al. 2001), and cell senescence (Chesnokova et al. 2007). Levels of PTTG1 expression are correlated with tumor invasiveness, recurrence, and poor prognosis, and PTTG1 has been identified as a key gene associated with tumor metastasis (Ramaswamy et al. 2003). However, no reports of a correlation between TERT and PTTG1 in the self-renewal of MSCs are available.
In the present study, we have established immortalized PDSCs (TERT+ cells) by transfection of the TERT gene into PDSCs (naive cells) by using the AMAXA system with a nucleofactor kit and compared the characteristics of naive cells and TERT+ cells. Furthermore, we have characterized the function of PTTG1 and the self-renewal mechanism of immortalized MSCs by down-regulation of PTTG1 with short interfering RNA (siRNA) in TERT+ cells. Finally, we have demonstrated the interaction between PTTG1 and TERT in the self-renewal of immortalized PDSCs.
Materials and methods
Cell culture
Placentas were considered normal when the pregnancy was free of medical, obstetrical, and surgical complications, and delivery occurred at term (gestation ≥ 37 weeks). All women who contributed to this study provided written informed consent prior to sample collection. The samples and their subsequent utilization for research purposes were approved by the Institutional Review Board of CHA General Hospital, Seoul, Korea. Naive PDSCs were harvested as described previously (Lee et al. 2010). Briefly, the amnion and the innermost membrane from the chorion and decidua of the placenta were removed. Cells scraped from the membrane were treated with 0.5 % collagenase IV (Sigma, St. Louis, Mo., USA) for 30 min at 37 °C. The harvested cells were cultured at 2 × 105 cells/cm2 in T25 flasks (BD Biosciences, San Jose, Calif., USA) in Ham’s F-12/DMEM supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin/streptomycin (P/S; all from GIBCO-BRL, Grand Island, N.Y., USA). Otherwise, TERT cells were immortalized (TERT+ cells) by using gene delivery systems with an AMAXA system. All cell culture media contained DMEM/F12 (GIBCO-BRL) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), 25 ng/ml fibroblast growth factor 4 (FGF4; Peprotech, N.J., USA), 1 μg/ml Heparin (Sigma), and 10 % FBS (GIBCO-BRL).
Generation of immortalized PDSCs by hTERT gene transfection
To induce the overexpression of the TERT gene, naive PDSCs (6 × 104 cells/cm2) were harvested and transfected by using the AMAXA system with a Human MSC nucleofactor kit (Lonza, N.J., USA). After electroporation of the hTERT gene into the naive PDSCs, we changed the medium every other day and observed changes in cell morphology. The cells were then maintained for 3 months and analyzed.
Analysis by reverse transcription plus the polymerase chain reaction
Total RNA was extracted from cells growing in 6-well plates with RNeasy plus mini kits (QIAgen, Valencia, Calif., USA). cDNA was synthesized by reverse transcription (RT) from total RNA (1 μg) with SuperScript III reverse transcriptase (Invitrogen, Carlsbad, Calif., USA) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplification was performed with specific primers (Table 1) in 25-μl reaction volumes containing 100 ng cDNA template, 2.5 U/μl DNA polymerase (Solgent, South Korea), and 200 μM dNTP. β-Actin was used as an internal control. cDNA was subjected to 35 cycles of amplification (20 s at 95 °C, 40 s at the appropriate annealing temperature as given in Table 1, and 1 min at 72 °C). Amplified PCR products were electrophoresed on 1.2 % agarose gels containing 1.5 μg/ml ethidium bromide and visualized under UV light.
Analysis of cell growth and cell cycle
To analyze the cell cycle, fluorescence-activated cell sorter (FACS) analysis was performed for naive PDSCs and TERT+ cells (1 × 106 cells). After being harvested, the cells were fixed in 70 % ethanol at room temperature for 10 min. RNase (0.5 μg/ml) and propidium iodide (50 μg/ml) were added to the cells, which were then incubated for 30 min at 37 °C. The treated cells were analyzed with a BD FACS Vantage SE Cell Sorter equipped with Becton and Dickson ModiFit LT software (BD Bioscience).
FACS analysis
To detect cell-surface antigens, we harvested naive PDSCs and TERT+ cells by using TrypLE Express with Phenol Red (GIBCO-BRL). Harvested cells were incubated in 100 μl phosphate-buffered saline (PBS) containing either mouse immunoglobulin G as a reference or specific concentrations of fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated antibodies (Table S1) at room temperature for 30 min. The samples were analyzed with a BD FACSVantage SE Cell sorter (Becton & Dickinson). The results were analyzed by using Cell-Quest software (Becton & Dickinson).
Differentiation of naive and immortalized PDSCs into adipogenic and hepatogenic lineages
Adipogenic differentiation
To induce adipogenic differentiation, naive PDSCs and TERT+ cells were plated at a density of 5 × 103 cells/cm2 in adipogenic induction medium containing 1 μM dexamethasone, 0.5 mM isobutyl methylxanthine, 0.2 mM indomethacin, 1.7 μM insulin (all from Sigma-Aldrich), 10 % FBS, and 1× penicillin-streptomycin. The growth medium was replaced twice each week. After 21 days, the cells were fixed with 4 % paraformaldehyde and incubated for 1 h with Oil Red O (Sigma-Aldrich), which stains lipids, in order to visualize lipid vesicles. Nuclei were counterstained with Mayer’s hematoxylin (Sigma-Aldrich) for 1 min.
Hepatogenic differentiation
To induce differentiation of naive PDSCs and TERT+ cells into hepatocytes, the cells were plated at a density of 2 × 104 cells/cm2 in basal medium (60 % DMEM-LG, 40 % MCDB201, 2 % FBS, 1 % penicillin-streptomycin) containing 20 ng/ml epidermal growth factor and 10 ng/ml basic FGF (bFGF) and grown to 60 % confluency. When the cells were 70–80 % confluent, the culture medium was replaced with early inducing medium to permit hepatic differentiation. For initiation, the cells were incubated for 7 days in basal medium supplemented with 10 ng/ml BMP4, 20 ng/ml HGF, 10 ng/ml bFGF, and 0.61 g/l nicotinamide. To progress to the maturation step, the cells were treated for another 2 weeks with maturation medium, which contained basal medium supplemented with 2 % FBS, 1 μmol/l dexamethasone, 1× ITS+ premix (insulin, transferrin, selenous acid, bovine serum albumin, linoleic acid), and 20 ng/ml oncostatin M. After incubation in the terminal medium for 7 days, hepatic differentiation was confirmed via indocyanine green (ICG) uptake by the hepatogenic induced cells. Subsequently, the hepatic induced cells were harvested to analyze hepatocyte-specific gene expression by RT-PCR.
Western blotting
Cells were washed with PBS and lysed in 100 μl cold RIPA buffer (Sigma) with a protease inhibitor cocktail (Roche, Mannheim, Germany). The cell lysate was centrifuged at 13,000g for 15 min at 4 °C. The supernatant was harvested, and its protein concentration was measured with a BCA protein assay kit (Pierce, Rockford, Ill., USA). For electrophoresis, 40 μg protein was dissolved in sample buffer (50 mM TRIS–HCl pH 6.8, 2 % SDS, 10 % glycerol, 1 % β-mercaptoethanol, 12.5 mM EDTA, 0.02 % bromophenol blue), boiled at 90 °C for 5 min, and separated on an 8 % SDS reducing gel. The separated proteins were transferred onto polyvinylidene difluoride membranes (Bio-rad, Hercules, Calif., USA) by using a trans-blot system (Bio-rad). The blots were blocked for 1 h in PBS containing 8 % non-fat dry milk (BD, Sparks, Md., USA) and 0.05 % Tween 20, at room temperature, and incubated at 4 °C overnight with primary antibodies (Table S2) in PBS plus Tween 20 (PBS-T) containing 2 % non-fat dry milk. The blots were incubated for 1 h with horseradish-peroxidase-conjugated secondary antibodies (1:10,000 dilution; Bio-rad) in PBS-T containing 2 % non-fat dry milk at room temperature. Then, the proteins were visualized with an enhanced chemiluminescence detection system (Amersham, Piscataway, N.J., USA).
Immunoprecipitation
The cells were scraped off the culture dish with 200 μl cold RIPA buffer (Sigma) containing protease inhibitor cocktail (Roche). The cell lysate was centrifuged at 13,000g for 15 min at 4 °C. The supernatant was harvested, and its protein concentration was measured with a BCA protein assay kit. For immunoprecipitation, 300 μg protein in RIPA buffer was incubated with an antibody at 2 μg per 300 μg total protein for 3–4 h at 4 °C. Then, a pre-mixture of protein and antibody was incubated with 20 μl of a 50 % slurry of protein-G-agarose beads (GenDEPOT) overnight at 4 °C with constant rotation. After centrifugation at 1000 rpm for 1 min at 4 °C, the supernatant was discarded, and the agarose-bead pellets were washed with PBS. After a final wash, the agarose-bead pellets were resuspended in a final volume of 20 μl with sample buffer (50 mM TRIS–HCl pH 6.8, 2 % SDS, 10 % glycerol, 1 % β-mercaptoethanol, 12.5 mM EDTA, 0.02 % bromophenol blue) and boiled at 90 °C for 5 min. Then, the agarose beads were removed by brief centrifugation, and the supernatant was loaded immediately onto an 8 % SDS-polyacrylamide reducing gel. Next, Western blotting was performed as described above.
ICG uptake assay
After the cultured cells had been washed with PBS, an ICG solution (100 mg/ml) was added to the plates at a final concentration of 1 mg ml−1 and incubated at 37 °C for 90 min. The plates were then rinsed with PBS and refilled with DMEM containing 10 % FBS. ICG was completely eliminated from the cells 72 h later.
TRAP assay
Telomerase activity was measured by using a PCR-based technique with a modified telomerase repeat amplification protocol (TRAP), which induces highly specific amplification of telomerase-mediated elongation products; the products were visualized by ethidium bromide staining. This technique permits highly specific amplification of telomerase-mediated elongation products under the following conditions: denaturation at 90 °C for 3 min, followed by 31 cycles of denaturation at 94 °C for 45 s, annealing at 53 °C for 45 s, and extension at 72 °C for 1 min. The product was loaded on polyacrylamide gels. After electrophoresis, the gel was stained with SYBR Green I. The sequences of the primers were as follows: TS primer, 5′-AATCCGTCGAGCAGAGTT-3′; CX primer, 5′-CCCTTACCCTTACCCTTACCCTAA-3′
Transfection of siRNA-PTTG1
For PTTG1 knock-down, 5 × 106 cells were plated in a 10-cm culture dish and cultured with 5 % CO2 at 37 °C. At 24 h later, the medium was removed from the culture dish, and 4 ml OPTI-MEM was added. The Lipofectamine 2000 (Invitrogen) protocol was followed for the next step. The cells were transfected with 100 nM siRNA-PTTG1 to down-regulate the PTTG1 gene and incubated for 48 h. Finally, the cells were harvested for analysis.
SA-β-galactosidase staining
The cells were washed with PBS, fixed for 3–5 min in 4 % formaldehyde, and incubated with fresh SA-β-gal staining solution containing 1 mg ml−1 X-gal, 40 mM citric acid, 40 mM sodium phosphate (pH 6.0), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2 at 37 °C for 12–16 h.
Statistics
The results are presented as means ± SD. Statistical significance was measured by multiple comparisons by using Student’s t-test with a significance level of P < 0.05.
Results
Characterization of immortalized PDSCs depends on TERT gene transfection
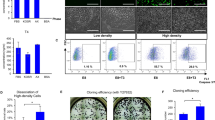
PDSCs immortalized by overexpression of hTERT (TERT+ cells) were generated with an AMAXA-nucleofactor kit (Lonza) for electroporation. After transfection of the TERT gene into naive PDSCs, their spindle-shape morphologies were altered into cuboidal shapes (Fig. 1a). Telomerase activity was not observed in naive PDSCs, similar to the negative control; however, the TERT+ cells exhibited strong telomerase activity at the same level as the positive control via the TRAP assay (Fig. 1b). Nevertheless, no differences in the expression of stemness markers were identified by RT-PCR analysis (Fig. 1c). Furthermore, the expression of surface markers of TERT+ cells was similar to that of naive PDSCs, although the percentages differed slightly. The analysis was negative for hematopoietic markers (e.g., CD34, CD45, and HLA-DR) and positive for non-hematopoietic markers (e.g., CD90, CD105, HLA-ABC, and HLA-G; Fig. 1d, e).
Comparison of the characteristics of naive placenta-derived mesenchymal stem cells (PDSCs; Naive) and PDSCs immortalized by overexpression of the hTERT (human telomerase reverse transcriptase) gene (TERT+). a Morphology of TERT+ cells was changed by immortalization. Bars 50 μm. b In vitro telomerase activities of naive PDSCs and TERT+ cells. TERT+ cells exhibited in vitro telomerase activity to the same extent as the positive control according to the telomere repeat amplification protocol (TRAP) assay. c Reverse transcription plus polymerase chain reaction (RT-PCR) analysis of stemness markers. β-Actin was used as an internal control. Both naive PDSCs and TERT+ cells exhibit a similar pattern of gene expression. d Immunophenotypes for hematopoietic and mesenchymal stem cells of naive PDSCs and TERT+ cells by flow cytometry. e Immunophenotypes for HLA-ABC, DR, and G of naive PDSCs and TERT+ cells by flow cytometry. The percentages of cells positive for the various cell surface markers were calculated by comparing the target protein signals with those in the isotype controls. f Cell cycle analysis by fluorescence-activated cell sorter (FACS) scan. S-phase, a criterion of proliferation, was increased in TERT+ cells compared with naive PDSCs. g Growth curve of naive PDSCs and TERT+ cells
By cell cycle analysis, the proportion of naive PDSCs and TERT+ cells in S-phase increased from 17.9 % to 41.1 %, respectively (Fig. 1f). Furthermore, the TERT+ cell count was significantly higher than the naive PDSC count after culture for the same length of time (P < 0.005, Fig. 1g). These results indicated that the overexpression of the hTERT gene in TERT+ cells allowed the cells to retain the properties of stem cells while exhibiting greater proliferation than naive cells.
In addition, TERT+ cells differentiated into adipocytes by incubation with adipogenic differentiation medium for 21 days after which time the adipocytes were detected by Oil-red O staining (Fig. 2a). The expression of lipoprotein lipase and PPAR-gamma, which are specific markers for adipocytes in differentiated TERT+ cells, also increased compared with naive PDSCs (Fig. 2b). Furthermore, TERT+ cells that were differentiated into hepatocytes were capable of detoxifying toxins as a representative hepatic function when they were exposed to ICG solution (Fig. 2c). By RT-PCR analysis, the expression of hepatocyte-related genes, including albumin and TAT (transactivator of transcription), increased after hepatogenic differentiation in naive PDSCs; however, no difference was seen in the expression of albumin and TAT between undifferentiated cells and differentiated cells. The expression of AFP (α-fetoprotein) in TERT+ cells decreased after hepatogenic differentiation (Fig. 2d). Although the efficiency with which TERT+ cells differentiated into hepatocytes was lower than that of naive PDSCs, we expect that TERT+ cells retain the potential to differentiate.
Differentiation of naive and TERT+ cells into adiopogenic and hepatogenic lineages. a Accumulation of oil droplets in differentiated naive PDSCs and TERT+ cells, as determined by Oil-Red O staining. Cells were counterstained with hematoxylin (undifferentiated states not shown). Bars 50 μm. b Adipogenic differentiation of naive PDSCs and TERT+ cells was confirmed by the detection of mRNA encoding the adipocyte markers lipoprotein lipase and PPAR-r. c Hepatogenic differentiation of naive PDSCs and TERT+ cells was revealed by the uptake of indocyanine green (undifferentiated states not shown). Bars 50 μm. d Hepatogenic differentiation of naive PDSCs and TERT+ cells was confirmed by RT-PCR (AFP, albumin, and TAT). β-Actin was used as an internal control
Correlation between cell survival and cell death in immortalized cells by TERT gene transfection
Important phenomena underlie the balance between cell survival and cell death including proliferation and differentiation. Therefore, we analyzed the roles of survival and death in cells immortalized by TERT through the analysis of several markers for stemness, cell cycle, and death. The expression of TERT and stemness markers including Oct-4 and Nanog was increased in TERT+ cells compared with naive PDSCs (Fig. 3a), even though the karyotype of the TERT+ cells was changed by the insertion of hTERT genes (Fig. S1). However, no difference was seen in the expression of SCF (stem cell factor). Compared with naive PDSCs, the expression of Cdk-4 and PTTG1 was increased in TERT+ cells, and the expression of p53, p21, and cyclin E1 was decreased. Interestingly, the expression of PTTG1 but not homologous PTTG2 was dramatically increased in TERT+ cells (Fig. 3b).
Analysis of the expression of self-renewal, cell cycle, anti-apoptotic, and autophagic proteins in naive PDSCs and TERT+ cells. a Expression of self-renewal markers was similar or increased in TERT+ cells compared with naive PDSCs. b In TERT+ cells, cell-cycle-related proteins were increased, and tumor-suppressing proteins were decreased. c In TERT+ cells, the anti-apoptotic protein Bcl-2 was increased, and apoptotic proteins were decreased. d Autophagic proteins phospho-mTOR and LC3 were increased in TERT+ cells. D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control
As shown in Fig. 3c, the expression of pro-apoptotic genes (e.g., Bax and Caspase-9) was decreased in TERT+ cells, except for the cleavage form PARP. The expression of Bcl-2, which is a representative anti-apoptotic factor, was dramatically increased (Fig. 3c). In addition, the expression of active type II of the lipidation of microtubule-associated protein light chain 3 (LC3), leading to the formation of a double membrane-limited autophagic vacuole or autophagosome in TERT+ cells was decreased via increased levels of phosphorylated mTOR (mammalian target of rapamycin), which is a negative regulator of type II LC3 (Fig. 3d). These findings indicate that immortalized PDSCs induce self-renewal activity through the enhancement of stemness and the cell cycle and decreases in cell death pathways, including apoptosis and autophagy.
Effect of down-regulation of PTTG1 by siRNA on the self-renewal activity of MSCs
As shown above, the expression of PTTG1 was dramatically increased in TERT+ cells. PTTG1 regulates the cell cycle by preventing separins from promoting sister chromatid separation during metaphase. To assess whether enhanced proliferation activity attributable to the overexpression of TERT was correlated with PTTG1 expression, both naive PDSCs and TERT+ cells were treated with siRNA-PTTG1, and the effect of PTTG1 was analyzed. To confirm the down-regulation PTTG1 caused by the treatment with siRNA, the expression of PTTG1 in both types of cells was analyzed. As shown in Fig. 4, the expression of PTTG1 in both types of cells was decreased by siRNA-PTTG1 treatment, and the expression of PTTG2 was also down-regulated by treatment with siRNA-PTTG1, because PTTG2 is homologous to PTTG1 (Fig. 4a). As shown in Fig. 1b, the telomerase activity of TERT+ cells was increased compared with naive PDSCs. To confirm the effect of PTTG1 on telomerase activity, cells were treated with siRNA-PTTG1, and their telomerase activity was analyzed. Telomerase activity was decreased in TERT+ cells after treatment with siRNA-PTTG1 compared with non-treated TERT+ cells (Fig. 4b, c). In addition, the expression of proliferation-related factors such as Oct-4, heat shock protein 90 (HSP90), and Ku70 was decreased in TERT+ cells because of the down-regulation of PTTG1. These results indicated that the expression of PTTG1 was closely related to telomerase activity and the control of proliferation in TERT+ cells. In addition, the expression of Cyclin E1 and p53 as cell cycle regulators was decreased by the knock-down of PTTG1; otherwise, the expression of Cdk-4 and p21 (Chesnokova et al. 2008) as cell cycle regulators was increased in TERT+ cells by the down-regulation of PTTG1 (Fig. 4d). No difference was observed in the expression of HSP70 in both types of cells, regardless of the down-regulation of PTTG1 (Fig. S2B).
Effects of pituitary tumor transforming gene 1 (PTTG1) expression in naive PDSCs and TERT+ cells on the survival and death of cells. a PTTG1 and PTTG2 expression were decreased in cells treated with siRNA-PTTG1, as assessed by RT-PCR analysis. b Cells with decreased PTTG1 expression were assayed for telomerase activity via the TRAP assay. c Expression of PTTG1, TERT, Oct-4, and Ku70 decreased because of the down-regulation of PTTG1, but expression of heat shock protein 90 (HSP90) was unchanged. d Cell cycle factors were decreased, and p21 was increased by knock-down of PTTG1. e Anti-apoptotic proteins were unchanged or slightly decreased in cells treated with siPTTG1, and apoptotic proteins were increased in cells treated with siPTTG1. f Autophagy activity was decreased after treatment with siPTTG1
Next, we analyzed whether PTTG1 expression could control proliferation activity in naive PDSCs and TERT+ cells by blocking apoptotic and autophagic pathways. No differences were found in the expression of Bad and Bax regardless of the down-regulation of PTTG1. However, the expression of caspase 9 and caspase 3 increased when PTTG1 was knocked down in both types of cells. Furthermore, the expression of Bcl-2 increased in cells treated with siRNA-PTTG1 (Fig. 4e). Analysis of the autophagic pathway revealed that the expression of phosphorylated mTOR and ATG5-12 increased in both types of cells when PTTG1 was down-regulated; however, type II LC3, an indicator of autophagy activity, was decreased (Fig. 4f). From these results, we suggest that decreased PTTG1 induces apoptosis rather than autophagy.
Induction of senescence in MSCs by the down-regulation of PTTG1
During serial expansion, aged MSCs are characterized by a flattened phenotype and increased β-galactosidase activity, resembling cellular senescence. Down-regulation of PTTG1 induced cellular senescence in naive PDSCs and TERT+ cells (Fig. 5a). To determine whether down-regulation of PTTG1 in both cell types affects the cell cycle, we conducted cell cycle analysis after treatment with siRNA-PTTG1. Down-regulation of PTTG1 induced a decrease of S-phase and senescence. In addition, the fraction of sub-G1 TERT+ cells was dramatically increased (Fig. 5b). To confirm the effect of PTTG1 on apoptosis, we assessed caspase-3/7 activity. No difference was noted in caspase-3/7 activity in naive PDSCs as a result of knock-down of PTTG1. However, caspase-3/7 activity in TERT+ cells was increased (Fig. 5c). These results indicate that PTTG1 expression involves the cellular senescence of MSCs and the apoptosis of immortalized MSCs attributable to hTERT overexpression, and that the dual roles of PTTG1 might be affected by its interaction with TERT.
Decreased PTTG1 results in the induction of cellular senescence. a Senescence-associated β-galactosidase activity at pH 6.0 (blue) was used as a marker of replicative senescence. Decreased PTTG1 in cells attributable to treatment with siPTTG1 eventually induced senescence. Bars 50 μm. b Cell cycle analysis by FACScan after treatment with siPTTG1. The S-phase as a criterion of proliferation was decreased in cells treated with siPTTG1. c Apoptosis analysis by using a caspase3/7 enzyme-linked immunosorbent assay. No difference was apparent in apoptotic activity in naive PDSCs following the down-regulation of PTTG1. However, apoptotic activity was increased in TERT+ cells treated with siPTTG1. *P value < 0.05 compared with treatment of siPTTG1
Functional interaction between TERT and PTTG1
To confirm the interaction of PTTG1 and TERT, we conducted immunoprecipitation experiments utilizing an anti-TERT antibody. TERT was found to interact with PTTG1 and HSP90, Ku70, and HSP70 (Fig. 6a, Fig. S2C). HSPs had previously been shown to interact with TERT (Haendeler et al. 2003), but the interaction between TERT and PTTG1 has not been characterized. We hypothesized that mediators between TERT and PTTG1 influence the dual function of PTTG1 in TERT+ cells. Interestingly, the Ku heterodimer has been reported to be associated with telomerase via interactions with hTERT and hPTTG (Chai et al. 2002). Based on these results, we performed immunoprecipitation by using an anti-Ku70 antibody and confirmed the interaction between PTTG1 and TERT. As shown in Fig. 6, the HSP90 and Ku70 proteins were found to interact with TERT, regardless of PTTG1 expression (Fig. 6a). In addition, Ku70 was found to interact with TERT, PTTG1, and HSP90 (Fig. 6b). Therefore, Ku70, TERT, and PTTG1 comprise a complex that affects cell cycle and self-renewal. Overexpression of TERT induces the expression of PTTG1, and the cell cycle and self-renewal activity are increased via the increased interaction between TERT and PTTG1. However, down-regulation of PTTG1 limits the potential for interaction with TERT. Therefore, apoptosis and senescence occur in cells in which PTTG1 is down-regulated (Fig. 7). Whether these phenomena are attributable solely to the interaction between these two proteins is not clear. Based on our results, we suggest that Ku70 is a mediator between TERT and PTTG1, because Ku70 interacts with both TERT and PTTG1.
Interaction of TERT and PTTG1 with HSP90 and Ku70 depends on PTTG1 expression. a TERT interacts with PTTG1, HSP90, and Ku70, as revealed by immunoprecipitation (IP) with an anti-TERT antibody. b Ku70 interacts with TERT, PTTG1, and HSP90, as determined by immunoprecipitation with an anti-Ku70 antibody
Representation of the regulatory mechanism of the balance between TERT and PTTG1 through association with Ku70. PTTG1 is overexpressed in TERT+ cells and interacts with TERT via Ku70. Changes in PTTG1 expression regulate cellular physiology, including self-renewal, apoptosis, and senescence, via the association of PTTG1 with Ku70 in TERT+ cells
Discussion
MSCs are able to self-renew and have the capacity to differentiate into three germ layers in vitro, including osteoblasts, adipocytes, and chondroblasts. Furthermore, MSCs are known to have immunosuppressive effects through various mechanisms, resulting in escape from the allogeneic host immunosurveillance system (Koc et al. 2000). Recently, other sources of MSCs have been isolated from other organs or tissues, including placenta, adipose tissues, cord blood, and the liver (Campagnoli et al. 2001; In’t Anker et al. 2004; Zuk et al. 2001). These sources have been examined as better therapeutic sources. In particular, MSCs derived from the placenta, which is involved in maintaining fetal tolerance, display immunomodulatory properties. Thus, placenta-derived MSCs are promising candidates for cell-based tissue engineering to repair or replace damaged tissues (Dennis et al. 2002). However, their efficacy remains controversial because of differences in the origins of MSCs and engraftment into damaged tissues (Parolini et al. 2008). Moreover, cell therapy by using MSCs, including BM-MSCs, is restricted by their limited self-renewal activity, because these cells are derived from adult organs and reflect the age of the donor. Therefore, alternative MSCs derived from other organs that are capable of overcoming the limitation of BM-MSCs are necessary for the clinical application of MSCs.
Many researchers have reported strategies for overcoming the limited self-renewal activity of MSCs by the manipulation of specific genes (e.g., hTERT and Bmi-1; Zhang et al. 2006). The human TERT gene, as a component of telomerase, maintains chromosomal integrity and genome stability through the synthesis of specific DNA repeats (Blackburn 2001; Nakamura and Cech 1998). Furthermore, it is the driving force in maintaining telomeres, which are composed of a short hexa-nucleotide sequence, TTAGGG. When hTERT is mutated, the resulting shorter telomere length is associated with premature aging diseases (Tsang et al. 2012). Most mammalian cells undergo limited cell division during long-term culture. This state of irreversible proliferative arrest is termed “replicative senescence”(Hayflick 1965).
In the present study, in order to overcome the limited life span and self-renewal activity of MSCs, we have established immortalized cells, denoted TERT+ cells, by inserting the hTERT gene into naive PDSCs by using the AMAXA gene delivery system. We have also characterized naive PDSCs compared with TERT+ cells. The characterization of TERT+ cells has revealed that proliferation, telomerase activity, and the expression of cell-cycle-related factors and autophagic makers in TERT+ cells are increased, whereas apoptosis is decreased. In addition, no difference has been seen in the differentiation potential between naive PDSCs and TERT+ cells, although TERT+ cells exhibit aneuploid karyotypes (Fig. S1). Interestingly, PTTG1 expression is significantly increased in TERT+ cells. By down-regulating PTTG1, the increase in cell-cycle-factors, self-renewal, and telomerase activity is reduced; however, the expression of apoptotic genes increases (Fig. 4). Furthermore, the decrease in PTTG1 induces cell senescence (Fig. 5). From these results, we conclude that PTTG1 is not the sole regulatory factor in TERT+ cells, and that some regulatory mediator(s) might exist between PTTG1 and TERT.
Cell cycle arrest has been reported to promote senescence through cyclin D, cyclin E (Liu et al. 2011), and cyclin-dependent kinases (Cdks), and the up-regulation of cyclin D1, Cdk-4, and Cdk-6 promotes the self-renewal of MSCs. In particular, cyclins D1/E1 increase the self-renewal activity of MSCs via the Ca2+/protein kinase C/mitogen-activated protein kinase (MAPK) and phosphatidyl-inositol-3-kinase pathways (Ryu et al. 2010). In the present study, we have found that the expression of cyclin E1 is increased by siRNA-PTTG1 treatment in both naive PDSCs and TERT+ cells (Fig. 4d).
PTTG1 is a cell-cycle-related factor, and up-regulated PTTG1 promotes cell proliferation by increasing the number of cells in S phase via the MAPK pathway; it regulates DNA repair and apoptosis via interactions with p53 and Ku (Romero et al. 2001). Functionally, PTTG1 regulates telomerase activity through interaction with telomerase to induce the proper assembly of the telomerase enzyme complex, including p23 (Forsythe et al. 2001). In addition, Ku is a heterodimeric protein that is composed of a ∼70 kDa subunit and an ∼80 kDa subunit and is involved in the regulation of telomere maintenance through binding the telomeric proteins TRF1, TRF2, and TERT (Tuteja and Tuteja 2000). Furthermore, Chai et al. (2002) have reported that Ku regulates telomerase activity by interacting with TERT. However, the interaction between TERT and PTTG1 has not been previously characterized, although interactions are known among TERT, HSP70, HSP90, and Ku70. We demonstrated an interaction between TERT and PTTG1 by immunoprecipitation (Fig. 6). Our results indicate that the expression of HSP70 and HSP90 is not altered in response to the down-regulation of PTTG1 in both cell types. These data indicate that PTTG1 does not directly affect the interaction among HSP70, HSP90, and TERT. Nevertheless, the expression of Ku70 decreases slightly when PTTG1 is down-regulated. These data indicate that Ku70 plays an important role as a regulatory mediator between PTTG1 and TERT. TERT interacts with Ku70, and Ku70 interacts with PTTG1; the resulting complex is translocated into the nucleus, leading to increases in the cell cycle, autophagy, and self-renewal activity. By contrast, decreased PTTG1 expression decreases the expression of Ku70 and HSP90. These environmental changes induce a change in the binding affinity between Ku70 and PTTG1. Furthermore, the interaction between TERT and Ku70 is also decreased by the decreased expression of Ku70. These changes induce apoptosis and cellular senescence as a phenomenon of aging.
Our findings suggest that the balance between TERT and PTTG1 attributable to efficient association with Ku70 might be important for the enhancement of the limited self-renewal activity of MSCs, although further study of the regulatory mechanism of Ku70 is needed. Moreover, this might facilitate the elucidation of the regulatory mechanism of self-renewal.
References
Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE (2011) Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474:399–402
Blackburn EH (2001) Switching and signaling at the telomere. Cell 106:661–673
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402
Campisi J, Dimri GP, Nehlin JO, Testori A, Yoshimoto K (1996) Coming of age in culture. Exp Gerontol 31:7–12
Chai W, Ford LP, Lenertz L, Wright WE, Shay JW (2002) Human Ku70/80 associates physically with telomerase through interaction with hTERT. J Biol Chem 277:47242–47247
Chesnokova V, Zonis S, Rubinek T, Yu R, Ben-Shlomo A, Kovacs K, Wawrowsky K, Melmed S (2007) Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res 67:10564–10572
Chesnokova V, Zonis S, Kovacs K, Ben-Shlomo A, Wawrowsky K, Bannykh S, Melmed S (2008) p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci U S A 105:17498–17503
Dennis JE, Carbillet JP, Caplan AI, Charbord P (2002) The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs 170:73–82
Forsythe HL, Jarvis JL, Turner JW, Elmore LW, Holt SE (2001) Stable association of hsp90 and p23, but not hsp70, with active human telomerase. J Biol Chem 276:15571–15574
Fridman AL, Tang L, Kulaeva OI, Ye B, Li Q, Nahhas F, Roberts PC, Land SJ, Abrams J, Tainsky MA (2006) Expression profiling identifies three pathways altered in cellular immortalization: interferon, cell cycle, and cytoskeleton. J Gerontol A Biol Sci Med Sci 61:879–889
Haendeler J, Hoffmann J, Rahman S, Zeiher AM, Dimmeler S (2003) Regulation of telomerase activity and anti-apoptotic function by protein-protein interaction and phosphorylation. FEBS Lett 536:180–186
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636
Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S (2000) Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet 355:716–719
Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA (2004) Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 6:543–553
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345
Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM, Kinzler KW, Vogelstein B, Lengauer C (2001) Securin is required for chromosomal stability in human cells. Cell 105:445–457
Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ (2011) Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res 346:53–64
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18:307–316
Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, Kim GI, Kwon SW, Hwang SG, Kim GJ (2010) Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem 111:1453–1463
Liu NA, Jiang H, Ben-Shlomo A, Wawrowsky K, Fan XM, Lin S, Melmed S (2011) Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc Natl Acad Sci U S A 108:8414–8419
Miura N, Nakamura H, Sato R, Tsukamoto T, Harada T, Takahashi S, Adachi Y, Shomori K, Sano A, Kishimoto Y, Ito H, Hasegawa J, Shiota G (2006) Clinical usefulness of serum telomerase reverse transcriptase (hTERT) mRNA and epidermal growth factor receptor (EGFR) mRNA as a novel tumor marker for lung cancer. Cancer Sci 97:1366–1373
Nakamura TM, Cech TR (1998) Reversing time: origin of telomerase. Cell 92:587–590
Napolitano MA, Cipollaro M, Cascino A, Melone MA, Giordano A, Galderisi U (2007) Brg1 chromatin remodeling factor is involved in cell growth arrest, apoptosis and senescence of rat mesenchymal stem cells. J Cell Sci 120:2904–2911
Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K, Nakamura T, Kiyono T, Toguchida J (2002) Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun 295:354–361
Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first International Workshop on Placenta Derived Stem Cells. Stem Cells 26:300–311
Pei L, Melmed S (1997) Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol 11:433–441
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33:49–54
Romero F, Multon MC, Ramos-Morales F, Dominguez A, Bernal JA, Pintor-Toro JA, Tortolero M (2001) Human securin, hPTTG, is associated with Ku heterodimer, the regulatory subunit of the DNA-dependent protein kinase. Nucleic Acids Res 29:1300–1307
Ryu JM, Lee MY, Yun SP, Han HJ (2010) High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J Cell Physiol 224:59–70
Saez C, Japon MA, Ramos-Morales F, Romero F, Segura DI, Tortolero M, Pintor-Toro JA (1999) hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene 18:5473–5476
Tong Y, Tan Y, Zhou C, Melmed S (2007) Pituitary tumor transforming gene interacts with Sp1 to modulate G1/S cell phase transition. Oncogene 26:5596–5605
Tsai CC, Chen CL, Liu HC, Lee YT, Wang HW, Hou LT, Hung SC (2010) Overexpression of hTERT increases stem-like properties and decreases spontaneous differentiation in human mesenchymal stem cell lines. J Biomed Sci 17:64
Tsang AR, Wyatt HD, Ting NS, Beattie TL (2012) hTERT mutations associated with idiopathic pulmonary fibrosis affect telomerase activity, telomere length, and cell growth by distinct mechanisms. Aging Cell 11:482–490
Tuteja R, Tuteja N (2000) Ku autoantigen: a multifunctional DNA-binding protein. Crit Rev Biochem Mol Biol 35:1–33
Wang Z, Yu R, Melmed S (2001) Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol 15:1870–1879
Zhang X, Soda Y, Takahashi K, Bai Y, Mitsuru A, Igura K, Satoh H, Yamaguchi S, Tani K, Tojo A, Takahashi TA (2006) Successful immortalization of mesenchymal progenitor cells derived from human placenta and the differentiation abilities of immortalized cells. Biochem Biophys Res Commun 351:853–859
Zou H, McGarry TJ, Bernal T, Kirschner MW (1999) Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285:418–422
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228
Author information
Authors and Affiliations
Corresponding authors
Additional information
Sang Shin Lee and Gi Jin Kim contributed equally to this work.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2011–0014098) and the Korea Research Foundation Grant funded by the Korean Government (MEST) (KRF-2011-0019610).
The authors of the manuscript declare no conflicts of interest.
Hyung-Jung Lee undertook the analysis of data, data interpretation, and manuscript drafting. Jong-Ho Choi carried out the collection and analysis of data. Jieun Jung made revisions and revised the data. Jin Kyeoung Kim performed the analysis of data, conception, and critical discussion. Sang Shin Lee was involved with critical discussion and financial support. Gi Jin Kim took part in conception and design, manuscript drafting, financial support, and final approval of manuscript.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lee, HJ., Choi, JH., Jung, J. et al. Changes in PTTG1 by human TERT gene expression modulate the self-renewal of placenta-derived mesenchymal stem cells. Cell Tissue Res 357, 145–157 (2014). https://doi.org/10.1007/s00441-014-1874-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1874-0