Abstract
The major molecular signals of pancreatic exocrine development are largely unknown. We examine the role of fibroblast growth factor 7 (FGF7) in the final induction of pancreatic amylase-containing exocrine cells from induced-pancreatic progenitor cells derived from human embryonic stem (hES) cells. Our protocol consisted in three steps: Step I, differentiation of definitive endoderm (DE) by activin A treatment of hES cell colonies; Step II, differentiation of pancreatic progenitor cells by re-plating of the cells of Step I onto 24-well plates at high density and stimulation with all-trans retinoic acid; Step III, differentiation of pancreatic exocrine cells with a combination of FGF7, glucagon-like peptide 1 and nicotinamide. The expression levels of pancreatic endodermal markers such as Foxa2, Sox17 and gut tube endoderm marker HNF1β were up-regulated in both Step I and II. Moreover, in Step III, the induced cells expressed pancreatic markers such as amylase, carboxypeptidase A and chymotrypsinogen B, which were similar to those in normal human pancreas. From day 8 in Step III, cells immunohistochemically positive for amylase and for carboxypeptidase A, a pancreatic exocrine cell product, were induced by FGF7. Pancreatic progenitor Pdx1-positive cells were localized in proximity to the amylase-positive cells. In the absence of FGF7, few amylase-positive cells were identified. Thus, our three-step culture protocol for human ES cells effectively induces the differentiation of amylase- and carboxypeptidase-A-containing pancreatic exocrine cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human embryonic stem (hES) cells derived from the inner cell mass of blastocysts of pre-implantation embryos can proliferate indefinitely and give rise to all cell types in the body. Human induced pluripotent stem (hiPS) cells are also pluripotent (Takahashi et al. 2007) and both types of stem cells have the potential to advance drug discovery and regenerative medicine. However, for hiPS cells, many issues such as immunogenicity (Zhao et al. 2011; Okita et al. 2011) and genomic integrity (Hong et al. 2012) remain unresolved. Therefore, we have used hES cells to establish a method by which pancreatic exocrine cells can be induced in steps that mimic development in the embryo.
The molecular mechanisms that regulate pancreatic acinar cell development remain unknown (Cleveland et al. 2012). Production of pancreatic cells from hES or hiPS cells has focused more on the differentiation of endocrine rather than exocrine cells (Chen et al. 2009; Zhang et al. 2009; Mfopou et al. 2010). The ability to derive pancreatic exocrine cells from hES cells would be a useful tool for elucidating underlying mechanisms of diseases such as pancreatic insufficiency, pancreatitis and pancreatic carcinoma.
We have previously demonstrated that mouse and human ES cells differentiate into mature pancreatic exocrine or endocrine cells through a well-defined developmental sequence. The first step is the formation of a definitive endoderm (DE), which then develops into common progenitor cells that express Pdx1 (Shirasawa et al. 2011a, 2011b). In turn, these cells differentiate to form the mature exocrine or endocrine cells. Differentiation of the DE is an important early stage in the induction of the pancreatic cell lineage (D’Amour et al. 2005). A recent study has shown that treatment with activin A and Wnt3a can effectively promote the development of definitive endodermal cells from hES cells (D’Amour et al. 2006). Retinoic acid (RA) signaling is required during pancreatic development, especially in the posterior foregut and midgut domains that express Pdx1 (Bayha et al. 2009). Additionally, a previous report has demonstrated that RA induces the efficient generation of Pdx1-positive cells from hES cells (Cai et al. 2010).
The factors that are effective in human exocrine cell differentiation are not known (Chen et al. 2009; Zhang et al. 2009). In the current study, we have focused on the differentiation of mature pancreatic exocrine cells that produce digestive enzymes. Fibroblast growth factor 7 (FGF7) is expressed in human embryonic pancreatic mesenchyme and is important for the proliferation, morphogenesis and cytodifferentiation of exocrine cells (Ye et al. 2005; Miralles et al. 1999). We have previously reported that FGF7, in combination with other inducing factors such as nicotinamide (NA) and glucagon-like peptide-1 (GLP-1), induces the differentiation of pancreatic exocrine cells from hES cells (Shirasawa et al. 2011b). FGF7 also promotes greater development of pancreatic progenitor cells (Shirasawa et al. 2011a). However, the role of FGF7 in inducing mature pancreatic exocrine cells has not been explored. Here, we show that FGF7 is effective in inducing pancreatic exocrine cell differentiation.
Materials and methods
hES cell culture and differentiation
This study was approved by the Shinshu University Institutional Review Board. Authors S.Y., A.M. and K.S. were approved by the Ministry of Education, Culture, Sports, 106 Science, and Technology of Japan to culture hES cells and they performed all of the cell cultures in this study. The hES cell line KhES-3 was purchased from Kyoto University in accordance with the Guidelines for Derivation and Utilization of Human Embryonic Stem Cells by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Undifferentiated hES cells were maintained on mitomycin C (Sigma, St. Louis, Mo., USA)-inactivated mouse embryonic fibroblasts (Invitrogen, Carlsbad, Calif., USA) in Dulbecco’s modified Eagle medium nutrient mixture F-12 (D-MEM/F-12; Gibco BRL, Rockville, Md., USA) supplemented with 20 % KnockOut serum replacement (Invitrogen), 100 μM nonessential amino acids (Gibco), 2 mM L-glutamine (Gibco), 100 μM 2-mercaptoethanol (Sigma) and 4 ng/ml basic FGF (bFGF; Invitrogen).
The goal of this study was to develop culture conditions that directed the differentiation of hES cells towards a pancreatic lineage and that ultimately resulted in the formation of cells that produced pancreatic enzymes. We achieved this goal by the stepwise culturing of hES cells with specific supplements provided at the appropriate times. The culture sequence was divided into three steps (Fig. 1). Step I led to the differentiation of the DE. hES cell colonies were treated with 100 ng/ml activin A (Sigma) and 25 ng/ml Wnt3a (R&D Systems, Minneapolis, Minn., USA) in RPMI medium (Gibco) supplemented with 2 mM L-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. After 24 h, the medium was switched to 100 ng/ml activin A in RPMI medium supplemented with 5 μg/ml insulin, 50 μg/ml transferrin, 30nM selenium chloride, 2 mM L-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin for 48 h.
Step II of the culture procedure resulted in the differentiation of pancreatic progenitor cells from the DE cells. The DE cells were re-plated onto 24-well plates and were treated with 1 μM all-trans RA (Sigma) in RPMI1640 medium supplemented with 2 % fetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin for 3 days.
Step III of the culture procedure achieved the final differentiation of cells containing pancreatic exocrine enzymes. The pancreatic progenitor cells were cultured in DMEM/F12 supplemented with 15 ng/ml FGF7 (R&D Systems), 10 mM NA (Sigma), 100 ng/ml GLP-1 (7–36 amide; Bachem, Torrance, Calif., USA), N2 supplement (Gibco), B27 supplement (Gibco), 50 U/ml penicillin and 50 μg/ml streptomycin. The medium was changed every other day for up to 15 days.
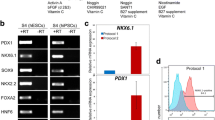
Representation of the culture protocol for differentiation of human undifferentiated embryonic stem cells (KhES-3; hESC) to pancreatic exocrine cells (Exocrine) via definitive endoderm (DE) and pancreatic progenitors (PP). Step I: Beginning with hESC, definitive endoderm (DE) was derived over a 3-day period by using a slightly modified version of a previously published protocol (Chen et al. 2009). During the first day, the inducing factors activin A and Wnt3a were present in the culture medium. During the next 2 days, only activin A was present. Step II: The DE cells were re-plated on 24-well plates at low or high density and treated with all-trans retinoic acid for an additional 3 days. Step III: The culture medium was changed to serum-free medium (N2, B27 in DMEM/F12 medium) supplemented with fibroblast growth factor 7 (FGF7), glucagon-like peptide 1 (GLP-1) and nicotinamide (NA). Cell markers: endodermal cell markers (Foxa2, Sox17), pancreatic progenitor cell marker (Pdx1) pancreatic exocrine cell marker (Amtlase)
Standard and real-time reverse transcription plus the polymerase chain reaction
Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions and complementary DNA (cDNA) was synthesized from total RNA by using a PrimeScript RT reagent kit (Perfect Real Time; TaKaRa Bio, Yokkaichi, Japan) for reverse transcription plus the polymerase chain reaction (RT-PCR). The cDNA was amplified by RT-PCR with specific primers (Table 1). For RT-PCR, the initial denaturation was for 10 min at 94 °C, followed by 35 cycles of heating at 94 °C (30 s), 53–65 °C (30 s) and 72 °C (30 s) and then, a final extension for 7 min at 72 °C. Real-time RT-PCR was performed by using a SYBR Premix Ex Taq (Perfect Real Time; TaKaRa Bio) in a Thermal Cycle Dice Real Time System (TaKaRa Bio). The cDNA was amplified by a 10-min initial denaturation at 95 °C, followed by 40 cycles of heating at 95 °C (5 s) and 60 °C (30 s). The expression of each gene was determined by real-time RT-PCR with specific primer sequences (Table 1). Expression values were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. First Choice human pancreas or salivary gland total RNA (Applied Biosystems, Carlsbad, Calif., USA) was used as a positive control for RT-PCR. For a negative control, distilled water was substituted for the mRNA template.
Immunofluorescence
To follow the incremental steps in the differentiation of mature pancreatic-like cells from undifferentiated hES cells, we analyzed, by immunohistochemistry, the presence of the following biomarkers for specific cell types: (1) Foxa2, a marker of endodermal cells; (2) Sox17, another marker of endodermal cells; (3) hepatic nuclear factor-1β (HNF1β), a marker of gut tube endoderm; (4) pancreatic duodenum homeobox-1 (Pdx1), a marker of pancreatic progenitor cells; (5) α-amylase, a marker of terminally differentiated pancreatic exocrine cells; (6) carboxypeptidase A (CPA), another marker of terminally differentiated pancreatic exocrine cells. Immunofluorescence staining procedures have been described in a previous report (Shirasawa et al. 2011a). Briefly, cultured cells were washed with phosphate-buffered saline (PBS), fixed in 4 % paraformaldehyde in PBS (pH 7.4) for 30 min, permeabilized with 0.1 % Triton X-100 in PBS and then treated with 1.5 % normal donkey or goat serum to block non-specific staining. After 30 min, the following diluted primary antibodies were added and incubated with the cells at 4 °C overnight: rabbit anti-Foxa2 (1:800; Cell Signaling Technology, Beverly, Mass., USA), mouse anti-Sox17 (1:100; R&D Systems), rabbit anti-HNF1β (1:100; Santa Cruz Biotechnology, Santa Cruz, Calif., USA), goat anti-PDX1 (1:200; R&D Systems), rabbit anti-α-amylase (1:500; Sigma) and goat anti-CPA (1:100; Santa Cruz Biotechnology). Following three washes with PBS, the samples were incubated with diluted secondary antibodies conjugated with either Alexa Fluor 488 or Alexa Fluor 568 and with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes, Eugene, Ore., USA). The specimens were observed by using a ZEISS Axio Observer Z1 (Carl Zeiss, Oberkochen, Germany) or ZEISS LSM 5 EXCITER confocal laser scanning microscope (Carl Zeiss).
To estimate the differentiation efficiency, the number of positive cells was determined by using the count function mode of the Axio Vision software on panoramic images (2.6 × 4.7 mm) obtained on the ZEISS Axio Observer Z1. The total number of cells was determined by DAPI nuclear staining. Data were determined as means ± SD obtained from at least three independent experiments.
Statistical analysis
Significant differences were analyzed by Student’s t-test with P < 0.05 being set as the critical level.
Results
Culture steps I and II: effect of inducing factors and influence of high-density culture
To induce the formation of DE, undifferentiated hES cells were treated with activin A and Wnt3a in culture Step I (Fig. 1). The morphology of the treated cells changed from undifferentiated hES cells, which were characterized with tightly packed colonies and a high nucleus-to-cytoplasm ratio (Fig. 2a), to cobblestone-like morphology, which was most evident at the periphery of the colonies (Fig. 2b, c). After the third day in culture Step I, many Sox17- and Foxa2-positive cells, characteristic of the DE, were present at the edges of colonies (Fig. 2d–f). A few of these cells were also present throughout the colonies (not shown). In control cultures not treated with activin A and Wnt3a, the expression of Sox17 and Foxa2 proteins was barely detected (Fig. 2g–i). Thus, the combination of activin A and Wnt3a induced DE differentiation.
Differentiation of hES cells into definitive endoderm (DE) in culture Step I. a Soon after the plating of the cells, a phase contrast image shows their typical undifferentiated morphology. b, c After 3 days in culture, the morphology changes to that of an induced hES cell colony (b) and, at higher magnification (c), reveals many cobblestone-like cells. d–f, g–i Double immunostaining shows that Sox17- and Foxa2-positive cells are present in the colonies after treatment with activin A and Wnt3a (Act(+), d–f), whereas many fewer double-positive cells are observed in non-treated colonies (Act(−), g–i)
Next, we hypothesized that cell-cell interaction might be important for the differentiation of mature cells, including pancreatic cells. Therefore, we investigated the effect of various cellular plating densities in culture Step II. In order to obtain low-density cultures in Step II, induced DE cells harvested from the 100-mm dishes in Step I were plated into 24-well plates. To obtain high-density cultures in Step II, the induced DE cells were plated into only 12 wells of the 24-well plate. Thus, in the high-density Step II cultures, the cell density was approximately twice that in the low-density Step II cultures. All of the re-plated cells were treated with RA for 3 days to induce pancreatic progenitor cells (Fig. 3a). After 3 days, the cells seeded at high density proliferated greatly and attained confluence (Fig. 3c). In contrast, the cells seeded at low density showed poor adhesion and generated only a few small colonies (Fig. 3b). In the high-density RA-treated cultures, HNF1β-positive gut tube endoderm cells were detected by immunohistochemistry (Fig. 3d). RT-PCR confirmed that the expression of the gut tube endoderm marker HNF1β was significantly greater in the high-density cultures (n = 3, P < 0.05, Fig. 3e). The expression of Pdx-1, a marker for pancreatic progenitor cells, was also higher in the high-density cultures but the difference was not statistically significant (n = 3, P = 0.0563, Fig. 3f).
Influence of cell seeding density in culture Step II. a Following culture Step I, the induced DE cells were re-plated and incubated with 1 μM retinoic acid (+RA) for 3 days. For low-density cultures, the DE cells from the 100-mm dishes of Step I were re-plated into 24-well plates. For high-density cultures, the cells were re-plated into only 12 wells of the 24-well plate and thus had approximately twice the cell density of the low-density Step II cultures. b,c Phase images showing cells re-plated at low density (b) and high density (c) and treated with RA (RA(+)) for 3 days. d Immunofluorescent staining showed hepatic nuclear factor-1β (HNF1β)-positive cells, indicating the presence of gut tube endoderm in Step II. e, f Real-time polymerase chain reaction (PCR) analysis of cells cultured for 3 days at low and high density with RA. g–j Real-time polymerase chin reaction (PCR) analysis showing the dynamics of mRNA expression for several key markers of pancreatic differentiation from human ES cells through Step I to Step II culture (g Goosecoid, h Foxa2, i Sox17, j HNF1β). *P < 0.05
During culture Steps I and II, the differentiation of DE and pancreatic progenitor cells was assessed by protein expression and real-time PCR analysis. The mRNA expression of key markers for pancreatic differentiation, such as the mesendodermal marker goosecoid (Fig. 3g), endodermal markers Foxa2 (Fig. 3h) and Sox17 (Fig. 3i) and gut tube endoderm marker HNF1β (Fig. 3j), indicated that hES cells were differentiated into the endodermal fate, representing important steps in pancreatic development. The expression of the mesendoderm marker, goosecoid, was up-regulated in culture Step I and remained unchanged or down-regulated to Step II. In contrast, the expression of endoderm markers Foxa2, Sox17 and gut tube endoderm marker HNF1β was up-regulated in both Steps I and II.
Culture step III: effect of growth factors on the differentiation of mature pancreatic exocrine cells
In culture Step III, the cells were cultured with growth factors FGF7, GLP-1 and NA to induce the differentiation of pancreatic exocrine cells (Fig. 1). Phase contrast images of control cells not treated with growth factors showed that they proliferated poorly and many cells were flattened and failed to form cell clusters (Fig. 4a–c). In contrast, cultures treated with growth factors FGF7, GLP-1 and NA formed multilayered cell clusters (Fig. 4d–f). Amylase-positive cells increased from day 8 (Fig. 4g, h) through day 15 (Fig. 4i, j). At the same time, small cells located near the larger amylase-positive cells stained positively for Pdx1, the pancreatic progenitor cell marker (Fig. 4h, j). This suggested that amylase-positive cells had originated from the Pdx1-positive cells developing near the edge of cell clusters. RT-PCR analysis demonstrated that the expression of pancreatic markers in the induced cells was similar to that in normal human pancreas, although the expression levels in induced cells were lower than those in human pancreas (Fig. 4k). The induced cells treated with FGF7, GLP-1 and NA consistently expressed pancreatic progenitor markers, such as Pdx1, Mist1 and P48 and mature pancreatic markers, such as amylase, CPA and chymotrypsin B (CTRB) but not in the cells cultured in serum-free medium in the absence of any inducing factors.
Differentiation of pancreatic exocrine cells by growth factors FGF7, GLP-1 and NA in culture Step III. Treatment with growth factors FGF7, GLP-1 and NA during culture Step III results in the differentiation of mature pancreatic amylase-containing cells. a–f Phase contrast images of Step III cultured at 8 days (a, d) and 15 days (b, c, e, f) show poor development of cells in serum-free (Serum Free; SF) medium that also lacked growth factors (a–c). Cells treated with growth factors (FGF7, GLP-1 and NA(+)) developed into multilayered clusters (d–f). Immunofluorescent staining for amylase and Pdx1 (arrows) on days 8 (D8; g, h) and 15 (D15; i, j) in cells treated with FGF7, GLP-1 and NA (FGF7(+)). Enlarged images (h, j) reveal amylase-positive cells located close to Pdx1-positive cells. Amylase-positive cells increase from day 8 (g) to day 15 (i) with the decreased expression of Pdx1. k At day 15 of culture Step III, reverse transcription plus PCR (RT-PCR) analysis showed that the expression of pancreatic markers in the induced cells was similar to that in normal human pancreas (Pdx-1, Mist1, P48 pancreatic progenitor cell markers, Amylase, CPA (carboxypeptidase A), CTRB (chymotrypsinogen B) differentiated exocrine cell markers, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) internal standard, SF serum-free and no growth factor treatment, (+) serum-free and with growth factor treatment, P.C. positive control, N.C. negative control)
Effect of FGF7 on hES-derived pancreatic exocrine differentiation
To determine the localization and to estimate the efficiency of differentiated cell production within the cell population, panoramic images of amylase-containing cells were obtained by immunohistochemistry (Fig. 5). After 15 days in culture Step III, significantly more amylase-positive cells were present in cultures treated with FGF7, GLP-1 and NA than in cultures treated with GLP-1 and NA alone or in cultures not receiving any growth factors (Fig. 5a–d). The amylase-positive cells were localized within or at the edges of the multilayered cell clusters (Fig. 5c). In contrast, only a few amylase-positive cells were seen in cultures treated with GLP-1 and NA but not FGF7 (Fig. 5b). Little cell proliferation occurred in the absence of FGF7, GLP-1 and NA treatment (Fig. 5a). At day 15 of the exocrine differentiation process, 12.2 ± 6.1 % of the cells in cultures with all three inducing factors expressed amylase, whereas this value was reduced to 4.2 ± 2.7 % of cells in cultures treated with GLP-1 and NA alone. For cells receiving no induction factors, only 0.7 ± 0.9 % expressed amylase protein (Fig. 5d). Thus, FGF7 played an important role in inducing mature pancreatic exocrine cells.
Effect of FGF7 on the differentiation of amylase-positive cells in culture Step III. a–c Panoramic images (2.6 × 4.7 mm) of immunofluorescently stained cells on day 15 of culture Step III. a Non-treated cells in serum-free (SF) culture. b Cells in SF culture treated with GLP-1 and NA but without FGF7 (FGF7(−)). c Cells in SF culture treated with GLP-1, NA and FGF7 (FGF7(+)). Bars 200 μm. d Efficiency of conversion to pancreatic exocrine cells as shown by the presence of amylase-positive cells. *P < 0.05
RT-PCR analysis with primers specific for pancreatic amylase and salivary amylase showed that pancreatic amylase mRNA was expressed in the induced cells but salivary amylase mRNA was not (Fig. 6a). Additional double-immunostaining revealed that amylase-positive cells also contained CPA, which is a pancreatic exocrine-specific digestive enzyme (Fig. 6b–j).
Characterization of amylase-positive cells on day 15 of culture Step III. a RT-PCR analysis showed that pancreatic amylase mRNA was present in the FGF7-induced cells (FGF7(+)) but salivary amylase mRNA was not detected (Sali human salivary gland mRNA, Pan human pancreas mRNA, N.C. negative control). b–j Immunofluorescent staining for pancreatic enzymes amylase and carboxypeptidase A (CPA) on day 15 (D15) in culture Step III (b–d and on day 22 (D22) in culture Step III (e–g). Boxed areas in e–g are shown at higher magnification in h–j
Discussion
In this study, we demonstrated that hES cells cultured in a stepwise fashion formed pancreatic exocrine cells when stimulated with FGF7 under the proper conditions. To prepare the cells for FGF7 stimulation, in culture Step I, hES cells were incubated with activin A and Wnt3a, a protocol known to induce DE cells efficiently (Thatava et al. 2011; Chen et al. 2012; Fu et al. 2011; D’Amour et al. 2005).
Culture of DE cells at moderately high density promotes the differentiation of Pdx1-positive cells by RA (Cai et al. 2010). In the present study, we demonstrated that, in culture Step II, the re-plated cell density influenced the differentiation efficiency from DE into pancreatic progenitor cells. We carried out two different density examinations. For high density, all cells from one 100 mm-dish were aliquoted into half the wells of a 24-well plate. For low density, they were aliquoted into all the wells of a 24-well plate. The differentiation efficiency was improved when the cells were cultured under the high-density conditions. Low cell density had a negative effect on pancreatic differentiation.
We found that RA induced the formation of Pdx1 cells and the same cells co-expressed HNF1β, a gut tube endoderm marker (Micallef et al. 2005). Expression of HNF1β, also known as vHNF1 (Coffinier et al. 1999), in the endoderm controls the generation of pancreatic precursors by sequential activation of HNF6 and Pdx1 (Poll et al. 2006). Therefore, we consider that HNF1β mRNA expression in culture Step II is important. Based upon the high expression of Pdx1 and HNF1β, the RA-induced cells were presumed to be pancreatic progenitor cells.
Previously, we developed differentiation protocols for pancreatic exocrine cells from mouse embryonic stem cells and hES cells (Shirasawa et al. 2011a, 2011b). Our induction protocol aimed to produce mature exocrine cells in culture Step III. FGF7, GLP-1 and NA were combined to stimulate the differentiation of pancreatic-enzyme-expressing cells from the RA-induced pancreatic progenitor cells. Amylase mRNA expression begins in an early stage of mouse development at embryonic day 14.5 and reaches the highest expression level compared with other secreted enzymes (Rovira et al. 2008). Therefore, we selected amylase as the principal marker for exocrine differentiation. In our method, amylase-positive cells appeared near Pdx1-positive cells at day 8 of culture Step III and afterward increased until day 15. The amylase-positive cells also expressed CPA similar to adult pancreas. Compared with control cells cultured without any inducing factors, the combination of FGF7, GLP-1 and NA efficiently induced the differentiation of exocrine cells. Moreover, FGF7 was a critical factor for exocrine differentiation in this protocol. In mouse ES cells, we previously determined that FGF7 induced differentiation of pancreatic progenitor cells but the effect on exocrine cell differentiation was not statistically significant (Shirasawa et al. 2011a). During rat pancreatic development in vitro, FGF7 promotes the proliferation of embryonic pancreatic epithelial cells and then further development into acinar exocrine cells (Miralles et al. 1999). Indeed, pancreatic development is achieved by signals, including FGF7, from the mesenchyme. This factor activates epithelial cell proliferation but represses development of the pancreatic epithelium into endocrine cells (Elqhazi et al. 2002). Moreover, in human embryonic mesenchyme, FGF7 induces the proliferation of epithelial cells (Ye et al. 2005). Our results are consistent with the importance of FGF7 for pancreatic exocrine development. In the absence of FGF7, few cells expressed amylase protein.
The amylase-positive cells were consistent with the pancreatic exocrine cell phenotype because of the presence of Pdx1-positive pancreatic progenitor cells in Step II cultures. However, hES-derived non-pancreatic cells were also present in the cultures. Amylase expression occurs not only in the pancreas but also in the salivary glands. Therefore, we constructed specific primers for both pancreatic and salivary amylase to ascertain whether the amylase-positive cells induced by our protocol were of pancreatic lineage. Pancreatic amylase mRNA but not salivary amylase mRNA, was detected by RT-PCR. Furthermore, immunofluorescence of exocrine markers indicated that amylase-positive cells also contained CPA, a pancreatic exocrine enzyme. These findings reveal that pancreatic exocrine differentiation was achieved by the stepwise culture of hES and required induction factor FGF7 in the final step.
In conclusion, we have induced hES cells to form DE and pancreatic progenitor cells, in a sequential manner, prior to a final differentiation into pancreatic exocrine cells. An understanding of the mechanisms of pancreatic exocrine development will be useful in the study of congenital pancreatic diseases and genetic disorders. In future work, we will clarify the individual roles of growth factors and improve the differentiation efficiency. The availability of differentiated pancreatic exocrine cells will be helpful in digestion assays and cytotoxicity evaluations.
References
Bayha E, Jørgensen MC, Serup P, Grapin-Botton A (2009) Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One 10:e5845
Cai J, Yu C, Liu Y, Chen S, Guo Y, Yong J, Lu W, Ding M, Deng H (2010) Generation of homogenous PDX1(+) pancreatic progenitors from human ES cell-derived endoderm cells. J Mol Cell Biol 2:50–60
Chen S, Borowial M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D (2009) A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5:258–265
Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK (2012) Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology 55:1193–1203
Cleveland MH, Sawyer JM, Afelik S, Jensen J, Leach SD (2012) Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells. Semin Cell Dev Biol 23:711–719
Coffinier C, Thépot D, Babinet C, Yaniv M, Barra J (1999) Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development 126:4785–4794
D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitve endoderm. Nat Biotechnol 23:1534–1541
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Aqulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401
Elqhazi L, Cras-Méneur C, Czernichow P, Scharfmann R (2002) Role for FGFR2IIIb-mediated signaling in controlling pancreatic endocrine progenitor cell differentiation. Proc Natl Acad Sci U S A 99:3884–3889
Fu S, Fei Q, Jiang H, Chuai S, Shi S, Xiong W, Jiang L, Lu C, Atadja P, Li E, Shou J (2011) Involvement of histone acetylation of Sox17 and Foxa2 promoters during mouse definitive endoderm differentiation revealed by microRNA profiling. PLoS One 6:e27965
Hong SG, Dunbar CE, Winkler T (2012) Assessing the risks of genotoxycity in the therapeutic development of induced pluripotent stem cells. Mol Ther 21:272–281
Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefantry AG, Stanley EG (2005) Retinoic acid induced Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes 54:301–305
Miralles F, Czernichow P, Ozaki K, Itoh N, Scharfmann R (1999) Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc Natl Acad Sci U S A 96:6267–6272
Mfopou JK, Chen B, Mateizel I, Sermon K, Bouwens L (2010) Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology 138:2233–2245
Okita K, Nagata N, Yamanaka S (2011) Immunogenicity of induced pluripotent stem cells. Circ Res 109:720–721
Poll AV, Pierreux CE, Lokmane L, Haumaitre C, Achouri Y, Jacquemin P, Rousseau GG, Careqhini S, Lemaiqre FP (2006) A vHNF1/TCF2-HNF6 cascade regulates the transcription factor network that controls generation of pancreatic precursor cells. Diabetes 55:61–69
Rovira M, Delaspre F, Massumi M, Serra SA, Valverde MA, Lloreta J, Dufresne M, Payré B, Konieczy SF, Savatier P, Real FX, Skoudy A (2008) Murine embryonic stem cell-derived pancreatic acinar cells recapitulated features of early pancreatic differentiation. Gastroenterology 135:1301–1310
Shirasawa S, Yoshie S, Yokoyama T, Tomotsune D, Yue F, Sasaki K (2011a) A novel stepwise differentiation of functional pancreatic exocrine cells from embryonic stem cells. Stem Cells Dev 20:1071–1078
Shirasawa S, Yoshie S, Yue F, Ichikawa H, Yokoyama T, Nagai M, Tomotsune D, Hirayama M, Sasaki K (2011b) Pancreatic exocrine enzyme-producing cell differentiation via embryoid bodies from human embryonic stem cells. Biochem Biophys Res Commun 410:608–613
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 30:861–872
Thatava T, Nelson TJ, Edukulla R, Sakuma T, Ohmine S, Tonne JM, Yamada S, Kudva Y, Terzic A, Ikeda Y (2011) Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther 18:283–293
Ye F, Duvillié B, Scharfmann R (2005) Fibroblast growth factors 7 and 10 are expressed in the embryonic pancreatic mesenchyme and promote the proliferation of embryonic pancreatic epitherial cells. Diabetologia 48:277–281
Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H (2009) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438
Zhao T, Zhang ZN, Rong Z, Xu Y (2011) Immunogenicity of induced pluripotent stem cells. Nature 474:212–215
Acknowledgment
The authors thank Mr. Kayo Suzuki and Dr. Kiyokazu Kametani (Research Center for Instrumental Analysis, Shinshu University) for their outstanding technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takizawa-Shirasawa, S., Yoshie, S., Yue, F. et al. FGF7 and cell density are required for final differentiation of pancreatic amylase-positive cells from human ES cells. Cell Tissue Res 354, 751–759 (2013). https://doi.org/10.1007/s00441-013-1695-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1695-6