Abstract
A new concept for wound therapy is the initiation of the regeneration of epidermal and dermal layers with appendages for skin function recovery. Bone-marrow-derived mesenchymal and epidermal stem cells (BMSCs and SSCs) are hypothesized to be able to home toward or to be transplanted to wound sites for skin repair and regeneration, but this awaits confirmation by further experimental and clinical evidence. In this study, the influence of the transplantation of BMSCs and SSCs with porous gelatin-β-tricalcium phosphate sponge as scaffolds on wound re-epithelization, collagen synthesis, skin tensile strength recovery, and skin appendage regeneration has been investigated. The transplantation of BMSCs or SSCs significantly accelerates wound re-epithelization, stimulates dermal collagen synthesis, and exhibits the trend to enhance the tensile strength recovery of skin. Furthermore, regenerative features of BMSCs and SSCs have been identified in activating blood vessel and hair follicle formation, respectively. These results not only provide experimental evidence for the application of BMSCs and SSCs as promising therapeutics for clinical wound treatment, but also display their characteristics in activating distinct skin appendage regeneration, which might have novel applications in skin tissue engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of novel strategies stimulating healing with skin appendage regeneration is an urgent goal for wound therapy (Diegelmann and Evans 2004). The absence of stem cells with regenerative properties has been identified as significantly slowing down the wound healing process (Velnar et al. 2009). The transplantation of stem cells to supplement the reduced numbers of stem cells for activating wound repair and regeneration is a new strategy receiving increasing interest (Peng et al. 2011). Two main sources of adult stem cells have been identified as being involved in skin tissue repair: (1) epidermal stem cells (SSCs), which are activated and migrate to the wound site for replenishing lost cells to aid re-epithelialization and to repair the damaged epithelium (Kamstrup et al. 2008); (2) bone-marrow-derived mesenchymal stem cells (BMSCs), which are believed to be able to mobilize from the bone marrow into the pool of circulating cells and then to home to the injury site within which they can regulate the proliferation and migration of cutaneous cells to reconstitute epidermis (Lau et al. 2009). However, until recently, most responses of BMSCs or SSCs to wounds have needed to be confirmed by further experimental results and clinical outcomes (Fu and Li 2009; Morasso and Tomic-Canic 2005). In particular, the slow in vitro propagation and the complexity of identification have greatly limited the investigation and application of SSCs (Barrandon 2007). Various surface antigens have been reported as molecular markers for SSCs (Nowak and Fuchs 2009; Ohyama et al. 2006). Among them, p63 is the first gene product that distinguishes SSCs from their transient amplifying progeny cells. The expression of p63 maintains the proliferative potential and terminal differentiation of SSCs (Pellegrini et al. 2011). CD34 is another typical surface marker for SSCs (Hoang et al. 2009; Jiang et al. 2010). In addition, CD71-dim cells from skin have been found to possess similar colony-forming efficiency but higher long-term growth potential than the remainder of the population (Webb et al. 2004). In the present study, P63briCD71dim or CD34briCD71dim has been used to identify isolated SSCs (Huang et al. 2009; Kim et al. 2004).
In addition, most cell delivery depends on systematic administration (Tark et al. 2010) or topical injection (Wang et al. 2011). Tissue engineering is a novel transplantation strategy that translates scientific knowledge into tangible products for advancing the repair and regeneration of tissues with biomaterials as a carrier. A simple way to apply cellular therapeutics at the wound site is by co-culturing the stem cells with biodegradable three-dimensional (3D) scaffolds that mimic the extracellular matrix, thereby forming a biomimetic system, and transplanting this system topically (Lee et al. 2011). We have previously optimized a 3D scaffold of gelatin sponge incorporating β-tricalcium phosphate (β-TCP), termed here GTS, within which BMSCs have been observed to proliferate and attach well (Mansbridge 2009; Takahashi et al. 2005a, 2005b; Weinand et al. 2010).
In this study, by determining the wound closure rate, dermal collagen synthesis, recovery of skin tensil strength, and histological recovery, we have investigated the influence of transplanted BMSCs or SSCs within GTS as carriers for wound healing and compared the characteristics of these two kinds of stem cells in skin regeneration.
Materials and methods
In vitro isolation and identification of BMSCs and SSCs
All experimental procedures were in accordance with the Zhejiang University guidelines for the welfare of experimental animals (Animal Experimentation Ethics Approval no. Zju2010-1-02-015). The isolation and culture of BMSCs was performed according to the method previously described (Liu et al. 2008). Three-week-old Sprague-Dawley male rats (50–60 g) were supplied by Zhejiang University Experimental Animal Center, China. Briefly, rat femurs were cut away from the epiphysis, and bone marrow was flushed out by using a syringe filled with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, USA), L-glutamine, penicillin (50 U/ml), and streptomycin (50 U/ml). The cell suspension was placed into 25-cm2 flasks (IWAKI Glass, Japan) and cultured at 37°C under 5% CO2. The medium was changed on day 4 of culture and every 3 days thereafter. When the cells of the first passage became sub-confluent, the cells were detached from the flask by using treatment, for 5 min at 37°C, with phosphate-buffered saline (PBS) containing 0.25% (wt) trypsin and 0.02% (wt) ethylene-diamine tetraacetic acid. Fourth passage cells at sub-confluence were used for all experiments.
Three-day-old rats (18–20 g) supplied by Zhejiang Academy of Medical Sciences, China were used for SSCs extraction. Briefly, skin tissue biopsy was obtained from the back of adult rats via plastic surgical procedures. The skin sample was sterilized with 75% ethanol, rinsed in PBS, minced into 2-mm-wide strips, and then treated with 0.25% dispase II overnight. The epidermis was mechanically separated from the dermis and then incubated in trypsin-EDTA (0.05%) at 37°C for 10 min to dissociate cells. After enzyme activity was blocked with keratinocyte serum-free medium (Gibco, USA) containing 10% FBS, the cells were suspended in medium. The cell suspension was filtered through a stainless steel mesh to remove residual tissues. The cells were collected by centrifugation for 5 min at 1,200 rpm and then plated onto 0.01% (g/g) collagen-type-IV-coated dishes at a cell density of 1×106/ml for 10 min at room temperature. The unattached cells were removed, and the rapidly adherent epidermal cells were cultured in KSFM supplemented with 100 IU/ml penicillin at 37°C in a humidified 5% CO2 atmosphere for 3 days before the medium was replaced. The medium was changed every other day. After the cells reached 80% to 90% confluence, they were subcultured by detaching them with trypsin-EDTA solution (Reiisi et al. 2010; Nowak and Fuchs 2009)
SSC identification was determined by immunocytochemical and flow cytometry analysis. Briefly, SSCs cultured on coverslips were fixed with 4% paraformaldehyde for 10 min at room temperature, blocked, and permeabilized with 0.3% Triton X-100 for 30 min. Primary antibody against p63 (1:25) was applied to the cells, which were incubated for 2 h at room temperature in a humidifier chamber followed by incubation with secondary antibody for 30 min according to the manufacturer’s protocol (BD Biosciences, USA). After staining with the secondary antibody, coverslips were inverted (cell-side down) and mounted with a mounting medium (PBS:glycerol at a ratio of 1:9). The cultured cells were examined under a fluorescent microscope. Negative isotype controls were used when imaging micrographs to ascertain that no false-positive staining had occured. In the flow cytometry assay, the stem cells were rinsed with 0.01 mol/l PBS. Endogenous peroxidases were blocked by incubating the cells with PBS containing 5% FBS (30 mins) and in a ice bucket for 10 mins. Polyoxymethylene (1 ml of a 4% solution) was then added to the cells for fixation for 30 min at room temperature. The fluorochrome-conjugated antibodies, namely fluorescein-isothiocyanate conjugated to p63 (FITC-p63), Percp-CD34, and FITC-CD71 (Santa Cruze, USA), were separately added to each 100 μl SSCs and incubates in an ice bucket for 30 min. To wash off excess antibody following staining, 1.5 ml PBS was added to each tube. Then, cells were centrifuged in a microfuge for 5 min at 2,000 rpm. The cells were resuspended in 1% paraformaldehyde (500 μl). Samples in which PBS was substituted instead of primary monoclonal antibodies were used as negative controls. Flow cytometry analysis was performed on a flow cytometer (BD Biosciences) and CellQuest software with 20,000 events being recorded for each sample. The negative controls (no antibody was added) were used as baselines, and the expression percentages of the tested markers were calculated. Experiments were repeated at least twice under the same conditions and settings.
Biological characterization of GTS
The fabrication and characterization of GTS and the cytotoxicity and adhesion property of the GTS to BMSCs were as reported previously (Takahashi et al. 2005a, 2005b). In this study, the biocompatibility of the GTS with the SSCs was investigated by testing the cytotoxicity of the GTS liquor to SSCs and the attachment of the SSCs to the GTS. The cytotoxicity test was carried out as follows. (1) The scaffold material was sterilized with ultraviolet light for 30 min and then cut into sheets with a size of 10 mm × 10 mm × 2 mm. (2) The scaffold sheets were extracted with SSCs culture medium, and all the liquor was collected for use. (3) For the MTT (3-[4,5-dimethyl-thiazolyl-2]-2,5-diphenol tetrazolium bromide) assay, 200 μl liquor/well was added to SSCs seeded at a density of 1×104 cells/well in 96-well plates, those wells containing cells and culture medium serving as negative control. (4) Liquor or culture medium was changed every 2 days. (5) The liquor/culture medium was replaced with culture medium containing MTT (0.5 mg/ml) at days 2, 4, 6, 8, and 10. After 4 h, the supernatant was aspirated, and 200 ml dimethyl sulfoxide (DMSO; Sigma, USA) was added to each well. The plate was micro-oscillated for 30 s, and then the absorbance at 570 nm was measured on a microplate reader. (6) The viability of the cells cultured in the GTS leaching liquor was calculated as the percentage of the negative controls by using the following equation: [(OD value of the sample well − OD value of the blank well)/(OD value of the negative-control well − OD value of the blank well)]×100%.
The attachment test was carried out as followings. (1) GTS was cut into discs of 6 mm in diameter and 3 mm in thickness. 2) One GTS coated with human-placenta-derived type IV collagen (0.01%, g/g) was placed in each well of 96-well plates, after which 0.1 ml SSC suspension (2×106cells/ml) was seeded into each well and co-cultured with the scaffold for 2 h. (3) The sponges homogeneously seeded with SSCs were then thoroughly washed with PBS to exclude non-adherent cells. (4) As a negative control, sponges after the 2 h of co-culture with the seeded cells were immediately replaced with culture medium containing MTT (0.5 mg/ml). After 4 h, the supernatant was aspirated, and 200 ml DMSO was added to each well. The plate was micro-oscillated for 30 s and tested for absorbance at 570 nm on a microplate reader. (5) Other cell-seeded sponges were further cultured, with the culture medium being changed every 2 days. (6) The culture medium was replaced with culture medium containing MTT (0.5 mg/ml) on days 2, 4, 6, 8, and 10. After 4 h, the supernatant was aspirated, and 200 ml DMSO was added to each well. The plate was micro-oscillated for 30 s, and the scaffold was removed. The supernatant was tested for absorbance at 570 nm on a microplate reader. (7) The amount of cells attached to GTS were calculated as the percentage of the negative controls by using the following equation: [(OD value of the sample well − OD value of the blank well)/(OD value of the negative control well − OD value of the blank well)]×100%.
Animal model and surgical procedures
Eight-week-old Sprague-Dawley rats (approximately 200 g) were supplied by Zhejiang University Experimental Animal Center, China. All of the experimental procedures on animals were in accordance with the Zhejiang University guidelines for the welfare of experimental animals (animal experimentation ethics approval no. Zju2010-1-02-015). The animals were anesthetized with 10% chloral hydrate. The hair on the back of each animal was clipped, and the skin was washed with providone-iodine solution. A sterile template measuring 10×10 mm was then placed on the skin, and the outline was traced by using a sterile fine felt-tipped pen. A skin excision of 10 mm × 10 mm was made by excising the skin within the confines of the square down to the level of subcutaneous panniculus carnosus (Dorsett-Martin 2004). The animals were then subjected to four different kinds of treatments: (1) receiving neither GTS nor cells (BC group); (2) receiving transplantation of GTS alone with 0.3 ml medium (GTS group); (3) receiving transplantation of GTS incorporating 0.3 ml SSC suspension at a density of 4×106/ml (SSG group); (4) receiving transplantation of GTS with 0.3 ml BMSC suspension at a density of 4×106/ml (BMG group). GTS with or without a cell suspension were pasted over the wound bed, and the wounds were further covered with sterile adhesive tegaderm dressings punctured with sterile needles to allow air exposure. After surgery, the animals were isolated, each to a cage. Fresh DMEM (0.3 ml with 10% FBS) or defined KSFM was added to the transplanted scaffolds with BMSCs or SSCs, and tegaderm dressings were replaced every 3 days. All animals were maintained under constant conditions (25±1°C) and had free access to standard diet and drinking water. Checking for post-surgery pain, distress, or complications was performed 24 h after surgery and daily thereafter. On postoperative day 10, rats in each group were killed, and the reconstituted skins were harvested for assays.
Tracking of transplanted stem cells
To track the integration of the grafted stem cells at the wound sites, cells were labeled with a fluorescent cell tracker, namely the red fluorescent dye, carbocyanine 1,1-dioctadecyl-1 conjugated to 3,30,30-tetramethylidocarboyanine perchlorate (CM-DiI, 1:100; Molecular Probes) before transplantation. Briefly, BMSCs or SSCs were harvested and resuspended in 2 ml PBS solution (pH 7.4) containing 4 μl CM-DiI solution (1 mg/ml) at a concentration of 1×106/ml for labeling at 37°C for 30 min and at 4°C for 15 min sequentially. After being labeled with CM-DiI, cells were washed twice with PBS before transplantation (Liu et al. 2011). Animals transplanted with these CM-Dil-labeled stem cells were harvested on day 5 for cell-tracking analysis.
Wound closure test
The progressive healing in various groups on days 3, 5, 7, and 10 was recorded by macroscopic observation. The wound closure percentages at various time points post-wounding were measured every 1–2 days from day 3 by copying the wounds with filter papers and calculating the weight percentage of filter papers as follows: wound closure percentage (%) = (area on day 0 − open area on day n)/area on day 0)×100.
Skin tensile strength measurement
The reconstituted skin was cut and excised for tensile strength determination at day 10 postwounding. Every strip of the newly formed skin was removed from each tested animal, and the cross-sectional area of each strip was determined with calipers within 10 min of harvest. The skin strips were individually mounted on a tensometer (Labthink, China) with a cross head speed of 25 mm/min, and the wound tensile strength was determined (Lauto et al. 2005). The resulting tensile strength values for three strips of each wound were averaged to arrive at a mean determination for each animal.
Masson’s trichrome staining and histological analysis
The cut reconstituted skin including full thickness skin layers (epidermis, dermis, and hypodermis) were fixed in 4% paraformaldehyde and processed according to routine light-microscopic tissue processing methods. The processed tissues were embedded in paraffin. Tissue sections (8 μm thick) were analyzed by Masson’s trichrome staining (Wang et al. 2008) and photographed by the Leica image analyzing system (Leica, Germany).
CD31 immunohistochemistry identification
Immunohistochemical identification of endothelial cells was performed by using a monoclonal antibody against CD31 (MEC13.3, BD Pharmingen, Calif., USA). Antibody binding was visualized via a three-step staining procedure by using a biotinylated polyclonal anti-rat Ig-G secondary antibody and the staining agents streptavidin horseradish-peroxidase and diaminobenzidine (DAB; Boshide, China). Capillary density was also assessed by counting the follicles staining for the endothelial antigen CD31 in high-power fields and deriving an average value (Kunz et al. 2006).
Statistical analysis
All values were expressed as means ± SD. Two-tailed Student’s t-tests were performed for data comparison of paired samples. One-way analysis of variance analysis was used for multiple group comparisons. A probability (P) value of <0.05 was considered to indicate statistical significance.
Results
Isolation and identification of BMSCs and SSCs
BMSCs were isolated from the bone marrow of Sprague-Dawley rats and expanded in vitro with our previously reported methods (Takahashi and Tabata 2003). With the protocols, BMSCs were obtained with high expression of CD29 and CD90 and low expression of CD45 (data not shown). BMSCs were maintained at 37°C under 5% CO2, and passage 4 was used for all experiments. Isolated SSCs were cultured and maintained at 37°C under 5% CO2, and passage 2 was used for all experiments. General cell morphology is shown in Fig. 1a, b. SSCs were positive for p63 (Fig. 1c) with an expression level of 48% (Fig. 1g vs negative control Fig. 1d) and expressed CD34 at 30% (Fig. 1h vs negative control Fig. 1e) indicating that 78% of the used cells were epidermal stem cells. In addition to the low expression of CD71 at 2.7% (Fig. 1i vs negative control Fig. 1f), these results together suggested the maintenance of stem cell phenotypes and the undifferentiation states of the stem cells.
Morphologies of the skin stem cells isolated from adult rat skins and molecular identification with several cellular surface antigens. a Cells attached to collagen type IV for 10 min, after sequential dissociation by 0.25% protease overnight at 4°C and 0.25% trypsin at 37°C, were round in shape, with a large nuclear to cytoplasmic ratio, and showed differences in sizes. b Cells were cultured for 10 days and kept a similar morphology, with some cells possessing fragmented nuclei. c Immunohistochemical staining of p63. d Negative control of fluorescein-isothiocyanate-p63 (FITC-p63). e Negative control of Percp-CD34. f Negative control of FITC-CD71. g Expression of FITC-p63 by SSCs. h Expression of Percp-CD34 by SSCs. i Expression of FITC-CD71 by SSCs. Bars 100 μm
Biological characterization of GTS
Scanning electron microscopy of GTS (Fig. 2a) further supplemented our previous GTS characterization with a pore size of 179.1±27.8 μm, 96% porosity, and a compression modulus of 1.1±0.2 (Takahashi et al. 2005b). The attachment and spread of SSCs to GTS at 6 h after seeding is shown in Fig. 2b. As demonstrated in Fig. 2c, the leaching liquors of the scaffold were not cytotoxic to SSCs within 10 days. The SSC attachment to GTS remained elevated in a time-dependent manner within 8 days and slightly decreased from day 8 to day 10 (Fig. 2d). These results not only provided evidence for the biosafety of the application of GTS for SSC growth, but also indicated the feasibility of utilizing GTS as a carrier for the transplantation of SSCs.
Tracking of transplanted stem cells
To confirm the engraftment of BMSCs and SSCs at wound sites upon transplantation, cells labeled with the fluorescent dye (CM-Dil) were tracked. As shown by fluorescence microscopy (Fig. 3), and in contrast to the blank control (Fig. 3a, no CM-Dil labeling), CM-Dil-labeled BMSCs (Fig. 3b) or SSCs (Fig. 3c) were detected with a diffused dispersion pattern in the new formed skins on the day 5 after transplantation. These results confirmed the efficiency of using GTS to transfer the stem cells to wound sites and provided evidence for the contribution of the transplanted stem cells to skin repair and regeneration.
The presence of transplanted carbocyanine 1,1-dioctadecyl-1 conjugated to 3,30,30-tetramethylidocarboyanine perchlorate (CM-DiI)-labeled BMSCs/SSCs was verified by red fluorescence in the frozen sections of the healed skins (yellow arrows). a Transplantation of bone-marrow-derived mesenchymal stem cells (BMSCs)/epidermal stem cells (SSCs) without CM-Dil labeling. b Transplantation of CM-Dil-labeled BMSCs. c Transplantation of CM-Dil-labeled SSCs. Bars 100 μm
Measurement of wound closure rate and skin tensile strength
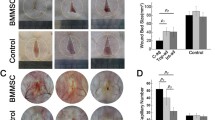
No postoperative adverse effects, e.g., infection, pyogenesis, or body fluid effusion, occurred in any of the animals throughout the experimental periods. Diet, drinking, and defecation were normal. Six rats were allocated to each group. These animals survived with stable bodyweights until the completion of the study. Progressive healing in the various groups on days 3, 5, 7, and 10 was recorded (Fig. 4). Wound closure was determined by the percentage of wound surface covered by healed skin (Fig. 5a). Compared with the blank control (BC group), the GTS alone expressed a promoting effect of 1.1– to 1.6-fold on wound closure (P<0.05). However, even compared with the GTS group, the transplantation of BMSCs/SSCs still exhibited dose-dependent accelerating effects on wound closure with an increase of 1.0– to 1.3-fold and 1.1– to 1.4-fold, respectively (P<0.05–0.01).
Skin tensile strength demonstrates the force per unit cross-sectional area needed to break the wound, thus reflecting the subdermal organization of the fibers in the newly deposited collagen. In the presence of an injury, a greater tensility during recovery translate into well-defined skin reformation. Compared with the BC group, the skin tensile strength of the GTS, BMG, and SSG groups expressed increasing trends on postoperative day 10 (Fig. 5b). Despite this, no difference was seen between the BMG/SSG/GTS groups and the negative blank control, and no significant difference existed among these groups with the normal positive control group for skin tensil strength.
Skin histological analysis with Masson’s trichrome staining
Skin histological analysis with Masson’s trichrome staining revealed a difference in the repair outcome. Masson’s trichrome staining of the healed skins on postoperative day 10 revealed re-epithelialization in all of the excisions (Fig. 6). Compared with the blank control (Fig. 6a, b), however, more extensive collagen deposition and thicker wavy collagen fibers were observed in all the GTS, BMG, and SSG groups (Fig. 6c–j). In addition to the increased collagen synthesis in all the GTS-incorporated groups, the collagen organization in the BMG and SSG groups was shown to be similar to that of normal skin, reflecting the different regulation of the transplanted stem cells on wound remodeling from that of GTS alone.
Masson’s trichrome staining of the healed skins at the postoperative day 10. a, b BC group (control). c, d GTS group. e, f BMG group. g, h SSG group. Note the scar tissue (areas left of yellow dashed lines), blood vessels (red arrows), and hair follicles (orange arrows). i, j Normal skin. Bars 100 μm
Using normal skin as a reference, we also observed that the healed skin in the BC and GTS groups displayed wide areas of dense dermis devoid of skin appendage with scar characteristics. In contrast, in the BMG and SSG groups, although some scar features remained (Fig. 6; areas below yellow dash lines), putative blood vessels (Fig. 6, red arrows) and hair follicles (Fig. 6, orange arrows) had appeared (Fig. 6, areas above yellow dashed lines), respectively. These results definitely showed the stimulating effects of BMG and SSG in regenerating blood vessel formation and hair follicle synthesis, respectively. Thus, the healing qualities of the BMG and SSG groups were superior to those of the BC and GTS groups.
CD31 immunohistochemistry identification
CD31 immunohistochemical identification is a reliable assay for observing angiogenesis at the molecular level. Figure 7 reveals the numbers and sizes of newly formed blood vessels in the tested groups. Compared with the blank control (BC group, Fig. 7a)/scaffold control (GTS group, Fig. 7b), in which almost no positive staining was found, many CD31-positive blood vessels with a similar size to that of normal skin were observed in the BMG group (Fig. 7c). Similar to the blank control and scaffold control, few vessels were identified in the SSG group (Fig. 7d).
CD31-positive cells are stained brown. Blood vessels composed of CD31-positive endothelial cells are indicated by black arrows. a BC group. b GTS group. c BMG group. d SSG group. e Normal skin. Bars 100 μm. f Quantification of blood vessels within the immunohistochemically stained sections (*P<0.05, **P<0.01, vs BC group)
The blood vessel numbers within the immunohisto-chemically identified sections, including that of the normal skin group (Fig. 7e) were also compared (Fig. 7f). In contrast to the blank control, the difference was statistically higher (P<0.05) in the BMG and normal skin groups, but not in the SSG group. These results provide evidence for the enhancement of BMSCs during blood vessel regeneration. A slight increasing effect of SSCs on wound angiogenesis might also exist.
Discussion
Adult skin consists of a keratinized stratified epidermis and an underlying thick layer of collagen-rich dermal connective tissue providing support and nourishment. Hairs and glands, the appendages of the skin, are derived from the epidermis but project deep into the dermal layer. In a full-thickness wound, skin tissue wounded deeply into the level of hair bulbs in the dermis exhibits a complete loss of hair follicles, and the repairing epithelium does not regenerate hair follicles and other epidermal appendage structures without external treatment (Martin 1997). Recent advances in stem cell biology have significantly improved our understanding of wound regeneration mechanisms. Adult stem cells are considered to be able to provide daughter cells to repopulate the lost or injured tissues and appendages. However, because of the slow growth and propagation of stem cells in culture, the experimental and clinical evidence for the potential of stem cells in wound treatment remains limited.
Currently, BMSCs are the adult stem cells receiving the most interest for regenerative medicine. However, most our understanding of the role of BMSCs in cutaneous homeostasis and wound healing has long been limited to the contribution of inflammatory cells. As the stem cells within skin, SSCs have been hypothesized as seed cells with a great potential for wound transplantation treatment. Nevertheless, until recently, the experimental and clinical evidence demonstrating the efficacy of SSCs for wound treatment has been scarce. Additionally, the influence of the BMSCs and SSCs with regard to skin appendage regeneration await clarification. The goal of this study has been to evaluate the efficiency of the transplantation of BMSCs/SSCs within a biodegradable gelatin scaffold as a carrier to stimulate wound healing and to compare the regenerative properties of these two kinds of adult stem cells. In particular, stem cells derived from skin or bone marrow have been isolated and propagated in culture environment. The cellular phenotypes of the isolated SSCs have been confirmed by their high expression of p63 and CD34 and low expression of CD71. GTS has been shown to be a the carrier with good biocompatibility and is efficient for the in vitro propagation and attachment of stem cells. With GTS as a topical carrier, the transplanted BMSCs and SSCs have been shown to be well engrafted into the newly formed host skin. We have evaluated several outcomes that are important in assessing successful wound healing and tissue response to transplants: (1) the wound healing closure rate (re-epithelization), (2) dermal collagen synthesis and skin tensile strength recovery, and (3) histological recovery with regard to skin appendage regeneration. The in vivo therapeutic potential of the transplanted BMSCs/SSCs with regard to skin healing and appendage regeneration has been investigated and compared.
First, we have shown that the transplantation of either BMSCs or SSCs into rat skin excision models can regenerate epidermis upon injury; this might be attributable to their differentiation into keratinocytes. Within 7 days post-wounding, the BMSCs exhibit a similar efficacy to that of SSCs in accelerating wound closure. However, the SSCs express a trend for stronger efficacy in accelerating wound closure than BMSCs after this period of post-wounding, despite that the closure rates in BMG and SSG showing no significant difference. This phenomenon is considered to be relevant to the complex fate of the transplanted stem cells at the wound site. These results provide direct evidence for the capacity of SSCs in reconstructing the epidermal barrier. Currently, controversy remains about the transdifferentiation potential of BMSCs into epithelial linages (Herzog et al. 2003). One of the important reasons for in the phenomena that we have observed might be the influence of the changed wound microenvironment, as the complex network of soluble and insoluble signals from the many cell types might direct BMSCs into differentiating into diverse cell phenotypes (Temple 2003). The significant acceleration on wound re-epithelization expressed by BMSCs in this study might be attributable to the direct contact of the transplanted BMSCs with the keratinocytes that are retained at the wound sites and that construct an environment advantageous for the directed differentiation of BMSCs into kerationcytes. This result with the BMSCs agrees with a recent report by Ma et al. (2011), who have reported that BMSCs contribute to accelerated wound closure and promote epithelial edge ingrowth.
The wound remodeling phase is known to be marked by the maturation of elements and associations of the extracellular matrix, leading to proteoglycan and collagen deposits in an attempt to regain normal tissue structure. The increase of collagen synthesis observed in the GTS, BMG, and SSG groups demonstrates the stimulation of both the GTS scaffold and/or BMSCs/SSCs on wound remodeling. In a recent study, the conditioned medium obtained from BMSCs has been reported to enhance significantly the in vitro cell survival ability of fibroblast cells and to promote the production or secretion of collagen, elastin, and fibronectin through the mechanism involving the suppression of matrix metalloprotease-1 expression and the inhibition of the accumulation of the oxidative stress of fibroblast cells (Young et al. 2010). These results might partly explain why the BMSCs can stimulate the in vivo collagen synthesis at the wound site observed in this study. Additionally, the enhancement of GTS and BMSCs/SSCs on wound remodeling is also indicated by the results of the skin tensile strength tests. Skin tensile strength depends on several factors, namely new collagen synthesis, matrix deposition, and cell migration (Wang et al. 2007). Accordingly, the regulation of the GTS and BMSCs/SSCs treatment on collagen synthesis and disposition might be one of the major mechanisms contributing to the increased skin tensile strength. However, notably, compared with the BMSCs/SSCs, the influence of GTS on wound remodeling might be attributable to the major component of the scaffold: gelatin is a hydrolyzed collagen that could improve the skin elasticity and biomechanics (Anthony and Mark 2007).
Together with the recovery of the epidermis and dermis, the regeneration of the skin appendages is a most interesting goal for wound treatment. In this study, we have shown that the reconstructed skin in the BMSC- or SSC-treated groups is closer to that of normal rat skin, in terms of its histological characteristics and skin appendage involvement. The pro-angiogenesis effects of BMSCs have been reported in abdominal wall hernia repair (Zhao et al. 2012), myocardial infarction (Kai and Helmut 2005), and ischemic injury (Wu et al. 2008), through the mechanism of differentation and/or growth factor secretion. Results of this study add evidence for the transdifferentiation potential of BMSCs into skin endothelia cell linages, which might have potential in diabetic ulcer treatment. In a recent study, the potential of BMSCs in combination with epidermal growth factor (EGF) as a stimulator in sweat gland regeneration in wounds (Huang et al. 2012) has been reported, reminding us of the wide regenerative potential of BMSCs in the regeneration of many manipulated tissues. Interesting results concerning transplanted SSCs lie in the induced regeneration of hair follicles. Despite some investigators concluding that epithelial stem cells are derived from hair follicular cells routinely (Pritinder 2006), the characteristics of epidermal stem cells, which include stem cells derived from both the epithelial basal layer and hair follicle bulge to skin cells linages deserve much more study. The results also remind us that the cotransplantation of a balanced cocktail of cells with differentiation properties, e.g., BMSCs and SSCs, might ultimately lead to a suitable wound treatment strategy. Currently, the favorable effects of cotransplantation of BMSCs with progenitor cells has been reported (Cristofanilli et al. 2011; Elizabeth et al. 2009; Wang et al. 2010), and most of the mechanisms have been explained on the basis of the functions of BMSCs as immunosuppressants (Yang et al. 2011), their expression of many molecules (Jin et al. 2011), and their stimulation of distinct paracrine cascades to enhance graft survival (Baron et al. 2010). However, in a pilot study aimed at investigating the potential benefit and side-effects of cotransplanted BMSCs related to their systemic immunosuppression in allogeneic hematopoietic stem cell transplantation, no evidence has been found for that BMSCs enhance hematopoietic recovery, and the cotransplantation of BMSCs results in disease relapse (Ning et al. 2008). Thus, the use of stem cells must be considered extremely carefully before a large-scale clinical trial is performed. The complex co-administration of two types of stem cells of different paracrine origins requires extreme caution, and the in vitro characterization of the two types of cells and the interference of differentiation and regenerative outcomes one with the other are preconditions for obtaining a confirmatory conclusion (Puymirat et al. 2009). In this study, problems of the combination of the two culture systems, namely DMEM with 10% FBS, L-glutamine, 50 U/ml penicillin, and 50 U/ml streptomycin for BMSCs, and the defined keratinocyte serum-free medium with 100 IU/ml penicillin for SSCs, which are two quite different culture systems, might significantly interfere with the growth, differentiation, and molecule expression of the stem cells. Additionally, one precondition for the good attachment of SSCs to the scaffold before transplantation is the pre-coating of the scaffold with collagen type IV, a condition that does not suit BMSCs. For cotransplantation, the problem of the pre-coating of the scaffold with a common protein that has good adhesion for both the BMSCs and SSCs has to be solved.
In summary, BMSCs and SSCs in rat skin excision models have been shown to have a similar capacity for regenerating an epidermis barrier upon injury. The promotion of BMSCs/SSCs with regard to dermal collagen synthesis and skin tensile strength recovery have also been demonstrated. Our histological analysis and molecular identification have revealed apparent angigoenesis and hair follicle regeneration in the BMSC- and SSC-treated groups, respectively, reflecting the distinct regenerative features of BMSCs and SSCs. In view of the wide differentiation potential of adult stem cells, together with the reduced ethical concerns relating to their use (Porada et al. 2006), we consider that SSCs/BMSCs will play a central role in future cell therapies for wounds. However, significant efforts should be directed toward translating BMSCs/SSCs research for use in human subjects. Species-specific differences between mice and humans in terms of wound healing and skin appendage biology warrant cautious interpretation of many animal-specific findings in the context of human wound healing.
References
Anthony DM, Mark WJ (2007) Tissue engineering of replacement skin, the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4:413–437
Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L et al (2010) Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant 16:838–847
Barrandon Y (2007) Genetic manipulation of skin stem cells, success, hope, and challenges ahead. Mol Ther 15:443–444
Cristofanilli M, Harris VK, Zigelbaum A, Goossens AM, Lu A, Rosenthal H et al (2011) Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev 20:2065–2076
Diegelmann RF, Evans MC (2004) Wound healing, an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
Dorsett-Martin WA (2004) Rat models of skin wound healing, a review. Wound Repair Regen 12:591–599
Elizabeth M, Winter MD, Angelique AM, Oorschot V, Hogers B, Graaf VD et al (2009) A new direction for cardiac regeneration therapy application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail 2:643–653
Fu X, Li H (2009) Mesenchymal stem cells and skin wound repair and regeneration, possibilities and questions. Cell Tissue Res 335:317–321
Herzog EL, Chai L, Krause DS (2003) Plasticity of marrow-derived stem cells. Blood 102:3483–3493
Hoang MP, Keady M, Mahalingam M (2009) Stem cell markers (cytokeratin 15, CD34 and nestin) in primary scarring and nonscarring alopecia. Br J Dermatol 160:609–615
Huang E, Lian X, Chen W, Yang T, Yang L (2009) Characterization of rat hair follicle stem cells selected by vario magnetic activated cell sorting system. Acta Histochem Cytochem 42:129–136
Huang S, Lu G, Wu Y, Jirigala E, Xu YG, Ma K et al (2012) Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 66:29–36
Jiang S, Zhao L, Purandare B, Hantash BM (2010) Differential expression of stem cell markers in human follicular bulge and interfollicular epidermal compartments. Histochem Cell Biol 133:455–465
Jin SO, Keung NK, Sung SA, Pennant WA, Kim HJ, Gwak SJ, Yoon do H, Lim MH, Choi BH, Ha Y (2011) Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant 20:837–849
Kai CW, Helmut D (2005) Mesenchymal stem cells for myocardial infarction. Circulation 112:151–153
Kamstrup M, Faurschou A, Gniadecki R, Wulf HC (2008) Epidermal stem cells—role in normal, wounded and pathological psoriatic and cancer skin. Curr Stem Cell Res 3:146–150
Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC (2004) Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci 61:2774–2781
Kunz P, Hoffend J, Altmann A, Dimitrakopoulou-Strauss A, Koczan D (2006) Angiopoietin-2 overexpression in Morris hepatoma results in increased tumor perfusion and induction of critical angiogenesis-promoting genes. J Nucl Med 47:1515–1524
Lau K, Paus R, Tiede S, Day P, Bayat A (2009) Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol 18:921–933
Lauto A, Hook J, Doran M, Camacho F, Poole-Warren LA (2005) Chitosan adhesive for laser tissue repair: in vitro characterization. Lasers Surg Med 36:193–201
Lee KB, Choi J, Cho SB, Chung JY, Moon ES, Kim NS, Han HJ (2011) Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res 29:1554–1562
Liu K, Liu R, Cao G, Sun H, Wang X, Wu S (2011) Adipose-derived stromal cell autologous transplantation ameliorates pulmonary arterial hypertension induced by shunt flow in rat models. Stem Cells Dev 20:1001–1010
Liu Y, Zhou H, Gao F (2008) Isolation and identification of stem cells from adult cashmere goat skin. Int J Dermatol 47:551–556
Ma K, Liao S, He LM, Lu J, Ramakrishna S, Chan CK (2011) Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Part A 17:1413–1424
Mansbridge JN (2009) Tissue-engineered skin substitutes in regenerative medicine. Curr Opin Biotechnol 20:563–567
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276:75–81
Morasso MI, Tomic-Canic M (2005) Epidermal stem cells, the cradle of epidermal determination, differentiation and wound healing. Biol Cell 97:173–183
Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J et al (2008) The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 22:593–599
Nowak JA, Fuchs E (2009) Isolation and culture of epithelial stem cells. Methods Mol Biol 482:215–232
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB et al (2006) Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 116:249–260
Pellegrini G, Dellambra E, Golisano O, Martinelli E (2011) p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA 98:3156–3161
Peng LH, Fung KP, Leung PC, Gao JQ (2011) Genetically manipulated adult stem cells for wound healing. Drug Discov Today 16:956–966
Porada CD, Zanjani ED, Almeida-Porad G (2006) Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther 1:365–369
Pritinder K (2006) Interfollicular epidermal stem cells, identification, challenges, potential. J Invest Dermatol 126:1450–1458
Puymirat E, Geha R, Tomescot A, Bellamy V, Larghero J, Trinquart L, Bruneval P, Desnos M, Hagège A, Pucéat M, Menasché P (2009) Can mesenchymal stem cells induce tolerance to cotransplanted human embryonic stem cells? Mol Ther 17:176–182
Reiisi S, Esmaeili F, Shirazi A (2010) Isolation, culture and identification of epidermal stem cells from newborn mouse skin. In Vitro Cell Dev Biol Anim 46:54–59
Takahashi Y, Tabata Y (2003) Homogeneous seeding of mesenchymal stem cells into nonwoven fabric for tissue engineering. Tissue Eng 9:931–938
Takahashi Y, Yamamotom M, Tabata Y (2005a) Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 26:4856–4865
Takahashi Y, Yamamoto M, Tabata Y (2005b) Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and b-tricalcium phosphate. Biomaterials 26:3587–3596
Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH (2010) Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 65:565–572
Temple S (2003) Stem cell plasticity—building the brain of our dreams. Nat Rev 2:513–520
Velnar T, Bailey T, Smrkolj V (2009) The wound healing process, an overview of the cellular and molecular mechanisms. J Int Med Res 37:1528–1542
Wang G, Ao Q, Gong K, Zuo HC, Gong Y, Zhang XF (2010) Synergistic effect of neural stem cells and olfactory ensheathing cells on repair of adult rat spinal cord injury. Cell Transplant 19:1325–1337
Wang W, Lin S, Xiao Y, Huang Y (2008) Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci 82:190–204
Wang Y, Nathanson L, McNiece IK (2011) Differential hematopoietic supportive potential and gene expression of stroma cell lines from midgestation mouse placenta and adult bone marrow. Cell Transplant 20:707–726
Wang Z, Gao Z, Shi Y, Sun Y (2007) Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthetic Surg 60:1193–1199
Webb A, Li A, Kaur P (2004) Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 72:387–395
Weinand C, Nabili A, Khumar M, Dunn JR, Ramella-Roman J, Jeng JC, Jordan MH, Tabata Y (2010) Factors of osteogenesis influencing various human stem cells on third-generation gelatin/β-tricalcium phosphate scaffold material. Rejuvenation Res 14:185–194
Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A et al (2008) Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant 16:993–1005
Yang HM, Cho MR, Sung JH, Yang SJ, Nam MH, Roh CR, Kim JM, Shin M, Song SH, Kwon CH, Joh JW, Kim SJ (2011) The effect of human fetal liver-derived mesenchymal stem cells on CD34+ hematopoietic stem cell repopulation in NOD/Shi-scid/IL-2Rã(null) mice. Transplant Proc 43:2004–2008
Young KJ, Jang YH, Yoo DR, Kim SN, Lee SK, Nam MJ (2010) Mesenchymal stem cells’ interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Rep Reg 18:655–661
Zhao Y, Zhang Z, Wang J, Yin P, Zhou J, Zhen M, Cui W, Xu G, Yang D, Liu Z (2012) Abdominal hernia repair with a decellularized dermal scaffold seeded with autologous bone-marrow-derived mesenchymal stem cells. Artif Organs 36:247–255
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the National Natural Science Foundation of China (Grants No: 81102393, 30873173), the 48th China Postdoctoral Science Foundation (Grant No: 420000-X91004), the Fundamental Research Funds for the Central Universities, China, the the Basic Research Funds for the Zhejiang University and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Rights and permissions
About this article
Cite this article
Peng, LH., Mao, ZY., Qi, XT. et al. Transplantation of bone-marrow-derived mesenchymal and epidermal stem cells contribute to wound healing with different regenerative features. Cell Tissue Res 352, 573–583 (2013). https://doi.org/10.1007/s00441-013-1609-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1609-7