Abstract
Considerable evidence indicates that the state of ocular connective tissues and their response in glaucomatous disease affect the degree of glaucoma damage. Both experimental and clinical data suggest that improved diagnostic and prognostic information can be derived from the assessment of the mechanical responsiveness of the sclera and lamina cribrosa to intraocular pressure (IOP). Controlled mutagenesis of the sclera has produced a mouse strain that is relatively resistant to increased IOP. Alteration of the baseline scleral state can be accomplished through either increased cross-linking of fibrillar components or their reduction. The sclera is a dynamic structure, altering its structure and behavior in response to IOP change. The biochemical pathways that control these responses are fertile areas for new glaucoma treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For over a century, we have recognized a link between the level of intraocular pressure (IOP) and glaucomatous optic nerve damage. In persons suffering abnormally high IOP, a change in optic nerve head (ONH) tissues described as excavation occurs, in parallel with progressive loss of visual field function. The change in the ONH is coincident with the death of retinal ganglion cells (RGC) and their axons in the retinal nerve fiber layer. No other retinal neurons die in glaucoma. When IOP is experimentally elevated in mammalian eyes, ONH and RGC changes occur similar to those in human disease. In every population-based survey for open angle glaucoma (OAG), 50%–80% of those with glaucoma damage have the same IOP levels seen in similarly aged normal persons. Thus, many of those with OAG have risk factors that make their ONH and RGC susceptible to damage at IOP levels that are tolerated without clinical disease by most individuals. Both the mean intraocular pressure (IOP) level (Bengtsson and Heijl 2005) and IOP fluctuation (Nouri-Mahdavi et al. 2004) are closely associated with incident human glaucoma and its progressive worsening. However, the risk factor for glaucoma damage is the level of IOP, not only elevated IOP. Furthermore, lowering of IOP slows the progressive loss of RGC in both animal and human glaucoma (Morrison et al. 1998; Heijl et al. 2002; Kass et al. 2002), irrespective of whether the initial IOP is above the normal level.
In experimental animal models, non-IOP-lowering neuroprotective treatments have been reported (Martin et al. 2003; McKinnon et al. 2002; Ji et al. 2004; Huang et al. 2005; Neufeld et al. 2002; Schwartz 2003; Nakazawa et al. 2006), largely aimed at slowing RGC body and axon death after injury has begun. Logically, neuroprotective treatments would be most effective at preventing RGC injury from entering an irretrievable phase by acting at the earliest stage of damage. Once RGC body or axon disruption begins, the restoration of normal RGC structure and function is less likely. The purpose of this report is to detail features of the ocular response to IOP (normal and elevated) likely to participate in glaucoma, to suggest potential candidate genes that might be diagnostically useful and to indicate a new therapeutic approach to altering these responses beneficially.
Ocular connective tissues mediate IOP effects
IOP-generated stress affects the ONH in at least two ways. First, a pressure differential occurs across the ONH (IOP minus retrobulbar optic nerve tissue pressure). Second, IOP-related stress in the eye wall (cornea and sclera) is transmitted to the ONH by circumferential (hoop) stress at its margins (Fig. 1). These stresses ultimately contribute to permanent deformation of ONH tissues, called excavation or cupping (Figs. 2 and 3), which is a key clinical feature of human glaucoma differentiating it from other optic neuropathies (Quigley et al. 1983). ONH deformation affects its RGC axons, astrocytes, blood vessels and (in non-human primates and human eyes) the ONH connective tissues. Associated with these alterations, anterograde and retrograde axonal transport at the ONH are interrupted in RGC. Coincident with this interruption, axon degeneration begins at the ONH and capillaries and glial defects are also observed at the same site. RGC death by apoptosis (Kerrigan et al. 1997; Quigley et al. 1995) is associated with axonal transport blockade in man (Quigley and Green 1979), monkey (Fig. 4; Gaasterland and Kupfer 1974) and rodent eyes at the ONH (Quigley et al. 1981) and intra-retinal events at the RGC body occur rapidly after initial axon injury.
IOP-related stress in the eye wall. a Scanning electron micrograph of human lamina cribrosa (optic nerve head) indicating the direction in which hoop stress acts on the structure (red arrows). b Representation of hoop stress (red arrows) in the sclera acting circumferentially, whereas the translaminar pressure difference between the intraocular pressure (IOP) and cerebrospinal fluid pressure (CSF) expressed at the retrolaminar optic nerve oppose each other to develop a second direction of stress on the optic nerve head (green arrows)
Scanning electron micrographs of the optic nerve head in human eyes. a Normal eye has lamina cribrosa inserting into the peripapillary sclera at a modest angle. b Glaucoma eye with excavation with expansion of scleral diameter and rotation of laminar insertion (orange arrows). Position of lamina is posterior and angled relative to normal appearance (blue lines). Bars 100 μm
Substantial evidence points to the central role of ocular connective tissues in mediating human glaucoma damage. The ONH zones in which physical deformation is greatest are those that suffer more RGC axon injury. The preferential loss of the RGC whose axons pass through the superior and inferior portions of the ONH explains the typical pattern of early to moderate visual field defects seen in glaucoma (Fig. 5; Quigley and Addicks 1981). Second, people with axial myopia are more susceptible to OAG (Boland and Quigley 2007) and their eyes have mechanical disadvantages in responding to the stress of IOP because of their larger globe diameter and thinner sclera. Third, hysteresis measured by an ocular response analyzer is a risk factor for OAG progression (Congdon et al. 2006). Fourth, in human OAG patients, scleral rigidity is estimated to be increased by two different indirect in vivo methods (Ebneter et al. 2009; Hommer et al. 2008). Fifth, in post-mortem inflation studies, the scleral stiffness of OAG eyes is greater than that of age-matched controls (Coudrillier et al. 2012). No doubt exists that vascular, glial, and immune factors contribute to RGC death in glaucoma. Axon injury and RGC loss in glaucoma are associated with disequilibrium in normal functions caused by direct fiber compression (Tan et al. 2006), failure of normal capillary nutrition (Grieshaber et al. 2007) and astrocyte activation (Tezel 2009). The contribution of IOP-generated stress to glaucoma is supported by abundant evidence and is potentially amenable to therapeutic intervention.
Lamina cribrosa in a scanning electron micrograp (a) showing that the larger dark pores between connective tissue beams are larger in the superior (blue dot) and inferior optic nerve head, corresponding to the portion of the nerve head through which axons pass that subserve the position of most typical visual field loss in glaucoma (here, the lower nasal field zone; b). Bar 300 μm
Studies of scleral anatomy and physiology are feasible and are highly relevant to events in the ONH. The connective and supporting tissues adjacent to RGC axons in the ONH make up the beams of the lamina cribrosa itself. However, the ONH is a complex and relatively small structure and so testing its internal mechanical behavior is only indirectly feasible (Yang et al. 2011). Biomechanical models (Burgoyne et al. 2005; Sigal et al. 2011) suggest that the IOP-generated force transmitted to the ONH at its periphery by scleral stress is a critical element in producing strain at the ONH (Sigal et al. 2005). Sigal et al. (2005) have reported that the behavior of the ONH is strongly dependent on the biomechanical properties of the peripapillary sclera. Acute deformation of the ONH and the consequent strain might be less dependent on translaminar pressure difference than on the indirect effects of IOP on the sclera, effects that are transferred to the ONH. The orientation of collagen and elastin within the ONH beams is entirely from periphery to center, not internal to external. Hence, either the major strain occurs from the scleral edge to the opposite scleral edge or the translaminar strain must be borne by fibers whose course is perpendicular to the direction of stress. Individual variations in the scleral state and its response are thus likely to be important glaucoma risk factors. Downs and co-workers have corroborated that the biomechanical response of the posterior sclera is an important determinant of strain at the ONH because of the tight coupling between the sclera and lamina (Girard et al. 2011a). Variations in scleral mechanical properties could be one explanation for the finding that half of those with open angle glaucoma (OAG) suffer injury in the normal IOP range (Quigley and Broman 2006). The candidates for risk factors and genes that explain glaucoma damage would therefore include both baseline scleral behavior and alterations in its behavior induced by IOP-related stress. Scleral responses to IOP might be both detrimental and beneficial to RGC survival. This review will present information on the sclera, namely anatomically, molecularly, physiologically and mechanically, by using data from experimental mice and monkeys and from human eyes.
Are animal models useful?
Mouse IOP elevation models generate data relevant to human glaucoma and provide research avenues not possible in monkey or human eyes. Mammalian eyes that are subjected to experimental IOP increase undergo neuronal, glial and associated tissue alterations (Fig. 6) that are phenotypically similar to human glaucoma (Morrison et al. 1990, 1997; Soto et al. 2011). Furthermore, lowering of IOP slows the progressive loss of RGC in both animal (Wong and Brown 2012) and human glaucoma. Whereas mouse eyes differ in details of ONH anatomy from primates, they share the site of glaucoma injury and the selective death of RGC. Jakobs and coworkers (Sun et al. 2009) have demonstrated that astrocytes in the mouse ONH simulate the structure of the collagenous lamina cribrosa in monkey and human eyes, potentially transferring scleral wall tension to ONH axons and capillaries. The mouse sclera has collagens, elastin and other molecules, as in the human sclera (Zhou et al. 2006), although its thickness and diameter are 10 times smaller than that of human eyes (Olsen et al. 1998). Biophysical analysis shows that the 3-mm-long mouse eye needs only one-eighth the scleral thickness of the 24-mm human eye to sustain similar biomechanical stress. An increase in axial length with chronic IOP increase is seen in the eyes not only of mouse but also of adult rats and monkeys and of human infants with chronic glaucoma. Thus, studies of experimental rodent glaucoma (Howell et al. 2012) probably provide relevant data on potential new diagnostic and therapeutic approaches in human eyes. Some experimental glaucoma model studies have artificially raised IOP in rodents by obstruction of aqueous outflow, whereas others have used genetically altered strains that develop IOP elevation (John et al. 1998; Mabuchi et al. 2004; Zhou et al. 2008; Mao et al. 2011; McDowell et al. 2012; Junglas et al. 2012; Senatorov et al. 2006). These mouse strains might be informative regarding not only the effect of IOP on RGCs but also the effect of modified connective tissues on susceptibility to damage. Studies of post-mortem human tissue document the actual disease but in human histological material, we cannot know the state of the sclera prior to glaucoma damage. Experimental monkey glaucoma has provided valuable information but non-human primates cannot be studied in large numbers and do not allow the study of the effects of the selective genetic alterations that are possible in mice. Experimental mouse glaucoma has begun to validate the role of scleral structure and its response to chronic IOP elevation in ways not possible with other approaches.
What scleral properties might be beneficial in glaucoma?
Macroscopic scleral properties
Although eyes of any size can suffer from glaucoma, people with larger eyes (typically with myopic refractive error) are more susceptible to OAG. A variety of reasons for this greater risk can be postulated but one of the more obvious is the fact, from simple physics, that a globe of larger diameter has greater stress in its wall than a smaller one, all other things being equal. Furthermore, things are known not to be equal, since myopic (axially longer) eyes have a thinner sclera. Again, if the tensile properties of two scleral walls are equal, a thinner sclera would be expected to have less ability to withstand the stress of IOP. Thus, a theoretical argument can be made that smaller eyes with a thicker sclera would be less likely to suffer injury. However, the composition of the sclera is complex at a microscopic level.
Microscopic fibrillar structure
The sclera consists in a variety of extracellular macromolecules, dominated by type 1 collagen. In addition, a variety of other collagens, elastin, proteoglycans and scleral fibroblasts occupy about 10% of the sclera by volume in quantitative histological examinations. The collagen is organized in layers or lamellae that have most fibers oriented in the same direction, with successive lamellae alternating between orientations of front to back, side to side, or oblique (Fig. 7). One analogy that decribes the general structure of this collagen is that of basket-weave. In the mouse, the orientation of fiber lamellae in the mid-sclera is more often anterior to posterior than equatorial or oblique, with about 30 successive lamellae. By contrast with the cornea or the ONH lamina cribrosa, which have uniform collagen fibril diameters, the fibril diameter distribution of the sclera is diverse and even varies between the outer, middle and inner scleras (nearest to the choroid; Fig. 8).
Transmission electron micrographs of CD1 mouse sclera tissue stained with tannic acid to intensify collagen fibrils. a Quantification of height and orientation of collagen bundles in the mid-sclera (color bars thickness of sequential lamellae). Bar 1 μm. b Collagen fibrils in a cross-section. Bar 100 nm
This general pattern of basket-woven lamellae is not the case in the peripapillary zone nearest the ONH. There, the fibrils are organized circumferentially around the ONH. Not only are the collagen fibrils oriented in this way but the peripapillary sclera has a complement of elastin, also running circumferentially (Fig. 9), whereas the remainder of the sclera has only minimal elastin (Quigley et al. 1983, 1991a, b; Hernandez et al. 1990; Yan et al. 2011). Detailed studies of the predominant regional orientation of collagen fibrils show this peripapillary ring (Pijanka et al. 2012). By contrast, within the ONH of the monkey or human eye, fibrils of both collagen and elastin course from one side to the other in straight lines (Fig. 10), probably in response to the stress generated in the peripapillary sclera to which the beams of the ONH connective tissue are attached. No fibrils are oriented from the inside to the outside of the eye in the perpapillary sclera or ONH. Thus, the resistance to translaminar stress in the ONH or to stress in this direction in the sclera must be borne by fibrils and lamellae that are perpendicular to this force.
Elastin distribution in a mouse peripapillary sclera. a Normal mouse optic nerve (ON) head peripapillary region, with a ring of elastin fibers. Luna stain. Bar 30 μm. b Elastin circumferentially arranged at the ON (arrows); note elastin bridging to pia mater (arrowhead). Bar 10 μm. c Confocal immunolabeling of elastin (white arrow) in a circumferential pattern at ONH. Bar 20 μm
Although fibrillar components of the sclera are prominently seen by light and electron microscopy, the other molecular species present are both diverse and potentially important elements of its structure and response to stress. Key glycosaminoglycans that are present include heparan sulfate, chondroitin sulfate, dermatan sulfate and keratan sulfate (Clark et al. 2011).
Glaucoma and sclera structural change
In adult OAG, the sclera does not dramatically alter its length or thickness. The same cannot be said of infants with glaucoma or experimental animals in which the length of the eye increases and the diameter of both the cornea and the ONH increase. The microscopic changes induced by experimental or human glaucoma in the peripapillary sclera and ONH are of interest in showing the response of the tissues to IOP-generated stress. The diameter distribution and orientation of fibrillar collagens in the ONH and peripapillary sclera are unchanged in human OAG eyes, although elastin is possibly degraded (Hernandez 1992) and definitely has an altered appearance (Fig. 11; Quigley et al. 1994) suggesting possible disruption of the intermolecular connections of elastin to the remainder of the connective tissue matrix.
Elastin bundles in the human lamina cribrosa. Luna stain, light microscopy. a Normal elastin in laminar beams is straight and aligned from one edge of the lamina to the other. b Glaucoma eye with elastin fibers that are wavy in appearance, perhaps indicating disinsertion from the connective tissue matrix as an effect of chronic damage. Bar 700 nm
In contrast to the sclera, the ONH undergoes dramatic stretching, deepening and widening in glaucoma. Indeed, the immediate peripapillary sclera is also altered, since the widening of the ONH involves lateral movement of the sclera. Recent studies of monkey eyes with chronic IOP elevation suggest that the process of early glaucoma damage includes the “recruitment” of connective tissue beams of the optic nerve posterior to the original lamina (Yang et al. 2011). These are beams that are connected to the sclera indirectly through the pia mater, as indicated by fibrillary connections from the peripapillary ring (Fig. 9b).
Age-related, ethnic and genetic differences in scleral composition might contribute to glaucoma susceptibility. As is well known, the axial length in older persons shortens and the sclera generally becomes thicker (Olsen et al. 1995). ONH and peripapillary scleral elastin differs between African-derived and European-derived individuals, perhaps representing a risk factor for higher OAG prevalence in people of African descent (Urban et al. 2007). Mutations in the lysyl oxidase-like protein 1 (LOXL1) gene are associated with exfoliation glaucoma (Thorleifsson et al. 2007), a subgroup in which differences from non-exfoliative eyes are found in posterior ocular connective tissues (Gottanka et al. 1997).
Biomechanical behavior of the sclera
Until recently, little information was available on the relevant mechanical behavior of the eye under normal conditions or with glaucoma. Early studies used uniaxial testing of an excised sclera between two metal grips (Downs et al. 2005; Woo et al. 1972; Spoerl et al. 2005) but this approach is unlikely to reproduce ideally the behavior of the intact sclera. More recently, methods have been developed to determine the relationship between stress generated by artificially elevated IOP and strain in intact posterior eye globes in vitro for eyes with either experimental glaucoma or induced myopia in mouse (Myers et al. 2010), tree shrew (Phillips et al. 2000), monkey (Downs et al. 2007; Girard et al. 2009, 2011b) and human (Coudrillier et al. 2012). The biomechanical response of connective tissue molecules in the sclera and ONH in glaucoma have been studied in monkeys with experimental glaucoma (Gottanka et al. 1997). Human glaucoma donor eyes with RGC loss were measurably stiffer than those of controls, as were experimental mouse and monkey glaucoma eyes. This suggests that the effect of IOP on the sclera leads to its stiffening. Whether human eyes would be more or less susceptible to glaucoma damage if they were more compliant at the baseline or stiffer at the baseline remains to be shown. One hypothesis is that a more compliant sclera increases susceptibility to RGC loss. Under this scenario, the finding of a stiffer sclera in post-mortem human glaucoma eyes after damage suggests that the stiffening is a protective response. We cannot determine, at this time, whether the stiffening in a human sclera has a beneficial or a detrimental effect. A potential alternative hypothesis is that eyes are more susceptible to glaucoma when they are stiffer and become even stiffer as a result of the disease. This scenario would be the case if stiffness of the sclera actually intensifies strain within the ONH. Either hypothesis is compatible with existing human data. Therefore, we urgently need to determine which of the two alternatives (stiffer sclera being overall beneficial or detrimental) is the case, since this would provide the direction in which therapeutic change in the sclera should go.
As with scleral anatomy, regional differences in mechanical properties are present in normal eyes and provide important clues as to the forces acting on the tissue. Models that attempt to describe scleral behavior require further development. Basic data are typically collected from the induced movement of particles sprayed onto the surface or of patterns shone onto the surface of the sclera. Each approach has value, although all of the in vitro models are carried out under less than natural conditions of temperature and humidity and under partial constraint of movement of some portion of the globe. Some models have assumed uniform scleral thickness or ignored thickness altogether, whereas others have assumed local isotropy (Boyce et al. 2007) or attempted to include the more likely situation of anisotropy (Lanir 1983). Indeed, recent human testing suggests significant anisotropy in the human sclera (B. Coudrillier, C. Boote, H.A. Quigley, T.D. Nguyen, in preparation).
Comparisons of the stress—strain relationship of regions of the sclera in mouse, monkey and human eyes agree that the greatest strain is in the peripapillary zone adjacent to the ONH (Coudrillier et al. 2012; Gottanka et al. 1997). Other regional differences, such as those between nasal/temporal or superior/inferior regions, might differ among individual humans or by species.
Scleral response to IOP-induced stress in experimental mouse glaucoma
We have produced an experimental glaucoma model in several strains of mice correlating the relative susceptibility to RGC loss with the findings in the sclera, including macroscopic and microscopic change, proteomic analysis and biomechanical behavior. In this model, consistent IOP is produced by obstruction of the aqueous humor outflow by injecting a mixture of 6-μm and 1-μm polystyrene beads and viscoelastic into the anterior chamber (Fig. 12; Cone et al. 2012) causing axial globe enlargement and selective RGC loss (Fig. 13). The method is based on an approach suggested by Sappington et al. (2010). We have identified differences among strains or genetic types of mice in susceptibility to RGC damage and have studied the way that the differences in scleral structure or response to glaucoma can be related to susceptibility. Our initial hypotheses include the rather simplistic idea that mice with larger eyes would be more susceptible to glaucoma, as are axially myopic humans.
Anterior segment of a young B6 (C57BL/6) mouse at 6 weeks after bead injection. Beads are seen as clear spheres because of the extraction of polystyrene during epoxy embedding (AC anterior chamber, R retina). Beads are found in the iris stroma, in the uveoscleral pathway between the ciliary body and the sclera (US) and in outflow channels equivalent to Schlemm’s canal (C). Toluidine blue (1%). Bar 70 μm
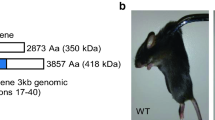
We identified that the albino CD1 strain was more susceptible to RGC loss than the B6 (C57BL/6) strain and that the DBA/2 strain is intermediate in sensitivity (Cone et al. 2010). One mouse strain with an induced mutation in collagen 8α2, a strain called Aca23 in which the eye is longer than in controls, has been found to be less susceptible to glaucoma damage than its wild type B6 base strain (Fig. 14; Steinhart et al. 2012). Extended IOP elevation in this model is accompanied by an increase in axial length and width (6% in CD1 and 10% in B6). Although the peripapillary sclera become thinner in both CD1 and B6 with glaucoma, most of the sclera is uniformly thinned in CD1 but actually thickens in B6. Testing of the inflation of enucleated mouse eyes in a mechanical apparatus showed that the peripapillary sclera in CD1 controls have significantly greater meridional strain than B6 mice and are different with respect to the ratios of meridional to circumferential strain from B6 mice, i.e., the CD1 mice exhibit anisotropy of the inflation response. In both CD1 and B6 mice, exposure to chronic IOP elevation results in stiffer pressure-strain responses. Whereas the Aca23 mutant mice have larger eyes than B6 controls, unlike the CD1 mice, their inflation responses are stiffer than those of B6. Furthermore, they lose few RGC at IOP exposures that damage significant numbers of RGC in their wild-type B6 littermates (Fig. 14). These findings suggest that large eye size alone is not the most important factor in glaucoma susceptibility in mice. Rather, a compliant baseline stress—strain response, anisotropy of mechanical response and scleral thinning with chronic glaucoma are features associated with greater damage. The uniformly increased scleral stiffness after glaucoma exposure in mice mimics findings in monkey (Gottanka et al. 1997; Downs et al. 2005) and human glaucoma eyes (Nguyen et al. 2013).
Microstructure of connective tissue
We have studied the microstructure of the sclera by combining measurements of the diffusion rates of fluorescently labeled dextran molecules of various diameters with obstruction scaling models (Danysh et al. 2010) in collaboration with Hopkins colleague, Justin Hanes. This method permits the evaluation of tissue microstructure that is not easily measured by classical fluid mechanics or elasticity measurement (Suh et al. 2005). Increased cross-linking (as envisioned here as a therapy) is known to be able to alter corneal and scleral permeability (Stewart et al. 2009). Since tiny 20-nm nanoparticles do not penetrate the mouse or rat sclera (Amrite et al. 2008), we have characterized the diffusion of fluorescently labeled dextrans of 20,000—70,000 molecular weight by using fluorescence recovery after photobleaching. Small regions are bleached during confocal microscopy and the re-entry of unbleached dextran from the periphery can be measured quantitatively to estimate diffusivity. Initial studies have shown that the peripapillary sclera has lower diffusivity than the mid-sclera and that CD1 mice have greater diffusivity than B6 mice (Fig. 15).
By uing the method of fluorescence recovery after photobleaching, the diffusivity of the sclera can be measured by the speed of return of fluoresceinated dextran molecules into a bleached zone by confocal microscopy. As shown here, the slowest return was in the peripapillary sclera of CD1 mice compared with the mid-sclera in CD1 mice, indicating a more compact connective tissue under normal circumstances near the optic nerve head (lowest curve). A significant difference was also found between mouse types, with a slower diffusivity in CD1 compared with B6 for the mid-sclera (upper two curves)
Proteomic changes
With co-investigator Gulgun Tezel, we have conducted the first proteomic studies that compare the sclera in normal and glaucoma mice (Tezel et al. 2005). Normal aging, diseases such as diabetes and chemical treatment can increase glycation cross-linking, altering the structure and mechanics of collagen-containing matrices (Tanaka et al. 1988), specifically increasing fibril diameter (Brummer et al. 2011; Malik et al. 1992) and increasing stiffness (Schultz et al. 2008; Hansen et al. 2009). Our initial studies show that, in both CD1 and B6 mice, the exposure of the sclera to chronic IOP elevation leads to significant increases in molecules that are important in the maintenance and remodeling of the sclera. These include thrombospondins 1 and 4, several myosin species, fibromodulin and heparin sulfate proteoglycan. An ingenuity analysis of proteomic data has suggested upregulation in the canonical pathways for integrin-linked kinase signaling and actin microskeleton signaling.
Scleral cell responses
The proteomic findings suggest that changes occur in the sclera quickly after IOP elevation, including features that indicate a substantial change in the activity of scleral fibroblasts. To investigate cellular changes, we have developed methods for the study of the whole sclera and its regions by confocal microscopy. The fibroblasts of the sclera make up about 15% by volume of its thickness in histological measurements. With exposure to elevated IOP, we find that no significant change takes place in the thickness or general orientation of scleral fibrillary elements or lamellae. Instead, it is the cell layers that expand. Cell division has been quantified by standard Ki67 labeling and found to increase in the glaucoma sclera and to be greater in the B6 strain that is less sensitive to glaucoma damage to RGC. Furthermore, labeling for α-smooth muscle actin is dramatically increased in the glaucoma sclera (Fig. 16). This anatomical evidence is consistent with the initial proteomic data, indicating a transition to the myofibroblast phenotype among scleral cells. The important role of the transition to myofibroblasts in the scleral response to experimental myopia in animals has been noted (McBrien et al. 2009; Summers Rada et al. 2006). Thus, therapeutic targets to alter the susceptibility to glaucoma damage might exist in pathways related to scleral remodeling.
Sclera whole-mounts at the peripapillary region were stained with the nuclear stain DAPI (blue) and labeled for alpha smooth muscle actin (green) and Ki67 (red) to identify cell division. a Untreated sclera. b Three-day-treated experimental glaucoma sclera. Ki67 labeling is greater in b, as is alpha smooth muscle actin labeling, indicating a transition to myofibroblasts. Bar 30 μm
Potential treatments for glaucoma that target the sclera
The evidence cited here points to the strong likelihood that the susceptibility to the effects of IOP in glaucoma can be altered by treatments that change the properties or responses of the sclera. We have previously suggested this approach and it is mentioned in recent reviews (Strouthidis and Girard 2013). The treatments could fall into two main areas: (1) change in the baseline state of the sclera so that its mechanical response to IOP is more favorable and (2) favorable changes in the cell-based biochemical processes of the sclera as it responds to IOP. We cannot as yet propose specific therapies, since the evidence does not fully support a direction for treatment. Rather, this review intends to support the need for research into these areas of potential therapy.
The mouse data suggest that eyes with mechanical responses that can be categorized as “stiffer” might be less susceptible to glaucoma damage. The characterization of such behavior as “stiff” is an oversimplification. Short-term and longer-term responses and regional responses in the zones of the sclera have been found and, for any given zone, anisotropy can be the case. Any specification of the features of a connective tissue is heavily dependent upon the conditions under which the tissue was tested. However, if we were to find, after further research, that eyes with a generally steeper stress—strain relationship are less likely to suffer injury at a given IOP, then treatments that alter the sclera in that direction would be beneficial. Increased cross-linking of the cornea in keratoconus through the use of riboflavin activated by ultraviolet light is being actively tested in humans (Wollensak et al. 2003) and can alter the stress—strain behavior, without significant damage to the retina or other ocular structures (Wollensak and Iomdina 2008). Use of this or similar approach for glaucoma was first suggested to Dr. Harry A. Quigley by Stephen Trokel, Columbia Univeristy. Application of an agent that would increase cross-linking of key molecules in the sclera could be accomplished with chemicals that do not require activation by ultraviolet light. These could be delivered by a subconjunctival injection in an outpatient setting. Theoretically, one treatment could accomplish the alteration of the stress—strain response of an eye to IOP, thereby permanently reducing glaucoma susceptibility.
The positive effects of increased scleral cross-linking of fibrillar elements might be mitigated by several factors. First, we are not at present certain that making the sclera “stiffer” would be clearly beneficial in human eyes. Further animal research is needed to show that proposed methods for altering the sclera would provide protection in mouse and other animal models. Reducing the strain in the peripapillary sclera, instead of protecting the eye, has been proposed to lead to a rigid perimeter for the ONH, potentially intensifying the translaminar pressure gradient and making the eye less safe. Second, the methods to alter the sclera must be shown to avoid off-target effects that counteract their benefit. For example, the cross-linking treatment could adversely impact the major ocular blood vessels that traverse the sclera or the extraocular muscles. Third, the precise degree of cross-linking needed might vary from person to person, requiring a method to estimate the extent of treatment or multiple small treatments. Methods that estimate the mechanical state of the sclera in the living eye would, in any case, be highly beneficial. These could use imaging technology with induced perturbations in IOP.
A beneficial effect on the sclera might be achieved not by stiffening but by producing a less stiff or a more elastic response. In this case, agents that affect collagens or non-collagenous elements in this direction should be sought. These could include enzymatic digestion with collagenase, elastase, chondroitinase, or hyaluronidase. Weinreb (2001) has suggested that scleral properties might be altered by the existing topical prostaglandin eye-drop treatment for glaucoma. Again, secondary detrimental effects and the degree of treatment would need to be assessed. Treatment with prostaglandin increases sclera permeability, improving uveoscleral outflow but with as yet unknown effects on mechanical behavior.
The second area for potential treatment is to alter the biochemical pathways that modulate the state of the sclera, most probably through acting on scleral fibroblasts. One example of such an approach is that taken with Marfan syndrome. In Marfan syndrome, the mutated site in fibrillin-1 produces its disruption of connective tissue by activating transforming growth factor β (TGFβ; Neptune et al. 2003; Ng et al. 2004), with consequent abnormalities in connective tissues, leading to aortic dissection, ocular lens dislocation and high myopia. Both gene expression and protein levels of TGFβ are elevated in OAG eyes in their human trabecular meshwork (Sethi et al. 2011) and ONH (Johnson et al. 2007; Pena et al. 1999; Zode et al. 2011; Kirwan et al. 2009). Our initial proteomic analyses in mouse glaucoma show greater than a two-fold increase in thrombospondin 1 and 4, which are activators of TGFβ. A TGFβ antagonist, losartan, reduces active TGFβ levels in a mouse model of Marfan syndrome, reversing the clinical abnormality of the aorta (Habashi et al. 2006, 2011). TGFβ is involved in scleral remodeling in experimental myopia in tree shrews (Jobling et al. 2004). Abnormal activation/inhibition of TGFβ in the sclera and ONH could increase susceptibility to IOP-induced stress and potentiate OAG damage. A treatment of this type might act as a beneficial modulator of the scleral response in glaucoma; however, further investigation needs to be undertaken to understand which subtypes of TGFβ are pertinent in glaucoma, specifically in the locations of interest: the anterior eye, the sclera and ONH. The pathways for change in the sclera require considerably more study before this can be advocated.
If we are to develop an approach to the modulation of the scleral response to IOP in glaucoma, a mode of delivery of the treatment would also need to be developed. For agents that are to alter the basic structure of the extracellular sclera, a direct application to the sclera would be the obvious choice. This could be by aqueous solutions applied subconjunctivally into the orbit. If a prolonged effect is needed, longer acting delivery methods by this route might include the drug being encapsulated in nanoparticles. For agents that could pass through the conjunctiva, local eye-drop application or sustained release from depot devices in the lacrimal canaliculus could be considered. However, we are unlikely to wish to expose the cornea to agents that could potentially be toxic or could reduce its clarity. Systemic delivery (such as through losartan pills) is feasible but might have general body side effects that are undesirable.
Concluding remarks
A new therapeutic approach to glaucoma is proposed that is based on the reduction in IOP-generated stress at the ONH by the alteration of the sclera. This treatment would aim to block initial injury to RGC axons at an earlier stage of RGC dysfunction than past proposed neuroprotective treatments. In determining which connective tissue molecules and their metabolism are most likely to be important in causing or preventing glaucoma injury, this research area will direct the search for candidate genes related to glaucoma damage and myopia.
References
Amrite AC, Edelhauser HF, Singh SR, Kompella UB (2008) Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis 14:150–160
Bengtsson B, Heijl A (2005) Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefe’s Arch Clin Exp Ophthalmol 243:513–518
Boland MV, Quigley HA (2007) Risk factors and open-angle glaucoma: concepts and applications. J Glaucoma 16:406–418
Boyce BL, Jones RE, Nguyen TD, Grazier JM (2007) Stress-controlled viscoelastic tensile response of bovine cornea. J Biomech 40:2367–2376
Brummer G, Littlechild S, McCall S, Zhang Y, Conrad GW (2011) The role of non-enzymatic glycation and carbonyls in collagen cross-linking for the treatment of keratoconus. Invest Ophthalmol Vis Sci 52:6363–6369
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT (2005) The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 24:39–73
Clark SJ, Keenan TDL, Fielder HL, Collinson LJ, Holey RJ, Merry CL, Kuppevelt TH van, Day AJ, Bishop PN (2011) Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Invest Ophthalmol Vis Sci 52:6511–6521
Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA (2010) Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res 91:415–424
Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA (2012) The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp Eye Res 99:27–35
Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA (2006) Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 141:868–875
Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD (2012) Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci 53:1714–1728
Danysh BP, Patel TP, Czymmek KJ, Edwards DA, Wang L, Pande J, Duncan MK (2010) Characterizing molecular diffusion in the lens capsule. Matrix Biol 29:228–236
Downs JC, Suh J-KF, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF (2005) Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci 46:540–546
Downs JC, Yang H, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF (2007) Three-dimensional histomorphometry of the normal and early glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. Invest Ophthalmol Vis Sci 48:3195–3208
Ebneter A, Wagels B, Zinkernagel MS (2009) Non-invasive biometric assessment of ocular rigidity in glaucoma patients and controls. Eye 23:606–611
Gaasterland D, Kupfer C (1974) Experimental glaucoma in the rhesus monkey. Invest Ophthalmol 13:455–457
Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC (2009) Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci 50:5226–5237
Girard MJA, Suh J-KF, Mottlang M, Burgoyne CF, Downs JC (2011a) Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthamol Vis Sci 52:5656–5669
Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC (2011b) Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci 52:5656–5669
Gottanka J, Flugel-Koch C, Martus P, Johnson DH, Lütjen-Drecoll E (1997) Correlation of pseudoexfoliative material and optic nerve damage in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci 38:2435–2446
Grieshaber MC, Mozaffarieh M, Flammer J (2007) What is the link between vascular dysregulation and glaucoma? Surv Ophthalmol 52 (Suppl 2):S144–S154
Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC (2006) Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312:117–121
Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedha D, Chen Y, Modiri AN, Judge DP, Dietz HC (2011) Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332:361–365
Hansen P, Hassenkam T, Svensson RB, Aagaard P, Trappe T, Haraldsson BT, Kjaer M, Magnusson P (2009) Glutaraldehyde cross-linking of tendon—mechanical effects at the level of the tendon fascicle and fibril. Connect Tissue Res 50:211–222
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, The Early Manifest Glaucoma Trial Group (2002) Reduction of intraocular pressure and glaucoma progression. Arch Ophthalmol 120:1268–1279
Hernandez MR (1992) Ultrastructural immunocytochemical analysis of elastin in the human lamina cribrosa. Changes in elastic fibers in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 33:2891–2903
Hernandez MR, Andrzejewska WM, Neufeld AH (1990) Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am J Ophthalmol 109:180–188
Hommer A, Fuchsjager-Mayr G, Resch H, Vass C, Garhofer G, Schmetterer L (2008) Estimation of ocular rigidity based on measurement of pulse amplitude using pneumotonometry and fundus pulse using laser interferometry in glaucoma. Invest Ophthalmol Vis Sci 49:4046–4050
Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW (2012) Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest 122:1246–1261
Huang W, Fileta JB, Dobberfuhl A, Filippopolous T, Guo Y, Kwon G, Grosskreutz CL (2005) Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci USA 102:12242–12247
Ji JZ, Elyaman W, Yip HK, Lee VW, Yick LW, Hugon J, So KF (2004) CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci 19:265–272
Jobling AL, Nguyen M, Gentle A, McBrien NA (2004) Isoform specific changes in scleral transforming growth factor beta expression and the regulation of collagen synthesis during myopia progression. J Biol Chem 30:18121–18126
John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR (1998) Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci 39:951–962
Johnson EC, Cepurna WO, Doser TA, Morrison JC (2007) Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci 48:3161–3177
Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bösl M, Bosserhoff A, Köstler J, Wagner R, Tamm ER, Fuchshofer R (2012) Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol 180:2386–2403
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK II, Wilson MR, Gordon MO (2002) The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120:701–713
Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME (1997) TUNEL-positive ganglion cells in human primary open angle glaucoma. Arch Ophthalmol 115:1031–1035
Kirwan RP, Wordinger RJ, Clark AF, O’Brien CJ (2009) Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol Vis 15:76–88
Lanir Y (1983) Constitutive equations for fibrous connective tissues. J Biomech 16:1–12
Mabuchi F, Lindsey JD, Aihara M, Mackey MR, Weinreb RN (2004) Optic nerve damage in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci 45:1841–1845
Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM (1992) Ageing of the human corneal stroma: structural and biochemical changes. Biochim Biophys Acta 1138:222–228
Mao M, Hedberg-Buenz A, Koehn D, John SWM, Anderson MG (2011) Anterior segment dysgenesis and early-onset glaucoma in nee mice with mutation of Sh3pxd2b. Invest Ophthalmol Vis Sci 52:2679–2688
Martin KRG, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW (2003) Gene therapy with brain-derived neurotrophic factor protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci 44:4357–4365
McBrien NA, Jobling AI, Gentle A (2009) Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci 86:E23–E30
McDowell CM, Luan T, Zhang Z, Putliwala T, Wordinger RJ, Millar JC, John SWM, Pang I-H, Clark AF (2012) Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Exp Eye Res 100:65–72
McKinnon SJ, Lehman DM, Tahzib NG, Ransom NL, Reitsamer HA, Liston P, LaCasse E, Li Q, Korneluk RG, Hauswirth WW (2002) Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther 5:780–787
Morrison JC, Dorman-Pease ME, Dunkelberger GR, Quigley HA (1990) Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol 108:1020–1024
Morrison JC, Moore CG, Deppmeier LMH, Gold BF, Meshul CK, Johnson EC (1997) A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res 64:85–96
Morrison JC, Nylander KB, Lauer AK, Cepurna WO, Johnson E (1998) Glaucoma drops control intraocular pressure and protect optic nerves in a rat model of glaucoma. Invest Ophthalmol Vis Sci 39:526–531
Myers KM, Cone FE, Quigley HA, Gelman SE, Pease ME, Nguyen TD (2010) The in vitro inflation response of mouse sclera. Exp Eye Res 91:866–875
Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI (2006) Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 26:12633–12641
Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC (2003) Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33:407–411
Neufeld AH, Das S, Vora S, Gachie E, Kawai S, Manning PT, Connor JR (2002) A prodrug of a selective inhibitor of inducible nitric oxide synthase is neuroprotective in the rat model of glaucoma. J Glaucoma 11:221–225
Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC (2004) TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114:1586–1592
Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Quigley HA (2013) Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest Ophthalmol Vis Sci (in press)
Nouri-Mahdavi K, Hoffman D, Coleman A, Liu G, Li G, Gaasterland D, Caprioli J (2004) Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 111:1627–1635
Olsen TW, Edelhauser HF, Lim JI, Geroski DH (1995) Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest Ophthalmol Vis Sci 36:1893–1903
Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF (1998) Human sclera: thickness and surface area. Am J Ophthalmol 125:237–241
Pena JDO, Taylor AW, Ricard CS, Vidal I, Hernandez MR (1999) Transforming growth factor isoforms in human optic nerve heads. Br J Ophthalmol 83:209–218
Phillips JR, Khalaj M, McBrien NA (2000) Induced myopia associated with increased scleral creep in the chick and tree shrew eyes. Invest Ophthalmol Vis Sci 41:2028–2034
Pijanka JK, Coudrillier B, Ziegler K, Sorensen T, Meek KM, Nguyen TD, Quigley HA, Boote C (2012) Quantitative mapping of collagen fiber orientation in non-glaucoma and glaucoma posterior human scleras. Invest Ophthalmol Vis Sci 53:5258–5270
Quigley HA, Addicks EM (1981) Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol 99:137–143
Quigley HA, Broman A (2006) The number of persons with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:151–156
Quigley HA, Green WR (1979) The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology 10:1803–1827
Quigley HA, Addicks EM, Green WR, Maumenee AE (1981) Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 99:635–649
Quigley HA, Hohman RM, Addicks EM, Massof RS, Green WR (1983) Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 95:673–691
Quigley HA, Dorman-Pease ME, Brown AE (1991a) Quantitative study of collagen and elastin of the optic nerve head and sclera in human and experimental monkey glaucoma. Curr Eye Res 10:877–888
Quigley HA, Brown A, Dorman-Pease ME (1991b) Alterations in elastin of the optic nerve head in human and experimental glaucoma. Br J Ophthalmol 75:552–557
Quigley HA, Pease ME, Thibault D (1994) Change in the appearance of elastin in the lamina cribrosa of glaucomatous optic nerve heads. Graefe’s Arch Clin Exp Ophthalmol 232:257–261
Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ (1995) Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci 36:774–786
Sappington RM, Carlson BJ, Crish SD, Calkins D (2010) The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci 51:207–216
Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM (2008) Structural factors that mediate scleral stiffness. Invest Ophthalmol Vis Sci 49:4232–4236
Schwartz M (2003) Neurodegeneration and neuroprotection in glaucoma: development of a therapeutic neuroprotective vaccine: the Friedenwald lecture. Invest Ophthalmol Vis Sci 44:1407–1411
Senatorov V, Malyuka I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, Tomarev S (2006) Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J Neurosci 26:11903–11914
Sethi A, Mao W, Wordinger RJ, Clark AF (2011) Transforming growth factor–β induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 52:5240–5250
Sigal IA, Flanagan JG, Ethier CR (2005) Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 46:4189–4199
Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC (2011) IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci 52:1896–1907
Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N (2011) Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest Ophthalmol Vis Sci 52:434–441
Spoerl E, Boehm AG, Pillunat LE (2005) The influence of various substances on the biomechanical behavior of lamina cribrosa and peripapillary sclera. Invest Ophthalmol Vis Sci 46:1286–1290
Steinhart MR, Cone FE, Nguyen C, Nguyen TD, Pease ME, Puk O, Graw J, Oglesby E, Quigley HA (2012) Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol Vis 18:1093–1106
Stewart JM, Schultz DS, Lee O-T, Trinidad ML (2009) Collagen cross-links reduce corneal permeability. Invest Ophthalmol Vis Sci 50:1606–1612
Strouthidis NG, Girard MJ (2013) Altering the way the optic nerve head responds to intraocular pressure—a potential approach to glaucoma therapy. Curr Opin Pharmacol 13:83–89
Suh J, Dawson M, Hanes J (2005) Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev 57:63–78
Summers Rada JA, Shelton S, Norton TT (2006) The sclera and myopia. Exp Eye Res 82:185–200
Sun D, Lye-Barthel M, Masland RH, Jakobs TC (2009) The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J Comp Neurol 516:1–19
Tan JCH, Kalapesi FB, Coroneo MT (2006) Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol 90:383–388
Tanaka S, Avigad G, Brodsky B, Eikenberry EF (1988) Glycation induces expansion of the molecular packing of collagen. J Mol Biol 203:495–505
Tezel G (2009) Fourth ARVO/Pfizer Ophthalmics Research Institute Conference Working Group. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci 50:1001–1012
Tezel G, Yang X, Cai J (2005) Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci 46:3177–3187
Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K (2007) Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 317:1397–1400
Urban Z, Agapova O, Hucthagowder V, Yang P, Starcher BC, Hernandez MR (2007) Population differences in elastin matauration in optic nerve head tissue and astrocytes. Invest Ophthalmol Vis Sci 48:3209–3215
Weinreb RN (2001) Enhancement of scleral macromolecular permeability with prostaglandins. Trans Am Ophthalmol Soc 99:319–343
Wollensak G, Iomdina E (2008) Crosslinking of scleral collagen in the rabbit using glyceraldehydes. J Cataract Refract Surg 34:651–656
Wollensak G, Spoerl E, Seiler T (2003) Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135:620–627
Wong AA, Brown RE (2012) A neurobehavioral analysis of the prevention of visual impairment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 53:5956–5966
Woo SL, Kobayashi AS, Schlegel WA, Lawrence C (1972) Nonlinear material properties of intact cornea and sclera. Exp Eye Res 14:29–39
Yan D, McPheeters S, Johnson G, Utzinger U, Vande Geest JP (2011) Microstructural differences in the human posterior sclera as a function of age and race. Invest Ophthalmol Vis Sci 52:821–829
Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF (2011a) Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci 52:7109–7121
Zhou J, Rappaport EF, Tobias JW, Young TL (2006) Differential gene expression in mouse sclera during ocular development. Invest Ophthalmol Vis Sci 47:1794–1802
Zhou Y, Grinchuk O, Tomarev SI (2008) Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci 49:1932–1939
Zode GS, Sethi A, Brun-Zinkernagel A-M, Chang I-F, Clark AF, Wordinger RJ (2011) Transforming growth factor-β2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis 17:1745–1758
Acknowledgments
The authors thank the members of their laboratory who contributed to the work presented here: Mary Ellen Pease, Ericka Oglesby, Matthew Steinhart and Cathy Nguyen. Faculty collaborators who provided important expertise included Thao (Vicky) Nguyen, Baptiste Coudrillier, Keith Meek, Craig Boote, Justin Hanes, Gulgun Tezel, Don Zack and Derek Welsbie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quigley, H.A., Cone, F.E. Development of diagnostic and treatment strategies for glaucoma through understanding and modification of scleral and lamina cribrosa connective tissue. Cell Tissue Res 353, 231–244 (2013). https://doi.org/10.1007/s00441-013-1603-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1603-0