Abstract
In plants, intercellular structures named plasmodesmata (PD) form a continuous cytoplasmic network between neighboring cells. PD pores provide channels for intercellular symplasmic (cell-to-cell) transport throughout most tissues of the plant body. Cell-defining proteins, such as transcription factors, and regulatory non-coding sequences, such as short interfering RNA, micro RNA, protein-encoding messenger RNAs, viroids, and viral RNA/DNA genomes move via PD channels to adjacent cells. PD-mediated intercellular transport of macromolecules is a regulated process depending on the tissue, developmental stage, and nature of the transported macromolecule. In this review, PD channels and their similarity to tunneling nanotubes present in animals are highlighted. In addition, homeodomain protein movement and cellular components regulating transport are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multicellular organisms have evolved various strategies for efficient communication between cells and tissues. This is evident from organisms in which there is a division of metabolism and function between tissues, as observed in higher plants and animals. Growth and differentiation are consequences of genetic programs and coordinated responses to internal and external stimuli by individual cells. Obviously, for the coordination of tissue-wide responses, intercellular communication is essential to integrate such stimuli in order to build and maintain a proper body plan. In general terms, this coordination takes place by the exchange of signal molecules. In plants, in addition to canonical receptor-mediated signaling (cell-cell signaling) across membrane boundaries, intercellular signaling occurs through membrane-lined plasmodesmatal microchannels (cell-to-cell signaling). This review focuses on the symplasmic pathway provided by plasmodesmata (PD) paralleling the symplasmic tunneling nanotube (TNT) pathway found in animals.
PD structure
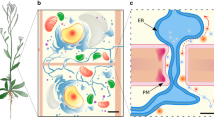
Eduard Tangl discovered the presence of connections between plant cells more than a century ago; by conventional microscopy, he detected channels between plant cells and postulated that they serve as communication and transport pathways (Tangl 1880). Based on modern electron microscopy imaging techniques, the PD microchannels have been shown to form de novo during cell division in the newly formed cell plate (primary PD; Hepler 1982). In addition, new PD appear later between non-clonal and clonal cells (secondary PD; Jones and Dropkin 1976). Secondary and primary PD appear morphologically similar in fully differentiated tissues: both classes of PD form higher order structures by building branches and forming a central expanded cavity at the junction of two neighboring cell walls, and both are outlined by the plasma membrane and contain an appressed membranous tube connected to the endoplasmic reticulum (ER; Fig. 1). Globular proteins seem to be present in the channel between the appressed ER and plasma membrane. These PD proteins have been suggested to form a helical structure with a width of approximately 5 nm forming a “nano” channel with a diameter of approximately 3 nm connecting the cytoplasm of the cells (Bayer et al. 2004; Waigmann et al. 1997; Overall and Blackman 1996; Ding et al. 1992; Burgess 1971). The appressed ER lumen appears to have a diameter of less than 2 nm (Robards and Lucas 1990). Between fully differentiated plant cells, several thousand PD channels are formed in clusters called pit fields (Faulkner et al. 2008). Thus, numerous PD build a symplasmic (continuous cytosol) and membranous network between neighboring cells forming a three-dimensional exchange system within and between tissues.
Representation of the complex membrane organization of a single plasmodesma (ER endoplasmic reticulum). Longitudinal (a) and cross section (b) of a plasmodesmal channel embedded in cell wall material formed between two neighboring cells. Note that the end-to-end distance of a plasmodesmata (PD) channel can be long (up to several micrometers) depending on the cell wall width. a, b Modified after Lucas et al. (2001)
Similarities and differences between TNTs and PD
The first evidence for tubular structures with a diameter of 50–200 nm connecting animal cells was found in cultured rat and human kidney cells by the group of Gerdes (Rustom et al. 2004) who named these channels TNTs and who have shown the filamentous-actin-dependent transfer of organelles via TNTs (Bukoreshtliev et al. 2009). TNTs are symplasmic tunnels formed by highly dynamic membranous protrusions connecting cells in the vicinity. They display a continuous membrane to both connected cells as seen in transmission electron micrographs. These “novel” channels seem to have similar functions and dimensions as PDs (see Fig. 1; Baluska et al. 2004; Ruiz-Medrano et al. 2004). Indeed, if PD had not been uncovered a century ago, they would most likely have been named nano-tunnels today.
Several shared features exist between PD and TNTs. The PD diameter is in the same range as that observed in TNTs and can vary from approximately 60 nm to several 100 nm in the region of the central cavity (see Fig. 1). The length of both PD and TNTs can be highly variable and can extend over the cell diameter of a connected cell. Small fluorescent tracer molecules and green fluorescent protein (GFP) move via PD and TNTs to adjacent cells. PD and TNTs can connect non-clonal cells as they can be formed de novo between various cell types. In plants, PD are observed between all juvenile cell types. In mature tissues, connectivity can be lost by PD modification, such as the connection between guard cells forming stomata and epidermal cells. In the plant vasculature, PDs are highly modified or degraded to build the special transport vessels of the phloem or xylem tube system, respectively. In cell cultures, TNTs are reported to connect fibroblasts, epithelial cells, immune cells (Davis and Sowinski 2008; Gerdes and Carvalho 2008), primary neurons, and astrocytes (Wang and Gerdes 2012). Confocal laser scanning microscopy imaging of living tissues has revealed that TNTs connect putative dendritic cells in the cornea of mice (Chinnery et al. 2008) and non-neural ectoderm cells in mouse embryos (Pyrgaki et al. 2010). Another similarity to the plant PD transport mechanism is found in the capacity of TNTs to facilitate cell-to-cell transport of viruses (Sowinski et al. 2008). Other transport features also seem to be shared between PD and TNTs. Uni-directional transport occurs from epidermal cells towards trichome cells via PD (Christensen et al. 2009; Fig. 2), as has also been observed between cultured normal rat kidney cells (Gurke et al. 2008).
Similarities of membranous connections and transport pathways between cells found in plants and animals. a Plants cells are symplasmically connected by multiple PD organized in pitfields. Experimental evidence supports a model whereby symplasmic transport occurs from one cell (Cell 1) to a connected neighboring cell (Cell 2) via PD microchannels. Although indicated in the drawing, delivery of homeodomain transcription factors bound to vesicles via a secretion mechanism independent of PD has not yet been observed. b, c Confocal laser scanning images of epidermal cells transiently expressing the viral movement protein (TMV-MP Tobacco mosaic virus movement protein) fused to red fluorescent protein (RFP, red; boxed area is shown at higher magnification right) or the homeodomain transcription factor (KN1) fused to green fluorescent protein (GFP, green). Both proteins move from cell to cell via PD and are found in association with PD structures (arrowheads) connecting epidermal cells. d Animal cells exchange macromolecules via the tunneling nanotube (TNT) pathway or via secretion/internalization. Evidence exists for homeodomain transcription factor movement via an unconventional secretion/internalization mechanism (Prochiantz and Joliot 2003). However, tubular connections build a symplasmic continuum between animal cells and provide a means of filamentous-actin-dependent transport of organelles. In a hypothetical line, vesicles (green) and TNTs might also facilitate transcription factor transport in animals. e Confocal laser scanning image of a TNT (arrowhead) formed between two animal cells (rat pheochromocytoma cells; PC12) stained with a fluorescent membrane marker. Right Wheat-germ agglutinin (WGA)-bound Alexa fluorescence (blue). f Representation of a cultured plant cell (protoplast) with a disrupted cell wall. Because of the presence of a viral movement protein (MP), the cells form membranous protrusions similar to TNTs found in the animal cells. The protrusions contain viral MP and fibers resembling a cytoskeleton. However, whether the structures solely contain viral MP or also cytoskeletal components is currently not known (Heinlein et al. 1998). g Example of protein movement via PD between two different cell types. The myb transcription factor CAPRICE fused to GFP (CPC-GFP, green) moves from a trichome cell into the underlying basement cells and suppresses trichome formation in neighboring cells. a, d Modified after Ruiz-Medrano et al. (2004)
In a hypothetical line, if a conserved tubule-forming mechanism exists in plants and animals, one would predict that a plant cell devoid of cell wall material might also build structures similar to that found in animal tissues. Indeed, plant protoplasts (cells lacking cell wall material) when producing a viral movement protein (MP) associated with PD form tubular structures protruding from the cell surface (Huang et al. 2000; Canto and Palukaitis 1999; Fig. 2). Although no evidence exists that such protoplastic tubules connect cells, they show striking parallels to TNTs. Both animal and plant cells might use a commonly conserved cellular mechanism to form connections in order to exchange macromolecules and organelles between cells.
Mobility and motifs of macromolecules transported via PD
One of the best-known functions of PD is that they facilitate the intercellular transport of small nutritional molecules such as amino acids and sugars; however, more recently, it has become obvious that macromolecules such as RNA and proteins also move from cell to cell via PD (Haywood et al. 2002; Oparka 2004; Tilsner et al. 2011; Lucas et al. 2009; Maule 2008; Barton et al. 2011; Genoves et al. 2011; Kaido et al. 2011; Lucas et al. 2001; Ruiz-Medrano et al. 2012). Proteins moving via PD are generally termed non-cell autonomous proteins (NCAPs). NCAPs are suggested to interact with PD components to increase the basic size exclusion limit of the PD pore as measured by metabolically inert fluorescent dextrans (Haywood et al. 2002). This increase in PD pore size is thought to facilitate the passage of endogenous RNA, proteins, and viral genomic information (Lucas and Gilbertson 1994; Kragler et al. 1998, 2000; Wolf et al. 1989, 1991). A number of mobile endogenous macromolecules have been suggested to be transported via PD and include transcription factors, mRNAs, and small RNAs such as micro RNA (miRNA) and small interfering RNA (siRNA) molecules, which function as signals that co-ordinate growth, regulate differentiation, and mediate systemic responses to environmental cues (Kim et al. 2001; Kim et al. 2003, 2005; Xu et al. 2011; Carlsbecker et al. 2010; Nakajima et al. 2001; Pant et al. 2008; Dunoyer et al. 2010a, 2010b). Interestingly, the intercellular transport of viral and endogenous macromolecules, such as viroid RNAs, viral MPs, and transcription factors, is selective and depends on unrelated motifs. For example, the intercellular transport capacity of mutant forms of viroid RNA lacking distinct specific hairpin structures changes in a tissue-specific manner (Qi et al. 2004). Moreover, structurally unrelated viral MPs and transcription factors, such as GRAS (named after GAI, RGA, and SCR) domain harboring SHORT ROOT or the homeodomain protein KNOTTED1 (KN1) move via PD, and their mobility varies depending on the nature of the tissue (Oparka et al. 1999; Kim et al. 2005b; Itaya et al. 1998; Zhong et al. 2008; Qi et al. 2004; Gallagher and Benfey 2009; Kim et al. 2002, 2003, 2005).
Even within a cell type, differences are observed; tobacco mosaic virus (TMV) uses subtypes of PD present within a cell and preferentially moves via branched PD (Oparka et al. 1999). In addition, unspecific transport of heterologous proteins via PD is regulated differently between cells of apical tissues. For example, the homeodomain transcription factor KN1 moves from the outer L1 layer of the apical meristem (stem cells) into the inner L2 and L3 cells but not vice versa (Kim et al. 2003, 2005). An example for developmental changes in PD connectivity is found during developmental transitions in embryos. Here, in early stages, PD allow the diffusion of GFP throughout the embryo, and in later stages, sub-domains corresponding to morphological regions of the plant are established with limited diffusion between cells (Kim and Zambryski 2005). In addition, PD permeability has recently been shown to be regulated depending on the redox status of the cells (Burch-Smith and Zambryski 2012; Barton et al. 2011).
Microinjection studies (Oparka 2004; Haywood et al. 2002; Ruiz-Medrano et al. 2004) have suggested that the transport of actively transported proteins via PD involves at least two consecutive processes. First, NCAPs are recognized by specific PD pathway receptors. Second, in the vicinity of the PD orifices, a predicted docking complex recognizes the presence of cargos and governs the passage of the cargo through PD. The predicted receptor(s) mediate structural modifications of the cargo and the diameter of PD pores (Haywood et al. 2002). In microinjection assays, a mobile protein such as the homeodomain protein KN1 seems to be unfolded, and the pore size is increased to allow passage (Kragler 2005; Kragler et al. 1998, 2000). In support of the results obtained by microinjection assays, a type II chaperonin complex containing the KN1 interacting protein CCT8 facilitates the proper refolding of KN1 after transport via PD in vivo (Xu et al. 2011). Most likely, the same chaperonine complex also facilitates viral transport in the recipient cells (Fichtenbauer et al. 2012).
Highly divergent motifs seem to mediate intercellular protein transport. Domain shuffling or mutant studies of mobile proteins, such as the homeodomain transcription factor KN1 (Kim et al. 2005; Lucas et al. 1995), the GRAS family protein SHR (Gallagher and Benfey 2009; Vaten et al. 2011), a subclass of phloem-specific HSC70 chaperones (Aoki et al. 2002), or membrane-associated PD–LOCATED PROTEIN 1 (Thomas et al. 2008), or of viral RNAs (Lough et al. 2006) have revealed highly variant transport motifs that are necessary and sufficient for entry into the intercellular PD transport pathway. For example, the homeodomain motif of KN1 and other closely related homeodomain transcription factors have been shown to mediate intercellular transport via PD. For SHR, domain shuffling experiments indicate that the presence of a GRAS domain and a functional nuclear localization signal seem to play a crucial role (Gallagher and Benfey 2009). For mobile HSC70 variants, deletion and shuffling experiments have shown that a small non-conserved C-terminal part (short variable region) of the chaperone is sufficient and necessary to mediate transport via PD. On the other hand, the unspecific PD-mediated allocation of foreign proteins such as free GFP and ER-localized GFP has been reported suggesting that open (gated) PD pores allow the diffusion of small proteins (Oparka and Roberts 2001; Oparka et al. 1999; Kim and Zambryski 2005; Kim et al. 2005a). Such diffusion of freely available macromolecules and specific motif-driven transport can be regulated by cell-wall-modifying enzymes found in close association with PD (Boyer and South 1989; Zavaliev et al. 2011; Rinne et al. 2011; Levy et al. 2007a, 2007b). Specific subsets of membrane-associated cell-wall-modifying enzymes such as 1,3-beta-glucanases or callose synthases regulate the accumulation of callose present at PD (Fig. 1). For example, stress-induced callose deposition reversibly plugs PD structures within minutes. Glucanase or callose synthase activity can cause an increase or decrease of pore size, enabling or restricting the intercellular transport of macromolecules, respectively (Levy et al. 2007a, 2007b; Rinne et al. 2001; Bucher et al. 2001; Benitez-Alfonso and Jackson 2009).
Studies of endogenous factors interacting with viral proteins, setting the stage for the transport of viral genomic information, have revealed a specific role of cytoskeleton-associated proteins and vesicular structures in intercellular transport. For example, viral produced MP has been shown to mediate the intercellular spread of the RNA genome of TMV. The TMV viral MP binds cooperatively to the viral RNA (Citovsky et al. 1990), associates to filamentous-actin, microtubule, ER, and PD pores. Association to filamentous-actin seems to serve as a pathway of the viral MP towards PD channels, whereas the localization to microtubules hinders the transport in the initial state of infection (Niehl and Heinlein 2011). Association to the ER establishes an assembly point to form a viral protein-RNA complex, which is guided most likely via the filamentous-actin to the PD pores (Heinlein 2002; Oparka 2004; Gillespie et al. 2002; Kawakami et al. 2004) .
However, despite a number of cellular components having been identified that are involved in intercellular transport via PD, we know surprisingly little about the motifs and receptors facilitating the transfer of proteins or RNA across the PD “nano”-channel.
Cell-to-cell transport of homeodomain transcription factors
The first plant endogenous protein to move via PD between cells was the homeodomain transcription factor KN1. Members of this protein family are essential for stem cell tissue maintenance in plants and have provided the initial evidence for an ever-increasing number of mobile transcription factors in plants (Lucas et al. 1995). Kim et al. (2003, 2002) have described the position-dependent intercellular movement of KN1 in transgenic Arabidopsis plants; KN1 fused to GFP moves from the inner layers of a leaf into epidermal cells, but not in the opposite direction. In support of a general mobility of a subclass of homeodomain proteins, the KN1 homologous Arabidopsis proteins STM and KNAT1 move out of the outermost L1 cell layer of the shoot apical meristem, and both trafficking proteins are able to complement a mutant stm allele. Consistent with the notion that KN1 intercellular transport is required for plant function, a cell-autonomous KN1 fusion protein expressed in the outermost L1 layer of the meristem cannot rescue an stm mutant. These results support the notion that the movement of homeodomain proteins is necessary to ensure the proper differentiation of plant stem cells.
The intercellular transport of homeodomain transcription factors also takes place between animal cells (Prochiantz and Joliot 2003). For example, the homeodomain transcription factor Engrailed acts as a non-cell autonomous signaling molecule that helps to specify crossvein formation on the wings of Drosophila melanogaster (Layalle et al. 2011). Another mobile Engrailed-like protein (En-2) present in Xenopus acts as a mobile signal involved in the proper formation of axons (Brunet et al. 2005). A further example of homeodomain protein transport in animals is found in the neuronal cells forming Antennapedia (Joliot et al. 1991). With respect to both Engrailed and Antennapedia, the mobility of cell-fate-deciding factors seems to depend on a so-called penetratin motif present in the homeodomain. These mobile transcription factors are thought not to utilize TNTs, as they seem to associate with vesicles that are secreted (Layalle et al. 2011; Maizel et al. 1999).
Interestingly, another similarity between animal and plant homeodomain transport has been found. The transport motif of animal homeodomain proteins is harbored in a similar structural context as plant homeodomain protein KN1. A mutation introduced in the proximity of the KN1 homeodomain motif (M6) or a deletion of the homeodomain disrupts its mobility in vivo (Lucas et al. 1995; Kim et al. 2005). Intriguingly, KN1 has also been shown to move between animal tissue-cultured cells (Prochiantz and Joliot 2003). So far, we do not know whether the transport mechanism used by Engrailed and Antennapedia or the TNTs are related to the PD-based transport system (Layalle et al. 2011). Nevertheless, this observation raises many questions regarding the mechanism recognizing and mediating homeodomain protein transport and the evolutionary relationship of the signal motifs.
More recent studies on two KN1-interacting proteins, namely MOVEMENT PROTEIN BINDING 2C (MPB2C) and CCT8 shed light on the mechanism of KN1 intercellular transport via PD. MPB2C is a plant-specific microtubule-associated factor initially identified as a viral MP interacting protein. MPB2C overexpression decreases the rate of viral infection spread, and MPB2C silencing increases the rate of viral infection spread. Interestingly, MPB2C also binds to the KN1 protein homeodomain region essential for mediating intercellular transport. As observed with the viral MP, the intercellular transport of KN1 and its relatives found in other plant species, such as the stem cell factors STM and KNAT1, can be negatively regulated by MPB2C (Winter et al. 2007). Because MPB2C is mainly expressed in the tissue neighboring the stem cell tissue present at the shoot apex, its function seems to restrict, with the support of other proteins, the presence of the intercellular transport activity of homeodomain proteins (F. Kragler, unpublished).
However, more experiments have to be performed to reveal the function of homeodomain protein transport via PD in plants. Moreover, we need to shown whether a specific PD-associated receptor protein can recognize a potential signal motif mediating the transport of a subclass of homeodomain proteins.
Intercellular transport of cell-fate-deciding proteins and miRNA
The best current examples for specific movement of cell-fate-deciding proteins are found in the pattern-forming WD40 protein called TRANSPARENT TESTA GLABRA 1 (TTG1), the myb-related genes TRYPTICHON (TRY) and CAPRICE (CPC; Fig. 2g; Wada et al. 1997, 2002), and the GRAS family gene SHORT ROOT (SHR; Nakajima et al. 2001; Sena et al. 2004; Helariutta et al. 2000). Both TTG1 and CPC/TRY decide epidermal hair patterns in leaves (Balkunde et al. 2010; Pesch and Huelskamp 2011), whereas SHR is essential for the formation of proper radial patterns in roots. Trichomes (leaf hair cells) form on Arabidopsis rosette leaves in a highly ordered fashion; CPC and TRY are expressed in trichome initial cells and, with other factors, determine the distance to the next trichome by suppressing trichome formation in neighboring cells. To illustrate further the complexity of the system, TTG1 protein is produced in all epidermal cells and acts earlier to define trichome cells. TTG1 moves via PD from surrounding epidermal cells into trichome initials (Bouyer et al. 2008). By this means, a gradient forms in cells around the trichome initials. Because the trichome-defining TTG1 factor is depleted around the trichome intials, no new trichomes can form in the neighboring cells. This ensures that no trichomes appear in close proximity of each other, and that a regular pattern is formed over the planar leaf surface.
Another example of a pattern-forming mechanism ocurring via intercellular signaling is found in the roots of plants. Correct radial patterning in Arabidopsis roots depends on the positional signal transmitted by SHR moving from the vascular cylinder to neighboring endodermal cells. In the endodermis, SHR by interacting with SCARECROW specifies endodermis identity (Sena et al. 2004; Nakajima et al. 2001). When expressed in other cell types, such as in phloem companion cells or in epidermal non-root hair cells, SHR does not move to neighboring cells. The radial pattern formed by SHR also underscores the complexity and specificity of this system, as it requires miRNA transport via PD. SHR protein moving into the endodermis activates SCARECROW expression. Together, these transcription factors specify the cortex-endodermal tissue and induce the expression of two miRNAs (miR165a and miR166b). These miRNAs are mobile and move most likely via PD into the vascular cylinder in which they inhibit the activity of specific homeodomain mRNAs and, by this means, promote the differentiation of a subset of vascular cells (Carlsbecker et al. 2010; Furuta et al. 2012; Vaten et al. 2011; Miyashima et al. 2011).
Are organelles transported via PD?
The function and mode of organelle transport between cells and the possible involvement of PD remain under discussion. Recent studies with grafting techniques have provided the first evidence for a transfer of whole organelles between plant cells. In grafting assays, plant tissues of two individual plants are fused to form a single chimeric plant body. This approach allows the screening of cellular components exchanged between genetically distinct cell types and has revealed that, after grafting, the chloroplasts of the tissue of one plant are transferred into the cells of the tissue of the other plant (Stegemann et al. 2012; Stegemann and Bock 2009). Despite the intercellular transfer of chloroplasts being limited to cells in close proximity to the graft junction, this finding establishes that whole organelles, including their genetic information, can be transferred between cells and can form new chimeric cell types.
Other cytoplasmic channels, which are in some aspects distinct from PD, have been observed between the meiotic cells present in anthers (plant male tissue containing cells undergoing meiosis). In transmission electron micrographs, wide open pores containing chromatin and organelles such as chloroplasts have been detected between meiotic cells (Mursalimov and Deineko 2011; Bellucci et al. 2003). Whether these structures devoid of ER components are related to or derived from PD is unknown. They have a large diameter of approximately 800 nm and are observed in extremely low frequencies between cells in somatic tissues. Some concerns exist as to whether these cytoplasmic channels are artifacts of fixation. However, in light of recent studies describing the exchange of chloroplasts between distinct cell types, we can reasonably assume that such structures are formed between cells and provide a pathway for the exchange of organelles and even chromosomes.
Concluding remarks
In a hypothetical cell line, one would predict that, if a pore-forming mechanism exists in plants, plant cells devoid of cell wall material might also build tubular structures similar to that in animal tissues. Indeed, plant protoplasts when producing viral MPs have the capacity to form tubular structures protruding from the cell surface (Fig. 2; Huang et al. 2000; Canto and Palukaitis 1999). Although no evidence exists that such protoplastic tubules connect cells, they show striking parallels to animal nanotubes and might represent a conserved mechanism establishing connections between cells and might fulfill demands to exchange signals in the form of specific macromolecules.
Despite striking parallels, more evidence is needed showing that the plant PD transport pathway is related to the TNTs used by animal cells. Obviously, the movement of transcription factors would provide a simple answer for a tissue to establish differentiation patterns. On the other hand, why are some homeodomain transcription factors mobile, whereas others that are closely related are not? How is their transfer regulated? These are intriguing questions that remain to be answered. A potential default intercellular transport pathway via diffusion for all cytosolic soluble proteins would impose a challenge for tissue differentiation and pattern formation. Thus, a delicate mechanism seems to have evolved in higher organisms with regard to the mobility and/or the presence of cell-fate-deciding factors.
Interestingly, despite several attempts to identify protein structures acting as PD targeting signals, a simple common sequence motif necessary and sufficient to guide heterologous proteins and RNA molecules through PD has not been determined. Based on knowledge gained by high-throughput screening combined with advanced in silico algorithms, the identification of PD targeting motifs and receptors might be feasible and will allow us to assign non-cell-autonomous functions to gene products.
Abbreviations
- CPC:
-
CAPRICE
- ER:
-
Endoplasmic reticulum
- GFP:
-
Green fluorescent protein
- HD:
-
Homeodomain protein
- KN1:
-
KNOTTED1
- miRNA:
-
Micro RNA
- MP:
-
Movement protein
- MPB2C:
-
MOVEMENT PROTEIN BINDING PROTEIN 2C
- NCAP:
-
Non-cell autonomous protein
- PD:
-
Plasmodesmata
- siRNA:
-
Small interfering RNA
- TMV:
-
Tobacco mosaic virus
- TNTs:
-
Tunneling nanotubes
- TTG1:
-
TRANSPARENT TESTA GLABRA 1
References
Aoki K, Kragler F, Xoconostle-Cazares B, Lucas WJ (2002) A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc Natl Acad Sci USA 99:16342–16347
Balkunde R, Pesch M, Hulskamp M (2010) Trichome patterning in Arabidopsis thaliana from genetic to molecular models. Curr Top Dev Biol 91:299–321
Baluska F, Hlavacka A, Volkmann D, Menzel D (2004) Getting connected: actin-based cell-to-cell channels in plants and animals. Trends Cell Biol 14:404–408
Barton DA, Cole L, Collings DA, Liu DY, Smith PM, Day DA, Overall RL (2011) Cell-to-cell transport via the lumen of the endoplasmic reticulum. Plant J 66:806–817
Bayer E, Thomas CL, Maule AJ (2004) Plasmodesmata in Arabidopsis thaliana suspension cells. Protoplasma 223:93–102
Bellucci M, Roscini C, Mariani A (2003) Cytomixis in pollen mother cells of Medicago sativa L. J Hered 94:512–516
Benitez-Alfonso Y, Jackson D (2009) Redox homeostasis regulates plasmodesmal communication in Arabidopsis meristems. Plant Signal Behav 4:655–659
Bouyer D, Geier F, Kragler F, Schnittger A, Pesch M, Wester K et al (2008) Two-dimensional patterning by a trapping/depletion mechanism: the role of TTG1 and GL3 in Arabidopsis trichome formation. PLoS Biol 6:e141
Boyer JN, South DB (1989) Seasonal changes in intensity of bud dormancy in loblolly pine seedlings. Tree Physiol 5):379–385
Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C (2005) The transcription factor Engrailed-2 guides retinal axons. Nature 438:94–98
Bucher GL, Tarina C, Heinlein M, Di Serio F, Meins F Jr, Iglesias VA (2001) Local expression of enzymatically active class I beta-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J 28:361–369
Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH (2009) Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett 583:1481–1488
Burch-Smith TM, Zambryski PC (2012) Plasmodesmata paradigm shift: regulation from without versus within. Annu Rev Plant Biol 63:239–260
Burgess JA (1971) Observations on structure and differentiation in plasmodesmata. Protoplasma 73:83–95
Canto T, Palukaitis P (1999) Are tubules generated by the 3a protein necessary for cucumber mosaic virus movement. Mol Plant Microbe Interact 12:985–993
Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J et al (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465:316–321
Chinnery HR, Pearlman E, McMenamin PG (2008) Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol 180:5779–5783
Christensen NM, Faulkner C, Oparka K (2009) Evidence for unidirectional flow through plasmodesmata. Plant Physiol 150:96–104
Citovsky V, Knorr D, Schuster G, Zambryski P (1990) The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell 60:637–647
Davis DM, Sowinski S (2008) Membrane nanotubes: dynamic long-distance connections between animal cells. Nature reviews. Mol Cell Biol 9:431–436
Ding B, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ (1992) Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell 4:915–928
Dunoyer P, Brosnan CA, Schott G, Wang Y, Jay F, Alioua A, Himber C, Voinnet O (2010a) An endogenous, systemic RNAi pathway in plants. EMBO J 29:1699–1712
Dunoyer P, Schott G, Himber C, Meyer D, Takeda A, Carington JC, Voinnet O (2010b) Small RNA duplexes function as mobile silencing signals between plant cells. Science 328:912–916
Faulkner C, Akman OE, Bell K, Jeffree C, Oparka K (2008) Peeking into pit fields: a multiple twinning model of secondary plasmodesmata formation in tobacco. Plant Cell 20:1504–1518
Fichtenbauer D, Xu XM, Jackson D, Kragler F (2012) The chaperonin CCT8 facilitates spread of tobamovirus infection. Plant Signal Behav 7:318–321
Furuta K, Lichtenberger R, Helariutta Y (2012) The role of mobile small RNA species during root growth and development. Curr Opin Cell Biol 24:211–216
Gallagher KL, Benfey PN (2009) Both the conserved GRAS domain and nuclear localization are required for SHORT-ROOT movement. Plant J 57:785–797
Genoves A, Pallas V, Navarro JA (2011) Contribution of topology determinants of a viral movement protein to its membrane association, intracellular traffic and viral cell-to-cell movement. J Virol 85:7797–7809
Gerdes HH, Carvalho RN (2008) Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol 20:470–475
Gillespie T, Boevink P, Haupt S, Roberts AG, Toth R, Valentine T, Chapman S, Oparka KJ (2002) Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of tobacco mosaic virus. Plant Cell 14:1207–1222
Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH (2008) Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res 314:3669–3683
Haywood V, Kragler F, Lucas WJ (2002) Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell 14 (Suppl):S303–S325
Heinlein M (2002) Plasmodesmata: dynamic regulation and role in macromolecular cell-to-cell signaling. Curr Opin Plant Biol 5:543–552
Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, Beachy RN (1998) Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10:1107–1120
Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101:555–567
Hepler PK (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111:121–133
Huang Z, Han Y, Howell SH (2000) Formation of surface tubules and fluorescent foci in Arabidopsis thaliana protoplasts expressing a fusion between the green fluorescent protein and the cauliflower mosaic virus movement protein. Virology 271:58–64
Itaya A, Woo YM, Masuta C, Bao Y, Nelson RS, Ding B (1998) Developmental regulation of intercellular protein trafficking through plasmodesmata in tobacco leaf epidermis. Plant Physiol 118:373–385
Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A (1991) Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA 88:1864–1868
Jones MG, Dropkin VH (1976) Scanning electron microscopy in nematode-induced giant transfer cells. Cytobios 15:149–161
Kaido M, Funatsu N, Tsuno Y, Mise K, Okuno T (2011) Viral cell-to-cell movement requires formation of cortical punctate structures containing red clover necrotic mosaic virus movement protein. Virology 413:205–215
Kawakami S, Watanabe Y, Beachy RN (2004) Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci USA 101:6291–6296
Kim I, Zambryski PC (2005) Cell-to-cell communication via plasmodesmata during Arabidopsis embryogenesis. Curr Opin Plant Biol 8:593–599
Kim I, Cho E, Crawford K, Hempel FD, Zambryski PC (2005a) Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc Natl Acad Sci USA 102:2227–2231
Kim I, Kobayashi K, Cho E, Zambryski PC (2005b) Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc Natl Acad Sci USA 102:11945–11950
Kim JY, Yuan Z, Cilia M, Khalfan-Jagani Z, Jackson D (2002) Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc Natl Acad Sci USA 99:4103–4108
Kim JY, Yuan Z, Jackson D (2003) Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 130:4351–4362
Kim JY, Rim Y, Wang J, Jackson D (2005) A novel cell-to-cell trafficking assay indicates that the KNOX homeodomain is necessary and sufficient for intercellular protein and mRNA trafficking. Genes Dev 19:788–793
Kim M, Canio W, Kessler S, Sinha N (2001) Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293:287–289
Kragler F (2005) Plasmodesmata: protein transport signals and receptors. In: Oparka K (ed) Plasmodesmata. Blackwell, Oxford, pp 53–72
Kragler F, Monzer J, Shash K, Xoconostle-Cazares B, Lucas WJ (1998) Cell-to-cell transport of proteins: requirement for unfolding and characterization of binding to a putative plasmodesmal receptor. Plant J 15:367–381
Kragler F, Monzer J, Xoconostle-Cazares B, Lucas WJ (2000) Peptide antagonists of the plasmodesmal macromolecular trafficking pathway. EMBO J 19:2856–2868
Layalle S, Volovitch M, Mugat B, Bonneaud N, Parmentier ML, Prochiantz A, Joliot A, Maschat F (2011) Engrailed homeoprotein acts as a signaling molecule in the developing fly. Development 138:2315–2323
Levy A, Erlanger M, Rosenthal M, Epel BL (2007a) A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J 49:669–682
Levy A, Guenoune-Gelbart D, Epel BL (2007b) Beta-1,3-glucanases: plasmodesmal gate keepers for intercellular communication. Plant Signal Behav 2:404–407
Lough TJ, Lee RH, Emerson SJ, Forster RL, Lucas WJ (2006) Functional analysis of the 5′ untranslated region of potexvirus RNA reveals a role in viral replication and cell-to-cell movement. Virology 351:455–465
Lucas WJ, Gilbertson RL (1994) Plasmodesmata in relation to viral movement within leaf tissue. Annu Rev Phytopathol 32:387–411
Lucas WJ, Bouché-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S (1995) Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270:1980–1983
Lucas WJ, Yoo BC, Kragler F (2001) RNA as a long-distance information macromolecule in plants. Nat Rev Mol Cell Biol 2:849–857
Lucas WJ, Ham BK, Kim JY (2009) Plasmodesmata—bridging the gap between neighboring plant cells. Trends Cell Biol 19:495–503
Maizel A, Bensaude O, Prochiantz A, Joliot A (1999) A short region of its homeodomain is necessary for engrailed nuclear export and secretion. Development 126:3183–3190
Maule AJ (2008) Plasmodesmata: structure, function and biogenesis. Curr Opin Plant Biol 11:680–686
Miyashima S, Koi S, Hashimoto T, Nakajima K (2011) Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 138:2303–2313
Mursalimov SR, Deineko EV (2011) An ultrastructural study of cytomixis in tobacco pollen mother cells. Protoplasma 248:717–724
Nakajima K, Sena G, Nawy T, Benfey PN (2001) Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413:307–311
Niehl A, Heinlein M (2011) Cellular pathways for viral transport through plasmodesmata. Protoplasma 248:75–99
Oparka KJ (2004) Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci 9:33–41
Oparka KJ, Roberts AG (2001) Plasmodesmata. A not so open-and-shut case. Plant Physiol 125:123–126
Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS et al (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97:743–754
Overall RL, Blackman LM (1996) A model of the macromolecular structure of plasmodesmata. Trends Plant Sci 1:307–311
Pant BD, Buhtz A, Kehr J, Scheible WR (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53:731–738
Pesch M, Huelskamp M (2011) Role of TRIPTYCHON in trichome patterning in Arabidopsis. BMC Plant Biol 11:130
Prochiantz A, Joliot A (2003) Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol 4:814–819
Pyrgaki C, Trainor P, Hadjantonakis AK, Niswander L (2010) Dynamic imaging of mammalian neural tube closure. Dev Biol 344:941–947
Qi Y, Pelissier T, Itaya A, Hunt E, Wassenegger M, Ding B (2004) Direct role of a viroid RNA motif in mediating directional RNA trafficking across a specific cellular boundary. Plant Cell 16:1741–1752
Rinne PL, Kaikuranta PM, van der Schoot C (2001) The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. Plant J 26:249–264
Rinne PL, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjarvi J, van der Schoot C (2011) Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23:130–146
Robards AW, Lucas WJ (1990) Plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol 41:369–420
Ruiz-Medrano R, Xoconostle-Cazares B, Kragler F (2004) The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr Opin Plant Biol 7:641–650
Ruiz-Medrano R, Kragler F, Wolf S (2012) Signaling and phloem-mobile transcripts. In: Huelskamp M, Kragler F (eds) Short and long distance signaling. Advances in Plant Biology, vol 3. Springer, Berlin, pp 151–177
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303:1007–1010
Sena G, Jung JW, Benfey PN (2004) A broad competence to respond to SHORT ROOT revealed by tissue-specific ectopic expression. Development 131:2817–2826
Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K et al (2008) Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature Cell Biol 10:211–219
Stegemann S, Bock R (2009) Exchange of genetic material between cells in plant tissue grafts. Science 324:649–651
Stegemann S, Keuthe M, Greiner S, Bock R (2012) Horizontal transfer of chloroplast genomes between plant species. Proc Natl Acad Sci USA 109:2434–2438
Tangl E (1880) Ueber offene Communicationen zwischen den Zellen des Endosperms einiger Samen. Jb Wiss Bot 12:170–190
Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ (2008) Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 6:e7
Tilsner J, Amari K, Torrance L (2011) Plasmodesmata viewed as specialised membrane adhesion sites. Protoplasma 248:39–60
Vaten A, Dettmer J, Wu S, Stierhof YD, Miyashima S, Yadav S et al (2011) Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell 21:1144–1155
Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277:1113–1116
Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K (2002) Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129:5409–5419
Waigmann E, Turner A, Peart J, Roberts K, Zambryski P (1997) Ultrastructural analysis of leaf trichome plasmodesmata reveals major differences from mesophyll plasmodesmata. Planta 203:75–84
Wang X, Gerdes HH (2012) Long-distance electrical coupling via tunneling nanotubes. Biochim Biophys Acta 1818:2082–2086
Winter N, Kollwig G, Zhang S, Kragler F (2007) MPB2C, a microtubule-associated protein, regulates non-cell-autonomy of the homeodomain protein KNOTTED1. Plant Cell 19:3001–3018
Wolf S, Deom CM, Beachy RN, Lucas WJ (1989) Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 246:377–379
Wolf S, Deom CM, Beachy R, Lucas WJ (1991) Plasmodesmatal function is probed using transgenic tobacco plants that express a virus movement protein. Plant Cell 3:593–604
Xu XM, Wang J, Xuan Z, Goldshmidt A, Borrill PG, Hariharan N et al (2011) Chaperonins facilitate KNOTTED1 cell-to-cell trafficking and stem cell function. Science 333:1141–1144
Zavaliev R, Ueki S, Epel BL, Citovsky V (2011) Biology of callose (beta-1,3-glucan) turnover at plasmodesmata. Protoplasma 248:117–130
Zhong X, Archual AJ, Amin AA, Ding B (2008) A genomic map of viroid RNA motifs critical for replication and systemic trafficking. Plant Cell 20:35–47
Acknowledgments
I apologize to all of my colleagues whose work was not mentioned or addressed in depth because of space limitations. I thank the anonymous reviewers for their helpful comments for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kragler, F. Plasmodesmata: intercellular tunnels facilitating transport of macromolecules in plants. Cell Tissue Res 352, 49–58 (2013). https://doi.org/10.1007/s00441-012-1550-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1550-1