Abstract
In the silkworm Bombyx mori, the diapause hormone-pheromone biosynthesis activating neuropeptide gene, DH-PBAN, is a neuropeptide gene that encodes a polypeptide precursor consisting in five Phe-X-Pro-Arg-Leu-NH2 (FXPRL) amide (FXPRLa) neuropeptides; DH (diapause hormone), PBAN (pheromone-biosynthesis-activating neuropeptide) and α-, β- and γ-SGNPs (subesophageal ganglion neuropeptides). These neuropeptides are synthesized in DH-PBAN-producing neurosecretory cells contained within three neuromeres, four mandibular cells, six maxillary cells, two labial cells (SLb) and four lateral cells of the subesophageal ganglion. DH is solely responsible, among the FXPRLa peptide family, for embryonic diapause. Functional differentiation has been previously suggested to occur at each neuromere, with the SLb cells releasing DH through brain innervation in order to induce embryonic diapause. We have investigated the immunoreactive intensity of DH in the SLb when thermal (25°C or 15°C) and light (continuous illumination or darkness) conditions are altered and following brain surgery that induces diapause or non-diapause eggs in the progeny. We have also examined the immunoreactivity of the other FXPRLa peptides by using anti-β-SGNP and anti-PBAN antibodies. Pupal SLb somata immunoreactivities seem to be affected by both thermal and light conditions during embryogenesis. Thus, we have been able to identify a close correlation between the immunoreactive intensity of neuropeptides and environmental conditions relating to the determination of embryonic diapause in B. mori.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seasonal polyphenisms are known to serve as a life-history strategy and are involved in the developmental phenotypic plasticity that has evolved for seasonal adaptation and the differential expression of alternative phenotypes from a single genotype, depending upon environmental conditions, including photoperiod, temperature, humidity and nutrition (West-Eberhard 2003; Gilbert and Epel 2009). Many insects exhibit seasonal changes in the color and structure of their bodies and wings and in the differentiation and maturation of their eggs (Tauber et al. 1986; Hodin 2009). However, the precise developmental processes and molecular mechanisms that are integrated with the environmental stimuli for seasonal polyphenisms remain unknown.
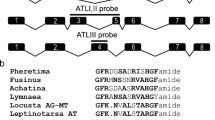
In the silkworm Bombyx mori, embryonic diapause is a unique process of seasonal polyphenism and is induced transgenerationally in the bivoltine strain. Progeny diapause is determined by the mother’s experience during embryonic development. For example, when eggs are incubated at 25°C, the resultant female moths are able to lay diapause eggs. In contrast, incubation at 15°C during the mother’s embryonic stage causes the resultant moths to lay non-diapause eggs (Watanabe 1924; Morita et al. 2003). If eggs are incubated at 20°C under continuous illumination or darkness, the resultant moths produce diapause or non-diapause eggs, respectively (Kogure 1933; Morita et al. 2003). The diapause hormone (DH) is a 24-amino-acid (aa) peptide amide that is responsible for embryonic diapause and functions by acting on a G-protein-coupled receptor in the developing ovaries during pupal-adult development in females (Yamashita 1996; Homma et al. 2006). DH-PBAN is a neuropeptide gene that encodes a polypeptide precursor consisting in five FXPRL amide (FXPRLa) neuropeptides; DH, PBAN (pheromone-biosynthesis-activating neuropeptide) and α-, β- and γ-SGNPs (subesophageal ganglion neuropeptides) in various insects including B. mori (Fig. 1a; Sato et al. 1993; Choi and Vander Meer 2009). DH-PBAN is exclusively expressed in eight pairs of neurosecretory cells. The DH-PBAN-producing neurosecretory cells (DHPCs) are located within the subesophageal ganglion (SG) in Bombyx (Sato et al. 1993, 1994; Shiomi et al. 2007). DHPCs are contained in three neuromeres, four mandibular cells (SMd), six maxillary cells (SMx), two labial cells (SLb) located along the ventral midline and four lateral cells (SL), and the locations of the DHPCs are conserved among insect species (Fig. 1c; Ichikawa et al. 1995; Davis et al. 1996; Sato et al. 1998). Immunohistochemical analysis has suggested that FXPRLa peptides are produced in the DHPCs and that their axonal projections reach the neurohemal organ, the corpus cardiacum, resulting in the release of these peptide hormones into the hemolymph (Ichikawa et al. 1995; Sato et al. 1998).
Representation of the biosynthesis of DH (diapause hormone), α-, β- and γ-SGNPs (subesophageal ganglion neuropeptides) and PBAN (pheromone-biosynthesis-activating neuropeptide) in Bombyx subesophageal ganglion (SG). a DH-PBAN cDNA encoding pre-prohormone undergoes post-translational processing via a series of enzymatic steps that cleave and further modify intermediate peptide substrates to yield the signal sequence (S.S), DH, α-, β- and γ-SGNPs and PBAN. b DH, α-, β-, γ-SGNPs, and PBAN neuropeptides belong to the FXPRLa peptide family that contains Phe-X-Pro-Arg (Lys)-Leu-NH2 at the C-terminus (underlined). c FXPRLa immunoreactivity has been detected in 16 DH-PBAN-producing neurosecretory cells (DHPCs) of SG; these cells are contained in three neuromeres, four mandibular cells (SMd), six maxillary cells (SMx), two labial cells (SLb) along the ventral midline, and lateral cells (SL). The SMd and SMx axons project to the neurohemal organ, viz. the corpus cardiacum, via the maxillary nerve (MxN). SLb and SL axons project to the corpus cardiacum via the circumoesophageal connective (CoC). We prepared antibodies against DH, β-SGNP and PBAN by using the 12-aa sequence located at the N-terminus as an antigen (gray shading in b)
A difference in the intensity of anti-FXPRLa immunostaining of SLb somata has been found between diapause-egg producers (DEP) and non-diapause-egg producers (NDEP) during pupal-adult development (Sato et al. 1998). Likewise, a significant difference occurs in the firing activity patterns of the SLb between DEP and NDEP and between SLb and other neuromeres in both DEP and NDEP (Ichikawa and Kamimoto 2003; Ichikawa 2003). Furthermore, the surgical ablation of the SLb somata during early pupal stages greatly impairs the preferential accumulation of 3-hydroxykynurenine in diapause eggs, whereas the ablation of the SMd and SMx clusters impairs pheromone production during the adult stage (Ichikawa et al. 1996). Innervation from the brain has also been speculated to control the release of DH (Fukuda 1952, 1953; Matsutani and Sonobe 1987; Shimizu et al. 1997). For example, removal of the brain or transection of the protocerebrum in the pupa of NDEP changes the pupa to a DEP (Shimizu et al. 1997). These surgical results suggest an involvement of the protocerebrum in the inhibitory control of DH secretion. Such observations strongly support the hypothesis that there is functional differentiation at each neuromere and that SLb release DH as regulated through brain innervation, resulting in the determination of embryonic diapause in Bombyx. Given these findings, we have investigated the immunoreactive intensity of DH in pupal SLb somata when the environmental conditions have been altered and when the brain has been surgically altered to induce diapause or non-diapause eggs in the progeny. We have also examined the immunoreactive intensity of each FXPRLa peptide by using anti-DH-, anti-β-SGNP- and anti-PBAN-specific antibodies. We have, by these means, identified a close correlation between the immunoreactive intensity of neuropeptides and the environmental conditions relating to the determination of transgenerational diapause in the silkworm B. mori.
Materials and methods
Insects
The polyvoltine (N4) and three bivoltine (Kosetsu, Daizo, and Kuroko) strains of B. mori were used. N4 strain eggs were incubated at 25°C under continuous darkness and the larvae were reared on an artificial diet (Kuwano-hana, JA Zennoh Gunma, Gunma, Japan). At the fifth instar stage, larvae were given fresh mulberry leaves. During the larval stages, animals were reared at 25-27°C under a 12-h light/dark cycle. Pupae were collected within 2 h after pupation (referred to as P0) in order to synchronize their subsequent development. Pupae were then maintained at 25°C. N4 moths laid only non-diapause eggs genetically, so that eggs never entered diapause at every generation. Bivoltine strain eggs were incubated under four different conditions: (1) at 25°C (25DD) or (2) at 15°C (15DD) under continuous darkness to obtain diapause or non-diapause eggs, respectively and (3) at 20°C under continuous illumination (20LL) or (4) at 20°C under continuous darkness (20DD) to obtain diapause or non-diapause eggs, respectively. The animals were then reared under larval-pupal and pupal-adult developmental conditions similar to those for the N4 strain. Pupal eyes became brown in color on day 4 (P4) after pupation and the pupae were normally eclosed on day 8 (P8) after pupation in all four strains, even when the eggs were kept under each of the different environmental conditions.
For transection of the brain, female pupae aged just after pupation until 2 h were anesthetized with diethyl ether. The brain was exposed by making a window on the ventral cuticle of the pupal head and the brain was cut along the midsagittal line (Fig. 6a) with fine forceps. The cuticle was then replaced and the cut edges were sealed with paraffin wax. These silkworms were maintained at 25°C throughout pupal-adult development.
Peptide synthesis and antibody production
Each peptide consisted in a 1- to 12-aa sequence from the N-terminal of DH, β-SGNP and PBAN (Fig. 1b) and was chemically synthesized with the cysteine residue in the C-terminus and purified by using reverse-phase high-performance liquid chromatography according to previously described methods (Imai et al. 1991). Each peptide was then conjugated with Imject Maleimide-Activated bovine serum albumin (BSA; Thermo Fisher Scientific, Rockford, Ill., USA) and used as the antigen. Blood samples collected from immunized mice were centrifuged and the resultant supernatant was collected as the antiserum. Each antiserum was assessed for its binding activity and cross-reactivity against mature peptides for DH, β-SGNP and PBAN by using the dot-immunobinding assay described below. These antisera were subsequently termed anti-DH[N], anti-β-SGNP[N] and anti-PBAN[N] antibodies, respectively. In addition, the antibody prepared previously (Shiomi et al. 1994; Sato et al. 1998) and shown to react with the C-terminal FXPRLa sequence in DH, α-, β-, γ-SGNP and PBAN, was termed anti-FXPRLa antibody in this study.
Dot-immunobinding assay
Sera immunoreactivity was detected with the dot-immunobinding assay (Hawks et al. 1982; Sato et al. 1998). Various amounts of synthetic DH, β-SGNP and PBAN were dotted onto nitrocellulose membranes (Advantec Toyo, Tokyo, Japan) and the dotted membranes were blocked with 5% skimmed milk in phosphate-buffered saline (PBS). The blots were then incubated with antiserum that had been serially diluted with PBS containing 0.2% Tween-20 (PBT). After a 2-h incubation, the membranes were washed with PBT and incubated for an additional 2 h with horseradish peroxidase (HRP)-linked anti-mouse IgG goat serum (Invitrogen, Carlsbad, Calif., USA) diluted 1:2000 with PBT. The washed membranes were developed by using the Konica Immunostain HRP IS-50B kit (Konica, Tokyo, Japan). We selected each antiserum from sera prepared from 20 mice, performed dot blots with 1 pmol of each mature peptide and checked the immunoreactivity of antiserum after serial dilutions. All four sera showing light immunoreactivity when the serum was diluted to 1:5000 were used in this study. In addition, we confirmed the immunological specificity of each antiserum by using the dot-immunobinding assay (data not shown). We did not observe any cross-reaction between each antiserum and other FXPRLa peptides.
Immunohistochemistry
Pupae were collected every 2 days (P2, P4 and P6) from just after pupation (P0) until day 8 of eclosion (A0) and the brain-SG complex was dissected at each time point. The immunoreaction procedures were adapted from Shiomi et al. (2003). Briefly, the brain-SG complex was dissected in fixative containing 4% paraformaldehyde, 7% picric acid, 10 mM MgCl2, 5 mM EGTA-NaOH and 0.5 M HEPES-NaOH (pH 6.9) and incubated at 4°C overnight. The fixed tissues were stored in 90% methanol containing 50 mM EGTA at -20°C until used. The fixed and stored tissues were hydrated through decreasing concentrations of methanol and washed with PBT. Tissue samples were soaked in PBS containing 2% Tween-20 over two nights, washed with PBT, blocked with PBT containing 5% heat-inactivated goat serum and 2% BSA, and incubated with anti-FXPRLa at a 1:5000 diltuion or with anti-DH[N], -β-SGNP[N] or -PBAN[N] at a dilution of 1:2500 at 4°C overnight. A signal was detected with Cy2-labeled goat anti-mouse IgG (Jackson ImmunoResearch Labs, West Grove, Pa., USA) diluted to 1:1500 and observed by using a Radiance 2000 confocal microscope (Bio-Rad, Hercules, Calif., USA). Confocal scans were performed under the same conditions for each silkworm strain and were in the range of Kr: 36.8-50.9, Iris: 4.8-5.7, Gain: 28.8-39.8 and Offset: -50 to -21.6. The sections presented in Fig. 6b were scanned with Kr: 7.8 or 54.6, Iris: 2.9 or 2.6, Gain: 16.9 or 26.3 and Offset: -1.7 or -18.5 for low-intensity and high-intensity scanning, respectively. Serial optical sections (n=16-21, 5.0 μm) were assembled into the combined images presented in Figs. 2, 3 and 4. NIH image 1.62 (http://rsb.info.nih.gov/nih-image/) was used to determine the relative fluorescence intensity of the SLb cells as the intensity of the mean pixel fluorescence for the individual somata (S). Fluorescence images were converted to grayscale and inverted into black and white images. An area adjacent to the area of interest (A) and an area from an image lacking a specimen (N) were scanned as the background signals. The relative fluorescence intensity was calculated as follows: relative fluorescence intensity (%) = 100×([(S)–(A)–(N)]/[(A)–(N)]) for the SLb of interest divided by ([(S)–(A)–(N)]/[(A)–(N)]) for the SLb of NDEP. Images were adjusted and assembled in Adobe Photoshop CS3 (Adobe Systems, San Jose, Calif., USA). The terminology for the Bombyx central nervous system was used according to that of Sato et al. (1998).
FXPRLa, DH, β-SGNP and PBAN immunoreactivity during the pupal-adult development of the polyvoltine strain (N4). The SG was dissected at 0 (P0), 2 (P2), 4 (P4) and 6 (P6) days after pupation, and 1 day immediately after adult eclosion (A0) and then subjected to immunohistochemistry with anti-FXPRLa (a-e), anti-DH[N] (f-j), anti-β-SGNP[N] (k-o), and anti-PBAN[N] (p-t) antibodies. Representative scanning images are presented (SMd mandibular cells, SMx maxillary cells, SLb labial cells, SL lateral cells, asterisks, arrowheads axons projecting from the SMx and SLb, respectively, double-headed arrows in a lateral arcuate tract that becomes confluent with the axons of the SMx and SMd, arrows SLb somata). Bar 100 μm
Effects of thermal conditions on anti-FXPRLa (a-f), anti-DH[N] (g-l), anti-β-SGNP[N] (m-r) and anti-PBAN[N] (s-x) immunoreactivity in the bivoltine strain (Kosetsu). During embryonic development, eggs were incubated at 25°C (25DD; d-f, j-l, p-r, v-x) or 15°C (15DD; a-c, g-i, m-o, s-u) under continuous darkness to induce diapause or non-diapause eggs, respectively. In the pupal stage, the SG was dissected out on 0 (P0) and 4 (P4) days after pupation and 1 day immediately after adult eclosion (A0) and then subjected to immunohistochemistry. Micrographs were obtained by using a confocal microscope operated under the same conditions for all specimens and representative scanning images are presented (SMd mandibular cells, SMx maxillary cells, SLb labial cells, SL lateral cells, arrows SLb somata). Bar 100 μm
Effects of light conditions on anti-DH[N] (a-d, i-l) and anti-β-SGNP[N] (e-h, m-p) immunoreactivity in the bivoltine strains Daizo and Kuroko. During embryonic development, eggs were incubated at 20°C under continuous light (20LL; c, d, g, h, k, l, o, p) or dark (20DD; a,b, e, f, i, j, m, n) conditions to induce diapause or non-diapause eggs, respectively. During the pupal stages, the SG was dissected on 0 (P0) and 4 (P4) days after pupation and then subjected to immunohistochemistry. Micrographs were obtained by using a confocal microscope operated under the same conditions for all specimens, and representative scanning images are presented (SMd mandibular cells, SMx maxillary cells, SLb labial cells, SL lateral cells, arrows SLb somata). Bar 100 μm
Sandwich enzyme-linked immunosorbent assay
For the measurement of DH content in the brain-SG complex, we used sandwich enzyme-linked immunosorbent assay performed according to the method described by Kitagawa et al. (2005) with some modifications. Briefly, the brain-SG complexes of five animals were dissected and suspended together in 250 μl ice-cold PBS. After homogenization with a plastic pestle, the tissue suspension was boiled for 5 min and cooled on ice for 10 min. Following centrifugation at 21,130g at 4°C, aliquots of the supernatant were examined in the assay. Immunoreactions were performed in 96-well micro-titer plates (Nunc no. 437111; Nunc, Roskilde, Denmark) with both anti-FXPRLa IgG and anti-DH[N] IgG prepared from the antisera used in this study. The chemiluminescence intensity was measured by means of a Luminescencer-JNRII (Atto, Tokyo, Japan).
Results
Developmental profiles of anti-FXPRLa, -DH[N], -β-SGNP[N] and -PBAN[N] antibody immunoreactivity in SG
We first investigated the immunoreactivity of the four antisera during the development of the polyvoltine strain, N4 (Fig. 2). Immunoreactivity to anti-FXPRLa antibody was clearly detected in the DHPCs, including the SMd, SMx, SLb along the ventral midline and SL on the day immediately after pupation (P0, Fig. 2a), similar to previously reported results (Fig. 1c; Sato et al. 1998; Shiomi et al. 2003). In addition, immunofluorescence was observed in the bundle of axons that extended laterally from the SMx and turned anteriorly (Fig. 2a-e, asterisks). The lateral arcuate tract became confluent with the axons of the SMd (Fig. 2a, double-headed arrows) and extended with them into the MxN. We also found that the axon from the SLb ran laterally and then formed a pair of longitudinal neurites (Fig. 2a-e, arrowheads). The longitudinal immunoreactive neurites that ascended anteriorly were found to contribute to the circumoesophageal connective. The somata of the SL extended neurites toward the midline and fused with the neurites arising from the SLb somata. With advancing pupal-adult development, intensely immunoreactive somata and axons of the DHPCs were detected at day 2 (P2) and day 4 (P4) after pupation (Fig. 2b, c). Branches of neurites from the SMx and SLb somata formed arborizations where those axons traversed in the SG. Thereafter, immunofluorescence intensity decreased leading up to adult eclosion (Fig. 2d). On the day of adult eclosion (A0), immunoreactive axons were only faintly observed in the SG (Fig. 2e).
The DHPCs were also clearly observed by using anti-DH[N] and anti-β-SGNP[N] antibodies. Changes in the intensity of somata and axon immunoreactivity to these antibodies during pupal-adult development were similar to those observed with the anti-FXPRLa antibody, although the immunoreactive axons were less intense (Fig. 2f-j, k-o). Immunoreactivity to the anti-PBAN[N] antibody was strikingly weaker, especially in the SLb and SL somata (Fig. 2p-t). Thus, the DH, β-SGNP and PBAN immunoreactivities were localized in all DHPCs, although the SLb and SL had strikingly reduced levels of PBAN immunoreactivity as compared with the other DHPCs in the SG. Furthermore, the developmental changes in these immunoreactivities were similar for the three peptides, with the most intense signals being detected in the middle pupal stages.
Immunoreactive intensity of pupal DHPCs in response to environmental conditions during embryonic development
Sato et al. (1998) demonstrated that, when using the anti-FXPRLa antibody, the staining intensity of SLb somata was selectively decreased over the course of pupal-adult development in DEP. The staining became extremely weak or undetectable at adult eclosion, although strong staining was continuously maintained in the SMd and SMx somata throughout metamorphosis. We confirmed these results in detail and examined whether each FXPRLa peptide responded to changes in the thermal and light conditions during embryonic development, by using the anti-DH[N], -β-SGNP[N] and -PBAN[N] antibodies (Figs. 3, 4, 5).
Relative fluorescence intensity of SLb immunoreactivity in pupal SG exposed to various environmental conditions during embryogenesis. The SG was dissected out on 0 days (0) and 4 days (4) after pupation. The relative fluorescence intensity of DH (a) and β-SGNP (b) in the SLb somata (shaded bars) is shown as the percentage compared with that in the pupa just after pupation (0) in the non-diapause egg producers (15DD, 20DD; unshaded bars); 15DD and 25DD represent the intensity of SLb immunoreactivity when eggs of the Kosetsu strain were incubated at 15°C under continuous darkness or at 25°C under continuous darkness, respectively, whereas 20DD and 20LL represent the intensity of SLb immunoreactivity when eggs of the Daizo strain were incubated at 20°C under continuous darkness or at 20°C under continuous light, respectively. Each bar represents the mean value of 10 samples ± SD. *Statistically significant differences at the 5% level
When the eggs were incubated under 25DD, 15DD, 20LL and 20DD conditions, adult females oviposited diapause, non-diapause, diapause and non-diapause eggs, respectively (data not shown). The typical staining patterns following changes in the thermal conditions are presented in Fig. 3. In the SG of the 25DD pupae, a marked reduction in FXPRLa, DH, β-SGNP, and PBAN immunoreactivity was observed in a pair of SLb somata on P4 and A0, although no differences were detected in immunoreactivity on P0. DH and β-SGNP immunoreactivity in the SLb somata was decreased to approximately half the original levels (51.4%, n=10; 40.3%, n=10) in P4 animals, respectively (Fig. 5a, b). No changes in other DHPC somata were observed for FXPRLa. In addition, no changes in staining intensity were found in any of the immunoreactive somata including the SLb from the 15DD silkworms throughout pupal-adult development (Figs. 3, 5a, b). Instead, the DH and β-SGNP immunoreactivity in the SLb somata was sustained at the levels observed at P0 in the P4 animals (105.3%, n=10; 124.1%, n=10), respectively (Fig. 5a, b). In addition, we observed robust immunoreactivity in the axons with arborization during the middle pupal stages for DH and β-SGNP in the 25DD silkworms, compared with that of the 15DD silkworms and N4 strain (Figs. 2, 3).
Next, we investigated DHPC immunoreactivity in pupal SG in response to altered light conditions during embryonic development (Fig. 4). In both the Daizo and Kuroko strains, anti-DH[N] immunoreactivity in all DHPCs somata was strongly observed in P0 under 20LL and 20DD conditions (Fig. 4a, c, i, k). However, the immunoreactivity of SLb somata was significantly decreased under 20LL conditions (38.79%, n=10), whereas no changes were observed under 20DD conditions (104.8%, n=0; Fig. 5). Likewise, anti-β-SGNP immunoreactivity in the SLb somata on P4 in 20LL silkworms was decreased to half its original level (54.76%, n=10) as compared with 20DD (118.4%, n=10; Fig. 5). Thus, pupal SLb somata immunoreactivity was affected by both thermal and light conditions during embryogenesis. These observations strongly support the previous observations that the SLb behave differently to SMd and SMx during the development of the neurosecretory system and that its activity is closely correlated to the determination of diapause type.
Effects of brain transection on DHPCs immunoreactivity
To investigate the effects of brain transection on SLb immunoreactivity, we cut the brain along the midsagittal line (see Fig. 6a) immediately after pupation in the polyvoltine strain, N4 and performed immunohistochemistry on P4. These animals oviposited the diapause eggs at a mean of 57±23% (n=25), whereas the control (sham-operated) animals oviposited non-diapause eggs (Fig. 6). Following immunohistochemistry with the anti-DH[N] and anti-β-SGNP[N] antibodies, SLb were detected in day 4 pupa control animals (Fig. 6b, c; control) similar to that observed in Fig. 2h, m. All DHPCs in the transected brain samples demonstrated decreases in immunoreactive intensity (data not shown). These changes were especially decreased in the SLb, so that this region was not visible under the same scanning conditions (Fig. 6d, e; Transection). In addition, DH content was drastically decreased in the SG following transection (Fig. 6f; n=10).
Effects of brain transection on DH and β-SGNP immunoreactivity in the SLb. a Pupal brain was transected along the midline (dotted line) just after pupation. Four days after pupation, the SG was dissected and then subjected to immunohistochemistry (SG subesophageal ganglion, SLb labial cells). b-e Immunofluorescence of the SLb somata was observed by using a confocal microscope operated under the same conditions for all specimens. Insets SLb observed by high-intensity scanning. The percentage of diapause eggs (%D) in the progeny is also presented (right). f DH content in SG was measured by the sandwich enzyme-linked immunosorbent assay. Each bar represents the mean value of 10 samples ± SD. *Statistically significant differences at the 5% level
Discussion
The immunolocalization of each FXPRLa was observed in all DHPCs with no sign of a particular expression pattern in distinct neuronal subsets, although the immunoreactive intensity differed in voltinisms. This result is unexpected, considering other examples of multiple peptides derived from a common precursor (e.g., FMRFamide peptides in Drosophila and pro-opiomelanocortin-derived peptides in vertebrates), where different peptides are typically expressed in distinct neuronal subsets (McCormick et al. 1999; Raffin-Sanson et al. 2003). Furthermore, the SLb and SL neurons have strikingly reduced levels of PBAN immunoreactivity as compared with the other neurons in the SG. Therefore, these results suggest novel processing of the precursor polyprotein and of the DH-PBAN precursor.
DH and other FXPRLa are multifunctional peptides that play a conserved role in the coordination of the life cycle among and within species. DH acts not only during diapause induction (Yamashita 1996) but is also thought to function during ecdysteroidgenesis in Bombyx (Watanabe et al. 2007). Furthermore, the neuropeptides of this family are involved in pheromone biosynthesis, cuticular tanning, myostimulation and pupariation behavior among various insects (Holman et al. 1986; Raina et al. 1989; Matsumoto et al. 1990; Zdarek et al. 1997). Moreover, hugin in Drosophila, a putative homolog of mammalian neuromedin U, encodes neuropeptides belonging to the pyrokinin/PBAN family, termed PK-2 and hugin-γ (Meng et al. 2002). PK-2 displays a moderate myostimulatory activity (Meng et al. 2002), whereas hugin acts in the modulation of feeding behavior in response to amino acid signals (Melcher and Pankratz 2005).
In this study, we have observed intense immunoreactivities to antibodies against each FXPRLa peptide in the DHPCs, except for the SLb somata during the middle pupal stage, irrespective of whether Bombyx is DEP or NDEP. The DH content in the brain-SG complex has previously been shown to decrease from the middle pupal stage in both diapause types (Kitagawa et al. 2005). Therefore, these results suggest that FXPRLa peptides are actively transported from the SG, although the precise developmental rate of FXPRLa biosynthesis remains unknown. These peptides act as hormones and neuromodulators that serve various functions during normal developmental processes accomplished by the inherent neuroendocrine system but not on developmental plasticity processes such as diapause induction in Bombyx.
In addition, we have also found that the immunoreactive intensities of antibodies against DH and other FXPRLa in the SLb are affected by alterations in environmental conditions including temperature and light changes during embryonic development. At the middle pupal stage, DH levels in the SG and SG-brain complex are markedly decreased in the DEP as compared with those of the NDEP (Kitagawa et al. 2005). Furthermore, significant morphological and functional differences in the three neuromeres of the DHPCs have been detected following surgical ablation experiments (Ichikawa et al. 1996) and electrophysiology experiments involving changes in firing activity patterns between DEP and NDEP (Ichikawa and Kamimoto 2003; Ichikawa 2003). These results suggest that the SLb is anatomically and functionally different from the SMd and SMx and is specialized for diapause determination. We consider that, in DEP, the transportation of DH and other FXPRLa from the SLb somata is most probably actively modified in the response to both temperature and light conditions during embryonic development and results in active release into the hemolymph during the middle pupal stages. As a consequence, DH is able to act on DH receptors expressed in the ovary but not other FXPRLa receptors (Homma et al. 2006; Watanabe et al. 2007), to induce diapause eggs in the progeny. Although the function of the active release from SLb of other FXPRLa remains unknown, we have revealed the close relationship between diapause type and FXPRLa-immunoreactive intensity in Bombyx.
To mediate different voltinism phenotypes in progeny, the nervous system might need to coordinate not only the operation of the neural circuits but also the short- and long-term alterations that occur within those circuits on brain plasticity, so that the regulation of dendritic growth, neural activity and neuromodulator release can be achieved. Surgical operation in the brain disrupts the control of diapause type in progeny and results in a drastic decrease in DH levels in the DHPCs. Although we do not know whether brain transection has a direct effect by disrupting the projection from decussating neurons that project to the SG and/or an indirect affect by disrupting the projection of other neurons that cross the brain midline, this result suggests that the neuron(s) projecting to the brain midline participate by DH accumulation in DHPCs, its release into the hemolymph through the neurohemal organ, viz. the corpus cardiacum, and the determination of diapause type.
In general, the developmental plasticity of life-history traits in organisms is thought to have evolved so as to integrate signals from the environment into the normal developmental processes via the transduction of transcriptional and neuroendocrine regulators (Gilbert and Epel 2008). In insects, hormones such as ecdysone, juvenile hormone and various neuropeptides switch developmental pathways in various polyphenisms, including chromatic adaptation and seasonal, caste and phase polyphenisms (Nijhout 1994). However, the mechanisms by which environmental signals are received by specific receptors in the sensory organs and the molecular mechanisms that underlie the transduction of those signals to the neuroendocrine systems on developmental plasticity in organisms remain unclear. In addition, the environmental agents that instruct a particular phenotype to emerge from the inherited repertoire of possible phenotypes must be elucidated. The present study might provide clues for solving the molecular mechanisms involved in the plasticity of neuroendocrine systems.
Abbreviations
- DEP:
-
Diapause-egg producers
- DH:
-
Diapause hormone
- DHPCs:
-
DH-PBAN-producing neurosecretory cells
- FXPRLa:
-
Phe-X-Pro-Arg-Leu-NH2
- MxN:
-
Maxillary nerve
- NDEP:
-
Non-diapause-egg producers
- PBAN:
-
Pheromone-biosynthesis-activating neuropeptide
- SG:
-
Subesophageal ganglion
- SGNP:
-
SG neuropeptide
- SLb:
-
Labial neuromere cell of the SG
- SL:
-
Lateral cell of the SG
- SMd:
-
Mandibular neuromere cell of the SG
- SMx:
-
Maxillary neuromere cell of the SG
References
Choi MY, Vander Meer RK (2009) Identification of a new member of the PBAN family of neuropeptides from the fire ant, Solenopsis invicta. Insect Mol Biol 18:161–169
Davis NT, Homberg U, Teal PE, Altstein M, Agricola HJ, Hildebrand JG (1996) Neuroanatomy and immunocytochemistry of the median neuroendocrine cells of the subesophageal ganglion of the tobacco hawkmoth, Manduca sexta: immunoreactivities to PBAN and other neuropeptides. Microsc Res Tech 35:201–229
Fukuda S (1952) Function of the pupal brain and suboesophageal ganglion in the production of non-diapause and diapause eggs in the silkworm. Ann Zool Jpn 25:149–155
Fukuda S (1953) Alteration of voltinism in the silkworms following transection of pupal esophageal connectives. Proc Jpn Acad 29:389–391
Gilbert SF, Epel D (2009) Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sinauer, Sunderland, Mass
Hawks R, Niday E, Gordon J (1982) A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem 119:142–147
Hodin J (2009) She shapes events as they come: plasticity in female insect reproduction. In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity of insects: mechanism and consequences. Science, Enfield, pp 423–521
Holman GM, Cook BJ, Nachman RJ (1986) Primary structure and synthesis of a blocked myotropic neuropeptide isolated from the cockroach, Leucophaea maderae. Comp Biochem Physiol [C] 85:219–224
Homma T, Watanabe K, Tsurumaru S, Kataoka H, Imai K, Kamba M, Niimi T, Yamashita O, Yaginuma T (2006) G protein-coupled receptor for diapause hormone, an inducer of Bombyx embryonic diapause. Biochem Biophys Res Commun 344:386–393
Ichikawa T (2003) Firing activities of neurosecretory cells producing diapause hormone and its related peptides in the female silkmoth, Bombyx mori. I. Labial cells. Zool Sci 20:971–978
Ichikawa T, Kamimoto S (2003) Firing activities of neurosecretory cells producing diapause hormone and its related peptides in the female silkmoth, Bombyx mori. II. Mandibular maxillary cells. Zool Sci 20:979–983
Ichikawa T, Hasegawa K, Shimizu I, Katsuno K, Kataoka H, Suzuki A (1995) Structure of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptide in the silkworm, Bombyx mori. Zool Sci 12:703–712
Ichikawa T, Shiota T, Shimizu I, Kataoka H (1996) Functional differentiation of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptide of the moth, Bombyx mori. Zool Sci 13:21–25
Imai K, Konno T, Nakazawa Y, Komiya T, Isobe M, Koga K, Goto T, Yaginuma T, Sakakibara K, Hasegawa K, Yamashita O (1991) Isolation and structure of diapause hormone of the silkworm, Bombyx mori. Proc Jpn Acad Ser B67:98–101
Kitagawa N, Shiomi K, Imai K, Niimi T, Yaginuma T, Yamashita O (2005) Establishment of a sandwich ELISA system to detect diapause hormone, and developmental profile of hormone levels in egg and subesophageal ganglion of the silkworm, Bombyx mori. Zool Sci 22:213–221
Kogure M (1933) The influence of light and temperature on certain characters of the silkworm, Bombyx mori. J Dept Agric Kyushu Imp Univ 4:1–93
Matsumoto S, Kitamura A, Nagasawa H, Kataoka H, Orikasa C, Mitsui T, Suzuki A (1990) Functional diversity of a neurohormone produced by the suboesophageal ganglion: molecular identity of melanization and reddish colouration hormone and pheromone biosynthesis activating neuropeptide. J Insect Physiol 36:427–432
Matsutani K, Sonobe H (1987) Control of diapause-factor secretion from the suboesophageal ganglion in the silkworm, Bombyx mori: the roles of the protocerebrum and tritocerebrum. J Insect Physiol 33:279–285
McCormick J, Lim I, Nichols R (1999) Neuropeptide precursor processing detected by triple immunolabeling. Cell Tissue Res 297:197–202
Melcher C, Pankratz MJ (2005) Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 3:e305
Meng X, Wahlstrom G, Immonen T, Kolmer M, Tirronen M, Predel R, Kalkkinen N, Heino TI, Sariola H, Roos C (2002) The Drosophila hugin gene codes for myostimulatory and ecdysis-modifying neuropeptides. Mech Dev 117:5–13
Morita A, Niimi T, Yamashita O (2003) Physiological differentiation of DH-PBAN-producing neurosecretory cells in the silkworm embryo. J Insect Physiol 49:1093–1102
Nijhout HF (1994) Insect hormones. Princeton University Press, Princeton
Raffin-Sanson ML, De Keyzer Y, Bertagna X (2003) Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol 149:79–90
Raina AK, Jaffe H, Kempe TG, Keim P, Blacher RW, Fales HM, Riley CT, Klun JA, Ridgway RL, Hayes DK (1989) Identification of a neuropeptide hormone that regulates sex pheromone production in female moths. Science 244:796–798
Sato Y, Oguchi M, Menjo N, Imai K, Saito H, Ikeda M, Isobe M, Yamashita O (1993) Precursor polyprotein for multiple neuropeptides secreted from the suboesophageal ganglion of the silkworm Bombyx mori: characterization of the cDNA encoding the diapause hormone precursor and identification of additional peptides. Proc Natl Acad Sci USA 90:3251–3255
Sato Y, Ikeda M, Yamashita O (1994) Neurosecretory cells expressing the gene for common precursor for diapause hormone and pheromone biosynthesis-activating neuropeptide in the suboesophageal ganglion of the silkworm, Bombyx mori. Gen Comp Endocrinol 96:27–36
Sato Y, Shiomi K, Saito H, Imai K, Yamashita O (1998) Phe-X-Pro-Arg-Leu-NH(2) peptide producing cells in the central nervous system of the silkworm, Bombyx mori. J Insect Physiol 44:333–342
Shimizu I, Aoki S, Ichikawa T (1997) Neuroendocrine control of diapause hormone secretion in the silkworm, Bombyx mori. J Insect Physiol 43:1101–1109
Shiomi K, Ishida Y, Ikeda M, Sato Y, Saito H, Imai K, Isobe M, Yamashita O (1994) Induction of non-diapause eggs by injection of anti-diapause hormone rabbit serum into the diapause type of the silkworm, Bombyx mori. J Insect Physiol 40:693–699
Shiomi K, Kajiura Z, Nakagaki M, Yamashita O (2003) Baculovirus-mediated efficient gene transfer into the central nervous system of the silkworm, Bombyx mori. J Insect Biotechnol Sericol 72:149–155
Shiomi K, Fujiwara Y, Yasukochi Y, Kajiura Z, Nakagaki M, Yaginuma T (2007) The Pitx homeobox gene in Bombyx mori: regulation of DH-PBAN neuropeptide hormone gene expression. Mol Cell Neurosci 34:209–218
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, New York
Watanabe K (1924) Studies on the voltinism of the silkworm, Bombyx mori. Bull Sericult Exp Sta (Tokyo) 6:411–455
Watanabe K, Hull JJ, Niimi T, Imai K, Matsumoto S, Yaginuma T, Kataoka H (2007) FXPRL-amide peptides induce ecdysteroidogenesis through a G-protein coupled receptor expressed in the prothoracic gland of Bombyx mori. Mol Cell Endocrinol 273:51–58
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, New York
Yamashita O (1996) Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J Insect Physiol 42:669–679
Zdarek J, Nachman RJ, Hayes TK (1997) Insect neuropeptides of the pyrokinin/PBAN family accelerate pupariation in the fleshfly (Sarcophaga bullata) larvae. Ann NY Acad Sci 814:67–72
Acknowledgments
We are indebted to the Division of Gene Research, Research Center for Human and Environmental Sciences, Shinshu University for providing the facilities for these studies. Fresh mulberry leaves were provided by Drs. Banno and Fujii of Kyushu University and by Dr. Maekawa of Ryukyu University during the winter season. We thank Dr. Yoshiomi Kato (International Christian University) for helpful comments on the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was funded by grants from the Research for the Future Program (JSPS-RFTF99L01203) and by a Grant-in-Aid (no. 20380033) from the Japanese Society for the Promotion of Science. Additional support was provided by Grants-in-Aid from the Global COE Program of the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Rights and permissions
About this article
Cite this article
Hagino, A., Kitagawa, N., Imai, K. et al. Immunoreactive intensity of FXPRL amide neuropeptides in response to environmental conditions in the silkworm, Bombyx mori . Cell Tissue Res 342, 459–469 (2010). https://doi.org/10.1007/s00441-010-1083-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1083-4