Abstract
Endocrine cells in the larval midgut of Drosophila melanogaster are recognized by antisera to seven regulatory peptides: the allatostatins A, B, and C, short neuropeptide F, neuropeptide F, diuretic hormone 31, and the tachykinins. These are the same peptides that are produced by the endocrine cells of the adult midgut, except for short neuropeptide F, which is absent in adult midgut endocrine cells. The anterior larval midgut contains two types of endocrine cells. The first produces short neuropeptide F, which is also recognized by an antiserum to the receptor for the diuretic hormone leucokinin. The second type in the anterior midgut is recognized by an antiserum to diuretic hormone 31. The latter cell type is also found in the junction between the anterior and middle midgut; an additional type of endocrine cell in this region produces allatostatin B, a peptide also known as myoinhibitory peptide. Both types of endocrine cells in the junction between the anterior and middle midgut can express the homeodomain transcription factor labial. The copper cell region contains small cells that either produce allatostatin C or a combination of neuropeptide F, allatostatin B, and diuretic hormone 31. The latter cell type is also found in the region of the large flat cells. The posterior midgut possesses strongly immunoreactive allatostatin C endocrine cells immediately behind the iron cells. In the next part of the posterior midgut, two cell types have been found: one produces diuretic hormone 31, and a second is strongly immunoreactive to antiserum against the leucokinin receptor and weakly immunoreactive to antisera against allatostatins B and C and short neuropeptide F. The last part of the posterior midgut again has two types of endocrine cells: those that produce allatostatin A, and those that produce tachykinins. Many of the latter cells are also weakly immunoreactive to the antiserum against diuretic hormone 31. As in the adult, the insulin-like peptide 3 gene appears to be expressed by midgut muscles, but not by midgut endocrine cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of the regulatory roles of endocrine cells in the gut of invertebrate species is of interest for fundamental and comparative reasons. We have suggested that the fruit fly Drosophila melanogaster is an excellent model for this purpose, as its genome has been sequenced (Adams et al. 2000), the identity of most, if not all, of its neuropeptides has been established (Baggerman et al. 2002, 2005; Liu et al. 2006), the receptors for many of these neuropeptides have been identified (e.g. Hauser et al. 2006), and this species can easily be genetically transformed, thus allowing powerful experimental techniques to be perfomed on this insect (Veenstra et al. 2008). We have recently described the localization of regulatory peptides in the midgut of adult Drosophila (Veenstra et al. 2008). As the fruit fly is a holometabolous species, it is entirely rebuilt during metamorphosis, and the larval gut is significantly different from the adult gut, including, as reported here, the organization of its endocrine cells.
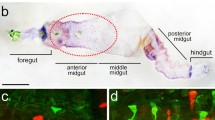
As in the adult, the gut is divided into three parts: the foregut, the midgut, and the hindgut. The midgut, the only part containing endocrine cells, consists of three different regions: the anterior, middle, and posterior midgut. At the beginning of the anterior larval midgut, four gastric caeca, which are blind-ending tubes connected to the main digestive tract, can be found. The anterior midgut starts as a broad, but rapidly narrowing, tube and sometimes resembles a funnel. The first part of the middle midgut is composed of the acid-secreting copper cells, which have a morphology similar to that of the parietal cells in the vertebrate stomach. The pH is less than 3 in this part of the midgut (Strasburger 1932; Dubreuil et al. 1998). During times of excess of dietary copper, these cells express the copper transporter ATP7 (Burke et al. 2008), which allows copper to accumulate inside the cells and to form a fluorescent complex with metallothionenin (McNulty et al. 2001). The individual copper cells are surrounded by interstitial cells. After this first part of the middle midgut, constituted by the copper and interstitial cells, we find the so-called large flat cells, which are followed by the iron cells. The pH is neutralized at the junction of the iron cells and the posterior midgut (Dubreuil et al. 1998). The posterior midgut is the largest section of the midgut and is generally presumed to be the part in which most of the absorption of nutrients takes place. However, the posterior midgut is not homogeneous, as for example, demonstrated by the expression of endogenous galactosidase activity in the posterior midgut (Becker et al. 1995).
Much work has been carried out on the embyronic development of the middle midgut, especially the genetic determination of the copper cells and the interstitial cells. This work has established the roles of the homeotic genes labial (lab) and defective proventriculus (dve) in the differentiation of the four different types of cells in the middle midgut (Hoppler and Bienz 1994, 1995; Nakagoshi et al. 1998; Dubreuil et al. 2001; Nakagoshi 2005).
In the adult, endocrine cells have been found to produce allatostatins A, B, and C, and neuropeptide F (NPF), the calcitonin-related diuretic hormone (DH31), and Drosophila tachykinins, whereas the Drosophila insulin 3 gene is expressed by some of the midgut muscles (Veenstra et al. 2008). I here describe the endocrine cells in the larval midgut, some of which appear to express lab, and which are perhaps derived from the same stem cells as those that generate the copper cells.
Materials and methods
Flies
Drosophila was maintained in the laboratory at 25°C in a 12-h dark/light cycle on standard corn-meal food. Wild-type flies were from a Canton Stock. NPF-gal4 flies (Wu et al. 2003) were a kind gift from Prof. Shen (Athens, Georgia, USA), DMS-gal4, DSK-gal4, Ilp3-gal4, and sNPF-gal4 were produced by the author as described previously (Park et al. 2008; Veenstra et al. 2008) and w;K5J2(p3.65-labGal4),UAS-H2B::YFP/[CyO] (Hirth et al. 2001) were kindly provided by Prof. Reichert (Basel, Switzerland). P{PZ}lab01241ry506/TM3,ryRKSb1 Ser1 (Bloomington stock number 11527) and cn1P{PZ}dve01738/CyO;ry506cn1 (Bloomington stock number 11073) stocks were obtained from the Bloomington Drosophila Stock Center (Bloomington, Indiana, USA). These stocks have P-element insertions in lab and dev, respectively, and carry an enhancer-sensitive P-lacZ fusion gene permitting nuclear LacZ expression.
Larvae used here were either wandering third instar larvae raised at 25°C or were feeding third instar larvae that were raised at 22°C and used between 90 and 100 h after egg laying. At least eight wandering and eight feeding larvae were analyzed for each antiserum; in many cases, the numbers of larvae used were much higher. A few adults were also used; these were between 5 and 14 days old.
Antisera and immunohistological procedures
The immunohistological methods were the same as those utilized in a previous publication (Veenstra et al. 2008); Table 1 lists the details of the antisera employed. Briefly, rabbit antisera and/or mouse monoclonal antibodies were used during overnight incubations on paraformaldehyde-fixed tissues, followed by overnight incubations with secondary antisera. For double-labeling with rabbit and mouse antibodies, secondary antisera with different fluorescent labels were selected. For double-labeling involving two different rabbit antisera, tissues were first incubated with an unlabeled rabbit antiserum, followed by a fluorescein- or Texas-red-labeled Fab-fragment, and subseqently an incubation with a fluorescein- or rhodamine-labeled purified anti-peptide IgG. Alternatively, some double-labeling involved two directly labeled IgGs. The labial antisera were visualized with DyLIght-594-labeled secondary antibodies. All secondary antisera and Fab fragments were obtained from Jackson Immunoresearch Europe (Suffolk, UK). In a few experiments, phalloidoin-rhodamine (Fluoprobes, Interchim, France) was used at a concentration of 0.2 U/ml.
Images were collected by using a SPOT 2.2.1 Digital Camera (Diagnostic Instruments) attached to an Reichert-Jung wide-field fluorescence microscope with classical rhodamine and fluorescein filters and SPOT imaging software. Images were enhanced by means of GIMP software (www.gimp.org).
Results
To facilitate the identification of the various parts of the larval midgut, established markers related to the transcription factors lab and dve were used. Lab is expressed in the copper cells, whereas dve is expressed in all four cell types of the larval middle midgut. To visualize the expression of lab, I used two lines of transgenic flies: w;K5J2(p3.65-labGal4),UAS-H2B::YFP/[CyO] and P{PZ}lab01241ry506/TM3,ryRKSb1Ser1. In the first line, the K5J2(p3.65-labGal4) transgene expresses gal4 under the control of the lab promotor, whereas the second transgene, viz., UAS-H2B::YFP, produces histone 2B extended with yellow fluorescent protein when gal4 is present, yielding a strongly fluorescent nuclear marker. The second line has a transposable element inserted into the lab gene and produces nuclear LacZ. Although the expression of these markers is similar, they are not identical, as demonstrated by recombining the K5J2(p3.65-labGal4),UAS-H2B::YFP and P{PZ}lab01241ry506 genes in a single individual (Fig. 1a1–3). The nuclear H2B::YFP yields a much stronger signal than nuclear LacZ immunoreactivity (Fig. 1a).
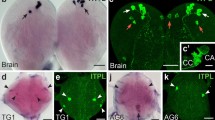
Immunohistological localization of regulatory peptides in the larval midgut in relation to the copper cells. All images are of whole-mounts examined on a wide-field fluorescence microscope; consequently, only part of the tissue is in focus. In all micrographs, anterior is left, and posterior is right. a Comparison of the expression of lab: a1 K5J2(p3.65-labGal4),UAS-H2B::YFP (green); a3 P{PZ}lab01241 after immunohistological detection of LacZ (red); a2 both methods combined. The most anteriorly localized cells are only labeled with H2B::YFP, whereas the most posteriorly located cells are labeled by both methods. Note the presence of small H2B::YFP-labeled nuclei in the anterior part. b Junction of the anterior to middle midgut showing the localization of allatostatin B/MIP-immunoreactive cells in a larva expressing H2B::YFP under control of the lab promotor: b1 allatostatin B/MIP-immunoreactivity (red); b2 H2B::YFP (green) and allatostatin B/MIP (red); b3 H2B::YFP (green). Note that several of the small H2B::YFP-labeled nuclei pertain to allatostatin B/MIP-immunoreactive endocrine cells. c Expression of both LacZ and H2B::YFP under the control of the lab promotor by means of the UAS-gal4 system: c2 the most anteriorly located cells labeled with H2B::YFP (green) also contain cytoplasmic LacZ-immunoreactivity (red in c1). These cells are in contact with one another and appear to form a ribbon (c1) that contains both small and large cells. d Relationship between H2B::YFP nuclear expression in p3.65-labGal4,UAS-H2B::YFP larvae and the morphology of the copper cells; d1 perisulfakinin antiserum in excess reveals the gross cellular morphology (red) and a few small NPF endocrine cells (arrowheads); d3 H2B::YFP nuclear expression (green); d2 merging the two images shows that the most anteriorly located cells having large nuclei labeled with H2B::YFP do not have the morphology of acid-secreting cells. e DH31 immunoreactivity (red) and H2B::YFP nuclear expression (green) in p3.65-labGal4,UAS-H2B::YFP larvae. Note that some of the small cells labeled by H2B::YFP are DH31 endocrine cells (arrowhead). f Photo-montage of the junction of the anterior and middle midgut in p3.65-labGal4,UAS-H2B::YFP larvae labeled with rhdodamine-labeled phalloidoin (red), which labels the gut musculature. Note the small bilateral suspension muscle (arrowhead), g Allatostatin B/MIP-immunoreactive cells in the midgut of a P{PZ}lab01241 larva. Note the presence of several large allatostatin B/MIP-immunoreactive endocrine cells (green) just before the middle midgut (the same cells as in b1) and fewer much smaller cells between the copper cells labeled by nuclear lacZ immunoreactivity (red). Bar 100 μm (a, b, d–g), 40 μm (c)
When visualizing the expression of lab by using the P{PZ}lab01241ry506 transgene, lab expression appears less robust than when using the combination of the K5J2(p3.65-labGal4) and UAS-H2B::YFP transgenes (Fig. 1b, c). In the latter, the nuclei of more cells are labeled, and some of these are much smaller (Fig. 1a, b, e). These smaller cells are endocrine cells producing allatostatin B/MIP (Fig. 1b) or DH31 (Fig. 1e). Not all the cells having a large H2B::YFP-labeled nucleus are copper cells. When the perisulfakinin antiserum is used at high concentrations, it labels the copper cells unspecifically, showing their morphology. When this is performed in w;K5J2(p3.65-labGal4),UAS-H2B::YFP/[CyO] larvae, one observes that the most anterior of the H2B::YFP labeled cells do not have the morphology of copper cells (Fig. 1d). The middle midgut region with cells expressing H2B::YFP under the control of the lab promotor can be divided into two parts: a posterior part in which H2B::YFP-labeled copper cells are tightly packed, and little space is occupied by the interstitial cells, and an anterior part in which the organization of these cells is often more chaotic, and the space that is occupied by the interstitial cells is larger. In both these regions, the large H2B::YFP-labeled cells also express the P{PZ}lab01241 transgene. In maggots that additionally have the UAS-LacZ transgene, one sometimes finds cells expressing cytoplasmic LacZ forming a meandering string of cells: most of them are large, but some of them are small (Fig. 1c).
Endocrine cells with small nuclei labeled with H2B::YFP are not limited to the anterior-middle midgut junction; such cells are also present in the copper cell region. Visualization of the H2B::YFP label in the latter is sometimes difficult, as their nuclei are much smaller than those of the copper cells, which are intensely fluorescent and often mask the fluorescence from the small nuclei. Although both endocrine cell types occurring in the copper cell region have been found to express H2B::YFP, the difficulty of detecting such expression makes it impossible to state whether it is common. Attempts to demonstrate labial immunoreactivity directly in these endocrine cells by using two different antisera failed; although labial immunoreactivity could be demonstrated in the nuclei of the copper cells, no immunoreactive signal was detected in the endocrine cells.
The musculature of the midgut is attached to two small suspension muscles just in front of the copper cells where the allatostatin B/MIP endocrine cells are located (Fig. 1f).
The location of the various endocrine cells are summarized in Table 2, Fig. 2. More detailed descriptions are given below.
Representation of the localization (dark and hatched areas) of paracrine and endocrine cells in the feeding third instar larva (AstA allatostatin A, AstB allatostatin B, AstC allatostatin C, sNPF short neuropepide F, NPF neuropeptide F, TK tachykinins, DH31 the Drosophila calcitonin-like diuretic hormone, AM anterior midgut, CC copper cell region, HG hindgut, IC iron cell region, J anterior-middle midgut junction, LFC large flat cell region, PM posterior midgut, dark areas regions where cells are most immunoreactive)
Immunostaining for DH31
Antisera against this peptide recognize endocrine cells localized throughout the midgut (Fig. 2). These cells are particularly abundant in the anterior midgut and the anterior-middle midgut junction (Figs. 1e, 4a2) but are also present in the middle and posterior midgut. The cells in the posterior midgut tend to be less immunoreactive. This distribution is similar in both feeding and wandering third instar larvae. However, the use of directly labeled antiserum reveals that the DH31 immunoreactivity is much diminished once the larva enters the wandering stage.
Immunostaining for allatostatins
Neuropeptides that inhibit the synthesis of juvenile hormone by the corpora allata are called allatostatins. Three different peptide families are known, which, in different insect species, inhibit the synthesis of juvenile hormone by the corpora allata. They are now commonly called allatostatins A, B, and C, although the allatostatins B are more correctly called myoinhibitory peptides (MIP), the name first given to them (Schoofs et al. 1991). Allatostatin-A-immunoreactive cells are found in the most caudal region of the posterior midgut and have previously been described in Drosophila (Yoon and Stay 1995). As in the adult (Veenstra et al. 2008), these cells have not been found to contain any other regulatory peptide. In the larval midgut, the allatostatin B/MIP-immunoreactive cells are present in various regions. The first group consists of between seven to 30 cells lying just before the copper cells of the middle midgut (Fig. 1b). These cells are different from the DH31 cells in the same area and are often the most strongly immunoreactive endocrine cells in the larval midgut, particularly in the wandering larva, where they are very large. Much smaller allatostatin B/MIP-immunoreactive endocrine cells are found in the region of the copper cells (Fig. 1g). These cells number up to 20 (usually fewer). They are small and are therefore easily overlooked, because they are often only weakly immunoreactive. They tend to be more numerous at the posterior part of the copper cell region. The third group of allatostatin-B/MIP cells occurs in the first half of the posterior midgut. These cells are less immunoreactive, and their density becomes less intense toward the middle of the posterior midgut.
Approximately 10–20 strongly allatostatin-C-immunoreactive endocrine cells are found immediately after the restriction separating the iron cells from the posterior midgut (Figs. 2, 4j), and a larger number of (usually much less immunoreactive) cells are present in the first half of the posterior midgut (Fig. 4f). The latter cells are the same as those expressing allatostatin B/MIP. In wandering larvae, a small number of allatostatin-C-immunoreactive cells are found between the copper cells; such cells are more rarely seen in the feeding larva. These cells are different from the allatostatin B/MIP-immunoreactive cells in the copper cell region. One rarely finds one or two allatostatin-C-immunoreactive endocrine cells in the junction of the anterior and middle midgut, a region in which one also finds the strongly immunoreactive allatostatin B/MIP endocrine cells. As in the adult, the territories of the allatostatin A and C endocrine cells in the posterior midgut are complementary. In the adult midgut, a few endocrine cells in the middle express both allatostatin A and C (Veenstra et al. 2008), but such cells could not be demonstrated convincingly in the larva.
Immunostaining for sNPF
As in the adult, the neurons of the hypocerebral ganglion contain strong immunoreactivity to Drosophila sNPF1 and sNPF3, the sNPF precursor, RFamide, and FMRFamide. In both adults and larvae, the same neurons are also labeled in transgenic flies of the sNPF-gal4/UAS-LacZ genotype. However, unlike in the adult, the larva also possesses, in the midgut, endocrine cells that contain immunoreactivity to the same peptide antisera as those that recognize the sNPF neurons in the hypocerebral ganglion (Fig. 3). These cells were usually only weakly immunoreactive to the Drosophila sNPF1 and sNPF precursor antisera but are well recognized by the antiserum to Drosophila sNPF3 and are often also recognized by the antisera to RFamide and FMRFamide. In particular, the sNPF (and FMRFamide) immunoreactivity of the cells in the posterior midgut in wandering larvae is extremely weak and often is not above background. All the sNPF-immunoreactive endocrine cells in the anterior midgut and in the region of the posterior midgut immediately after the iron cells are also recognized by an antiserum to the leucokinin receptor (Fig. 4c). This immunoreactivity is robust and much more intense than that in the stellate cells of the Malpighian tubules, which in my hands are hardly labeled. Unlike the immunoreactivity with the neuropeptide antisera (i.e., RFamide, FMRFamide, sNPF1, sNPF3, and sNPF precursor), which is variable and often weak, particularly in the posterior midgut, the immunoreactivity with the leucokinin receptor antiserum is highly reproducible. As this antiserum was not included in our previous study (Veenstra et al. 2008), it was tested on the adult midgut in Drosophila, but no immunoreactivity was found, consistent with the apparent absence of expression of the leucokinin receptor in the adult midgut as reported by the Fly Atlas project (Table 4; Chintapalli et al. 2007). In the wandering larvae, the leucokinin-receptor-immunoreactive endocrine cells in the posterior midgut are also recognized by the antisera to allatostatin B/MIP and allatostatin C (Fig. 4e1, e2). Although allatostatin B/MIP-immunoreactive endocrine cells are, on occasion, also found in feeding larvae in the same area of the midgut, their weak and inconsistent immunoreactivity has not allowed me to demonstrate convincingly that these cells are also sNPF-immunoreactive. However, this seems likely, as they appear to be the same cells that express both sNPF and allatostatin B/MIP immunoreactivities in wandering larvae. The sNPF-immunoreactive endocrine cells in the anterior and posterior midgut are not LacZ-immunoreactive in sNPF-gal4/UAS-LacZ larvae.
Photo-montage of the immunohistological localization of sNPF precursor immunoreactivity in the anterior midgut of a feeding third instar larva. Strongly immunoreactive cell bodies occur in the hypocerebral ganglion with their axons running over the proventriculus and the first part of the anterior midgut, which also contains immunoreactive endocrine cells. Bar 100 μm
Peptide immunoreactivity in the midgut of the larva of the fruit fly (anterior is left, and posterior is right). a LacZ (green in a1) and DH31 immunoreactivity (red in a2) in a NPF-gal4/UAS-LacZ larva. Note that the large DH31-immunoreactive endocrine cells (*), located in the anterior midgut do not express LacZ immunoreactivity, whereas the smaller LacZ-immunoreactive endocrine cells (**) in the anterior-middle midgut junction are recognized by the DH31 antiserum, as are the smaller cells (***) located between the copper cells. b Allatostatin B/MIP (green in b1) and DH31 immunoreactivity (red in b2) colocalize in the same cells in the middle midgut. c Leucokinin receptor immunoreactivity (green in c1) and sNPF-precursor immunoreactivity (red in c2) occur in the same endocrine cells of the anterior midgut. d LacZ immunoreactivity (green in d1) and FMRFamide immunoreactivity (red in d2) in a NPF-gal4/UAS-LacZ larva. Note that the large LacZ-immunoreactive endocrine cells (**), which are located in the anterior-middle midgut junction, do not express FMRFamide immunoreactivity, whereas the smaller cells (***) located between the copper cells are FMRFamide-immunoreactive. e Beginning of the posterior midgut showing leucokinin-receptor-immunoreactive endocrine cells (green in e1) and allatostatin-C-immunreactive cells (red in e2). Note that the most anteriorly located allatostatin-C-immunoreactive endocrine cells do not express the leucokinin receptor, whereas those located slightly more posteriorly (arrowheads) are less immunoreactive toward the allatostatin C antiserum but are also recognized by the antiserum to the leucokinin receptor. f Photo-montage of the first part of the posterior midgut showing the localization of allatostatin-C-immunoreactive endocrine cells (red) and the midgut muscles expressing the ilp3 gene as suggested by LacZ immunoreactivity (green) in an ilp3-gal4/UAS-LacZ larva. Note the strongly allatostatin-C-immunoreactive neurons (red) at the begining of the posterior midgut (the same cells as in j) and the weaker immunoreactive cells (green) in the second part of the posterior midgut. Planes of focus are slightly different. g Allatostatin-A-immunoreactive endocrine cells in the posterior midgut. h, i High magnifications of individual NPF-producing cells as detected by LacZ immunoreactivity in NPF-gal4/UAS-LacZ larvae. Note the basolateral extensions in both cells. j Allatostatin B/MIP immunoreactivity (green) and LacZ immunoreactivity (red) in a P{PZ}dve01738/CyO larva. Strongly immunoreactive allatostatin C endocrine cells are located immediately posterior of the iron cells. Bar 100 μm (a–g, j), 40 μm (h, i)
Immunostaining for NPF
In the larval middle midgut of NPF-gal4/UAS-LacZ flies, some LacZ-immunoreactive endocrine cells are also labeled by the RFamide and FMRFamide-antisera and are thus likely to contain NPF (Figs. 2, 4d1, d2). The latter are all located in the regions of the copper cells and of the large flat cells but are absent in the region of the iron cells and from the anterior and posterior midgut. The (FM)RFamide cells in the region of the large flat cells are not open endocrine cells; sometimes, they have basolateral extensions (e.g. Fig. 4h, i), and a variable number of them often lie in a straight line. These peculiarities makes them easy to recognize. The LacZ-immunoreactive cells in NPF-gal4/UAS-LacZ larvae between the copper and large flat cells are also immunoreactive with the antiserum to DH31 (Fig. 4a1, a2) and with the antiserum to allatostatin B/MIP (Fig. 4b1, b2), but these cells are different from the allatostatin-C-immunoreactive cells.
In NPF-gal4/UAS-LacZ larvae, LacZ-immunoreactive endocrine cells are also present in the anterior-middle midgut junction (Fig. 4a1, d1). However, these cells are neither recognized by the RFamide antiserum nor the FMRFamide antiserum but are immunoreactive toward the DH31 antiserum (Fig. 4a1, d1); these are the DH31-immunoreactive endocrine closest to the copper cells, and the ones that are labeled with H2B::YFP in p3.65-labGal4;UAS-H2B::YFP larvae. Interestingly, LacZ-immunoreactive endocrine cells are also found in the same region in sNPF-gal4/UAS-LacZ and DSK-gal4/UAS-LacZ larvae, but these LacZ-immunoreactive endocrine cells are not recognized by antisera to RFamide, FMRFamide, sNPF1, sNPF3, sNPFprecursor, or perisulfakinin in either of these types. Nevertheless, as in the NPF-gal4/UAS-LacZ larvae, the LacZ-immunoreactive endocrine cells in the anterior-middle midgut junction in sNPF-gal4/UAS-LacZ and DSK-gal4/UAS-LacZ larvae are immunoreactive toward the DH31 antiserum. Thus, these DH31 endocrine cells express three transgenes driven by the (partial) promotors of three different neuropeptide genes, but the expression of the peptides normally produced by the genes under the control of these promotors cannot be detected in those cells.
Immunostaining for sulfakinin and myosuppressin
As in the adult, no sulfakinin-like immunoreactivity was found in the midgut endocrine cells of larvae, when using the perisulfakinin antiserum in concentrations at which it is specific for sulfakinins in the central nervous system. At higher concentrations, the same cells that are labeled with the RFamide and FMRFamide antisera are immunoreactive.
No expression of myosuppressin was found in endocrine cells in the larval midgut, either by looking for LacZ-immunoreactivity in DMS-gal4/UAS-LacZ flies or by using the SchistoFLRFamide 1 antiserum, which is relatively specific for myosuppressin in Drosophila (Park et al. 2008; Veenstra et al. 2008).
Immunostaining for Drosophila tachykinins
Tachykinin-immunoreactive endocrine cells have been found in the caudal half of the posterior midgut, where they tend to be concentrated near the junction with the hindgut. Unlike the situation in the adult, where tachykinin-producing endocrine cells are also present in the anterior midgut, such cells are absent from this region in the larval midgut. These cells are the same as those described by Siviter et al. (2000). Some of the tachykinin endocrine cells in the posterior midgut are also immunoreactive with DH31 in both feeding and wandering larvae. As the DH31 immunoreactivity in the posterior midgut is weak, it is not clear whether all tachykinin immunoreactive endocrine cells contain DH31.
Immunostaining for PDF
In the adult, axons from neurons in the thoracicoabdominal ganglion have been found to innervate the most caudal part of the posterior midgut. In the larva, similar (probably the same) neurons project axons onto the hindgut, but these do not reach the midgut.
Immunostaining for insulin
As in the adult, neither the antiserum to Drosophila insulin-like peptide 2 (ilp2) nor that against Drosophila insulin-like peptide 3 (ilp3) recognized endocrine cells in the larval midgut. Expression of the Drosophila ilp3 gene was demonstrated in the adult by using transgenic flies with the ilp3 promotor to drive the expression of gal4. We used the same protocol to localize the expression of the ilp3 in the larvae, which was similar to that in the adult. LacZ-immunoreactivity in ilp3-gal4/UAS-LacZ larvae was found in longitudinal midgut muscles of the caeca and circular muscles of the anterior midgut and the first part of the posterior midgut (Fig. 4f).
Other antisera
The antisera to adipokinetic hormone, capa 2, capa precursor, corazonin, crustacean cardioactive peptide, SIFamide, leucokinin, Manduca allatotropin, and proctolin did not reveal any immunoreactivity in the larval midgut, even though all these antisera, with the exception of that to Manduca allatotropin, were immunoreactive in the larval central nervous system.
Data from the Fly Atlas project
The expression of the Drosophila genes in the adult midgut as reported by the Fly Atlas project (Chintapalli et al. 2007) allowed us to confirm the expression of the neuropeptide genes as found by immunohistology. The recent addition of several larval tissues allows the same for the larval midgut, and in Table 3, the relative expression patterns in the midgut of the known Drosophila neuropeptide genes are compared. The data indicate that the relative expression of allatostatin A is similar in the midgut of larvae and adults, whereas the expression of allatostatin C, NPF, and tachykinin is lower, and that of allatostatin B, DH31, and sNPF is higher in the larva than they are in the adult.
The data from the Fly Atlas also allow one to identify putative target tissues of the midgut endocrine cells by determining which tissues express their receptors. In Table 4, the expression of neuropeptide receptors of interest in the midgut, Malpighian tubules, and the hindgut are compared between larvae and adults.
Of the ten neuropeptide receptors found in the Malpighian tubules in at least three out of the four experiments in the adult and/or larvae, six have virtually the same level of expression in the larva as in the adult, whereas the expression of one of the tachykinin receptors is four times higher in the larva than in the adult, and the expression of the receptors for DH31, CAPA, and NPF are significantly lower in the larva. Notably the relative expression of the DH31 receptor in the Malpighian tubules in the larva is only about 3% of that in the adult. The relative expression of neuropeptide receptors in the midgut shows greater similarity between larvae and adults; the expression of the receptor encoded by CG12370, which is probably a DH44 receptor, in the larva is about one third its expression in the adult, whereas the leucokinin receptor is detectably expressed in the larva, but not the adult. The latter results confirm the immunohistological data on the leucokinin receptor reported here. The most salient differences in neuropeptide receptor expression between larvae and adults in the hindgut concerns the receptor for DH31, which is significantly lower in the larva, and that of the leucokinin receptor, which is more than ten-fold higher in the larva than in the adult.
Discussion
An illustration showing that the expression of lab in midgut cells that are much smaller than the average copper cell (see Fig. 8C in Dubreuil et al. 2001) suggests that this transcription factor might be expressed in endocrine cells in the larval midgut. Although I have been unable to demonstrate labial immunoreactivity in the endocrine cells in the junction between the anterior and middle midgut, the expression of H2B::YFP in endocrine cells in this region of the midgut in K5J2(p3.65-labGal4),UAS-H2B::YFP/[CyO] larvae suggests this to be the case. Thus, not only the copper cells, but also developing endocrine cells probably express this homeodomain transcription factor of the Antennapedia complex. Lab is well known for its function in development (Angelini and Kaufman 2005), including that of the nervous system, notably the tritocerebrum (Hirth et al. 1998), and of the midgut, where it induces the development of the copper cells (Hoppler and Bienz 1994, 1995). The observation that cells that express gal4 under the control of the lab promotor sometimes form a meandering ribbon is suggestive of a lab-expressing stem cell that might give rise to both endocrine and copper cells. However, this is not the only possible explanation for the putative expression of lab in these endocrine cells.
As is known from the vertebrate literature, developing endocrine cells in both the pancreas and the intestine often transiently express neuropeptide genes other than those that will be expressed by the mature endocrine cell (Alpert et al. 1988; Schonhoff et al. 2004). Sometimes, the peptide products of these genes are transiently present in these endocrine cells, whereas in other cases, only mRNA can be detected, or the transient activation of such neuropeptide genes is detected by transgenes (Schonhoff et al. 2004). A similar transient expression of the sNPF, NPF, and DSK genes in developing endocrine cells of the anterior-middle midgut junction might explain why endocrine cells in this region of the midgut express transgenes under the control of promotors of these three different neuropeptide genes, whereas the expression of the corresponding neuropeptides is undetectable in the same cells. Although a transient expression of a neuropeptide gene might yield too little neuropeptide to be detectable, similar quantities of gal4 are likely to be sufficient to activate the UAS-LacZ transgene and lead to significant and easily detectable quantities of beta-galactosidase.
As in the adult, the neuropeptide genes found to be expressed by immunohistological techniques in the larvae are the same as those that are given in the Fly Atlas (Chintapalli et al. 2007). Thus, the absence of immunoreactivity against antisera to adipokinetic hormone, capa 2 (which recoginzes not only the capa peptides, but also pyrokinins and eclosion triggering hormones; Kean et al. 2002), capa precursor, corazonin, crustacean cardioactive peptide, SIFamide, leucokinin, and proctolin in the larval midgut is not surprising, as the expression of these genes has not been found in the Fly Atlas (Table 3). The antiserum to Manduca allatotropin has been included, because homologous peptides are present in other insect species, including mosquitoes (Veenstra and Costes 1999), and in moths allatotropin inhibits midgut ion transport (Lee et al. 1998). However, the recent identification of an allatotropin receptor (Yamanaka et al. 2008) that does not have a homolog in Drosophila (unpublished observations) makes it unlikely that the fruit fly has an allatotropin gene. The apparent expression of bursicon-A and ion transport peptide has been previously discussed (Veenstra et al. 2008).
As judged by the number of immunoreactive cells, the midgut expression of NPF, tachykinin, and allatostatin C is lower in the larva than in the adult, whereas on the same criteria, the expression of the sNPF, DH31, and allatostatin B/MIP is higher in the larva than in the adult. These observations are in agreement with the data from the Fly Atlas (Table 3). The expression of the leucokinin receptor in the larval midgut, but not the adult midgut, is similarly corroborated by the data from the Fly Atlas (Table 4).
The detection of gene expression as used by the Fly Atlas is, in principle, highly specific, even though false positives are possible (e.g. Veenstra et al. 2008), whereas the immunoreactivity of many antisera, particularly those against small amidated neuropeptides such as the antisera to (FM)RFamide used here, depend on the correct processing of the neuropeptide precursors. Nevertheless, it is premature to assume that the peptides themselves are present in the midgut; biochemical evidence for this will be needed.
Of the five Drosophila neuropeptide genes that produce N-terminally extended RFamides, i.e., the sulfakinins, dromyosuppressin, the FMRFamides, NPF, and sNPFs, only the NPF gene has been found to be expressed in endocrine cells of the adult midgut (Veenstra et al. 2008). However, in the larva, we have also detected endocrine cells producing sNPF. These cells react with antisera specific for Drosophila sNPF3 and its precursor and with the less specific antisera to sNPF1, RFamide, and FMRFamide. These cells have not been found when using sNPF-gal4 transgenic flies. It is likely that the sequence of the sNPF gene used to drive the expression of gal4 is insufficient to achieve the same expression as native sNPF. Notably, the 25,000-bp intron of sNPF, which is absent from the transgene, might contain regulatory elements. The expression of this transgene in the central nervous system is also much less extensive than the staining observed by the sNPF-specific antisera (unpublished observations). Nevertheless, there seems to be little doubt that these midgut endocrine cells produce authentic sNPF, as two of three antisera (one against the precursor and one against Drosophila sNPF3) are highly specific and consistently label the same neurons (e.g., Johard et al. 2008; Veenstra et al. 2008); these same two antisera also label the same endocrine cells in the anterior and the beginning of the posterior midgut. Interestingly, sNPF immunoreactivity in the wandering larva is much less pronounced, whereas the sNPF endocrine cells in the posterior midgut in wandering larvae appear to make more allatostatin B/MIP, suggesting that, during development, the ratio in which these two peptides are produced changes.
We have found no evidence for the expression of myosuppressin or sulfakinins in the larval midgut. Although we have detected endocrine cells expressing the DSK-gal4 transgene, these cells never show any immunoreactivity toward either RFamide, FMRFamide, or perisufakinin antisera, whereas the same cells also express other transgenes driven by neuropeptide promotors. We can thus reasonably conclude that, in the larva as in the adult, dms and dsk are not expressed in the midgut. The expression of NPF in the midgut seems to be much less important in the larva than in the adult; furthermore, whereas NPF endocrine cells also produce tachykinins in the adult, NPF colocalizes with allatostatin B/MIP and DH31 in the larva.
In the larval midgut, the expression of tachykinins is limited to the last half of the posterior midgut, whereas in the adult, they are also present in the anterior and the middle midgut. Although the functions of neither sNPF nor the tachykinins are well understood, the sNPF innervation of the proventriculus suggests a role in muscle contractions similar to that ascribed to the tachykinins (e.g., Siviter et al. 2000); perhaps sNPFs released by the endocrine cells in the anterior midgut in the larva play the same role as the tachykinins in the adult midgut (see also Onken et al. 2004).
Allatostatin B/MIP endocrine cells are difficult to detect in the adult midgut (Veenstra et al. 2008). In the larva, on the contrary, allatostatin B/MIP-immunoreactive cells are prominent. The largest allatostatin B/MIP cells are those found just in front of the copper cells. The size of these cells and their strong immunoreactivity suggests that they release much more peptide than the tiny allatostatin B/MIP-immunoreactive cells found between the copper cells themselves. These large allatostatin B/MIP-producing cells might release their product as a hormone, whereas the small cells between the copper cells are possibly paracrine in nature. The latter and the small allatostatin C endocrine cells in the same region are reminiscent of the somatostatin paracrine cells in the vertebrate stomach, suggesting that one of the effects of allatostatin B/MIP and/or C may be to inhibit acid secretion by the copper cells. If this were so, one would expect that, once the large endocrine cells release their products, allatostatins B and/or C would similarly diminish acid secretion. If allatostatin C were responsible for the inhibition of acid secretion by the copper cells, it would have the same function as somatostatin, its vertebrate homolog (Veenstra 2009).
Whereas DH31 endocrine cells in the adult are located exclusively in the caudal half of the posterior midgut, such endocrine cells are distributed throughout the midgut in the larva and are most abundant and immunoreactive in the anterior midgut, the same part of the midgut in which sNPF-producing endocrine cells are found that express the leucokinin receptor. Thus, the larval anterior midgut may play an important role in the regulation of water balance. We have suggested previously that diuretic hormones in the midgut probably serve a local digestive function (Veenstra et al. 2008). If a maggot were feeding on highly diluted food, it would take large amounts of hydrogen ions to acidify its meal. In such cases, it might be advantageous to concentrate the food before acidifying it. If so, it would make sense for the anterior midgut to move water into the hemolymph, and its physiological regulation might be mediated by the endocrine cells in this region.
In the adult, DH31 endocrine cells are only found in the last half of the posterior midgut, whereas the DH31 receptor is abundantly expressed in the Malpighian tubules of the adult (Veenstra et al. 2008), and DH31 is known to stimulate fluid secretion by this tissue (Coast et al. 2001). Thus, the evidence suggests that those cells that also produce tachykinins stimulate fluid secretion by the Malpighian tubules. In the larval midgut many more endocrine cells produce DH31, although the expression of the DH31 receptor in the Malpighian tubules is much less important. Nevertheless, evidence points again to the DH31/tachykinin-immunoreactive endocrine cells in the last half of the posterior midgut as being important regulators of fluid secretion, because the larval Malpighian tubules abundantly express one of the two tachykinin receptors, and the only known cells producing tachykinins that are likely to be released into the hemolymph are those endocrine cells in the posterior midgut. Thus, these cells probably have the same effect on fluid secretion by the Malpighian tubules in both larvae and adults, even though the hormones through which this is achieved might be somewhat different.
The endocrine cells in the posterior part of the midgut could have the same or similar functions in both larvae and adults: one type producing tachykinins and DH31 to stimulate fluid secretion by the Malpighian tubules and the midgut, and a second type producing allatostatins A to inhibit fluid secretion. If nutrients in this part of the gut were abundant, there would be no need to remove more water from the midgut, nor a need to move the gut contents into the next part of the digestive tract, and allatostatins A might be released. If, however, nutrients were present in a diluted watery environment, the DH31 in cells might be released in order to concentrate these nutrients, allowing more efficient digestion and absorption. Although speculative, such a scheme would be consistent with the observation that, in cockroaches, allatostatin A stimulates both the production and release of protease and α-amylase in the midgut (Fuse et al. 1999; Sakai et al. 2006), whereas in cockroach hemolymph the concentrations of tachykinins are significantly increased in starved animals (Winther and Nässel 2001; Pascual et al. 2008).
References
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YHC, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Miklos GLG, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies A, de Pablos B, Delcher A, Deng ZM, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, D Chen unn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong FC, Gorrell JH, Gu ZP, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston DA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke ZX, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai ZW, Lasko P, Lei YD, Levitsky AA, Li JY, Li ZY, Liang Y, Lin XY, Liu XJ, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RDC, Scheeler F, Shen H, Shue BC, Siden-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AHH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang GG, Zhao Q, Zheng LS, Zheng XQH, Zhong FN, Zhong WY, Zhou XJ, Zhu SP, Zhu XH, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC (2000) The genome sequence of Drosophila melanogaster. Science 287:2185–2195
Agricola H-J, Bräunig P, Meissner R, Nauman W, Wollweber L, Davis N (1995) Colocalization of allostatin-like immunoreactivity with other neuromodulators in the CNS of Periplaneta americana. In: Elsner N, Menzel R (eds) Learning and memory. Thieme, Stuttgart, p 616
Alpert S, Hanhan D, Teitelman G (1988) Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell 53:295–308
Andriès JC, Belemtougri G, Tramu G (1991) Multiple peptide immunoreacivities in the nervous system of Aeschna cyanea (Insecta, Odonata). Histochem 96:139–148
Angelini DR, Kaufman TC (2005) Comparative developmental genetics and the evolution of arthropod body parts. Annu Rev Genet 39:95–119
Baggerman G, Cerstiaens A, De Loof A, Schoofs L (2002) Peptidomics of the larval Drosophila melanogaster central nervous system. J Biol Chem 277:40368–40374
Baggerman G, Boonen K, Verleyen P, De Loof A, Schoofs L (2005) Peptidomic analysis of the larval Drosophila melanogaster central nervous system by two-dimensional capillary liquid chromatography quadrupole time-of-flight mass spectrometry. J Mass Spectrom 40:250–260
Becker MN, Brenner R, Atkinson NS (1995) Tissue-specific expression of a Drosophila calcium-activated potassium channel. J Neurosci 15:6250–6259
Boer HH, Schot LPC, Veenstra JA, Reichelt D (1980) Immunocytochemical identification of neural elements in the central nervous systems of a snail, some insects, a fish, and a mammal with an antiserum to the molluscan cardio-excitatory tetrapeptide FMRF-amide. Cell Tissue Res 231:21–27
Burke R, Commons E, Camakaris J (2008) Expression and localisation of the essential copper transporter DmATP7 in Drosophila neuronal and intestinal tissues. Int J Biochem Cell Biol 40:1850–1860
Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA, Spana EP, Davies SA, Dow JA (2002) The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol 205:3799–3807
Chen Y, Veenstra JA, Davis NT, Hagedorn HH (1994) A comparative study of leucokinin-immunoreactive neurons in insects. Cell Tissue Res 276:69–83
Chintapalli VR, Wang J, Dow JAT (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39:715–720
Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA (2001) The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol 204:1795–1804
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by antiserum against synthetic PDH. Cell Tissue Res 250:377–387
Dubreuil RR, Frankel J, Wang P, Howrylak J, Kappil M, Grushko TA (1998) Mutations of a spectrin and labial block cuprophilic cell differentiation and acid secretion in the middle midgut of Drosophila larvae. Dev Biol 194:1–11
Dubreuil RR, Grushko T, Baumann O (2001) Differential effects of a labial mutation on the development, structure, and function of stomach acid-secreting cells in Drosophila melanogaster larvae and adults. Cell Tissue Res 306:167–178
Fuse M, Zhang JR, Partridge E, Nachman RJ, Orchard I, Bendena WG, Tobe SS (1999) Effects of an allatostatin and a myosuppressin on midgut carbohydrate enzyme activity in the cockroach Diploptera punctata. Peptides 20:1285–1293
Grimmelikhuijzen CJP, Graff D (1986) Isolation of pyroGlu-Gly-Arg-Phe-NH2 (Antho-RFamide), a neuropeptide from sea anemones. Proc Natl Acad Sci USA 83:9817–9821
Hauser F, Williamson M, Cazzamali G, Grimmelikhuijzen CJP (2006) Identifying neuropeptide and protein hormone receptors in Drosophila melanogaster by exploiting genomic data. Brief Funct Genomic Proteomic 4:321–430
Hirth F, Hartmann B, Reichert H (1998) Homeotic gene action in embryonic brain development of Drosophila. Development 125:1579–1589
Hirth F, Loop T, Egger B, Miller DFB, Kaufman TC, Reichert R (2001) Functional equivalence of Hox gene products in the specification of the tritocerebrum during embryonic brain development of Drosophila. Development 128:4781–4788
Hoppler S, Bienz M (1994) Specification of a single cell type by a Drosophila homeotic gene. Cell 76:689–702
Hoppler S, Bienz M (1995) Two different thresholds of wingless signalling with distinct developmental consequences in the Drosophila midgut. EMBO J 14:5016–5026
Isabel G, Martin JR, Chidami S, Veenstra JA, Rosay P (2005) AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am J Physiol Regul Integr Comp Physiol 288:R531–R538
Johard HA, Enell LE, Gustafsson E, Trifilieff P, Veenstra JA, Nässel DR (2008) Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: relations to extrinsic neurons expressing different neurotransmitters. J Comp Neurol 507:1479–1496
Johnson EC, Bohn LM, Taghert PH (2004) Drosophila CG8422 encodes a functional diuretic hormone receptor. J Exp Biol 207:743–748
Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JAT (2002) Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 282:R1297–R1307
Lee KY, Horodyski FM, Chamberlin ME (1998) Inhibition of midgut ion transport by allatotropin (Mas-AT) and Manduca FLRFamides in the tobacco hornworm Manduca sexta. J Exp Biol 201:3067–3074
Liu F, Baggerman G, D’Hertog W, Verleyen P, Schoofs L, Wets G (2006) In silico identification of new secretory peptide genes in Drosophila melanogaster. Mol Cell Protoemics 5:510–522
McNulty M, Puljung M, Jefford G, Dubreuil RR (2001) Evidence that a copper-metallothionein complex is responsible for fluorescence in acid-secreting cells of the Drosophila stomach. Cell Tissue Res 304:383–389
Meier S, Sprecher SG, Reichert H, Hirth F (2006) Ventral veins lacking is required for specification of the tritocerebrum in embryonic brain development of Drosophila. Mech Dev 123:76–83
Nakagoshi H (2005) Functional specification in the Drosophila endoderm. Dev Growth Differ 47:383–392
Nakagoshi H, Hoshi M, Nabeshima Y, Matsuzaki F (1998) A novel homeobox gene mediates the Dpp signal to establish functional specificity within target cells. Genes Dev 12:2724–2734
Onken H, Moffett SB, Moffett DF (2004) The anterior stomach of larval mosquitoes (Aedes aegypti): effects of neuropeptides on transepithelial ion transport and muscular motility. J Exp Biol 207:3731–3739
Park D, Veenstra JA, Park JH, Taghert PH (2008) Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS ONE 3(3):e1896. doi:10.1371/journal.pone.0001896
Pascual N, Maestro JL, Chiva C, Andreu D, Belles X (2008) Identification of a tachykinin-related peptide with orexigenic properties in the German cockroach. Peptides 29:386–392
Radford JC, Davies SA, Dow JAT (2002) Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 277:38810–38817
Reichwald K, Unnithan GC, Davis NT, Agricola H, Feyereisen R (1994) Expression of the allatostatin gene in endocrine cells of the cockroach midgut. Proc Natl Acad Sci USA 91:11894–11898
Sakai T, Satake H, Takeda M (2006) Nutrient-induced α-amylase and protease activity is regulated by crustacean cardioactive peptide (CCAP) in the cockroach midgut. Peptides 27:2157–2164
Schonhoff SE, Giel-Moloney M, Leiter AB (2004) Minireview: development and differentiation of gut endocrine cells. Endocrinology 145:2639–2644
Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A (1991) Isolation, identification and synthesis of locustamyoinhibiting peptide (LOM-MIP), a novel biologically active neuropeptide from Locusta migratoria. Regul Pept 35:111–119
Schoofs L, Holman GM, Paemen L, Veelaert D, Amelinckx M, De Loof A (1993) Isolation, identification, and synthesis of PDVDHFLRFamide (SchistoFLRFamide) in Locusta migratoria and its association with the male accessory glands, the salivary glands, the heart, and the oviduct. Peptides 14:409–421
Siviter RJ, Coast GM, Winther AM, Nachman RJ, Taylor CA, Shirras AD, Coates D, Isaac RE, Nässel DR (2000) Expression and functional characterization of a Drosophila neuropeptide precursor with homology to mammalian preprotachykinin A. J Biol Chem 275:23273–23280
Strasburger M (1932) Bau, Funktion und Variabilität des Darmtractus von Drosophila melanogaster. Z Wiss Zool 140:539–649
Terhzaz S, Rosay P, Goodwin SF, Veenstra JA (2007) The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem Biophys Res Commun 352:305–310
Veenstra JA (2009) Allatostatin C and its paralog allatostatin double C: the arthropod somatostatins. Insect Biochem Mol Biol 39:161–170. doi:10.1016/j.ibmb.2008.10.014
Veenstra JA, Costes L (1999) Isolation and identification of a peptide and its cDNA from the mosquito Aedes aegypti related to Manduca sexta allatotropin. Peptides 20:1145–1151
Veenstra JA, Davis NT (1993) Localization of corazonin in the nervous system of the cockroach Periplaneta americana. Cell Tissue Res 274:57–64
Veenstra JA, Hagedorn HH (1993) A sensitive enzyme immuno assay for Manduca allatotropin and the existence of an allatotropin-immunoreactive peptide in Periplaneta americana. Arch Insect Biochem Physiol 23:99–109
Veenstra JA, Lau GW, Agricola HJ, Petzel DH (1995) Immunohistological localization of regulatory peptides in the midgut of the female mosquito Aedes aegypti. Histochem Cell Biol 104:337–347
Veenstra JA, Agricola HJ, Sellami A (2008) Regulatory peptides in the fruit fly midgut. Cell Tissue Res 234:499–516
Winther AM, Nässel DR (2001) Intestinal peptides as circulating hormones: release of tachkinin-related peptide from the locust and cockroach midgut. J Exp Biol 204:1269–1280
Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P (2003) Developmental control of foraging and social behavior by the Drosophila neurpeptide Y-like system. Neuron 39:147–161
Yamanaka N, Yamamoto S, Žitňan D, Watanabe K, Kawada T, Satake H, Kaneko Y, Hiruma K, Tanaka Y, Shinoda T, Kataoka H (2008) Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS ONE 3(8):e3048. doi:10.1371/journal.pone.0003048
Yoon JG, Stay B (1995) Immunocytochemical localization of Diploptera punctata allatostatin-like peptide in Drosophila melanogaster. J Comp Neurol 363:475–488
Acknowledgements
I am grateful to Heinrich Reichert, Ping Shen, and Paul Taghert for generously sending various fly lines, to Frank Hirth, Cok Grimmelikhuijzen, Liliane Schoofs, Julian Dow, René Feyereisen, and Heinrich Dircksen for sharing valuable antisera, to Jean-Luc Morel for an aliquot of his rhodamine-labeled phalloidoin, to Venkat Chintapalli, Jing Wang, and Julian Dow for the timely addition of larval tissues to the Fly Atlas, and to two anonymous reviewers for constructive criticism of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veenstra, J.A. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res 336, 309–323 (2009). https://doi.org/10.1007/s00441-009-0769-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0769-y