Abstract
The effects of starvation on cell death in the midgut of Periplaneta americana were studied histochemically and ultrastructurally. TUNEL assays showed that cell death began to increase in the columnar cells and nidi, the nests of stem cells and newborn cells from 2 weeks of starvation. A significant increase in cell death occurred in the nidi after 4 weeks of starvation. Cockroaches starved for 4 weeks showed active-caspase-3-like immuno-reactivity both in the columnar cells and nidi, whereas control cockroaches that were fed for 4 weeks showed this reactivity only in the apical cytoplasm of columnar cells. Electron microscopy revealed no chromatin condensation in the nucleus of columnar cells of cockroaches, whether fed or starved for 4 weeks. Starved cockroaches exhibited many small vacuoles in the cytoplasm of some columnar cells and “floating” organelles including nuclei in the lumen. A 4-week starvation induced the appearance of cytoplasmic fragmentation and secondary lysosomes in the nidi. Each fragment contained nuclear derivatives with condensed chromatin, i.e. apoptotic bodies. Mitotic cells were found in some, but not all nidi, even within the same starved sample. Fragmentation was not observed in the nidi of control cockroaches. Thus, starvation increases cell death not only in the columnar cells, but also in the nidi. The cell death in the nidi is presumably apoptosis executed by caspase 3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starvation exerts fatal stress on insects and affects various aspects of their life, such as behavior, development, reproduction, and hormone secretion (Nijhout 1975; Barcay and Bennett 1991; Chapman and Partridge 1996; Shintani et al. 2003; Chen and Gu 2006). The intestinal tract of an insect is also affected by starvation in terms of size and cell proliferation activity. We have observed starved male cockroaches for 4 weeks and demonstrated that starvation reduces tissue size and cell proliferation in the midgut of Periplaneta americana but that this rebounds after refeeding (Park and Takeda 2008). We have also reported that the midgut of cockroaches starved for 4 weeks shows the subsidence of the muscle layers into the epithelium in a complex manner, with many small condensed nuclei in the nidi, nests of stem cells and newborn cells. Apoptosis often exhibits nuclear condensation with chromatin concentration (Kerr et al. 1972; Clarke 1990). In the intestinal tracts of mammals, terminal deoxynucleotidyl-transferase-mediated nick-end labeling (TUNEL) assays have demonstrated that starvation stimulates apoptosis (Boza et al. 1999; Jeschke et al. 2000; Chappell et al. 2003), but the appearance of apoptosis in crypts (the sites of cell proliferation) under starvation has not been studied sufficiently (Premoselli et al. 1998). These findings have lead to the hypothesis that starvation induces cell death not only in the columnar cells, but also in the nidi of the cockroach midgut. The cell death in the nidi might involve apoptosis.
In this study, we have investigated the effects of starvation on cell death in the midgut epithelium of P. americana by using the TUNEL method. Immunohistochemistry for active-caspase 3 and electron microscopy have been employed to determine whether the cell death in the nidi is apoptosis. To clarify the relationship between cell death and cell proliferation, and the general events in the midgut under starvation, we have followed the same time schedule as that in our previous experiments on cell proliferation in cockroaches under starvation for 4 weeks (Park and Takeda 2008).
Materials and methods
Insects
Stock colonies of Periplaneta americana were maintained en masse on an artificial diet (MF, Oriental Yeast, Tokyo, Japan) and water provided ad libitum at 25°C. Newly emerged adult males collected from the colonies were kept in plastic containers (30.0×16.5×13.5 cm) for 1 week. Each container housed 15–18 cockroaches that had constant access to the artificial diet and water on a light-dark regimen of 12 h light:12 h darkness.
Starvation stress
Cockroaches were transferred from their containers to small plastic cups (10.0 cm diameter, 4.5 cm high) and kept individually for several weeks at 25°C under the same light-dark cycle. For starvation experiments, the cockroaches were kept without food, and the control cockroaches were maintained with food. Water was always accessible to the cockroaches.
Preparation of tissue sections
The midgut of each fed and starved cockroach was dissected in phosphate-buffered saline (PBS, pH 7.4) at 4°C under CO2 anesthesia. After dissection, the midgut was stretched on a rubber mat and then covered with 4% paraformaldehyde (PFA) in Milloning’s phosphate buffer (M-PBS) at pH 7.4. After 5 min, the tissues were transferred into a bottle containing fresh PFA and kept for 4 h at 4°C. Following fixation, the tissues were dehydrated in an ethanol series, cleared in xylene, embedded in paraffin, and kept overnight at 60°C. Sections of 8 μm thickness were cut from the paraffin blocks on a rotary microtome, mounted on 3-aminopropyltriethoxysilane-coated glass slides, and dried overnight at 37°C.
Histochemistry for TUNEL assay
The ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (S7101, Chemicon International) was used to microscopically examine cell death in the midgut of P. americana by using the TUNEL assay. Dewaxed sections were rinsed in PBS for 5 min, incubated with Proteinase K (20 μg/ml, Sigma, in PBS) for 15 min at room temperature, rinsed in distilled water (2×2 min each), incubated with 3% H2O2 solution for 5 min, and rinsed in PBS for 5 min. Terminal deoxynucleotidyl transferase (TdT) was then overlaid onto the sections, which were incubated in a humidified chamber at 37°C for 1 h. The reaction was stopped in a stop solution. After being rinsed in PBS (3×5 min each), the sections were incubated with digoxigenin antibody for 30 min at room temperature. Following further rinses in PBS (3×5 min each), the sections developed a brown color in 3, 3′-diaminobenzidine (DAB) solution. Negative nuclei were counterstained in blue with hematoxylin. Sections for the negative control were also incubated with reaction buffer, but without TdT.
Quantification of TUNEL-positive signals
To compare the incidence of cell death in the midgut between the fed and starved cockroaches, the density of positive signals of TdT in the columnar cells or the nidi was calculated from the TUNEL-assay data. The numbers of intense positive signals in the columnar cells or the nidi were counted by using a microscope (BX50, Olympus Corporation, Tokyo, Japan). The area of columnar cells or nidi was determined by a point-counting method (Weibel 1979, 1980). A transparent sheet with a grid lattice of 1855 (35×53) points was placed on the photographed images of midgut sections (DP70; Olympus, Tokyo, Japan), and then the number of intersecting points was determined in order to measure the areas. The density of positive signals in the columnar cells or in the nidi is shown both as per section and as per 1000 square micrometers. The mean values derived from 4–6 samples are given with the standard error of the mean. The starved group was compared statistically with the fed controls at the same time points by the Mann-Whitney’s U-test (StatView 5.0 software Japanese version, SAS Institute, USA). Statistical difference was defined as P<0.05.
Immunohistochemistry for active-caspase 3
Sections from the midgut of cockroaches fed for 4 weeks or starved for 4 weeks were dewaxed in xylene, passed through an ethanol series, and then rinsed in PBS twice for 5 min each. The sections were incubated in 0.01 M citrate buffer, pH 6.0, at 80°C for 1 h. Thereafter, they were placed at room temperature for 2 h. The sections were rinsed in distilled water (2×5 min each) and in PBS with 0.5% Tween 20 (PBT) for 5 min and then incubated with blocking solution (PBT containing BSA) for 1 h at room temperature. After this blocking step, the sections were incubated with affinity-purified rabbit antibody against active-caspase 3 (AB3623; Chemicon International; diluted 1:50 in blocking solution) overnight at 4°C, rinsed in PBT (3×5 min each), and then incubated with biotinylated anti-rabbit IgG (Vectastain ABC Kit PK-6101; Vector Laboratories, Burlingame, USA; diluted 1:200 in blocking solution) for 1 h at room temperature. Subsequently, the sections were rinsed in PBT (3×5 min each), incubated with avidin-horseradish peroxidase complex (Vectastain ABC Kit PK-6101) for 30 min at room temperature., rinsed with PBT, and then exposed to DAB solution for 20 min. Positively reacting areas of the sections became brown in color. The sections were also counterstained with hematoxylin. Sections used for the negative control were also incubated with blocking solution but without the primary antibody.
Electron microscopy
The midgut dissected from cockroaches fed or starved for 4 weeks was fixed in 2.5% glutaraldehyde (GA) in M-PBS for 5 min, after which it was cut into small pieces of 2 mm in length, transferred into a new bottle, and fixed in fresh GA at 4°C overnight. The samples were rinsed in M-PBS (3×10 min each), fixed with buffered 1% OsO4 for 1 h at 4°C, rinsed in distilled water for 1 min, dehydrated in ethanol, and embedded in Quetol 812 resin mixture. Ultrathin sections were cut from the resin blocks with an ultra microtome MT-1 (Ivan Sorvall) by using a histo-diamond knife or diamond knife (Diatome, Switzerland). The sections were stained with 4% uranyl acetate for 10 min and lead citrate for 10 min and observed by using a Hitachi H-7100 electron microscope at 75 kV.
Results
Structure of the cockroach midgut
The midgut of P. americana consists of two layers (Fig. 1a). The outer layer is connective tissue composed of muscles and collagen fibers. The inner layer is an epithelium containing three different cell types: columnar cells, endocrine or paracrine cells, and stem cells. The columnar cells have microvilli facing the lumen and are cylindrical in shape. Nidi are located at the basal side of the epithelium at regular intervals. The midgut of starved cockroach has a smaller diameter to that of fed cockroach (data not shown).
Localization and quantification of TUNEL-positive signals in the midgut of fed or starved cockroaches. a Cockroaches fed for 4 weeks show positive signals in the nuclei (black arrows) and in the apical cytoplasm (white arrow) of the columnar cells (cc columnar cell, l lumen, ml muscle layer, mv microvilli, nd nidus). b Cockroaches starved for 4 weeks show positive signals not only in the columnar cells (arrow), but also in the nidi (arrowheads). Bars 50 μm. c, e Densities of the positive signals in the columnar cells begin to increase after 2 weeks of starvation. d, f Densities of the positive signals in the nidi begin to increase after 2 weeks of starvation and show a significant increase after 4 weeks of starvation. c, d Density/section. e, f Density/1000 μm2 (open circles fed cockroaches, filled circles starved cockroaches, n=4-6). Data are presented as means±SEM. *Significantly different from fed controls at the same time point, P<0.05
TUNEL assay
Two different types of positive signals from the TdT enzyme were observed in the columnar cells: one in the nuclei (black arrows in Fig. 1a) and the other in the apical cytoplasm (white arrow in Fig. 1a). The sizes of positive signals in the apical cytoplasm were the same as or smaller than those of the nuclei in the columnar cells. Cockroaches starved for 3 or 4 weeks showed positive signals not only in the columnar cells, but also in the nidi (arrowheads in Fig. 1b). The midgut of both fed and starved cockroaches, when incubated without TdT enzyme solution, showed no signals (data not shown).
Quantitative analyses based both density/section and density/1000 μm2 gave essentially similar results. Starvation increased TUNEL-positive signals both in the columnar cells and in the nidi. In the columnar cells, the density of the positive signals began to exceed that of the fed cockroaches after 2 weeks of starvation (Fig. 1c, e). In the nidi, the density of the signals in the fed cockroaches remained at low values, but that of the starved cockroaches began to increase after 2 weeks and showed significant increases after 4 weeks of starvation (P<0.05; Fig. 1d, f).
The midgut of cockroaches starved for 4 weeks was further examined immunohistochemically by targeting active-caspase 3 and by using electron microscopy to clarify the features of cell death in the nidi under starvation. The midgut of cockroaches fed for 4 weeks was also examined as a control.
Immunohistochemistry for the occurrence and localization of active-caspase 3
The midgut of cockroaches fed for 4 weeks showed active-caspase-3-like immunohistochemical reactivity (caspase-3-ir) only in the apical cytoplasm of columnar cells (arrows in Fig. 2a). The sizes of the immuno-positive granules were the same as or smaller than the sizes of the nuclei in the columnar cells. Cockroaches starved for 4 weeks showed caspase-3-ir not only in the columnar cells, but also in the nidi (arrow and arrowheads in Fig. 2b). Caspase-3-ir in the nidi occurred as granules or was localized in the cytoplasm around the nucleus. The midgut of both fed and starved cockroaches incubated without the antibody showed no reactivity (data not shown).
Active-caspase-3-like immunohistochemical reactivity in the the midgut of cockroaches fed or starved for 4 weeks (cc columnar cell, l lumen, ml muscle layer, mv microvilli, nd nidus). a Cockroaches fed for 4 weeks show positive reactivity as granules but only in the apical cytoplasm of columnar cells (arrows). b Cockroaches starved for 4 weeks show positive reactivity not only in the columnar cells (arrow), but also in the nidi (arrowheads). Bars 50 μm
Ultrastructural observation of the midgut
Columnar cells of cockroaches fed for 4 weeks had rich cytoplasm with abundant rough endoplasmic reticulum (rER), mitochondria, and various sizes of secondary lysosomes (Fig. 3a). Some secondary lysosomes contained the debris of organelles (Fig. 3b). The nidi consisted of multilayered packed cells with a small volume of cytoplasm, which contained many mitochondria, little rER, and few secondary lysosomes (Fig. 3c). Connective tissue lay beneath the basal side of the epithelium. The tissues were composed of collagen fibers, ground substances, fibroblasts, muscles, and tracheae. Smooth basal laminae were deposited between the epithelium and the connective tissue.
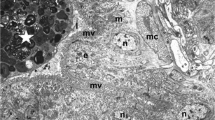
Columnar cells and nidi in the midgut of cockroaches fed for 4 weeks. a, b Columnar cells have a large cytoplasmic area with abundant rough endoplasmic reticulum (rer), mitochondria, and various sizes of secondary lysosomes (sl), some of which exhibit the debris of organelles (arrow in b; nc nucleolus, nu nucleus, mv microvilli). c The nidi (nd) consist of aggregates of multilayered small cells. The basal laminae are smooth at the basal side of the nidi (cf collagen fiber, mc muscle, t trachea). Bars 5 μm (a, c), 2 μm (b)
The majority of columnar cells of cockroaches starved for 4 weeks did not show significant structural differences from those of cockroaches fed for 4 weeks (Fig. 4a). They also had rER and various sizes of secondary lysosomes containing the debris of organelles in their apical cytoplasm (arrow in Fig. 4a), but some columnar cells of the starved cockroaches did not show any rER in their cytoplasm, which contained many small vacuoles (Fig. 4b, c). Other columnar cells collapsed, and their organelles including the nucleus, mitochondria, and denatured rER were discharged into the lumen (Fig. 4d). The nuclei in the lumen (Fig. 4d) seemed to be intact, but euchromatin was rarely observed in these nuclei, and the heterochromatin was more condensed than that in situ in the fed (Fig. 3a) and starved (Fig. 4a) cockroaches. The nidi of the starved cockroaches showed different structures from those of the fed animals. The papilla-shaped nidi exhibited many secondary lysosomes, cytoplasmic fragments, aggregated intracellular tubules, and wider intercellular spaces between the cells (Fig. 4e, f). The cytoplasmic fragments had split nuclei with condensed chromatin (arrows in Fig. 4e). Moreover, mitotic division was observed in some nidi, but not all, even in the same starved sample (Fig. 4g). Basal laminae of the starved cockroaches were more complex and rougher than those of the fed animals (Fig. 4e).
Columnar cells and nidi in the midgut of cockroaches starved for 4 weeks. a Intact columnar cells possess rough endoplasmic reticulum (rer), mitochondria, and various sizes of secondary lysosomes (sl) containing cell debris (arrow). b Affected columnar cells have many small vacuoles in their cytoplasm. c Higher magnification of the boxed area in b. d The nuclei (nu), mitochondria (black arrows), and denatured rough endoplasmic reticulum (arrowheads) discharged from the collapsed columnar cells are found in the lumen (l) above the microvilli (mv). e Papilla-shaped nidi (nd) possess many cytoplasmic fragments containing split nuclei with condensed chromatin (white arrows) and a large volume of aggregated intracellular tubules. The basal laminae are more complex than those of the fed cockroaches. f, g Secondary lysosome (sl) and mitotic division are seen in other nidi (cf collagen fiber, mc muscle). Bars 5 μm (a, b, d, e), 2 μm (c, f, g)
Discussion
Cockroaches starved for 4 weeks showed a significantly higher density of TUNEL-positive signals (Fig. 1d, f) and cytoplasmic fragments containing nuclear derivatives with condensed chromatin in the nidi (Fig. 4e). However, none of these cytoplasmic fragments were observed in the nidi of continuously fed cockroaches (Fig. 3c). The morphological features of these fragments were similar to those of apoptotic bodies shown by Kerr et al. (1972). These results lead to the conclusion that the TUNEL-positive signals in the nidi of starved cockroaches represent apoptotic bodies (Fig. 1b), namely starvation-induced apoptosis in the nidi of the midgut. The appearance of caspase-3-ir in the nidi of cockroaches starved for 4 weeks shows that apoptosis appearing in the nidi under starvation is caspase-dependent. Generally, the caspase family induces the condensation of chromatin, the condensation and blebbing of cytoplasm, and the internucleosomal degradation of DNA. The caspase family is divided into two categories: initiator caspases (caspase 8 and 9) and effector caspases (caspase 3 and 7). Extracellular apoptotic signals are mediated by caspase 8, whereas internal apoptotic signals derived from mitochondria are mediated by caspase 9. Both signals activate effector caspases such as caspase 3. Activated caspase 3 induces apoptotic features and demarcates the “point of no return”. Therefore, this caspase is called executioner caspase (Lockshin and Zakeri 2004). Further experiments are required to clarify whether caspase 8 or 9 induces apoptosis in the nidi under starvation.
An increase in the density of TUNEL-positive signals in columnar cells under starvation represented an increase of cell death in columnar cells (Fig. 1c, e). However, immunohistochemistry for active-caspase 3 and electron microscopy did not show the appearance of apoptosis in the columnar cells, either in the fed cockroaches or in those starved for 4 weeks. Caspase-3-ir was observed in the midgut of both the fed and the starved cockroaches as granules in the apical cytoplasm of columnar cells (Fig. 2a, b). TUNEL-positive signals (white arrow in Fig. 1a) and secondary lysosomes containing debris of organelles (Figs. 3a, b, 4a) were occasionally seen at similar positions. Caspase 3 may be related to the digestion of cell debris containing nuclear fragments in the secondary lysosomes of columnar cells because caspase 3 is involved in digestion of the cytoskeleton under metamorphosis (Martin and Baehrecke 2003). Columnar cells in the midgut of the fed or starved cockroaches do not contain nucleus with markedly condensed chromatin (Figs. 3a, Fig. 4a). Endo and Nishiitsutsuji-Uwo (1982) have found dark intact columnar cells containing dark nucleus without markedly condensed chromatin in the midgut of P. americana and concluded that these are degenerating cells that will eventually be discharged into the lumen. The dark cells might be undergoing apoptosis because similar dark cells with degenerating cytoplasm and nuclei have been defined as apoptotic in the villus tips of the small intestine in mice (Potten and Allen 1977). Some columnar cells of the starved cockroaches lose rER and exhibit many small vacuoles in their non-dark cytoplasm (Fig. 4b, c). Our electron-microscopic observations have revealed “floating” organelles including nuclei and degenerated rER in the lumen (Fig. 4d). The small vacuoles are probably degenerated rER that might change into swollen rER in the lumen after the columnar cells have collapsed and the vacuoles are discharged into the lumen (Fig. 4d). The observed “floating” nuclei in the lumen are presumably not be derived from the cell death as reported by Endo and Nishiitsutsuji-Uwo (1982) because the nuclei are not dark and seem to be intact (Fig. 4d). These results show the possibility that starvation induces cell death that is not derived from dark columnar cells. However, the appearance and the features of the cell death of columnar cells in insect midgut have not been studied sufficiently, even under fed conditions. Further qualitative and quantitative experiments are required with electron microscopy.
The midgut of starved cockroaches shows reduced cell proliferation activity and increased cell death (Park and Takeda 2008; Fig. 1c-f). These events under starvation reduce the total cell population and save energy consumption in the midgut. The cell debris, including the apoptotic bodies that appear after cell death, might induce the appearance of secondary lysosomes and supply energy to engulfing cells (Fig. 3b and Fig. 4a, f). The appearance of apoptotic bodies in nidi after 4 weeks of starvation (Fig. 4e) may represent the last means of supplying energy for the nidi, as mitosis is sustained even under 4 weeks of starvation (Park and Takeda 2008; Fig. 4g). The nidi show high cell proliferation after refeeding (Park and Takeda 2008). The saving and production of energy by reducing cell proliferation and increasing cell death are probably survival strategies in the midgut of starved cockroaches. These strategies may allow the midgut to maintain the minimum ability of digestion and cell proliferation in order to speed the recovery of normal function as soon as the animal is refed.
Caterpillars metabolize glucose derived from food into glycogen and then store it in their fat body. During short-term starvation, the stored glycogen is metabolized into trehalose and then released into the hemolymph to maintain the required level of trehalose. However, if starvation continues for a long term, the levels begin to decline because of the shortage of glycogen (Bede et al 2007). This result may explain the reduced cell proliferation activity and increased cell death in the midgut of P. americana after 2 weeks of starvation (Park and Takeda 2008; Fig. 1c-f). During the first week of starvation, cockroaches use nutrients stored in their fat body to sustain the normal digestive condition of the midgut. However, the nutrients are used up after 2 weeks of starvation, and then the midgut must embark on a new survival strategy: the reduction of cell proliferation and increase in cell death. Further starvation probably forces additional mobilization of energy derived, even from the nidi, by apoptosis. Improved definition of the chronological effects of starvation on the fat body and the relationships between the fat body and other organs including the midgut should clarify the hierarchical defense mechanisms of insects under starvation, from optimistic conditions to emergency situations.
References
Barcay SJ, Bennett GW (1991) Influence of starvation and lighting on the movement behavior of the German cockroach (Blattodea: Blattellidae). J Econ Entomol 84:1520–1524
Bede JC, McNeil JN, Tobe SS (2007) The role of neuropeptides in caterpillar nutritional ecology. Peptides 28:185–196
Boza JJ, Moennoz D, Vuichoud J, Jarret AR, Gaudard-de-Weck D, Fritsche R, Donnet A, Schiffrin EJ, Perruisseau G, Ballevre O (1999) Food deprivation and refeeding influence growth, nutrient retention and functional recovery of rats. J Nutr 129:1340–1346
Chapman T, Partridge L (1996) Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci 22:755–759
Chappell VL, Thompson MD, Jeschke MG, Chung DH, Thompson JC, Wolf SE (2003) Effects of incremental starvation on gut mucosa. Dig Dis Sci 48:765–769
Chen CH, Gu SH (2006) Stage-dependent effects of starvation on the growth, metamorphosis, and ecdysteroidogenesis by the prothoracic glands during the last larval instar of the silkworm, Bombyx mori. J Insect Physiol 52:968–974
Clarke PG (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 181:195–213
Endo Y, Nishiitsutsuji-Uwo J (1982) Fine structure of developing endocrine cells and columnar cells in the cockroach midgut. Biomed Res 3:637–644
Jeschke MG, Debroy MA, Wolf SE, Rajaraman S, Thompson JC (2000) Burn and starvation increase programmed cell death in small bowel epithelial cells. Dig Dis Sci 45:415–420
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Lockshin AD, Zakeri Z (2004) When cells die. II. A comprehensive evaluation of apoptosis and programmed cell death. Wiley-Liss, New York
Martin DN, Baehrecke EH (2003) Caspase function in autophagic programmed cell death in Drosophila. Development 131:275–284
Nijhout HF (1975) A threshold size for metamorphosis in the tobacco horn worm, Manduca sexta (L.). Biol Bull 149:214–225
Park MS, Takeda M (2008) Starvation suppresses cell proliferation that rebounds after refeeding in the midgut of the American cockroach, Periplaneta americana. J Insect Physiol 54:386–392
Potten CS, Allen TD (1977) Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res 60:272–277
Premoselli F, Sesca E, Binasco V, Caderni G, Tessitore L (1998) Fasting/re-feeding before initiation enhances the growth of aberrant crypt foci induced by azoxymethane in rat colon and rectum. Int J Cancer 77:286–294
Shintani Y, Munyiri F, Ishikawa Y (2003) Change in significance of feeding during larval development in the yellow-spotted longicorn beetle, Psacothea hilaris. J Insect Physiol 49:975–981
Weibel ER (1979) Practical methods for biological morphometry. Stereological Methods, vol 1. Academic Press, London
Weibel ER (1980) Theoretical foundations. Stereological Methods, vol 2. Academic Press, London
Acknowledgements
We thank the staff of the Park Laboratory (Cell Structure and Regulation) for advice and technical assistance with electron-microscopic observations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, M.S., Park, P. & Takeda, M. Starvation induces apoptosis in the midgut nidi of Periplaneta americana: a histochemical and ultrastructural study. Cell Tissue Res 335, 631–638 (2009). https://doi.org/10.1007/s00441-008-0737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0737-y