Abstract
Parkinson’s disease (PD) is the most common movement disorder. The neuropathology is characterized by the loss of dopamine neurons in the substantia nigra pars compacta. Transplants of fetal/embryonic midbrain tissue have exhibited some beneficial clinical effects in open-label trials. Neural grafting has, however, not become a standard treatment for several reasons. First, the supply of donor cells is limited, and therefore, surgery is accompanied by difficult logistics. Second, the extent of beneficial effects has varied in a partly unpredictable manner. Third, some patients have exhibited graft-related side effects in the form of involuntary movements. Fourth, in two major double-blind placebo-controlled trials, there was no effect of the transplants on the primary endpoints. Nevertheless, neural transplantation continues to receive a great deal of interest, and now, attention is shifting to the idea of using stem cells as starting donor material. In the context of stem cell therapy for PD, stem cells can be divided into three categories: neural stem cells, embryonic stem cells, and other tissue-specific types of stem cells, e.g., bone marrow stem cells. Each type of stem cell is associated with advantages and disadvantages. In this article, we review recent advances of stem cell research of direct relevance to clinical application in PD and highlight the pros and cons of the different sources of cells. We draw special attention to some key problems that face the translation of stem cell technology into the clinical arena.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the most common movement disorder. About 1% of the population older than 60 years is affected. Although a number of brain regions are affected in PD, the neuropathology that has received the most attention is the loss of dopamine (DA) neuron in the substantia nigra pars compacta. This leads to a decrease of DA in the striatum. The patients exhibit bradykinesia, rigidity, and tremor (Samii et al. 2004). Currently, there are three main approaches to treat PD; drug therapy, deep brain stimulation (DBS), and cell transplantation.
Most patients start with medication that enhances dopaminergic neurotransmission in the failing nigrostriatal system, such as L-3,4-dihydoroxyphenylalanine (L-dopa) and DA agonists. In the long-term, however, drug treatment loses its efficacy (wearing-off phenomenon), and the patient develops dramatic fluctuations in motor function, despite high blood levels of L-dopa (on-off phenomenon), and drug-induced involuntary movements (dyskinesia). With continued disease progression, many patients also suffer from neuropsychiatric symptoms.
Surgical treatment with DBS can partially circumvent these problems. Especially stimulations in the subthalamic nucleus (STN) can improve motor function and reduce off-time dyskinesias and medication usage (Pahwa et al. 2006). The key to the beneficial outcome of DBS is to select the patients carefully. A robust preoperative response to L-dopa is a good predictor of successful outcome of DBS in the STN. In general, DBS is the most effective in PD patients that are at Hoehn and Yahr stage 2–4 and display intact cognition (Samii et al. 2004). As with any neurosurgical procedure, DBS is also associated with certain risk, such as infection, intracranial hemorrhage, and seizures. Some patients also develop post-operative depression. On the whole, however, serious adverse events are relatively rare with DBS (Umemura et al. 2003; Kenney et al. 2007). Whereas DBS can provide long-term symptomatic relief and is definitely an important addition to the therapeutic arsenal in PD, the procedure neither slows down disease progression, nor is reparative in nature.
Outline of various types of donor cells for transplantation
Three major sources of cells have been considered as donor cells for PD (Fig. 1, Table 1). The first source is represented by the cells of the embryonic/fetal ventral mesencephalon (VM) as presently used in the clinical trials. The second and third sources are various types of stem cells. Stem cells are defined as undifferentiated cells that are capable of self-renewal and that can differentiate into different cell types, i.e., they are multipotent (Gage 2000). Stem cell research has received much attention, both in scientific circles and also in the media, because they show potential for being applied in regenerative medicine. The second candidate donors for PD are thus the neural stem cells (NSC), which can be derived from embryonic/fetal or adult brains. This stem cell type is most probably restricted to differentiating along neural cell lineages, i.e., into neurons, astrocytes, or oligodendrocytes. The third potential source of donor tissue is represented by embryonic stem cells (ESC) obtained from the inner cell mass of the blastocyst. This cell type is pluripotent and can theoretically produce all tissues in the body. A number of human ESC lines have been established during the last decade, and technologies to control and direct their differentiation have emerged. Another possibility is the use of somatic stem cells obtained from tissues outside the central nervous system. These cell types are derived from specific mature tissues, such as bone marrow and skin, and from umbilical cord (Li et al. 2001; Joannides et al. 2004; Fu et al. 2006). Several well-debated studies over the past 8 years have claimed that these somatic stem cells retain a remarkable plasticity and can “transdifferentiate” into cell types not normally found in the tissues in which they reside (Priller et al. 2001; Cogle et al. 2004). There is still no strong evidence that these stem cells can really differentiate into neurons and function as such in vivo, and certainly, no demonstration has shown that they can differentiate into DA neurons. Should there be a breakthrough in this research field and should somatic stem cells from non-neural sources prove able to differentiate into DA neurons, major implications would have to be addressed. Such cells would definitely be useful for the therapy of PD and could provide an ethically uncontroversial and inexhaustible source of donor cells that could even be obtained from the patients themselves (i.e., autologous grafting). At least for now, however, the former three cell types, namely VM tissue, NSC, and ESC, have more possibilities for clinical application. Therefore, we will focus on these three sources of cells in this review.
Strategy of cell therapy for Parkinson’s disease (PD). Three types of cells are considered as possible donor sources of transplantation for PD: embryonic/fetal ventral mesencephalic cells, neural stem cells, and embryonic stem cells. The primary goal is to obtain high enough numbers of DA-neuron progenitors that are functional in the host brain and have no risk of tumor formation
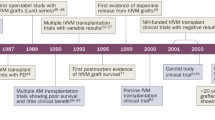
Embryonic/fetal VM transplantation
After several years of systematic animal studies (Brundin and Hagell 2001), the first clinical trial with cell transplantation in PD was performed in 1987. The graft tissue was derived from human VM of aborted embryos (Brundin et al. 1987). Up to now, about 400 PD patients worldwide have received this treatment. In several small open-label trials, some grafted patients have exhibited dramatic improvements in general performance, increased speed of movement, and reduction of rigidity. 18F-fluorodopa and 11C-raclopride positron emission tomography (PET) scans have indicated long-lasting survival and functionality of the grafts in those patients (Brundin et al. 2000b; Hagell and Brundin 2001; Hauser et al. 1999; Mendez et al. 2000). However, two double-blind placebo-controlled trials sponsored by the National Institutes of Health have failed to improve symptoms at the primary endpoints. In addition, some patients have developed graft-induced dyskinesias (GID; Freed et al. 2001; Olanow et al. 2003). A subsequent reevaluation of some of the open-label trials has revealed that the patients in these studies also occasionally develop involuntary movements that persist in the absence of L-dopa therapy (Hagell and Cenci 2005). As a consequence of the unexpectedly poor outcome of the controlled trials and the observations of GID, neural transplantation in PD has virtually been halted. Nevertheless, these reports have initiated useful discussions regarding the future of neural transplantation as a whole. For example, crucial technical factors for successful cell transplantation and how to avoid side effects such as severe GID are areas of active debate. Two main topics are in focus, i.e., patient selection and surgical issues. Disease stage and preoperative response to L-dopa are thought to be important predictors of transplant outcome (Olanow et al. 2003, 2004; Freed et al. 2004). Recently, several different genetic forms of PD have been identified (Hardy et al. 2006), even though familial PD patients represent a minority of all PD cases. The emerging complexity of PD genetics suggests that the various grafted PD patients have suffered from diverse underlying pathogenetic processes. Thus, not all patients suffering from PD might be suitable for cell therapy. In the first reported double-blind trial, younger patients (less than 60 years old) exhibited a better response to the grafts than older subjects (Freed et al. 2001). Recent animal experimental studies (Breysse et al. 2007) and these clinical data indicate that more severe pathology at baseline reduces the chances of a successful outcome after transplantation. Several surgical aspects have also varied between studies, e.g., tissue preparation, number of cells transplanted, graft implantation techniques, and immunosuppression regimens. In some studies, solid pieces of embryonic donor tissue have been grafted, whereas in others, the tissue has been mechanically dissociated into a cell suspension before injection. Most trials have used freshly dissected fetal VM tissue. However, VM tissue was stored in cell culture for up to 4 weeks before transplantation in the “Denver-Columbia” trial (Freed et al. 2001). The number of donor embryos used for one side of the putamen and/or caudate nucleus has ranged from 1 to 8 (Hagell and Brundin 2001). In many open-label trials, combinations of different immunosuppressive drugs have been given to the patients for more than 6 months after surgery. In the “Tampa-Mount Sinai” trial, the second published double-blind trial, cyclosporine A was given alone and only for 6 months post-surgery (Olanow et al. 2003). Interestingly, this study showed that the grafted patients started losing the functional benefit of the grafts 6 months after transplantation, which implied that immune rejection might occur once immunosuppression is stopped. Some patients from the “Tampa-Mount Sinai” patients died several years after surgery because of unrelated causes. Interestingly, the grafts contained many normal-looking DA neurons that were, however, typically surrounded by numerous immunocompetent cells that may be indicative of an immune response (Kordower et al. 1997). By contrast, the “Denver-Columbia” trial did not use any immunosuppression at all, which may have contributed to the poor survival of the grafts. Only 7,000 to 40,000 surviving tyrosine hydroxylase (TH)-immunopositive neurons per side were observed in one of the cases (Freed et al. 2001). This is far below the minimal number (>100,000) of TH-immunopositive neurons per side of the brain suggested to be necessary to elicit a good clinical response (Hagell and Brundin 2001). As mentioned briefly above, GIDs have also become an important issue that jeopardize the continued development of not only neural transplantation in PD, but also the future use of stem cells in PD. Virtually all patients on anti-Parkinson medication eventually develop L-dopa-induced dyskinesias. The frequency of GIDs in grafted patients is much lower, i.e., fewer than 50% of patients exhibit some involuntary movements, which only trouble a small minority. Therefore, we could argue that GIDs should not be viewed as a greater source of concern than L-dopa-induced dyskinesias. The fundamental difference, however, is that GIDs do not disappear simply by changing or removing the medication, but potentially they represent a permanent change of brain function. As is also largely the case for L-dopa-induced dyskinesias, the mechanisms underlying GID are still unknown. Rats grafted with VM from embryos can exhibit abnormal involuntary movements in response to amphetamine (Lane et al. 2006; Carlsson et al. 2006) or L-dopa (Maries et al. 2006). Interestingly, these movements primarily occur in rats that have received L-dopa prior to grafting and have displayed L-dopa-induced involuntary movements. Both the size of the implants and their precise location within the striatum seem to influence the risk of drug-induced abnormal involuntary movements (Lane et al. 2006; Carlsson et al. 2006; Maries et al. 2006).
Taken together, these data suggest that cell transplantation has great potential to provide symptomatic relief in PD, but that many issues need to be clarified, and that techniques accordingly should be optimized before this method can be developed into a therapy. Further clinical trials are definitely needed to address some of these issues. Moreover, relying on tissue from aborted embryos/fetuses is not sustainable in the long run, and an alternate source of donor cells needs to be developed.
In this context, the strategy of using stem cells as a source of cells for transplantation in PD is both theoretically feasible and attractive from both practical and ethical standpoints.
Neural stem cells
The term “neural stem cell” (NSC) has been used loosely to describe cells that can generate neural tissue or are derived from the neural systems. In this review, we use the term NSC to define a multipotent cell type that is derived from neural tissues and is committed to the neural linage. This type of cells is found in the developing nervous system and in the mature brain. In human adult brain, NSCs are enriched in the subventricular zone and subgranular zone of the hippocampal dentate gyrus (Eriksson et al. 1998; Roy et al. 2000; Sanai et al. 2004). Recently, the human equivalent of the rodent rostral migratory stream has been described, suggesting that neurogenesis also takes place in the adult human subventricular zone, and that the newly born neurons migrate to the olfactory bulb (Curtis et al. 2007). Thus, there are two potential sources of human NSCs for cell therapy: immature brain tissue (e.g., from an aborted embryo) or adult brain tissues (e.g., from a patient’s brain). One potential advantage of NSCs over ESCs is that they are less prone to form tumors after transplantation. Importantly, NSCs appear to be genomically more stable than ESCs, which often exhibit chromosomal changes (Cowan et al. 2004; Maitra et al. 2005; Herszfeld et al. 2006; Draper et al. 2004). Nevertheless, NSCs can exhibit signs of senescence (e.g., telomere shortening) after repeated passaging (Ostenfeld et al. 2000).
Regional specificities of neural stem cells
Typically, NSCs proliferate in a culture medium supplemented with specific mitogenic growth factors, such as basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF; Gage et al. 1995; Ostenfeld and Svendsen 2004). Regardless of which part of the immature central nervous system from which they are derived, NSCs proliferate in response to similar growth factors. However, they are not all equivalent to each other, so that they maintain certain specific properties related to their origins, even after being maintained for prolonged periods in vitro (Hitoshi et al. 2002; Ostenfeld et al. 2002; Parmar et al. 2002). Consequently, when exposed to optimal culture conditions, NSC obtained from VM produce more DA neurons than NSC from other parts of the central nervous system (Ostenfeld et al. 2002; Horiguchi et al. 2004; Schwarz et al. 2006). Apart from regional specificity, the proportion of DA neurons generated from NSCs is also highly dependent upon the age of donor tissue, the length of culture in vitro, and details of the culture conditions (Jensen and Parmar 2006). In general, the proportion of NSCs that differentiates into DA neurons is low. The number of TH-immunopositive cells is less than 2% out of the total number of cells, even if the NSCs are derived from the rat embryonic midbrain and are genetically modified to express transcription factors associated with DAergic differentiation (Svendsen et al. 1997; L. Roybon et al., in preparation). Attempts to generate large quantities of DA neurons from NSCs originally obtained from the embryonic human nervous system have not provided more encouragement (Christophersen et al. 2006; Yang et al. 2004). With regard to NSCs harvested from the adult human nervous system, the generation of DA neurons is apparently even more difficult, and to date, there is no convincing demonstration that functional DA neurons have been obtained from such NSCs.
Protocols for inducing DA neurons from neural stem cells
A combination of defined soluble factors is one of the most common approaches to promote the differentiation of DA neurons from NSCs. Carvey et al. (2001) have reported that a combination of interleukin-1 (IL-1), interleukin-11 (IL-11), leukemia inhibitory factor, and glial cell line-derived neurotrophic factor (GDNF) yields TH expression in 20%–25% of cells derived from rodent mesencephalic progenitors. By treating the cells with these factors, the authors have generated and expanded a clone in which 98% of the cells express TH. Transplants of these cells are able to reverse asymmetric motor behavior in the hemiparkinsonian rat model, indicating that the cells are functional DA neurons. Despite the apparent success of this culture protocol, it is not widely used by other groups.
As mentioned above, there are no reports of culture protocols supporting the generation of large numbers of DA neurons from human NSCs derived from the immature brain (Christophersen et al. 2006; Yang et al. 2004). We have previously demonstrated that a cocktail of several factors, e.g., acidic fibroblast growth factor, forskolin, phorbol 12-myristate 13-acetate, dibuturyl cyclic AMP, GDNF, insulin-like growth factor-I, and IL-1α, induce the differentiation of neural progenitors into DA neurons. The efficiency is still very limited and only 4%–10% of the cells are TH-immunopositive at the end of the differentiation period (Christophersen et al. 2006).
For successful transplantation in PD, the donor neurons should not only express TH, but the neurons should also be of the midbrain DA neuronal phenotype that is found in the substantia nigra pars compacta (A9 region). TH-immunopositive cells are also found in the olfactory bulb, and these cells co-express γ-aminobutyric acid, which A9 neurons in substantia nigra do not. According to studies of VM transplants in animals (Thompson et al. 2005) and PD patients (Mendez et al. 2005), only A9 DA neurons can effectively innervate the striatum. Grafts of primary midbrain tissue contain a mixture of DA neurons, including those derived from the A9 and those coming from the adjacent ventral tegemental area (A10). Thus, the distinction of A9 DA neurons from both olfactory bulb and A10 DA neurons is highly important and possible to achieve by using multiple markers. The A9 DA neurons express a unique G-protein-coupled inwardly rectifying potassium channel (Girk2) and are negative for calbindin that, on the other hand, is expressed in the ventral tegmental DA neurons (A10). In order to verify that neurons are truly of the A9 DA neuronal phenotype, they should express additional marker proteins, such as Nurr1, Pitx3, Engrailed1/2 (En1/2), Lmx1a, Lmx1b, and DA transporter (Andersson et al. 2006).
The delivery of genes encoding transcription factors has also been pursued to regulate the differentiation of DA neurons. Rat forebrain precursors have been reported to differentiate into DA neurons in vitro merely after being genetically modified to co-express Nurr1, Sonic hedgehog (SHH), and Bcl-XL (Nurr1/SHH/Bcl-XL) or Nurr1 and the proneural bHLH transcription factor Mash1 (Nurr1/Mash1). Such precursors engineered with Nurr1/SHH/Bcl-XL or Nurr1/Mash1 also survive grafting into the striatum and reverse asymmetric motor behavior in rats with unilateral 6-hydroxydopamine (6-OHDA)-lesions of the nigrostriatal pathway (C.H. Park et al. 2006). However, in our own recent studies, we have not found a similar approach to be as effective. We used retroviral vectors to transduce rat embryonic neural stem cells with one of several key midbrain transcription factors (Lmx1a, Msx1, neurogenin 2, and Pitx3). Individually, none of these genes increased the proportion of cells that differentiated into DA neurons, and overexpressing Lmx1a in combination with any one of the others also did not improve the differentiation into DA neurons (L. Roybon et al., in preparation). Others have reported that, if Nurr1 is overexpressed in neural stem cells, around 5% of the transduced cells will express TH, but that the simultaneous overexpression of Neurogenin2 has no additional effect in this regard (H.J. Kim et al. 2007; Andersson et al. 2007). We believe that neural stem cells harvested from the mid-late stage embryonic brain have passed the developmental window when they are able to “drive” into a specific neuronal phenotype. We need to develop protocols that more closely mimic the conditions in the developing brain. In such protocols, several transcription factors should be expressed in a carefully co-ordinated fashion, and the stem cells could differentiate into specific types of neurons. Furthermore, additional problems need to be addressed in order to transfer the technologies developed in rodent cells to clinical use. Generally, human NSCs display a longer doubling time than those of rodents. In addition, the telomeres of human NSCs are significantly shorter than their rodent counterparts (12 kb vs. 40 kb), which suggests that they will reach senescence more rapidly (Ostenfeld et al. 2000).
An alternative approach to obtaining large numbers of transplantable human DA neurons is to immortalize NSCs or partially committed progenitors by genetic modification (Liste et al. 2004; Paul et al. 2007). Human progenitors derived from 8-week-old VM have been immortalized by the regulated expression of v-myc. Under certain culture conditions, around 19% of these cells develop into TH-immunopositive neurons (Lotharius et al. 2002; Paul et al. 2007). When these cells are grafted into the brains of immunosuppressed hemiparkinsonian rats, however, they either die or cease to express TH (Paul et al. 2007). The study of this type of immortalized cell line is, of course, worthwhile for basic research. On the other hand, such lines are unlikely to be used clinically for safety reasons, even if the issue of survival of the TH neurons is solved.
Risk of tumor formation of NSCs
Although cultured NSCs are often found still to divide at the time of transplantation, tumor formation after grafting to the brain has not been reported. However, recent studies have demonstrated similarities between NSCs and glioma stem cells; this indicates that NSCs can potentially form tumors after transplantation. Both glioma stem cell and NSC can form spheres, express the surface molecule CD133, and differentiate into many different cell types (Zhang et al. 2006; Bao et al. 2006; Sim et al. 2006). Therefore, one has to be cautious when considering NSCs as a potential source of donor tissue for transplantation in PD patients, as these cells may still retain the potential to generate tumors. Culture protocols will almost definitely have to eliminate dividing NSC cells from cell populations intended for transplantion into patients.
Embryonic stem cells
The first ESC line was established from the inner cell mass of a mouse blastocyst in 1981 (Evans and Kaufman 1981; Martin 1981). It took an additional 17 years before the first human ESC was established (Thomson et al. 1998). The findings that ESCs proliferate readily and are pluripotent make them an interesting potential source of DA neurons for transplantation in PD.
Modes of protocols for inducing DA neurons
One method to induce a neural fate in ESCs utilizes the so-called “default pathway” (Fig. 2). This means that embryonic ectoderm will become neuroectoderm when transforming growth factor-β (TGF-β) family signaling is inhibited (Wilson and Edlund 2001). Several groups have shown that Noggin, a bone morphogenic protein antagonist, increases neural differentiation from human ESCs (Pera et al. 2004; Ben-Hur et al. 2004; Sonntag et al. 2007; Iacovitti et al. 2007). Two main approaches are commonly used to generate DA neurons from ESCs. One protocol starts by the generation of embryoid bodies, from which neural progenitors are selected. These are subjected to several defined growth factors in order to stimulate their differentiation into DA neurons (Lee et al. 2000). The other type of protocol is based on co-culturing the ESCs on special forms of feeders (Kawasaki et al. 2000; Barberi et al. 2003; Yue et al. 2006; Ueno et al. 2006).
-
a)
Regarding soluble factors that promote differentiation of ESCs into DA neurons, specific midbrain factors, such as SHH, FGF8, and ascorbic acid, were originally shown to be able to induce the development of DA neurons from murine ESCs (Lee et al. 2000). A wide range of factors applied in different combinations has subsequently been reported to promote further the development of DA neurons from mouse or monkey ESCs. They include IL1-β, GDNF, neurturin, TGF-β3, dibutyryl cyclic AMP, vitamin B12, brain-derived neurotrophic factor, neurotrophin3, FGF20, and TGF-α (Rolletschek et al. 2001; C.H. Park et al. 2005; Yamazoe et al. 2006; Takagi et al. 2005; S. Park et al. 2004). The downstream signaling pathways used by these factors to induce a DAergic phenotype are not well understood.
-
b)
Regarding the effects of feeders on ESCs, several types of cells have been reported to have similar inducing activities. The original finding was that PA6 mouse stromal cells promote the differentiation of mouse ESCs into DA neurons (Kawasaki et al. 2000). Subsequently, other types have been reported to exert similar effects, namely bone marrow stromal (MS5) cells, Sertoli cells, and amniotic membrane (Barberi et al. 2003; Yue et al. 2006; Ueno et al. 2006). Human fetal midbrain astrocytes immortalized by overexpression of telomerase have recently been reported to facilitate the development of DA neurons from human ESCs (Roy et al. 2006). The advantage of all these co-culture techniques is that they are simple and fast. One disadvantage is that the cellular mechanisms underlying the effects of the feeder cells are completely unknown. Therefore, it is difficult to devise rational strategies to optimize the results. Moreover, several of the feeder cells are non-human in origin, which precludes their use in clinical protocols.
-
c)
Genetic modification of ESCs is another approach to promote their differentiation into DA neurons. Specific transcription factor genes have been overexpressed in ESCs, in attempts to differentiate the pluripotent cells into DA neurons. Several kinds of genes have been tested in murine ESCs, e.g. Nurr1, Pitx3, Mash1, Lmx1a, and Msx1 (J.H. Kim et al. 2002; Chung et al. 2005; Kanda et al. 2004; Andersson et al. 2006). Nurr1 has long been known to be crucial for DA neuron development. Mice that are null mutants for Nurr1 lack a normal substantia nigra (Zetterstrom et al. 1997). Therefore, the overexpression of Nurr-1 has been extensively tested as a strategy to increase the numbers of DA neurons obtained from ESCs (J.H. Kim et al. 2002; Chung et al. 2002; D.W. Kim et al. 2006; Martinat et al. 2006). Notably, even in non-neuronal cells, Nurr1 can induce the expression of TH, aromatic amino acid decarboxylase, retinaldehyde dehydrogenase, and calbindin (Sonntag et al. 2004). In ESCs, the overexpression of Nurr1 alone is not sufficient, additional factors such as SHH and FGF8 being required to induce proper midbrain DA neurons. Recently, Andersson et al. (2006) have shown that the overexpression of Lmx1a itself generates proper midbrain DA neurons; they have identified, two genes, Lmx1a and Msx1, as major upstream regulators of DA neuron specification. Expression of Lmx1a induces the maturation of mouse ESC into ventral midbrain DA neurons when FGF2, FGF8, and low concentrations of SHH are added to the medium.
Dopamine neurons differentiated from human ESCs in vitro when co-cultured on PA6 stromal cells for 3 weeks. Immunohistochemical staining with antibodies against tyrosine hydroxylase (TH; red) and beta-tubulin type III (TuJ1; green) shows that many of the double-positive cells are DA neurons. Bar 200 μm
The best protocol for inducing DA neurons so far
In murine ESCs, the highest percentage of TH-immunopositive cells (about 90%) among beta-tubulin type III (TuJ1)-positive cells has been achieved by a protocol involving the combination of Nurr1 overexpression, PA6 co-culture, and soluble factors (SHH, FGF8, ascorbic acid; D.W. Kim et al. 2006). A similar protocol with a combination of co-culture (MS5 or astrocyte) and soluble factors (SHH, FGF8) has generated 64%–79% of TH-expressing cells among TuJ1-immunopositive cells from human ESCs (Perrier et al. 2004; Roy et al. 2006). Interestingly, transduction of Lmx1a in the mouse ESC-derived neural progenitor cells alone can enhance the generation of midbrain DA neurons in the presence of FGF2, FGF8, and SHH to a higher percentage (about 60% colonies are positive for TH), even under feeder-free conditions, indicating that Lmx1a plays a key role in the differentiation of DA neurons with a midbrain identity (Andersson et al. 2006).
As mentioned in the section describing NSCs (see above), the full characterization of the phenotype of DA neurons is also important. Immunopositivity for TH is a prerequisite if the cell is going to be relevant for transplantation studies, but this is not sufficient. The cells should also express additional markers (mentioned previously) characteristic of midbrain A9 DA neurons.
Grafting cells derived from mouse and monkey ESCs
DA neurons derived from murine ESCs have been frequently reported to survive transplantation into the striatum of immunosuppressed rats with a unilateral 6-OHDA lesion of the nigrostriatal pathway. In some cases, the grafts promote behavioral recovery in the recipients (J.H. Kim et al. 2002; Nishimura et al. 2003; Barberi et al. 2003; Baier et al. 2004; Rodriguez-Gomez et al. 2007). Most of the studies have focused on drug-induced rotation, but some reports also describe improved spontaneous neurological functions in the grafts (J.H. Kim et al. 2002). Primate ESCs can also differentiate into DA neurons when co-cultured with PA6 feeder cells (Kawasaki et al. 2002). Monkeys with lesions of the DA system, following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration, have been reported to recover with regard to motility and posture when grafted with DA neurons derived from primate ESCs. The same monkeys also exhibit increased 18F-flurodopa uptake in the striatum on positron emission tomography scans after transplantation (Takagi et al. 2005); histological examination has revealed an average of around 2,100 surviving TH-immunopositive grafted neurons on each side of the brain. In comparison with the post-mortem analyses of the clinical studies of embryonic/fetal mesencephalic transplantations (see above), the number of surviving grafted DA neurons is much lower in monkeys. Nevertheless, this study (Takagi et al. 2005) is the first to report functional effects of grafted neurons derived from primate ESCs.
Grafting cells derived from human ESC
Several groups have reported that human ESCs can differentiate into DA neurons in vitro (Schulz et al. 2004; Zeng et al. 2004; Perrier et al. 2004; S. Park et al. 2004; C.H. Park et al. 2005; Ben-Hur et al. 2004; Yan et al. 2005; Sonntag et al. 2007; Ueno et al. 2006; Brederlau et al. 2006; Roy et al. 2006; Iacovitti et al. 2007). This has been documented by using immunocytochemistry, reverse transcription/polmyerase chain reaction, electrophysiology, and measurements of DA by high performance liquid chromatography. However, the evidence that DA neuron derived from human ESC can effectively reduce neurological deficits following transplantation into rats is sparse. The majority of the few reports that have directly addressed this issue have either described no effects (Brederlau et al. 2006; C.H. Park et al. 2005) or only small changes following grafting (Ben-Hur et al. 2004). Specifically, Ben-Hur and coworkers (2004) have induced neural progenitors by treatment with Noggin in human ESCs, with 0.56% of the cells expressing TH. The authors describe some reductions in amphetamine- and apomorphine-induced rotation in addition to improvements of stepping adjustments and forelimb placing. Histological analysis has revealed that each graft contains an average of 389 TH-immunopositive neurons (only 0.18% of the total number of surviving human cells). The small number of DA neurons in relation to the relatively large grafts implies that the data should be interpreted with some caution and that it is not absolutely certain that the observed functional changes are attributable to the grafted DA neurons. A recent paper has reported not only the survival of large numbers of grafted DA neurons derived from human ESCs, but also functional recovery in hemiparkinsonian rats; the DA neurons were obtained from human ESCs by using a combination of soluble factors (SHH and FGF8) and co-culture with immortalized human midbrain astrocytes (Roy et al. 2006). However, several unusual features regarding the nature and speed of functional recovery in this study have been discerned. We have suggested that the behavioral effects of the grafts are not attributable to DA release but arise because the ESC-derived implants grow extensively and damage the host striatum (Christophersen and Brundin 2007). The authors have subsequently published a corrigendum (Goldman et al. 2007) stating that the grafts were not as large as they had originally reported in their paper. There remain, however, several issues regarding the functional evaluation of the grafts that weaken claims that the transplants cause functional recovery. For example, an intraventricular route of 6-OHDA administration was used, giving rise to only partial striatal DA depletion. This level of depletion is not certain to elicit robust rotational behavior in response to apomorphine. The dose of apomorphine was fifty-fold higher than that normally used to evaluate supersensitive DA receptors (Marshall and Ungerstedt 1977). The authors (Roy et al. 2006) claim that the apomorphine-induced asymmetry recovers rapidly and completely, which has never been reported before with other types of grafts (Brown and Dunnett 1989; Herman et al. 1988). Amphetamine-induced turning behavior, which is a more reliable indicator of graft function, has not been reported by Roy et al. (2006). Changes in spontaneous behavior (the “stepping test”) were reported to occur on the same side of the body of the rat in which the unilateral lesion and grafts were located, which is also at variance with all prior literature (Olsson et al. 1995). Taken together, the study by Roy et al. (2006) does not convincingly show that the grafted human ESC-derived cells can affect behavior in a DA-dependent fashion.
With the exception of the study by Roy et al. (2006), the survival of human ESC-derived neurons is poor following transplantation. Whereas the transplanted grafts can still contain numerous neurons that express a marker for neuronal nuclei (NeuN; Schulz et al. 2004; C.H. Park et al. 2005; Brederlau et al. 2006), the number of TH-immunopositive neurons is several-fold lower than would be expected based on earlier intracerebral transplantation research. Either the human ESC-derived DA neurons die during the transplantation procedure or they down-regulate TH expression once they are grafted. Differences seem to exist between embryonic/fetal mesencephalon-derived progenitors, which typically survive at a rate of around 10% (Brundin et al. 2000a), and human ESC-derived progenitors/neurons. Indeed, the instability of stem-cell-derived DA neuron is a problem for both human ESCs and NSCs (Ostenfeld et al. 2000; C.H. Park et al. 2005; Zeng et al. 2004; Christophersen et al. 2006; Schulz et al. 2004). Moreover, genetically modified human midbrain progenitors also appear to suffer from the drawback of unstable TH expression (Paul et al. 2007). Thus, the progenitors differentiate into neurons and seem to survive transplantation well, but the subpopulation that is TH-immunopositive no longer remains in the grafts. The mechanisms underlying this selective vulnerability or instability of phenotype are not understood. Possibly, the cells have not matured sufficiently and can somehow “dedifferentiate”, thus returning to a more immature state. Further studies are needed to devise strategies to resolve this important issue.
Genetic instability in human ESCs
Although ESCs have several features that make them a promising cell source for cell therapy, they also exhibit several unique problems. One such issue is that ESCs are genetically unstable. Karyotypic changes in several human ESC lines have been reported (Cowan et al. 2004; Maitra et al. 2005; Herszfeld et al. 2006; Draper et al. 2004). These abnormalities often make them proliferate more rapidly and shorten the doubling times of the cells. The changes commonly occur in chromosomes 12 and 17. Chromosomal abnormalities are also common in human embryonal carcinoma cell lines. These abnormalities might affect the differentiation of the cells and thereby the results after transplantation, although this remains to be clarified. Certainly, the likelihood of tumor formation after grafting is probably influenced by chromosomal abnormalities.
Tumor and teratoma formation after transplantation
One advantage of ESCs is that they are readily expanded (Fig. 3). Human ESCs double every 20–72 h (Amit et al. 2000; Cowan et al. 2004; Herszfeld et al. 2006). In comparison, NSCs have doubling times of 60–106 h (Mori et al. 2006; Horiguchi et al. 2004). The high proliferative capacity of ESCs may also be a disadvantage in some circumstances, as they could continue to grow rapidly after being grafted into the brain. Some human ESC-derived grafts have been reported to form tumor-like structures consisting of neuroepithelial cells, rather than teratomas that contain tissues from all germ layers (Roy et al. 2006).
Tumorigenicity of human embryonic stem cells. Hematoxylin and eosin staining. Tumor formation was detected 2 weeks after transplantation of undifferentiated human ES cells (HUES-3) into the rat striatum (inset). Cells appeared immature and generated a large mass with some rosette formations indicating that undifferentiated ESCs can survive and continue to divide (higher magnification of boxed area in inset). For clinical use, the undifferentiated ESCs must be eliminated from the donor population prior to transplantation. Bar 200 μm
The pluripotency of ESCs can pose an additional practical problem. It is often difficult to exclude undifferentiated ESCs and non-neural cells completely from an ESC culture. Because undifferentiated ESC can generate teratomas after transplantation, this risk also applies to intracerebral implants of ESC-derived cells (Thinyane et al. 2005; Fukuda et al. 2006; Chung et al. 2006; Bjorklund et al. 2002). Several strategies have been tried to eliminate pluripotent or partially differentiated ESCs in order to avoid tumor formation. Fluorescence-activated cell sorting has been employed in two studies describing the protocols for selecting neural precursors from mixed populations including undifferentiated ESCs, viz., from ESCs derived from Sox1-green fluorescent protein (GFP) knock-in mice; the sorted Sox1-positive cells do not form tumors after intracerebral transplantation, whereas grafted Sox1-negative cells frequently generate large tumors (Fukuda et al. 2006; Chung et al. 2006). Another approach to reduce the risk of teratoma formation is to induce the selective death of undifferentiated cells. For example, the ceramide analog N-oleoyl serinol (S18) can induce apoptosis in undifferentiated cells that express Oct-4 and prostate apoptosis response-4. After neural induction from murine ESCs, treatment with S18 increases the proportion of Nestin-positive progenitors and decreases tumorigenicity (Bieberich et al. 2004). Another approach is to manipulate Cripto. This is one of the EGF-CFC signaling molecule family proteins and plays an important role in the neural differentiation of ESCs and tumor generation after transplantation. It is expressed in the inner cell mass and trophoblast cells of the mouse blastocyst and in a wide range of epithelial cancers. When low numbers of Cripto knockout (Cr−/−) ESCs are transplanted into the striatum of rats with 6-OHDA lesions, they differentiate into DA neurons without forming tumors. On the other hand, wild-type ESCs frequently generate teratomas under the same conditions (Parish et al. 2005).
Undifferentiated ESC do not always cause tumor after transplantation into the brain. Small numbers of undifferentiated mouse ESCs have been shown to differentiate into DA neurons after being dissociated into a single cell suspension and grafted to the striatum of imunosuppressed rats. In 56% of the recipients, grafts survive transplantation with no signs of tumors 14–16 weeks after surgery, whereas 20% of the animals exhibit prominent teratomas, and 24% of them exhibit no graft survival (Bjorklund et al. 2002). The dissociation of the cells into a single cell suspension has been suggested to be important in promoting their “default” differentiation into neurons, as opposed to retaining their pluripotency. Apparently, the local environment at the site of transplantation also influences the risk of teratoma formation. When human ESCs are grafted into the developing brains of rat embryo (embryonic day 14), they do not generate tumors but migrate, differentiate into neurons, and form a chimeric brain with the host neurons (Muotri et al. 2005). The immune system is also likely to affect the risk of tumor growth from transplanted ESCs. Generally, allografts yield tumors more frequently than xenografts (Erdo et al. 2003). In summary, when evaluating the risk for tumor generation, we need to take the following sorts of factors into consideration: contamination of undifferentiated cells; karyotypic changes in the ESC-derived donor cells; number of cells transplanted; state of the host environment (e.g., mature versus developing or inflamed versus quiescent); host immune response.
The risk of tumor formation from stem-cell-derived grafts is one of the major hurdles that prevent clinical application in PD. Therefore, more research is clearly needed to unravel mechanisms underlying tumor formation from ESCs. Until these are fully understood, methods that exclude dividing cells and select only post-mitotic DA neurons might be the strategies that can bring us closest to clinical application.
Other problems related to ESCs
Another issue, which is particularly difficult to overcome regarding the use of human ESC-derived neurons for clinical transplantation, is that of animal contaminants in the culture protocols. Typically, human ESCs are cultured on mouse feeder cells with animal-derived “serum replacements”. Exposing human ESCs to animal derivatives leads them to incorporate N-glycolyl-neuraminic acid residues (Neu5Gc) that are present in most mammals, except humans. Humans have circulating antibodies against Neu5Gc (Martin et al. 2005). Several attempts have been made to establish methods to culture and differentiate human ESCs under “feeder-free” and “xeno-free” conditions by using only human-derived products (Klimanskaya et al. 2005; Mallon et al. 2006; Iacovitti et al. 2007; Ellerstrom et al. 2006).
Human ESCs are also inherently connected to ethical issues because of their origin in a blastocyst, and the potential that the technology can be used to clone human beings. Recently, the International Society for Stem Cell Research has published ethical guidelines for human ESC research (Daley et al. 2007). Researchers should retain open communication with the general public and policy makers with regard to the direction to be taken concerning stem-cell-based therapies. With the brain being a particularly controversial transplantation site from an ethical standpoint, dialogue with society is extremely important for scientists working on stem cell therapies for PD.
ES-like cells from reprogrammed somatic cells
In 2006, a Japanese group succeeded in inducing pluripotent stem cells from mouse fibroblasts by retrovirally transducing the cells with the four transcription factors Oct3/4, Sox2, c-Myc, and Klf4. They called the resulting cells “induced pluripotent stem (iPS) cells” and demonstrated that they exhibited certain features of pluripotent ESC, including neural differentiation (Takahashi and Yamanaka 2006). This strategy can be classified as a “de-differentiation” or “reprogramming” approach. Recently, independent studies have confirmed the initial study and carried the findings one step further (Maherali et al. 2007; Okita et al. 2007; Wernig et al. 2007). For example, iPS cells derived from fibroblasts clearly have the ability to become incorporated into a blastocyst and to generate chimeric mouse offspring.
This groundbreaking work suggests that it will be possible to generate pluripotent cells directly from patients’ own somatic cells in the future. Tests of whether the iPS cell technique can be applied to human cells, and whether the resulting cells can be differentiated into DA neurons will be of great interest. If these tests provide positive results, several practical issues are likely to emerge, such as the need to control the proliferative activities of these cells. This, however, does not distract from the fact that iPS cells are en extremely exciting future option for cell therapy in PD.
Concluding remarks
The backbone of stem cell therapy for PD is the knowledge and experience obtained from embryonic/fetal VM transplantation in the context of both basic research and clinical studies. The central concept of mesencephalic tissue transplantation is that the donor cells include post-mitotic progenitors with the phenotype of the DA neurons of the substantia nigra pars compacta (A9). Thus, the first step to a new cell therapy in PD is to generate these cells from stem cells, instead of from embryonic/fetal brains. The cells generated from stem cells have to be safe and have no risk of generating tumors after transplantation. We also need (1) to devise better criteria regarding patient selection; (2) to establish the optimal numbers of cells to inject; (3) to find a mild and yet effective mode of immunosuppression; (4) to learn how to avoid unwanted side-effects, such as GIDs. Thus, many problems remain to be solved before we have a successful clinical protocol for stem cell therapy in PD. An important issue for clinical application will be the balance of benefit and safety. Nevertheless, continued research on the cell transplantation strategy should be encouraged because some PD patients have shown dramatic improvement of their symptoms following embryonic/fetal mesencephalic transplantation. Taking all the data together, we believe that stem cell therapy will become one of the treatment options for PD patients in the future.
References
Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA (2000) Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 227:271–278
Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J (2006) Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124:393–405
Andersson EK, Irvin DK, Ahlsio J, Parmar M (2007) Ngn2 and Nurr1 act in synergy to induce midbrain dopaminergic neurons from expanded neural stem and progenitor cells. Exp Cell Res 313:1172–1180
Baier PC, Schindehutte J, Thinyane K, Flugge G, Fuchs E, Mansouri A, Paulus W, Gruss P, Trenkwalder C (2004) Behavioral changes in unilaterally 6-hydroxy-dopamine lesioned rats after transplantation of differentiated mouse embryonic stem cells without morphological integration. Stem Cells 22:396–404
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 21:1200–1207
Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE (2004) Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells 22:1246–1255
Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG (2004) Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol 167:723–734
Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 99:2344–2349
Brederlau A, Correia AS, Anisimov SV, Elmi M, Roybon L, Paul G, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY (2006) Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 24:1433–1440
Breysse N, Carlsson T, Winkler C, Bjorklund A, Kirik D (2007) The functional impact of the intrastriatal dopamine neuron grafts in parkinsonian rats is reduced with advancing disease. J Neurosci 27:5849–5856
Brown VJ, Dunnett SB (1989) Comparison of adrenal and foetal nigral grafts on drug-induced rotation in rats with 6-OHDA lesions. Exp Brain Res 78:214–218
Brundin P, Hagell P (2001) The neurobiology of cell transplantation in Parkison’s disease. Clin Neurosci Res 1:507–520
Brundin P, Strecker RE, Lindvall O, Isacson O, Nilsson OG, Barbin G, Prochiantz A, Forni C, Nieoullon A, Widner H et al (1987) Intracerebral grafting of dopamine neurons. Experimental basis for clinical trials in patients with Parkinson’s disease. Ann N Y Acad Sci 495:473–496
Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF (2000a) Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant 9:179–195
Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Bjorklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O (2000b) Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain 123:1380–1390
Carlsson T, Winkler C, Lundblad M, Cenci MA, Bjorklund A, Kirik D (2006) Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis 21:657–668
Carvey PM, Ling ZD, Sortwell CE, Pitzer MR, McGuire SO, Storch A, Collier TJ (2001) A clonal line of mesencephalic progenitor cells converted to dopamine neurons by hematopoietic cytokines: a source of cells for transplantation in Parkinson’s disease. Exp Neurol 171:98–108
Christophersen NS, Brundin P (2007) Large stem cell grafts could lead to erroneous interpretations of behavioral results? Nat Med 13:118–119
Christophersen NS, Meijer X, Jorgensen JR, Englund U, Gronborg M, Seiger A, Brundin P, Wahlberg LU (2006) Induction of dopaminergic neurons from growth factor expanded neural stem/progenitor cell cultures derived from human first trimester forebrain. Brain Res Bull 70:457–466
Chung S, Sonntag KC, Andersson T, Bjorklund LM, Park JJ, Kim DW, Kang UJ, Isacson O, Kim KS (2002) Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci 16:1829–1838
Chung S, Hedlund E, Hwang M, Kim DW, Shin BS, Hwang DY, Jung Kang U, Isacson O, Kim KS (2005) The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci 28:241–252
Chung S, Shin BS, Hedlund E, Pruszak J, Ferree A, Kang UJ, Isacson O, Kim KS (2006) Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem 97:1467–1480
Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scott EW (2004) Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 363:1432–1437
Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP, Wang S, Morton CC, McMahon AP, Powers D, Melton DA (2004) Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med 350:1353–1356
Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, Roon-Mom WM van, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS (2007) Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315:1243–1249
Daley GQ, Richter LA, Auerbach JM, Benvenisty N, Charo RA, Chen G, Deng HK, Goldstein LS, Hudson KL, Hyun I, Junn SC, Love J, Lee EH, McLaren A, Mummery CL, Nakatsuji N, Racowsky C, Rooke H, Rossant J, Scholer HR, Solbakk JH, Taylor P, Trounson AO, Weissman IL, Wilmut I, Yu J, Zoloth L (2007) Ethics. The ISSCR guidelines for human embryonic stem cell research. Science 315:603–604
Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW (2004) Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 22:53–54
Ellerstrom C, Strehl R, Moya K, Andersson K, Bergh C, Lundin K, Hyllner J, Semb H (2006) Derivation of a xeno-free human embryonic stem cell line. Stem Cells 24:2170–2176
Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, Hossmann KA, Trapp T (2003) Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab 23:780–785
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344:710–719
Freed CR, Breeze RE, Fahn S, Eidelberg D (2004) Preoperative response to levodopa is the best predictor of transplant outcome. Ann Neurol 55:896
Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS (2006) Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells 24:115–124
Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M, Sasai Y, Hashimoto N (2006) Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells 24:763–771
Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438
Gage FH, Ray J, Fisher LJ (1995) Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci 18:159–192
Goldman SA, Roy NS, Beal MF, Cleren C (2007) Large stem cell grafts could lead to erroneous interpretations of behavioral results? Nat Med 13:118–119
Hagell P, Brundin P (2001) Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol 60:741–752
Hagell P, Cenci MA (2005) Dyskinesias and dopamine cell replacement in Parkinson’s disease: a clinical perspective. Brain Res Bull 68:4–15
Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A (2006) Genetics of Parkinson’s disease and parkinsonism. Ann Neurol 60:389–398
Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW (1999) Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 56:179–187
Herman JP, Lupp A, Abrous N, Le Moal M, Hertting G, Jackisch R (1988) Intrastriatal dopaminergic grafts restore inhibitory control over striatal cholinergic neurons. Exp Brain Res 73:236–248
Herszfeld D, Wolvetang E, Langton-Bunker E, Chung TL, Filipczyk AA, Houssami S, Jamshidi P, Koh K, Laslett AL, Michalska A, Nguyen L, Reubinoff BE, Tellis I, Auerbach JM, Ording CJ, Looijenga LH, Pera MF (2006) CD30 is a survival factor and a biomarker for transformed human pluripotent stem cells. Nat Biotechnol 24:351–357
Hitoshi S, Tropepe V, Ekker M, Kooy D van der (2002) Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain. Development 129:233–244
Horiguchi S, Takahashi J, Kishi Y, Morizane A, Okamoto Y, Koyanagi M, Tsuji M, Tashiro K, Honjo T, Fujii S, Hashimoto N (2004) Neural precursor cells derived from human embryonic brain retain regional specificity. J Neurosci Res 75:817–824
Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M (2007) A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: studies in vitro and in vivo. Brain Res 1127:19–25
Jensen JB, Parmar M (2006) Strengths and limitations of the neurosphere culture system. Mol Neurobiol 34:153–161
Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S (2004) Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet 364:172–178
Kanda S, Tamada Y, Yoshidome A, Hayashi I, Nishiyama T (2004) Over-expression of bHLH genes facilitate neural formation of mouse embryonic stem (ES) cells in vitro. Int J Dev Neurosci 22:149–156
Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y (2000) Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28:31–40
Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S, Nakatsuji N, Sasai Y (2002) Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci USA 99:1580–1585
Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, Jankovic J (2007) Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg 106:621–625
Kim DW, Chung S, Hwang M, Ferree A, Tsai HC, Park JJ, Chung S, Nam TS, Kang UJ, Isacson O, Kim KS (2006) Stromal cell-derived inducing activity, Nurr1, and signaling molecules synergistically induce dopaminergic neurons from mouse embryonic stem cells. Stem Cells 24:557–567
Kim HJ, Sugimori M, Nakafuku M, Svendsen CN (2007) Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol 203:394–405
Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418:50–56
Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R (2005) Human embryonic stem cells derived without feeder cells. Lancet 365:1636–1641
Kordower JH, Styren S, Clarke M, DeKosky ST, Olanow CW, Freeman TB (1997) Fetal grafting for Parkinson’s disease: expression of immune markers in two patients with functional fetal nigral implants. Cell Transplant 6:213–219
Lane EL, Winkler C, Brundin P, Cenci MA (2006) The impact of graft size on the development of dyskinesia following intrastriatal grafting of embryonic dopamine neurons in the rat. Neurobiol Dis 22:334–345
Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD (2000) Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 18:675–679
Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M (2001) Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett 316:67–70
Liste I, Garcia-Garcia E, Martinez-Serrano A (2004) The generation of dopaminergic neurons by human neural stem cells is enhanced by Bcl-XL, both in vitro and in vivo. J Neurosci 24:10786–10795
Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P (2002) Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem 277:38884–38894
Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K (2007) Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1:55–70
Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A (2005) Genomic alterations in cultured human embryonic stem cells. Nat Genet 37:1099–1103
Mallon BS, Park KY, Chen KG, Hamilton RS, McKay RD (2006) Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol 38:1063–1075
Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K (2006) Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis 21:165–180
Marshall JF, Ungerstedt U (1977) Striatal efferent fibers play a role in maintaining rotational behavior in the rat. Science 198:62–64
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78:7634–7638
Martin MJ, Muotri A, Gage F, Varki A (2005) Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 11:228–232
Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U, Wang H, Abeliovich A (2006) Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci USA 103:2874–2879
Mendez I, Dagher A, Hong M, Hebb A, Gaudet P, Law A, Weerasinghe S, King D, Desrosiers J, Darvesh S, Acorn T, Robertson H (2000) Enhancement of survival of stored dopaminergic cells and promotion of graft survival by exposure of human fetal nigral tissue to glial cell line-derived neurotrophic factor in patients with Parkinson’s disease. Report of two cases and technical considerations. J Neurosurg 92:863–869
Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O (2005) Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 128:1498–1510
Mori H, Ninomiya K, Kino-oka M, Shofuda T, Islam MO, Yamasaki M, Okano H, Taya M, Kanemura Y (2006) Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res 84:1682–1691
Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH (2005) Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci USA 102:18644–18648
Nishimura F, Yoshikawa M, Kanda S, Nonaka M, Yokota H, Shiroi A, Nakase H, Hirabayashi H, Ouji Y, Birumachi J, Ishizaka S, Sakaki T (2003) Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells 21:171–180
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54:403–414
Olanow CW, Freeman TB, Kordower JH (2004) Preoperative response to levodopa is the best predictor of transplant outcome (author reply). Ann Neurol 55:896–897
Olsson M, Nikkhah G, Bentlage C, Bjorklund A (1995) Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15:3863–3875
Ostenfeld T, Svendsen CN (2004) Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells 22:798–811
Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN (2000) Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol 164:215–226
Ostenfeld T, Joly E, Tai YT, Peters A, Caldwell M, Jauniaux E, Svendsen CN (2002) Regional specification of rodent and human neurospheres. Brain Res Dev Brain Res 134:43–55
Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte-Stewart H, Hallett M, Miyasaki J, Stevens J, Weiner WJ (2006) Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66:983–995
Parish CL, Parisi S, Persico MG, Arenas E, Minchiotti G (2005) Cripto as a target for improving embryonic stem cell-based therapy in Parkinson’s disease. Stem Cells 23:471–476
Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH (2005) In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem 92:1265–1276
Park CH, Kang JS, Shin YH, Chang MY, Chung S, Koh HC, Zhu MH, Oh SB, Lee YS, Panagiotakos G, Tabar V, Studer L, Lee SH (2006) Acquisition of in vitro and in vivo functionality of Nurr1-induced dopamine neurons. FASEB J 20:2553–2555
Park S, Lee KS, Lee YJ, Shin HA, Cho HY, Wang KC, Kim YS, Lee HT, Chung KS, Kim EY, Lim J (2004) Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci Lett 359:99–103
Parmar M, Skogh C, Bjorklund A, Campbell K (2002) Regional specification of neurosphere cultures derived from subregions of the embryonic telencephalon. Mol Cell Neurosci 21:645–656
Paul G, Christophersen NS, Raymon H, Kiaer C, Smith R, Brundin P (2007) Tyrosine hydroxylase expression is unstable in a human immortalized mesencephalic cell line—studies in vitro and after intracerebral grafting in vivo. Mol Cell Neurosci 34:390–399
Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C (2004) Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist Noggin. J Cell Sci 117:1269–1280
Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L (2004) Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 101:12543–12548
Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW, Dirnagl U (2001) Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J Cell Biol 155:733–738
Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD (2007) Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson’s disease. Stem Cells 25:918–928
Rolletschek A, Chang H, Guan K, Czyz J, Meyer M, Wobus AM (2001) Differentiation of embryonic stem cell-derived dopaminergic neurons is enhanced by survival-promoting factors. Mech Dev 105:93–104
Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA (2000) In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med 6:271–277
Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA (2006) Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12:1259–1268
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363:1783–1793
Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740–744
Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG (2004) Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells 22:1218–1238
Schwarz SC, Wittlinger J, Schober R, Storch A, Schwarz J (2006) Transplantation of human neural precursor cells in the 6-OHDA lesioned rats: effect of immunosuppression with cyclosporine A. Parkinsonism Relat Disord 12:302–308
Sim FJ, Keyoung HM, Goldman JE, Kim DK, Jung HW, Roy NS, Goldman SA (2006) Neurocytoma is a tumor of adult neuronal progenitor cells. J Neurosci 26:12544–12555
Sonntag KC, Simantov R, Kim KS, Isacson O (2004) Temporally induced Nurr1 can induce a non-neuronal dopaminergic cell type in embryonic stem cell differentiation. Eur J Neurosci 19:1141–1152
Sonntag KC, Pruszak J, Yoshizaki T, Arensbergen J van, Sanchez-Pernaute R, Isacson O (2007) Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the BMP antagonist Noggin. Stem Cells 25:411–418
Svendsen CN, Caldwell MA, Shen J, Borg MG ter, Rosser AE, Tyers P, Karmiol S, Dunnett SB (1997) Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Exp Neurol 148:135–146
Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N (2005) Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 115:102–109
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Thinyane K, Baier PC, Schindehutte J, Mansouri A, Paulus W, Trenkwalder C, Flugge G, Fuchs E (2005) Fate of pre-differentiated mouse embryonic stem cells transplanted in unilaterally 6-hydroxydopamine lesioned rats: histological characterization of the grafted cells. Brain Res 1045:80–87
Thompson L, Barraud P, Andersson E, Kirik D, Bjorklund A (2005) Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci 25:6467–6477
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Ueno M, Matsumura M, Watanabe K, Nakamura T, Osakada F, Takahashi M, Kawasaki H, Kinoshita S, Sasai Y (2006) Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proc Natl Acad Sci USA 103:9554–9559
Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, Baltuch GH (2003) Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg 98:779–784
Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature: e-published ahead of printing
Wilson SI, Edlund T (2001) Neural induction: toward a unifying mechanism. Nat Neurosci 4 (Suppl):1161–1168
Yamazoe H, Kobori M, Murakami Y, Yano K, Satoh M, Mizuseki K, Sasai Y, Iwata H (2006) One-step induction of neurons from mouse embryonic stem cells in serum-free media containing vitamin B12 and heparin. Cell Transplant 15:135–145
Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC (2005) Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23:781–790
Yang M, Donaldson AE, Marshall CE, Shen J, Iacovitti L (2004) Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant 13:535–547
Yue F, Cui L, Johkura K, Ogiwara N, Sasaki K (2006) Induction of midbrain dopaminergic neurons from primate embryonic stem cells by coculture with Sertoli cells. Stem Cells 24:1695–1706
Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS, Freed WJ (2004) Dopaminergic differentiation of human embryonic stem cells. Stem Cells 22:925–940
Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276:248–250
Zhang QB, Ji XY, Huang Q, Dong J, Zhu YD, Lan Q (2006) Differentiation profile of brain tumor stem cells: a comparative study with neural stem cells. Cell Res 16:909–915
Acknowledgements
The authors’ own research on stem cells is supported by the following grants: The Swedish Research Council; Swedish Parkinson Foundation; Torsten och Ragnar Söderbergs stiftelser; Stiftelsen Olle Engkvist Byggmästare; Konung Gustaf V:s och Drottning Victorias Stiftelse; Swedish Brain Foundation; Swedish Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morizane, A., Li, JY. & Brundin, P. From bench to bed: the potential of stem cells for the treatment of Parkinson’s disease. Cell Tissue Res 331, 323–336 (2008). https://doi.org/10.1007/s00441-007-0541-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0541-0