Abstract
New neurons continue to be generated in two privileged areas of the adult brain: the dentate gyrus of the hippocampal formation and the olfactory bulb. Adult neurogenesis has been found in all mammals studied to date, including humans. The process of adult neurogenesis encompasses the proliferation of resident neural stem and progenitor cells and their subsequent differentiation, migration, and functional integration into the pre-existing circuitry. This article summarizes recent findings regarding the developmental steps involved in adult hippocampal neurogenesis and the possible functional roles that new hippocampal neurons might play.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult hippocampal neurogenesis is neuronal development under the conditions extant within the adult brain. Because adult neurogenesis originates from neural precursor cells in the adult hippocampus, it provides an intriguing example of the way that new neurons develop within an essentially non-neurogenic environment. Generally, the century-old presumption of “no new nerve cells after birth” and the notion of limited regenerative capacity of the mammalian brain (e.g., Ramón y Cajal 1928) are accepted as requiring modification. As originally described by Josef Altman in 1965 (Altman and Das 1965) and rediscovered in the 1990s, two privileged areas of the adult mammalian brain are the exceptions to the rule that neurogenesis exclusively occurs during development: the dentate gyrus of the hippocampal formation and the system of the subventricular zone (SVZ)/olfactory bulb. In the dentate gyrus, the excitatory principle neuron, viz., the granule cell, continues to be generated throughout life from neural stem and progenitor cells in the subgranular zone (SGZ). Neural stem and progenitor cells also reside and proliferate in the SVZ, and their progeny migrate via the rostral migratory stream to the olfactory bulb where they differentiate into two types of interneurons. Adult neurogenesis has been found in all mammals studied to date, including humans (Eriksson et al. 1998).
In the early studies, tritiated thymidine was used to birthmark and label proliferating cells in the adult brain, followed by autoradiographic detection (Altman and Das 1965; Kaplan and Hinds 1977). One problem at that time was that the neuronal phenotype of adult-generated cells could not be unequivocally demonstrated. Later, the presence of the polysialylated form of neural cell adhesion molecule (PSA-NCAM) was found in the dentate gyrus (Seki and Arai 1993a, b) and further supported the notion of ongoing neuronal development in this part of the adult brain. In the 1990s, bromodeoxyuridine (originally applied to developmental studies) was first used for the study of adult neurogenesis. In conjunction with immunohistochemistry and confocal microscopy, this has allowed us to determine the cellular identity of adult-born cells.
In the meantime, a detailed picture of the steps and processes involved in the generation of new neurons in the adult dentate gyrus has emerged (reviewed in Kempermann et al. 2004; Abrous et al. 2005; Ming and Song 2005; Lledo et al. 2006). The process of adult hippocampal neurogenesis encompasses the slow proliferation of early stem or progenitor cells, the subsequent faster proliferation of more restricted progenitors (expansion phase), the selection for survival or elimination of young cells, the integration of the surviving cells into the pre-existing neuronal network, and lastly the later phases of postmitotic development that include gradually increasing neuronal connectivity and changes in physiological neuronal properties.
SGZ: neurogenic niche in adult hippocampus
Neurogenesis in the adult dentate gyrus originates from a precursor population that resides in the SGZ, a thin band of tissue between the granule cell layer and the hilus. Most of the cell proliferation occurs here. Neural stem or progenitor cells also exist in brain areas whose entire neuronal populations are generated during intrauterine development (Palmer et al. 1999). In other words, in non-neurogenic areas, resident neural stem cells do not make use of their potential. However, when transplanted into an area that is permissive for neuronal development, such as the dentate gyrus, their potential can be utilized (Suhonen et al. 1996; Shihabuddin et al. 2000). Therefore, regulatory environmental cues independent of the stem cell must play an important role in allowing neurogenesis to occur in neurogenic areas. Alternatively, neurogenesis from resident stem cells may be actively suppressed in non-neurogenic areas. Along these lines, constitutive bone morphogenic protein (BMP) signaling has been shown to direct adult neural precursor cells toward a glial fate, and the presence of BMP antagonists in neurogenic areas may account for neurogenic permissiveness (Lim et al. 2000). The particular microenvironment, with all its constituents, that is permissive for neurogenesis is referred to as the “neurogenic niche” or “neural stem cell niche”.
The neural stem cell niches in the adult brain contain various cell types and tissue components, including stem/progenitor cells and their progeny, astrocytes, oligodendroglia, microglia, immune cells, and the vasculature (Mercier et al. 2002). Proliferative “hot spots” are often located close to the vasculature, and therefore, important regulation for neurogenesis has been suspected to come from blood vessels (Palmer et al. 2000). This is in agreement with findings from other tissues that suggest a prominent role for the vasculature and its basement membrane in maintaining tissue-specific stem cell niches (for a review, see Nikolova et al. 2007). Vascular endothelial growth factor (VEGF) is one of the trophic factors that is vasculature-derived and is a potent regulator of adult neurogenesis (Jin et al. 2002; Schanzer et al. 2004). Recent data indicate that, within the vasculature, endothelial cells provide important cues for the neural stem cell niche (Wurmser et al. 2004). Surprisingly, Wurmser et al. (2004) also suggest that neural stem or progenitor cells might in turn contribute to the maintenance and development of the local vasculature by producing endothelial cells, but this observation awaits confirmation.
The local astrocytic population probably plays an important role in creating neurogenic permissiveness in the SGZ niche. Hippocampus-derived astrocytes, but not astrocytes from the spinal cord, have been demonstrated to regulate neuronal differentiation from neural stem cells or progenitor cells in vitro (Song et al. 2002). Some of the astrocyte-derived factors that perhaps mediate these effects have been identified (Barkho et al. 2006). In vivo, proliferating cells tend to be located in close proximity to astrocytes (Shapiro et al. 2005; Plumpe et al. 2006).
Hippocampal precursor cells
An elegant series of experiments from Arturo Alvarez-Buylla’s group established the astrocytic nature of hippocampal neural precursor cells. Proliferating hippocampal cells were ablated with an antimitotic agent, and the first cell cohort to reappear expressed glial fibrillary acidic protein (GFAP), an astrocytic marker (Seri et al. 2001). In another set of experiments, avian virus receptor (AVR) was targeted to glial cells (receptor expression was controlled by the GFAP or nestin promoter). This system allowed the selective introduction of a reporter gene into AVR-expressing cells (glial cells). The finding that the reporter gene first appeared in glial cells and only later was found in neurons further demonstrated the developmental potential of some hippocampal astrocytes (Seri et al. 2001, 2004).
Hippocampal precursor cells were first isolated from the embryonic brain by Jasodarah Ray in 1993 (Ray et al. 1993) and from the adult brain by Theo Palmer in 1995 (Palmer et al. 1995). Adult hippocampal precursors were found to be multipotent, giving rise to neurons, astrocytes, and oligodendrocytes in vitro (Palmer et al. 1995, 1999). The report of “stem cells” was later disputed (Seaberg and van der Kooy 2002; Bull and Bartlett 2005), and hippocampal precursor cells, in contrast to cells from the SVZ, were argued to be lineage-restricted progenitors with limited self-renewal. However, by using a microdissection protocol in combination with an enrichment procedure that overcame the issue of sub-critical culture densities, the existence of multipotent stem cells with multi-lineage potential was confirmed for the adult murine dentate gyrus (Babu et al. 2007).
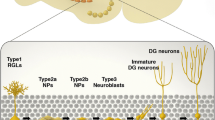
Type 1 cells
Schematics visualizing the developmental steps of adult hippocampal neurogenesis have been devised and presented previously, and the interested reader is referred to that material (Kempermann et al. 2004; Steiner et al. 2006). Adult hippocampal neurogenesis originates from cells with morphological and functional characteristics of glial cells. Type 1 cells (corresponding to the vertical astrocytes in the publications by Alvarez-Buylla) constitute the resident early precursor population that shares morphological and antigenic features with radial glia. Glial features also characterize neural stem cells in the embryonic brain (Noctor et al. 2001, 2002; Heins et al. 2002; Malatesta et al. 2003) and the other neurogenic area in the adult brain, the SVZ (Doetsch et al. 1999; Scheffler et al. 2005). The soma of type 1 cells is triangular-shaped and located in the SGZ. Type 1 cells usually extend a strong apical process into the molecular layer and may contact blood vessels through vascular end feet (Filippov et al. 2003). Type 1 cells are abundant in the SGZ but rarely divide. They express astrocytic marker GFAP and intermediate filament nestin, but not the calcium-binding protein S100β, which is expressed in a distinct postmitotic astrocytic population (Seri et al. 2001; Steiner et al. 2004). Many type-1 cells express the radial glia marker BLBP and stem cell protein Sox2 (Steiner et al. 2006). Electrophysiologically, type 1 cells display classical astrocytic features, such as passive membrane properties (Filippov et al. 2003; Fukuda et al. 2003).
Type 2 cells
Type 1 cells give rise to fast proliferating intermediate precursors (type 2 cells and type 3 cells). Most of the expansion of the pool of newly born cells occurs during the stage of the type 2 cell. Type 2 cells are morphologically distinct from type 1 cells: their processes are short and horizontally oriented. The type 2 cells also show overlap in glial (BLBP, nestin) and neuronal marker (DCX and PSA-NCAM) expression. They have a small soma and an irregularly shaped nucleus and lack the strong apical process of type 1 cells. The developmentally younger subtype (type 2a) is positive for nestin, whereas type 2b cells in addition show the first antigenic signs of neuronal differentiation, most notably NeuroD and Prox1. The electrophysiologic properties of type 2 cells are in accord with this. Those that presumably correspond to the glia-like cells show the passive features of astrocytes. However, many (presumably the type-2b cells) display “complex” membrane features; in occasional type 2 cells, sodium currents can be found (Filippov et al. 2003). At the level of the type 2 cells, the first observed synaptic input is gamma aminobutyric acid (GABA)-ergic (Tozuka et al. 2005; Wang et al. 2005). Initially, cells respond to diffuse GABA and later to synaptic GABA. GABAergic innervation at this stage promotes further maturation, and GABA has an excitatory influence on these early cells. Proliferation of type 2 cells is positively regulated by physical exercise (Kronenberg et al. 2003) and presumably other non-specific stimuli.
Type 3 cells
The type 3 cell stage is one of transition from a slowly proliferating “neuroblast” to a postmitotic immature neuron. Under normal circumstances, type 3 cells show only little proliferative activity. Seizures, however, can dramatically increase the proliferation of type 3 cells (Jessberger et al. 2005). Type 3 cells invariably express markers of the neuronal lineage (DCX, PSA-NCAM, NeuroD, Prox1) and lack glial markers. DCX expression shows almost complete overlap with PSA-NCAM and spans a developmental period that comprises the type 3 stage, the exit from the cell cycle, and the initial 2–3 weeks of postmitotic neuronal development (Brandt et al. 2003; Rao and Shetty 2004; Couillard-Despres et al. 2005; Plumpe et al. 2006). The highly variable morphology of type 3 cells reflects this developmental transition: type 3 cell processes have various lengths and complexities, and the orientation of their processes increasingly changes from horizontal to vertical. Radial migration into the granule cell layer to the final destination of the young neuron also occurs on the level of the type 3 cell. Exit from the cell cycle mostly occurs at the type 3 cell stage and coincides with the transient expression of the calcium-binding protein calretinin (Brandt et al. 2003).
Selection
Just as in embryofetal and early postnatal brain development, the majority of adult-born cells is quickly eliminated through apoptosis (Biebl et al. 2000; Kuhn et al. 2005). Elimination of cells seems to occur after exit from the cell cycle but still within the first 2 weeks (at the level of DCX- and calretinin-expressing cells). Activation of N-methyl-D-aspartate (NMDA) receptors on developing neurons plays a role in the regulation of survival (Tashiro et al. 2006). The proportion of cells recruited into function is variable, but generally, a large surplus of progenitors is created through proliferation, and only a small fraction of those cells is selected for long-term survival (Kempermann et al. 2003). The proliferative activity of precursor cells is thus a poor predictor of net neurogenesis. Most of the variability in net neurogenesis can be explained by variable selection for survival (Kempermann et al. 2006). The new neurons are added to the dentate gyrus, rather than replacing old granule cells, and lead to the overall growth of the structure with time (Crespo et al. 1986). Growth of the dentate gyrus appears to occur mostly in the first year of rodent life (Altman and Das 1965; Bayer et al. 1982; Boss et al. 1985) and is substantially slowed down or may even stop later on, because of the dramatic age-dependent decrease of hippocampal neurogenesis.
The survival of new neurons is positively regulated by cognitively stimulating factors, such as living in an enriched environment (Kempermann et al. 1997) and, in some studies, even by short behavioral training known to impose particular demands on hippocampal function (Gould et al. 1999a; Dobrossy et al. 2003). However, the results are not unequivocal and require critical interpretation (Ambrogini et al. 2004b; Ehninger and Kempermann 2006; Mohapel et al. 2006).
Development of the immature neuron to a mature granule cell
Calretinin is exchanged for calbindin at 2–3 weeks after cell division, thus labeling a population of postmitotic immature neurons. During the stage of calretinin expression, large parts of the development and elaboration of the dendritic tree and axon elongation toward CA3 occur. Axon outgrowth is initiated before synaptic contacts on the dendritic tree are made (Zhao et al. 2006). The axons of new neurons grow along the mossy fiber tract toward CA3 (Hastings and Gould 1999) where they terminate in synapse- and interneuron-rich structures, the so-called boutons. Retroviral labeling studies have shown that axonal contact in CA3 is made as early as 10 days after cell division, whereas dendritic spines first appear about a week later and continue to be formed over a period of months (Zhao et al. 2006). Excitatory input onto new granule cells is largely complete by approximately 2 months after cell division (Toni et al. 2007).
Various approaches have been taken to demonstrate the functional integration of new granule cells into the local circuitry. Trans-synaptic labeling studies with a pseudorabies virus have demonstrated that new neurons establish synaptic contacts with axons coming from the entorhinal cortex and with CA3 pyramidal neurons (Carlen et al. 2002). The behavioral and pharmacological induction of immediate early gene expression in new neurons has revealed an increasing responsivess to synaptic stimulation that parallels the maturation of adult-born cells (Jessberger and Kempermann 2003). Across the entire population, kainic acid injection does not lead to IEG expression 2 weeks after BrdU, but induced IEG expression occurs in about 50% of new neurons at 4 weeks after BrdU and in about 80% of new granule cells at 7 weeks after BrdU. Retroviral labeling experiments and the availability of transgenic animals expressing green fluorescent protein (GFP) from various cell-type-specific promoters have permitted the physiological characterization of developing cells at various maturational stages. Data from early precursor cells have been derived from nestin-GFP reporter mice (Filippov et al. 2003; Fukuda et al. 2003). A transgenic line that expresses GFP from the pro-opiomelanocortin promoter has been used to study the properties of postmitotic cells (Overstreet et al. 2004). In addition, a line expressing GFP from the DCX promoter has been generated (Couillard-Despres et al. 2006).
Synaptic input onto new cells is initially only GABAergic and excitatory (Tozuka et al. 2005). At about 2 weeks after cell division, glutamatergic inputs appear, dendritic spines are formed, and GABAergic input becomes inhibitory. GABAergic input drives the neuronal maturation and integration of young cells (Ge et al. 2006).
Young granule cells progress through a phase of unique electrophysiological properties. They display lower activation thresholds and a higher resting membrane potential and undergo long-term potentiation more easily (Wang et al. 2000; van Praag et al. 2002; Ambrogini et al. 2004a; Schmidt-Hieber et al. 2004). Differences in NMDA receptor composition contribute to the different plasticity profile of younger and older granule cells (Ge et al. 2007). The lower activation and plasticity threshold of young granule cells is probably responsible for the preferential recruitment of these cells into functional circuits (Kee et al. 2007). At about 7 weeks after cell division, granule cells are physiologically indistinguishable from their older neighbors (van Praag et al. 2002). Compatible with this finding, the elaboration of the excitatory input seems to be largely completed 60 days after cell division (Toni et al. 2007).
Functional relevance of new hippocampal neurons
The data discussed above clearly demonstrate that adult-born hippocampal granule cells have neuronal electrophysiological features, make synaptic contacts to pre- and postsynaptic partners, respond to synaptic stimulation, and are integrated into the hippocampal circuitry. However, the incorporation of adult hippocampal neurogenesis into current concepts of hippocampal network function and behavior remains challenging. Available experimental animal data deal with the regulation of hippocampal neurogenesis through hippocampus-dependent behavior, address the correlation of neurogenesis with behavior, and ask whether the ablation of neurogenesis interferes with hippocampus-dependent behavioral tasks. Several theoretical concepts regarding the role of new neurons in hippocampal function have been proposed and will be briefly discussed below.
The regulation of the survival of hippocampal progenitor cells by experience and cognitively challenging environmental factors sparked the interest in the behavioral function of new granule cells (Kempermann et al. 1997). Subsequently, a learning paradigm (Morris water maze) that imposes particular functional demands on the hippocampus has been shown also selectively to increase the survival of hippocampal progenitors, whereas a control version of the task that shares performance factors but is hippocampus-independent (visible platform) does not (Gould et al. 1999a). In other studies (Ehninger and Kempermann 2006; Mohapel et al. 2006), this effect has not been seen, possibly because of confounding task factors that are strong regulators of hippocampal neurogenesis, such as stress (Gould and Tanapat 1999) and physical activity (van Praag et al. 1999).
A positive correlation between hippocampal neurogenesis and learning-related parameters in the Morris water maze has been found at least in some studies (Kempermann and Gage 2002; Drapeau et al. 2003).
In order to test whether hippocampal neurogenesis is required for hippocampus-dependent learning, various approaches have been taken to ablate dividing cells in the hippocampus. Blockade of neurogenesis has been achieved with pharmacological, radiological, and genetic strategies, generally with high efficacy. However, any lack in the specificity of the manipulations can complicate the interpretation of the results. In the first study of this kind (Shors et al. 2001), dividing hippocampal cells were ablated with a methylating agent (methylazoxymethanol acetate; MAM). This treatment impaired the learning of a hippocampus-dependent version of the eye-blink conditioning task but left the learning of a hippocampus-independent version of the task unaffected (Shors et al. 2001). Other hippocampus-dependent tasks, however, are not impaired by MAM treatment (Shors et al. 2002). One possible explanation for this and related findings (e.g., Saxe et al. 2006) is that hippocampal neurogenesis is selectively involved in only some forms of hippocampal learning. Whether this hypothesis holds as more blockade of neurogenesis studies are published remains to be seen. Santarelli et al. (2003) have chosen irradiation of the SGZ to block neurogenesis and shown that the intact SGZ is required for the anxiolytic action of an antidepressant drug. The attribution of this behavioral effect to a blockade of neurogenesis is complicated by the finding that SGZ irradiation leads to a pronounced local inflammatory response (Monje et al. 2003). Recently, genetic strategies have been used in mice to ablate the hippocampal precursor population (e.g., Garcia et al. 2004). The behavioral characterization of such a mouse line has revealed a deficit in some hippocampus-dependent behavioral tasks, but not others (Saxe et al. 2006).
Thus, at least some studies suggest a direct role of hippocampal neurogenesis in hippocampus-dependent behaviors, particularly hippocampus-dependent learning and memory.
Several (not necessarily mutually exclusive) models regarding the role of neurogenesis in hippocampal function have been discussed:
-
New neurons are directly involved in the temporary hippocampal storage of memory (Gould et al. 1999b). According to this view, newly born neurons are directly involved in the transient storage of hippocampus-dependent memories. These memories gradually become independent of the hippocampus and are subsequently stored in other parts of the brain, such as the neocortex. This concept is not consistent with the fact that hippocampus-dependent memories can be acquired throughout life, whereas neurogenesis levels are low in old age. In one version of this view, the transient nature of the hippocampus dependency of the memory is matched with the transient existence of adult-born hippocampal neurons. This idea is, however, inconsistent with the findings that new granule cells persist and are added to the pre-existing older granule cells rather than replacing them (Kempermann et al. 2003).
-
New neurons are not directly involved in the storage of hippocampus-dependent memories. Instead, the refinement of the hippocampal circuitry through the addition of new neurons to the dentate gyrus reflects an adaptive process to functional demands, such that hippocampal information-processing capacity is optimized for future memory storage (Kempermann 2002). This view is consistent with the finding that new neurons are added to the dentate gyrus throughout life, rather than replacing older granule cells, and that the mossy fiber tract continues to grow throughout adult life.
-
New neurons are added to the hippocampal network to avoid catastrophic interference (Wiskott et al. 2006). According to this idea, the increasing connectivity of a network with increasing experience and stored memories eventually leads to “over-connectivity”, the degradation of specific association, and the collapse of network function. The addition of new elements to the network can increase storage capacity and prevent catastrophic interference.
-
Adult-born neurons are directly involved in hippocampal memory traces and play a role in establishing temporal contiguity between events. Enhanced plasticity of young granule cells allows for the preferential association of representations that are closely related in time (Aimone et al. 2006).
-
Hippocampal neurogenesis is involved in forgetting (Feng et al. 2001). Replacement of older granule cells by new ones clears out old memories and provides storage capacity for new memories, analogous to overwriting a storage device. This theory is not consistent with the findings that new neurons are added to the dentate gyrus, rather than replacing old granule cells, and that neurogenesis is low in old age. Furthermore, the concept of active hippocampal “forgetting” largely lacks empirical support.
Concluding remarks
The developmental steps that are involved in the process of adult neurogenesis and that lead from hippocampal precursor cells to new neurons are now fairly well understood. A clearer overall picture has emerged, although questions remain. The functional significance of adult nuerogenesis and its impact on the circuit and ultimately behavioral level still need to be better understood.
References
Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569
Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9:723–727
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335
Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R (2004a) Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res 1017:21–31
Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R (2004b) Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci Lett 359:13–16
Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G (2007) Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE 2:e388
Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X (2006) Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev 15:407–421
Bayer SA, Yackel JW, Puri PS (1982) Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216:890–892
Biebl M, Cooper CM, Winkler J, Kuhn HG (2000) Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett 291:17–20
Boss BD, Peterson GM, Cowan WM (1985) On the number of neurons in the dentate gyrus of the rat. Brain Res 338:144–150
Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, Behrens W von der, Kempermann G (2003) Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci 24:603–613
Bull ND, Bartlett PF (2005) The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci 25:10815–10821
Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J (2002) Functional integration of adult-born neurons. Curr Biol 12:606–608
Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14
Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, Aigner L (2006) Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci 24:1535–1545
Crespo D, Stanfield BB, Cowan WM (1986) Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Exp Brain Res 62:541–548
Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN (2003) Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry 8:974–982
Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716
Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA 100:14385–14390
Ehninger D, Kempermann G (2006) Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes Brain Behav 5:29–39
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317
Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ (2001) Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32:911–926
Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G (2003) Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci 23:373–382
Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T (2003) Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci 23:9357–9366
Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV (2004) GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 7:1233–1241
Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439:589–593
Ge S, Yang CH, Hsu KS, Ming GL, Song H (2007) A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54:559–566
Gould E, Tanapat P (1999) Stress and hippocampal neurogenesis. Biol Psychiatry 46:1472–1479
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999a) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265
Gould E, Tanapat P, Hastings NB, Shors TJ (1999b) Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci 3:186–192
Hastings NB, Gould E (1999) Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol 413:146–154
Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M (2002) Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci 5:308–315
Jessberger S, Kempermann G (2003) Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci 18:2707–2712
Jessberger S, Romer B, Babu H, Kempermann G (2005) Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol 196:342–351
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99:11946–11950
Kaplan MS, Hinds JW (1977) Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197:1092–1094
Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362
Kempermann G (2002) Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 22:635–638
Kempermann G, Gage FH (2002) Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci 16:129–136
Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495
Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003) Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130:391–399
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452
Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH (2006) Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci USA 103:780–785
Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463
Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J (2005) Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci 22:1907–1915
Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A (2000) Noggin antagonizes BMP signaling to create a niche for adult neurogenesis.Neuron 28:713–726
Lledo PM, Alonso M, Grubb MS (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7:179–193
Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M (2003) Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37:751–764
Mercier F, Kitasako JT, Hatton GI (2002) Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188
Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250
Mohapel P, Mundt-Petersen K, Brundin P, Frielingsdorf H (2006) Working memory training decreases hippocampal neurogenesis. Neuroscience 142:609–613
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765
Nikolova G, Strilic B, Lammert E (2007) The vascular niche and its basement membrane. Trends Cell Biol 17:19–25
Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409:714–720
Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR (2002) Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci 22:3161–3173
Overstreet LS, Hentges ST, Bumaschny VF, Souza FS de, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M (2004) A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci 24:3251–3259
Palmer TD, Ray J, Gage FH (1995) FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6:474–486
Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH (1999) Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci 19:8487–8497
Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494
Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Romer B, Rodriguez GR, Kronenberg G, Kempermann G (2006) Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci 7:77
Praag H van, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270
Praag H van, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034
Ramón y Cajal S (1928) Degeneration and regeneration of the nervous system. Hafner, New York
Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234–246
Ray J, Peterson DA, Schinstine M, Gage FH (1993) Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA 90:3602–3606
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809
Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103:17501–17506
Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG (2004) Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 14:237–248
Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA (2005) Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci USA 102:9353–9358
Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187
Seaberg RM, Kooy D van der (2002) Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci 22:1784–1793
Seki T, Arai Y (1993a) Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res 17:265–290
Seki T, Arai Y (1993b) Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 13:2351–2358
Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160
Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A (2004) Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478:359–378
Shapiro LA, Korn MJ, Shan Z, Ribak CE (2005) GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res 1040:81–91
Shihabuddin LS, Horner PJ, Ray J, Gage FH (2000) Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci 20:8727–8735
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376
Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12:578–584
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G (2004) Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46:41–52
Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G (2006) Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54:805–814
Suhonen JO, Peterson DA, Ray J, Gage FH (1996) Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383:624–627
Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH (2006) NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442:929–933
Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, Praag H van, Martone ME, Ellisman MH, Gage FH (2007) Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 10:727–734
Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47:803–815
Wang LP, Kempermann G, Kettenmann H (2005) A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci 29:181–189
Wang S, Scott BW, Wojtowicz JM (2000) Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42:248–257
Wiskott L, Rasch MJ, Kempermann G (2006) A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16:329–343
Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Lie DC, Gage FH (2004) Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430:350–356
Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehninger, D., Kempermann, G. Neurogenesis in the adult hippocampus. Cell Tissue Res 331, 243–250 (2008). https://doi.org/10.1007/s00441-007-0478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0478-3