Abstract
The freshwater fish Serrasalmus spilopleura (piranha) has a continuous type of reproduction; gametes are constantly produced and released during the reproductive cycle. The testes do not undergo seasonal morphological changes but exhibit two constant regions throughout the year: the medullar region (involved with spermatogenesis) and the cortical region (involved with spermiation and sperm storage). We have evaluated the ultrastructure of the Leydig cells and the activity of 3β-HSD (an essential enzyme related to steroid hormone biosynthesis) and acid phosphatase (AcPase; lysosomal marker enzyme) in these two regions. The activity of 3β-HSD is stronger in the medullar region, and the Leydig cells in this region have a variety of cytological features that reflect differences in hormone synthesis and/or that could be linked to steroidogenic cells under various degrees of hormonal activity. In the cortical region, 3β-HSD activity is weak and the Leydig cells exhibit signs of degeneration, as confirmed by their ultrastructure and intense AcPase activity. These degenerative signs are indicative of cytoplasmic remodelling to degrade steroidogenic enzymes, such as 3β-HSD, that could lead to senescence or even to autophagic cell degeneration. S. spilopleura thus constitutes an interesting model for increasing our understanding of steroidogenesis control in freshwater teleost fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The freshwater teleost, Serrasalmus spilopleura (Ostariophysi, Characiformes, Characidae), also known as the pirambeba (piranha), is an endemic species in South America basins, with good adaptation to lentic environments (Leão 1996; Schleser 1997). This species is not migratory and is commonly found in Brazilian reservoirs, where its reproduction is continuous and frequent in the raining seasons (Lamas and Godinho 1996; Fujihara 1997). The males exhibit continuous spermatogenesis, in which spermatozoa are constantly produced and released throughout the reproductive cycle. Their testes do not undergo cyclical morphological changes but possess a medullar region and a cortical region that remain in a constant state throughout the year (R.H. Nóbrega, unpublished). In the medullar region, the germinal epithelium is constituted by a “continuum” of cysts at various stages of spermatogenesis. This type of epithelium is defined as continuous (Grier 2002; Lo Nostro et al. 2003) and is responsible for sperm production (active spermatogenesis). In the cortical region, the germinal epithelium is predominantly composed of Sertoli cells separated by scattered germ cell cysts, a morphology that results from spermiation (release of spermatozoa into testicular lumen) without cyst replacement. This type of epithelium is defined as discontinuous (Grier 2002; Lo Nostro et al. 2003); it ceases to produce sperm and becomes involved in sperm storage.
In contrast to the continuous spermatogenesis of S. spilopleura, most fish exhibit discontinuous spermatogenesis (seasonal breeders) in which spermatozoa are present in the testes only during a particular season of the year (for a review, see Cinquetti and Dramis 2003). In seasonal breeding teleosts, Leydig cells are well known to undergo cyclical changes during the annual reproductive cycle (Van den Hurk et al. 1978; Shanbhag and Nadkarni 1979; Borges Filho 1987; Cauty and Loir 1995; Civinini et al. 2001; Cinquetti and Dramis 2003; Lo Nostro et al. 2004). During testicular maturation in these seasonal fishes, the Leydig cells possess strong activity for 3β-HSD (an essential enzyme related to steroid hormone biosynthesis), a well-developed smooth endoplasmic reticulum, and numerous mitochondria with a dense matrix and tubular cristae. On the other hand, at the end of the reproductive cycle of these types of fish, the Leydig cells show a reduced 3β-HSD activity accompanied by cytoplasmic changes, such as damaged mitochondria (swollen and with a clear matrix), intense vacuolization and an increased number of lysosomes (Van Vuren and Soley 1990; Cauty and Loir 1995; Cinquetti and Dramis 2003).
No studies have as yet focused on the changes that occur in Leydig cells during continuous spermatogenesis in fishes. Morphological and enzyme studies are necessary for an accurate evaluation of testicular histoarchitecture and steroidogenesis and, subsequently, of the endocrine control of reproduction in fishes with continuous spermatogenesis. In this study, we have evaluated the ultrastructure of Leydig cells and their 3β-HSD activity in the medullar (predominantly involved with spermatogenesis) and cortical (predominantly involved with spermiation and sperm storage) regions of S. spilopleura. In addition, ultrastructural cytochemistry for acid phosphatase (AcPase) has been performed on the medullar and cortical region in order to evaluate degenerative signs in Leydig cells (such as an increased number of lysosomes).
Materials and methods
Animals
Adult S. spilopleura (males) were collected monthly in the Piracicaba river (Santa Maria da Serra, São Paulo State, Brazil) throughout the period of November 2003 to April 2005 (n = 65). They were anaesthetized with 0.1% benzocaine, weighed, measured (standard and total lengths) and killed by decapitation (according to the institutional animal care protocols and approval). Their testes were quickly removed, weighed and fixed by immersion for each of the following methods.
Histology
Testes were fixed in 2% glutaraldehyde and 4% paraformaldehyde in Sørensen’s phosphate buffer (0.1 M, pH 7.2) for at least 24 h, dehydrated and embedded in resin (Technovit 7100, Jung HistoResin) according to normal histological procedures. Sections (3 μm thick) were cut, stained with periodic-acid-Schiff’s (PAS)/haematoxylin/Metanil yellow (Quintero-Hunter et al. 1991) and documented by using a computerized image analyser (Leica Qwin 2.5).
Enzyme histochemistry for 3β-HSD
The in situ localization of 3β-HSD in S. spilopleura testis was conducted by enzyme histochemistry according to a modified protocol of Borges Filho (1987) and Lo Nostro and collaborators (2004). Testicular fragments of S. spilopleura were cryopreserved in a sucrose solution, immediately frozen in liquid nitrogen and stored at −80°C until used. The frozen material was sectioned at 5 μm and incubated in the dark at 37°C for 2 h in a medium containing 11.9 ml phosphate buffer (PB; 0.1 M, pH 7.6), 900 μl nitro blue tetrazolium (NBT; prepared by dissolving 1.0 mg NBT in 1 ml distilled water), 600 μl substrate (prepared with 4 mg dehydroepiandrosterone dissolved in 1 ml dimethylsulphoxide), 20.0 mg NAD+. After incubation, slides were washed in PB, lightly fixed with formol Ca+2 and mounted in gelatin. Steroid substrate or NAD+ was omitted from control samples.
Transmission electron microscopy
Testis fragments were fixed in 2% glutaraldehyde and 4% paraformaldehyde in Sørensen’s phosphate buffer (0.1 M, pH 7.2) for at least 24 h. The material was post-fixed in 1% osmium tetroxide (in the same buffer) for 2 h in the dark, contrasted en bloc with an aqueous solution of 5% uranyl acetate for 2 h, dehydrated and embedded in Araldite. Testes were sectioned, contrasted with a saturated solution of uranyl acetate in 50% alcohol and lead citrate and documented by using a Philips-CM 100 transmission electron microscope.
Ultrastructural cytochemistry for AcPase
Testis fragments were briefly fixed in 1% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h at 4°C, rinsed in the same buffer and incubated at 37°C for 1 h in a medium containing 25 mg cytidine-5′-monophosphate, 12 ml distilled water, 10 ml 0.05 M acetate buffer (pH 5.0), 3 ml 1% lead nitrate (Pino et al. 1981). After incubation, the material was once more fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2), post-fixed in the dark for 2 h in 1% osmium tetroxide in the same buffer, rinsed again several times in sodium cacodylate buffer, contrasted en bloc with 2% uranyl acetate for 2 h, dehydrated in acetone, embedded in Araldite, sectioned and observed unstained by using a Phillips-CM 100 transmission electron microscope. Negative control samples were incubated in medium without substrate (cytidine-5′-monophosphate).
Results
Testicular structure and enzyme histochemistry for 3β-HSD
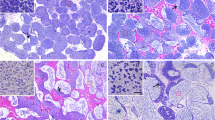
S. spilopleura has an anastomosing tubular testis that exhibits two constant regions throughout the reproductive cycle: the medullar region and the cortical region (Fig. 1). In the medullar region, the interstitium is conspicuously enlarged around the germinal compartment, which has a reduced testicular lumen (Fig. 1). In contrast, the cortical region has a less prominent interstitium and an enlarged testicular lumen filled with spermatozoa (Fig. 1).
Cross section of the testis of S. spilopleura. Two regions can be seen: medullar (M) and cortical (C). The cortical region consists of a network of large anastomosing tubules (AT) that are filled with sperm. The testis is attached to the coelomic cavity by a mesorchium (MS). Staining: periodic-acid-Schiff’s (PAS)/haematoxylin/Metanil yellow. Bar 200 μm
The enzymatic reaction for 3β-HSD occurs in the cytoplasm of the Leydig cells, which exhibit distinct patterns in the medullar and cortical regions (Fig. 2). The Leydig cells from the medullar region show a strong reaction for 3β-HSD (Fig. 2a) and preferentially accumulate in the angular interstices between three or more tubules (Fig. 2a,b). The 3β-HSD reaction is also positive on the wall of the cysts (Fig. 2b). In the cortical region, Leydig cells have a weak diffuse reaction for 3β-HSD within the interstitial compartment (Fig. 2c,d).
Histochemical reaction for 3β-HSD. a, b A strong enzymatic reaction is found in the interstitial compartment (IC) of the medullar region in which the germinal compartment (GC) is surrounded by a continuous germinal epithelium (CGE). b A weak reaction for 3β-HSD (arrowhead) is seen on the wall of the cysts. c, d The reaction to 3β-HSD is weak and diffuse in the IC of the cortical region in which the germinal epithelium is discontinuous (DGE). Bars 100 μm (a, c), 50 μm (b, d)
Ultrastructure of Leydig cells
In the medullar region, the germinal epithelium is continuous and constituted by cysts at different stages of spermatogenesis (Fig. 3a). In the interstitium, Leydig cells are commonly found near to blood vessels (Fig. 3a). Their nucleus is slightly eccentric with an irregular shape and contains a diffuse heterochromatin and an evident nucleolus (Fig. 3b). The cytoplasm displays well-developed smooth endoplasmic reticulum (SER) and numerous mitochondria with tubular cristae (Fig. 3b). Lipid droplets have not been seen in the cytoplasm of the Leydig cells (Fig. 3b). Apart from these characteristics, which they share, Leydig cells display the follow variations in their ultrastructure: (1) some show numerous large mitochondria that have a dense matrix and tubular cristae (Fig. 3b–d); (2) others present numerous mitochondria (with a moderately electron-dense matrix) and dilated perinuclear cisternae connected to an highly vesicularised and dilated SER (Fig. 3e); (3) some contain dilated SER cisternae and mitochondria that become gradually larger (Fig. 3f); (4) some can also present abundant SER and a few mitochondria that have a moderately electron-dense matrix and tubulo-vesicular cristae (Fig. 4a,b); (5) some contain numerous dense mitochondria (Fig. 4c). Associations between Leydig cells and granulocytes and between Leydig cells and amyelinic nerves have also been documented in the interstitial compartment of this region (Fig. 4d).
Ultrastructure of Leydig cells in the medullar region. a Note the continuous germinal epithelium (CGE), Sertoli cell (SE), germ cells (GC), spermatogonia division (arrowhead), spermatozoa (SZ), cyst (CY). In this region, Leydig cells (LE) display various features (see LE*). Other structures include blood vessels (BV), a myoid cell (MY) and erythrocytes (ER). b Leydig cell (LE) exhibiting abundant smooth endoplasmic reticulum (SER) and numerous mitochondria (M) with tubular cristae (N nucleus, NU nucleolus, CF collagen fibres). c Higher magnification of Leydig cell (NE nuclear envelope). d Detail of the mitochondria boxed in c; they have a dense matrix and closed tubular cristae. e Leydig cell exhibiting numerous mitochondria and dilated SER (arrowheads). Note the nuclear pores (arrows). f Detail of Leydig cell cytoplasm with dilated SER and enlarged mitochondria. Bars 5.5 μm (a), 1 μm (b–f)
Ultrastructure of Leydig cells in the medullar region. a Leydig cell (N nucleus, NE nuclear envelope) with a few mitochondria (M) and a well-developed smooth endoplasmic reticulum (SER). b Higher magnification of the mitochondria boxed in a (arrow dense deposit in the mitochondrial matrix, arrowhead SER arranged in a sacular structure surrounding the mitochondria). c Note that the mitochondria have a dense matrix and closed tubular cristae (CF collagen fibres). d Associations between Leydig cells (LE), granulocytes (GR) and an unmyelinated nerve (AN). Note the Schwann cell nucleus (SCH). Bars 1 μm
In the cortical region, the germinal epithelium is predominantly discontinuous being constituted of Sertoli cells separated by scattered developing cysts (Fig. 5a). In this region, Leydig cells have an irregular nucleus that becomes denser and contains clumps of heterochromatin (Fig. 5a,b). The cytoplasm possesses dense bodies and vacuoles of variable shape and electron density (Figs. 5b,c). In the mitochondria, the matrix becomes electron-lucent and the cristae tend to become less dense (Fig. 5b,f). In others, the mitochondria tend to swell (Fig. 5c), showing electron-lucent cristae that may be associated with myelin figures (Fig. 5d). The SER is more scarce and scattered (Fig. 5e). Some cytoplasmic fragments of Leydig cells can be found in the interstitium of this region (Fig. 6a,d). Monocytes inside blood vessels (Fig. 6b,c) and associations between macrophages and Leydig cells are commonly found in the interstitium of the cortical region (Fig. 6a,d).
Ultrastructure of Leydig cells in the cortical region. a Note the discontinuous germinal epithelium (DGE), spermatozoa (SZ), multivesicular body (MB), myoid cell (MY), interstitial compartment (IC), Sertoli cell (SE) and the disorganized cytoplasm (asterisk) of a Leydig cell (LE). b Detail of Leydig cell showing a dense nucleus (N) and vacuolated cytoplasm (arrowheads). In the mitochondria (M), the matrix is electron-lucent and the cristae are less dense. c In other Leydig cells, the mitochondria seem to swell (SER smooth endoplasmic reticulum, NE nuclear envelope, arrowhead dense body). d Higher magnification of mitochondria boxed in c. A myelin figure (double arrow) can be found in association with the mitochondrial matrix. e Detail of the disorganized Leydig cell cytoplasm. f Mitochondrial cristae are less dense (arrows). Bars 2.32 μm (a), 1 μm (b–f)
a Interstitial compartment (IC) of the cortical region (GE germinal compartment, SE Sertoli cell, MY myoid cell, LE Leydig cell, BV blood vessel, MC macrophage, asterisk some cytoplasmic fragments of Leydig cells found in the IC). b Blood vessel with erythrocytes (ER) and a monocyte (MO). Note the nucleus (N), nuclear pore (arrow), swollen mitochondria (M), smooth endoplasmic reticulum (SER), endothelial cell (EN), collagen fibres (CF) and fibroblast (F). cDouble arrows indicate associations between a monocyte (MO) and endothelial cells (EN). d In the cortical region, associations between macrophages (MC) and Leydig cells (LE) are common. Note the damaged mitochondria (double arrowheads) and vacuolated cytoplasm (double asterisks). Bars 2.32 μm (a), 1 μm (b–d)
Ultrastructural cytochemistry for AcPase
In the medullar region, the reaction to AcPase is weak and occurs only in the Leydig cells nucleus. In contrast, Leydig cells of the cortical region exhibit a strong reaction to AcPase in the nucleus, mitochondria and in some vesicles (Fig. 7a–d). The largest vesicles, which are strongly AcPase-positive, are lysosomes, whereas the smallest, which show a weak reaction, are vesicles containing hydrolytic enzymes (Fig. 7b,c). Gradually, the mitochondria and other organelles are enveloped by an endomembranous system (vacuoles) to which AcPase vesicles (lysosomes and smallest vesicles) tend to fuse (Fig 7c,d). AcPase activity is also found in some mitochondria (Fig. 7d).
Ultrastructural cytochemistry for AcPase. a Strong reaction was found in the nucleus (N) and lysosomes (arrowheads) of cortical Leydig cells (LE) but not in the mitochondria (M). b Detail of Leydig cell cytoplasm. Note that vacuoles (V) fuse to each other (single asterisks) or directly to mitochondria (double asterisk). Small vesicles (arrows) and lysosomes (white arrowheads) fuse to large vacuoles and/or mitochondria. c The smallest vesicles and the large vacuoles (V) appear to be fusing (arrows). Some vacuoles are full of dense reaction product (arrowheads). d Some mitochondria (M) have AcPase activity (double white arrow). Fusion among the vacuoles to envelope the organelles can also be seen (asterisks). e Weak reaction in the Leydig cell nucleus. f Strong reaction in the multivesicular body (MB) and lysosome (arrowhead) of Sertoli cells (SE). Bars 1 μm
Discussion
As in other teleosts, Leydig cells of S. spilopleura are located in the interstitial compartment, singly or in small groups, and usually lie near to blood vessels (Pudney 1996, 1999). In S. spilopleura, the steroidogenic potential of Leydig cells has been confirmed by their ultrastructure, which is typical of steroid-producing cells (a well-developed SER and numerous mitochondria with tubular cristae), and by enzymatic histochemistry for 3β-HSD. The activity of 3β-HSD differs along the testicular regions of S. spilopleura. In the medullar region, which is mainly involved in spermatogenesis, 3β-HSD activity is strong, corroborating that spermatogenesis is highly dependent on sexual steroid hormones (Schulz and Miura 2002; Miura and Miura 2003; Miura et al. 2006). In contrast, in the cortical region, which is mostly responsible for spermiation and sperm storage, the 3β-HSD reaction is weak and is dispersed through the interstitium, suggesting a downregulation of 3β-HSD activity. Miura and collaborators (2003, 2006) have recently shown that progestins play an important role in spermiation and sperm maturation in the Japanese eel (a teleost fish). Thus, although further investigation is needed, the decrease in 3β-HSD activity in the Leydig cells from the cortical region can be postulated as being attributable to an accumulation of 17α-hydroxyprogesterone (negative feedback), which is the precursor of progestins (for a review, see Miura et al. 2006).
Our findings are also in agreement with histochemical studies of seasonal breeding teleosts showing that 3β-HSD activity increases during maturation (spermatogenesis) and decreases after spermiation, becoming almost imperceptible in testicular regression (Van den Hurk et al. 1978; Shanbhag and Nadkarni 1979; Borges Filho 1987; Cinquetti and Dramis 2003; Lo Nostro et al. 2004).
Leydig cells undergo ultrastructural changes along the testicular regions of S. spilopleura. However, in the medullar region, Leydig cells with different features are seen in the interstitium. Despite the steroidogenic characteristics that they share, medullar Leydig cells exhibit variations in their ultrastructure, especially in the nucleus (shape and condensation), mitochondria (number, size, electron density and arrangement of cristae), SER (volume and degree of dilation) and perinuclear space (distance between the membranes of the nuclear envelope). These cytological features are similar to those found in the interrenal cells of Gasterosteus aculeatus (Civinini et al. 2001), suggesting that these characteristics reflect differences in hormone synthesis and/or might be linked to steroidogenic cells under different degrees of hormonal activity.
Of note, steroidogenic enzymes are localized partly in mitochondria and partly on the SER membranes (for a review, see Akingbemi et al. 1999). After the mitochondrial step in the cholesterol side cleavage, the synthesis of sex hormones is performed on SER membranes on which 3β-HSD, a key enzyme in the biosynthesis of steroid hormones (for reviews, see Haider and Servos 1998; Haider 2004), is localized. Thus, the stronger 3β-HSD activity found in the medullar region might be correlated with those Leydig cells that have abundant dilated SER. The expansion of the perinuclear space is also seen in these cells and is considered to be a reflex of endoplasmic reticulum activity; under hormonal stimuli, the endoplasmic reticulum expands and, in consequence, the perinuclear space becomes highly dilated (for a review, see Carvalho 2001).
In the cortical region, Leydig cells show signs of degeneration, such as a more condensed nucleus, damaged mitochondria (swollen, electron-lucent matrix, degraded or less-dense cristae, associations with myelin figures), lysosomes, myelin figures, dense bodies and vacuolization. These characteristics have also been documented in Leydig cells at the end of the reproductive cycle of some seasonal teleosts (Van Vuren and Soley 1990; Cauty and Loir 1995; Cinquetti and Dramis 2003) and during the steroidogenic cycle of G. aculeatus interrenal cells (Civinini et al. 2001). Some authors have reported that, in cells under stress-induced conditions, the mitochondria undergo structural and functional alterations, leading to the formation of megamitochondria, also called giant mitochondria or swollen mitochondria (Wakabayashi 1999; Bottone et al. 2006; Yoon et al. 2006). This mitochondrial characteristic is considered as a marker of the senescence or death of cells. This cytological feature of swollen mitochondria is also observed in Leydig cells of the cortical region, suggesting a moment of “crisis” in the cellular cycle that is overcome or that precedes senescence or cell death.
The degenerative signs in the cortical Leydig cells have been confirmed by the strong activity of AcPase. The AcPase reaction occurs in the nucleus, in lysosomes, in vesicles containing hydrolytic enzymes and in mitochondria. Recently, AcPase activity has been reported in the nucleus indicating that AcPase could have cell-signalling functions, through the dephosphorylation of specific nuclear components, which would mean that AcPase controls gene expression (Cruz Landim et al. 2002; Custodio et al. 2004). In S. spilopleura, the AcPase reaction in the nucleus can be interpreted as a variation of the nuclear functional state, being related to the changes occurring in the cortical Leydig cells. The intense AcPase activity found in the cytoplasm (lysosomes, vesicles containing hydrolytic enzymes and autophagosomes) might be correlated with the low 3β-HSD activity of the cortical region, suggesting an involvement of the endosome-lysosome system in the degradation of some of the steroidogenic enzymes, such as 3β-HSD. Cavaco and collaborators (1999) have also reported lysosomes and autophagosomes in the Leydig cells of young Clarias gariepinus treated with androgens and attribute their presence to cytoplasmic remodelling to diminish the number of mitochondria and to degrade enzymes of the steroidogenic pathway in response to negative feedback.
Associations between cortical Leydig cells and macrophages are also evident in S. spilopleura. In mammals, macrophage-derived factors seem to modulate steroidogenic activity in Leydig cells (for a review, see Gaytan et al. 1994). Studies of mammals have demonstrated that pro-inflammatory cytokines produced by macrophages inhibit androgen synthesis in Leydig cells by diminishing the activity of the enzymes of the steroidogenic pathway (Sun and Risbridger 1994; Watson et al. 1994; Hales 1996). In teleosts, Lister and Van der Kraak (2002) have demonstrated that tumour necrosis factor α, which is produced by macrophages, inhibits testosterone synthesis at several enzyme sites and have suggested that the regulation of steroidogenesis in fish, as in mammals, involves multiple paracrine and autocrine factors. Therefore, although further investigation is needed, cortical Leydig cells might be regulated in a paracrine manner and, in response to negative feedback, they might undergo cytoplasmic remodelling involving the endosome-lysosome system to degrade certain steroidogenic enzymes, such as 3β-HSD. The morphological and enzymatic changes occurring in these cells might be interpreted as a sign of cellular remodelling leading to senescence or even to autophagic cell degeneration.
The main ultrastructural and enzymatic changes occurring in Leydig cells throughout the continuous spermatogenesis of S. spilopleura is summarized in Fig. 8. However, these results give rise to questions, such as (1) do those Leydig cells with characteristics of degeneration die or do they undergo cytoplasmic remodelling in response to a negative feedback; (2) if Leydig cells undergo cytoplasmic remodelling, do they recover their steroidogenic potential; (3) if Leydig cells die, how are they renewed and what types of cell are their precursors? Since S. spilopleura has different testicular regions, it is an interesting model for studying these questions and for understanding steroidogenesis control in freshwater teleost fish.
Changes in ultrastructure and enzymes in Leydig cells during the continuous spermatogenesis of S. spilopleura. Status of spermatogenesis: a–c active spermatogenesis; d spermiation and sperm storage. a A medullar Leydig cell (N nucleus, NU nucleolus) initially has few mitochondria (M) and a well-developed smooth endoplasmic reticulum (SER). b Mitochondria (M) later become more numerous and larger with a dense mitochondrial matrix. c The SER and the perinuclear space become highly dilated. d In the cortical region, the nucleus (N) of the Leydig cell is dense and the cytoplasm becomes vacuolated (V). In some mitochondria (M), the matrix is electron-lucent and the cristae tend to become less dense. The mitochondria can also swell and show myelin figures (arrowheads) associated with the matrix. Increased numbers of lysosomes (LY) are present. The transition between each phase of steroidogenic cycle is indicated by open arrows. Bottom Strong reaction (++++), weak reaction (+), weak reaction only in the nucleus (+*)
Reference
Akingbemi BT, Ge R-S, Hardy MP (1999) Leydig cells. In: Knobil E, Neill JD (eds) Encyclopedia of reproduction, vol 2. Academic Press, San Diego, pp 1021–1033
Borges Filho OF (1987) Caracterização dos estádios de maturação e correlação com avaliações histoquímico-enzimáticas e ultra-estruturais das células endócrinas testiculares, durante o ciclo reprodutivo do Prochilodus scrofa - Steindachner 1881. Tese de Doutorado, Instituto de Biociências da Universidade de São Paulo, São Paulo
Bottone MG, Soldani C, Fraschini A, Alpini C, Croce AC, Bottiroli G, Pellicciari C (2006) Enzyme-assisted photosensitization with rose Bengal acetate induces structural and functional alteration of mitochondria in HeLa cells. Histochem Cell Biol, DOI 10.1007/s00418-006-0235-9
Carvalho HF (2001) Envoltório nuclear. In: Carvalho HF, Recco-Pimentel S (eds) A Célula 2001. Editora Manole, Tamboré-Barueri, Brazil, pp 77–87
Cauty C, Loir M (1995) The interstitial cells of the trout testis (Oncorhynchus mykiss): ultrastructural characterization and changes throughout the reproductive cycle. Tissue Cell 27:383–395
Cavaco JEB, Van Blijswijk B, Leatherland JF, Goos HJT, Schulz RW (1999) Androgen-induced changes in Leydig cell ultrastructure and steroidogenesis in juvenile African catfish, Clarias gariepinus. Cell Tissue Res 297:291–299
Cinquetti R, Dramis L (2003) Histological, histochemical, enzyme histochemical and ultrastructural investigations of the testis of Padogobius martensi between annual breeding seasons. J Fish Biol 63:1402–1428
Civinini A, Padula D, Gallo VP (2001) Ultrastructural and histochemical study on the interrenal cells of the male stickleback (Gasterosteus aculeatus, Teleostei), in relation to the reproductive annual cycle. J Anat 199:303–316
Cruz Landim C, Reginato RD, Moraes RL, Cavalcante VM (2002) Cell nucleus activity during post-embryonic development in Apis mellifera L. (Hymenoptera: Apidae). Intranuclear acid phosphatase. Genet Mol Res 1:131–138
Custodio AM, Goes RM, Taboga SR (2004) Acid phosphatase activity in gerbil prostate: comparative study in male and female during postnatal development. Cell Biol Int 28:335–344
Fujihara CY (1997) Dinâmica populacional de Serrasalmus spilopleura, Kner 1860 no reservatório de Jurumirim (rio Paranapanema, SP): aspectos do crescimento, estrutura populacional, reprodução e nutrição. Dissertação de Mestrado, Instituto de Biociências, UNESP, Botucatu, SP, Brasil
Gaytan F, Bellido C, Aguilar E, Van Rooijen N (1994) Requirement for testicular macrophages in Leydig cell proliferation and differentiation during prepubertal development in rats. J Reprod Fertil 102:393–399
Grier HJ (2002) The germinal epithelium: its dual role in establishing male reproductive classes and understanding the basis for indeterminate egg production in female fishes. In: Creswell RL (ed) Proceedings of the Fifty-third Annual Gulf and Caribbean Fisheries Institute, November 2000. Mississippi/Alabama Sea Grant Consortium, Fort Pierce, pp 537–552
Haider SG (2004) Cell biology of Leydig cells in the testis. Int Rev Cytol 233:181–241
Haider SG, Servos G (1998) Ultracytochemistry of 3β-hydroxysteroid dehydrogenase in Leydig cell precursors and vascular endothelial cells of the postnatal rat testis. Anat Embryol 198:101–110
Hales DB (1996) Leydig cell-macrophage interactions: an overview. In: Payne AH, Hardy MP, Russell LD (eds) The Leydig cell. Cache River, Vienna, Ill., pp 452–475
Lamas IR, Godinho AL (1996) Reproduction in the piranha Serrasalmus spilopleura, a neotropical fish with an unusual pattern of sexual maturity. Environ Biol Fishes 45:161–168
Leão ELM (1996) Reproductive biology of piranhas (Teleostei, Characiformes). In: Val AL, Almeida-Val VMF, Randall DJ (eds) Physiology and biochemistry of fishes of the Amazon. Instituto Nacional de Pesquisas da Amazonia, Manaus, Brazil, pp 31–41
Lister A, Van der Kraak G (2002) Modulation of goldfish testicular testosterone production in vitro by tumor necrosis factor α, interleukin-1β, and macrophage conditioned media. J Exp Zool 292:477–486
Lo Nostro F, Grier H, Andreone L, Guerrero GA (2003) Involvement of the gonadal germinal epithelium during sex reversal and seasonal testicular cycling in the protogynous swamp eel, Synbranchus marmoratus Bloch 1795 (Teleostei, Synbranchidae). J Morphol 257:107–126
Lo Nostro FL, Antoneli FN, Quagio-Grassiotto I, Guerrero GA (2004) Testicular interstitial cells, and steroidogenic detection in the protogynous fish, Synbranchus marmoratus (Teleostei, Synbranchidae). Tissue Cell 36:221–231
Miura T, Miura CI (2003) Molecular control mechanisms of fish spermatogenesis. Fish Physiol Biochem 28:181–186
Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C (2006) Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Proc Natl Acad Sci USA 103:7333–7338
Pino RM, Pino LC, Bankston PW (1981) The relationships between the Golgi apparatus, GERL, and lysosomes of fetal rat liver Kupffer cells examined by ultrastructural phosphatase cytochemistry. J Histochem Cytochem 29:1061–1070
Pudney J (1996) Comparative cytology of the Leydig cell. In: Payne AH, Hardy MP, Russell LD (eds) The Leydig cell. Cache River, Vienna, Ill., pp 98–142
Pudney J (1999) Leydig and Sertoli cells, nonmammalian. In: Knobil E, Neill JD (eds) Encyclopedia of reproduction, vol 2. Academic Press, San Diego, pp 1008–1020
Quintero-Hunter I, Grier H, Muscato M (1991) Enhancement of histological detail using Metanil yellow as counterstain in periodic acid/Schiff’s hematoxylin staining of glycol methacrylate tissue sections. Biotech Histochem 66:169–172
Schleser DM (1997) Piranhas: everything about origins, care, feeding, diseases, breeding, and behavior. Barron’s Educational Series, New York
Schulz RW, Miura T (2002) Spermatogenesis and its endocrine regulation. Fish Physiol Biochem 26:43–56
Shanbhag AB, Nadkarni VB (1979) Histological and histochemical studies on the testicular cycle of a fresh water teleost Channa gachua (Hamilton). Anat Anz 146:381–389
Sun XR, Risbridger GP (1994) Site of macrophage inhibition of luteinizing hormone-stimulated testosterone production by purified Leydig cells. Biol Reprod 50:363–367
Van den Hurk R, Peute J, Vermeij JAJ (1978) Morphological and enzyme cytochemical aspects of the testis and vas deferens of the rainbow trout, Salmo gairdneri. Cell Tissue Res 186:309–325
Van Vuren JHJ, Soley JT (1990) Some ultrastructural observations of Leydig and Sertoli cells in the testis of Tilapia rendalli following induced testicular recrudescence. J Morphol 206:57–63
Wakabayashi T (1999) Structural changes of mitochondria related to apoptosis: swelling and megamitochondria formation. Acta Biochim Pol 46:223–237
Watson ME, Newman RJ, Payne AM, Abdelrahim M, Francis GL (1994) The effect of macrophage conditioned media on Leydig cell function. Ann Clin Lab Sci 24:84–95
Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G (2006) Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol 209:468–480
Acknowledgements
We thank the Centro de Microscopia Eletrônica and the Laboratório de Reprodução de Peixes Neotropicais, Departamento de Morfologia, IBB, UNESP, Botucatu, for the use of their facilities. We are also grateful to Maria Helena Moreno for help with the electron micrographs, to Antonio Vicente Salvador and Sueli Cruz Michelin for histological support and to Drs. Fabiana Lo Nostro, Denise Vizziano, Daniela Carvalho dos Santos and Maeli Dal Pai Silva for suggestions regarding the histochemical reactions.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo (03/11078-9 and 04/01262-0).
Rights and permissions
About this article
Cite this article
Nóbrega, R.H., Quagio-Grassiotto, I. Morphofunctional changes in Leydig cells throughout the continuous spermatogenesis of the freshwater teleost fish, Serrasalmus spilopleura (Characiformes, Characidae): an ultrastructural and enzyme study. Cell Tissue Res 329, 339–349 (2007). https://doi.org/10.1007/s00441-006-0377-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0377-z