Abstract
We previously reported that fibroblast growth factor 2 (FGF2) facilitated the differentiation of transplanted bone marrow cells (BMCs) into hepatocytes. Our earlier study also demonstrated that administration of FGF2 in combination with bone marrow transplantation (BMT) synergistically activated tumor necrosis factor-alpha signaling and significantly improved liver function and prognosis more than BMT alone. However, the way that it affected the extracellular matrix remained unclear. Here, we investigated the effect of FGF2 treatment together with BMT on liver fibrosis in mice treated with carbon tetrachloride (CCl4). Transplantation of BMCs and concurrent treatment with FGF2 caused a statistically significant reduction in CCl4-induced liver fibrosis that was accompanied by strong expression of matrix metalloproteinase 9 as compared with FGF2-only treatment or BMT alone. Moreover, in this process, the proliferation of bone-marrow-derived cells was accelerated without causing apoptosis. Thus, the administration of FGF2 in combination with BMT synergistically improves CCl4-induced liver fibrosis in mice. This treatment has the potential of being an effective therapy for patients with liver cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of reports have recently demonstrated the capacity of bone marrow cells (BMCs) to differentiate into a variety of cell lineages, including hepatocytes (Theise et al. 2000; Orlic et al. 2001; Ferrari et al. 1998; Lagasse et al. 2000; Krause et al. 2001; Kotton et al. 2001; Petersen et al. 1999; Okamoto et al. 2002), leading to the idea that BMCs might be an attractive cell source for regenerative therapy of the liver. In previous work, we have developed and reported an in vivo mouse model, the green fluorescent protein/carbon tetrachloride (GFP/CCl4) model, which has made possible the monitoring of BMC differentiation into hepatocytes (Terai et al. 2003). Using this model system, we have shown that transplanted GFP-positive BMCs populate a liver damaged by continuous injection of CCl4. Moreover, the transplanted BMCs differentiate into albumin-producing hepatocytes via Liv2-positive hepatoblast intermediates (Terai et al. 2003), and bone marrow transplantation (BMT) results in elevated serum albumin levels, reduced liver fibrosis, and improvement in the survival rate of CCl4-treated mice (Sakaida et al. 2004). Taken together, these results suggest that BMT might be an effective treatment for patients with liver failure.
In our model system, we have also found that fibroblast growth factor 2 (FGF2) is the most important growth factor for the differentiation of BMCs into hepatocytes. Further, the administration of FGF2 in combination with BMT synergistically activates tumor necrosis factor α (TNFα) signaling and significantly improves liver function and prognosis (Ishikawa et al. 2006). However, the way that FGF2 treatment together with BMT affects the extracellular matrix (ECM) is not well understood. During liver regeneration, hepatocyte proliferation in conjunction with inflammatory cytokines (such as TNFα) and various growth factors is thought to control ECM remodeling. Recent studies have also demonstrated that proteinases, such as matrix metalloproteinases (MMPs), play important roles not only in ECM remodeling (Bezerra et al. 1999), but also in hepatocyte proliferation (Mars et al. 1996; Kim et al. 1997). Furthermore, TNFα has been reported to promote ECM degradation, by involving MMP9 induction through the activation of the nuclear factor κ B (NFκB) pathway (Serandour et al. 2005). Our previous evaluations of several MMPs and of tissue inhibitors of metalloproteinases (TIMPs) by using DNA-microarray and immunohistochemistry have also revealed that MMP9 is one of the important fibrolysis inducers in our model (Sakaida et al. 2004; Omori et al. 2004). Here, we investigate the effect of FGF2 treatment with BMT on CCl4-induced liver fibrosis in mice and on the proliferation and death of bone-marrow-derived cells.
Materials and methods
Experimental protocol
We previously developed and reported an in vivo mouse model, the GFP/CCl4 model (Terai et al. 2003). Six-week-old C57 BL/6 female mice (Japan SLC, Shizuoka, Japan) were injected intraperitoneally with CCl4 (1.0 ml/kg body weight) twice a week for 4 weeks to induce persistent liver damage. The first day after this treatment, 1 × 105 GFP-positive BMCs were isolated from C57 BL/6 Tg14 (act-EGFP) OsbY01 mice (GFP-transgenic mice; provided by Dr. Masaru Okabe, Genome Research Center, Osaka University, Osaka, Japan) and transplanted with or without 30 μg/kg recombinant FGF2 (rFGF2; R&D Systems, Minneapolis, Minn., USA) slowly, by using a 31G needle and Hamilton syringe, via the tail vein as previously described (Terai et al. 2003; Ishikawa et al. 2006). The same dose of CCl4 was subsequently injected twice a week to maintain persistent liver damage, and individual mice were killed at 2 weeks post-transplantation. Mice with CCl4-induced chronic liver damage were divided into four treatment groups: (1) CCl4 group (no treatment control); (2) rFGF2 group (rFGF2 treatment without BMT); (3) BMC group (BMT without rFGF2 treatment); (4) BMC + rFGF2 group (BMT with rFGF2 treatment).
Analysis of liver fibrosis
Liver samples were obtained from six mice per group and examined histologically for liver fibrosis by using picro-Sirius red staining of sections of paraffin-embedded material as previously described (Sakaida et al. 1999, 2004). The samples were quantified by means of a Provis microscope (Olympus, Tokyo, Japan) equipped with a charge-coupled device camera, and the red area (the fibrotic area) was assessed by computer-assisted image analysis with MetaMorph software (Universal Imaging, Downingtown, Pa., USA). The mean value of 30 random fields per group was compared as the expressed percent area of liver fibrosis.
Immunohistochemical staining
Liver samples from six mice per group were examined by an immunohistochemical non-fluorescent staining method as previously described (Shinoda et al. 1992). A total of 30 random fields per group were analyzed independently, and the percentage of stained area was calculated by using MetaMorph software. We examined the expression of several factors with antibodies against GFP (Santa Cruz Biotechnology, Calif., USA), Ki-67 (Santa Cruz Biotechnology), alpha-smooth muscle actin (αSMA; Dako, Kyoto, Japan), MMP9 (Santa Cruz Biotechnology), CD11b (BD Pharmingen, Tokyo, Japan), and Ly-6G and Ly-6C (Gr-1; BD Pharmingen, Tokyo, Japan). In addition, we detected GFP and Ki-67 or MMP9, αSMA and MMP9, and MMP9 and CD11b or Gr-1 immunofluorescently with the following secondary antibodies: Alexa Fluor R-488 and R-568 donkey anti-goat IgG (H + L) conjugates and Alexa Fluor R-488 goat anti-rabbit IgG (H + L) conjugate (Molecular Probes, Eugene, Ore., USA). The analysis was performed as previously described (Terai et al. 2003; Sakaida et al. 2004; Ishikawa et al. 2006).
Western blot analysis of MMP9
To obtain protein, isolated pure BMCs were subjected to homogenization with lysis buffer (20 mM TRIS-HCl pH 7.5, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride) and then centrifuged. A total of 100 μg protein was analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting. Protein was electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Calif., USA). The PVDF membrane was incubated with goat anti-mouse MMP9 antibody (1:500 dilution), washed, and then incubated with an anti-goat IgG conjugated to horseradish peroxidase (Amersham Biosciences, N.J., USA; 1:4000 dilution). Bands were visualized with an enhanced chemiluminescence (ECL) Western blotting detection system (Amersham Biosciences). We also performed immunoblot analysis with phosphate-buffered saline as a negative control. All experiments were repeated independently at least three times with reproducible results.
Analysis of apoptosis in liver
Frozen sections were prepared from liver samples from six mice per group, and apoptotic cells in the frozen sections were detected by using the apoptosis in situ detection kit (Wako, Osaka, Japan) based on the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUDT) nick end labeling (TUNEL) procedure. In the assay, the addition of fluorescein-dUDT to the 3′-terminals of apoptotically fragmented DNA with TdT was followed by immunochemical detection with horseradish-peroxidase-conjugated anti-fluorescein and diaminobenzidine as a substrate. The proportion of stained area was calculated by using MetaMorph software in a total of 30 random fields per group. We compared the mean value in each group as the level of apoptosis. We also determined whether GFP-positive cells corresponded to TUNEL-positive cells by using contiguous sections from the animals of the BMC + rFGF2 group. In addition, we immunostained for GFP and stained for TUNEL in CCl4 mice (as a control). In this staining system, positive cells were brown in color.
Statistical analysis
Values are shown as mean ± SD. Data were analyzed with the Fisher exact test. A P-value of <0.05 was considered statistically significant.
Ethical considerations
These experiments were reviewed by the Committee of Animal Experiment Ethics at the Yamaguchi University School of Medicine and were carried out under the guidelines for animal experiments at Yamaguchi University School of Medicine (no. 105).
Results
Analysis of liver fibrosis with Sirius red staining
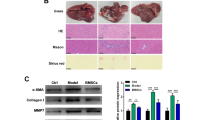
We performed Picro-Sirius red staining to assess the level of liver fibrosis. Continuous CCl4 treatment for 6 weeks induced cirrhosis of the liver. The level of liver fibrosis changed little in the rFGF2-only group, was reduced to some degree in the BMT-alone group, and was lowest in the BMC + rFGF2 group (P < 0.05, Figs. 1a, top row, 2). These results suggested that administration of FGF2 with BMT most significantly reduced liver fibrosis in CCl4-treated mice.
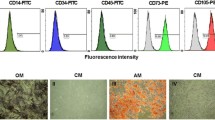
a Sirius red staining and immunohistochemical detection of MMP9. The amount of Sirius-red-stained fibers decreases, and MMP9 expression is elevated after administration of FGF2 in combination with BMT, with significant differences. Note that GFP-positive bone-marrow-derived cells secret MMP9. Top row a–d Sirius red staining. Middle row e–h Immunohistochemistry of MMP9. Bottom row i Immunofluorescent double-staining of GFP and MMP9. j Immunofluorescent double-staining of αSMA and MMP9. k Immunofluorescent double-staining of Gr-1 and MMP9. l Immunofluorescent double-staining of CD11b and MMP. a, e CCl4 group; transplantation of neither BMCs nor rFGF2. b, f rFGF2 group; rFGF2-only treatment without BMT. c, g BMC group; BMC-only transplantation without rFGF2. d, h BMC + rFGF2 group; transplantation of both BMCs plus rFGF2 treatment. i Merged image of GFP (green) and MMP9 (red). j Merged image of MMP9 (green) and αSMA (red). k Merged image of MMP9 (green) and Gr-1 (red). l Merged image of MMP9 (green) and CD11b (red). i–l Any co-expression is in yellow. ×40 (a–h), ×400 (i–l). b Western blot analysis of MMP9. Isolated pure BMCs express MMP9: left lane isolated BMCs, right lane phosphate-buffered saline (PBS) as a negative control (N.C.)
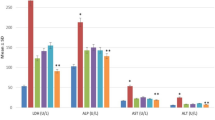
Percentage of stained area after Sirius red staining and MMP9 staining. In the BMC + rFGF2 group, the percentage of Sirius red stained fiber is lowest, and that of MMP9 stained area is highest among the four groups, with significant differences. The expression level of MMP9 is in inverse proportion to the amount of fiber. #No significant difference compared with the value of CCl4 group (P > 0.05). *Significant difference compared with the value of CCl4 group (P < 0.05). **Significant difference compared with the value of BMC group (P < 0.05)
Analysis of fibrolysis with MMP9 expression
We next detected MMP9 immunohistochemically to investigate the mechanism of fibrolysis. As shown in Figs. 1a, middle row, 2, BMT-alone elevated MMP9 expression to some degree, but the elevation was highest in mice treated with BMC + rFGF2 (P < 0.05). Additionally, the distribution of MMP-9 protein was similar to that of GFP in the sections, as previously reported (Sakaida et al. 2004). Moreover, co-expression of GFP and MMP9 was detected by using immunofluorescent double-staining (Fig. 1a, bottom row, i). On the other hand, we could detect a little co-expression of αSMA and MMP9 (Fig. 1a, bottom row, j), but the co-expression of both αSMA and MMP9 was weaker than that of both GFP and MMP9 (Fig. 1a, bottom row, i, j). MMP9 expression in neutrophils and macrophages was also assessed by using GR-1 (as a marker of neutrophils; Fleming et al. 1993) and CD11b (as a marker of macrophages; Springer et al. 1979). We failed to detect co-expression of MMP9 and Gr-1 or of MMP9 and CD11b (Fig. 1a, bottom row, k, l). These results suggested that neutrophils and macrophages did not express MMP9, but that isolated pure BMCs and transplanted BMCs secreted MMP9 (Fig. 1a, bottom row, i, 1b). The percentages of area stained after Sirius red staining and after staining for MMP9 expression are presented in Fig. 2. Of the four treatments, FGF2 treatment together with BMT significantly elevated MMP9 expression and induced the secretion of MMP9 by bone-marrow-derived cells.
Analysis of cell proliferation with Ki-67 staining
We performed immunohistochemical analysis of Ki-67 to investigate cell proliferation in the liver. The percentage area stained for Ki-67 in the BMC group was significantly higher than that in the CCl4 and rFGF2 groups (P < 0.05). Moreover, the administration of FGF2 in combination with BMT caused a statistically significant elevation in the expression of Ki-67 as compared with the BMC-alone group (P < 0.05, Figs. 3a–d, 4). In addition, after immunofluorescent double-staining for GFP and Ki-67, we could detect co-expression of the markers in both the BMC and BMC + rFGF2 groups (Fig. 3e). Together, these analyses showed that the expression of Ki-67 was highest in the BMC + rFGF2 group, and that GFP-positive transplanted BMCs expressed Ki-67.
Expression of Ki-67 as revealed by immunohistochemistry. Ki-67 expression is significantly elevated after administration of FGF2 in combination with BMT in comparison with the other three groups, and GFP-positive transplanted BMCs express Ki-67. a CCl4 group; transplantation of neither BMCs nor rFGF2. b rFGF2 group; rFGF2-only treatment without BMT. c BMC group; BMC-only transplantation without rFGF2. d BMC + rFGF2 group; transplantation of BMCs and rFGF2 treatment. e Double-fluorescent merged image of GFP (green) and Ki-67 (red), with co-expression resulting in yellow. ×40 (a–d), ×100 (e)
Percentage of stained area after staining for Ki-67. BMT-alone (BMC) elevates expression of Ki-67, but the percentage of the Ki-67-stained area in the BMC + rFGF2 group is highest among the four groups. #No significant difference compared with the value of the CCl4 group (P>0.05). *Significant difference compared with the value of the CCl4 group (P < 0.05). **Significant difference compared with the value of the BMC group (P < 0.05)
Analysis of apoptosis with TUNEL staining
We further analyzed the incidence of apoptosis in the liver, apoptosis being reportedly regulated by TNFα. We detected apoptotic cells in frozen sections of liver tissue with the TUNEL assay. No TUNEL-positive cells were found in liver samples from normal mice (data not shown); however, apoptosis was detected in livers treated with CCl4 to induce persistent liver damage. In contrast, rFGF2-only treatment, BMC-only transplantation, and the combined treatment (BMC transplantation plus rFGF2) resulted in no statistically significant differences in the percentages of TUNEL-positive areas (P > 0.05, Fig. 5). We also checked the location of apoptotic cells in the tissue sections and determined whether GFP-positive cells (stained brown) corresponded to TUNEL-positive cells by using contiguous sections. No obvious difference in the distribution of apoptotic cells was detected among the four groups (Fig. 5). In addition, the GFP-positive region was different from the TUNEL-positive region in the BMC + rFGF2 group (Fig. 6). These data indicated that the proportion and distribution of TUNEL-positive apoptotic cells were almost the same in all four groups (CCl4, rFGF2, BMC, BMC + rFGF2), and that treatment did not cause apoptosis of GFP-positive bone-marrow-derived cells.
TUNEL staining in the CCl4, rFGF2, BMC, and BMC + rFGF2 groups The proportion and distribution of TUNEL-positive apoptotic cells are almost same in all four groups. a CCl4 group; transplantation of neither BMCs nor rFGF2. b rFGF2 group; rFGF2-only treatment without BMT. c BMC group; BMC-only transplantation without rFGF2. d BMC + rFGF2 group; transplantation of both BMCs and rFGF2. ×100
Detection of GFP and TUNEL staining in the CCl4 group and BMC + rFGF2 group. GFP-positive cells do not correspond to TUNEL-positive cells in continuous sections from liver samples taken from the BMC + rFGF2 group. GFP and TUNEL-positive cells are stained brown. Top row CCl4 group; transplantation of neither BMCs nor rFGF2 (control group). Bottom row BMC + rFGF2 group; transplantation of BMCs and treatment with rFGF2. a Immunohistochemistry for GFP (circles GFP-positive bone-marrow-derived cells). b TUNEL staining (arrows TUNEL-positive cells). x100
Discussion
Here, we report that the administration of FGF2 in combination with BMT results in collagen fiber degradation, improvement in CCl4-induced liver fibrosis, and strong expression of MMP9. In addition, the present study shows that the combined treatment is more effective in reducing liver fibrosis than BMT alone. A number of studies have demonstrated the stimulatory effect of growth factors and cytokines on MMP9 expression in various normal and cancer cells. FGF2 has been reported to induce MMP9 secretion in MCF-7 breast cancer cells (Liu et al. 2002), and TNFα via the NFκB-mediated pathway induces MMP9 in normal human bronchial epithelial cells (Hozumi et al. 2001). In addition, TNFα promotes a type of ECM degradation that involves MMP9 induction through the activation of the NFκB pathway (Serandour et al. 2005). The promoter of MMP9, like other members of the MMP family, contains multiple transcription-factor-binding sites, including NFkB and AP-1 elements, consistent with induction by a variety of cytokines, growth factors, and oncogenes (Emonard and Hornebeck 2000). Our previous study has suggested that transplantation of BMCs and concurrent treatment with rFGF2 has a synergistic effect that induces TNFα signaling (Ishikawa et al. 2006). In the present study, administration of FGF2 in combination with BMT has been found significantly to reduce liver fibrosis and to elevate MMP9 expression in comparison with other treatments. Additionally, the expression level of MMP9 is inversely related to the amount of fiber deposition. On the other hand, FGF2 treatment alone has no significant effect on ECM degradation and MMP9 induction. Thus, we suppose that the enhanced improvement in CCl4-induced liver fibrosis by the combined treatment is closely related to MMP9 activation and is attributable to the FGF2-induced increase in MMP9 expression in the transplanted BMCs. As shown in Fig. 1, transplanted BMCs but not neutrophils and macrophages (Fig. 1a, bottom row, k, l) express MMP9. On the other hand, αSMA-positive cells (activated hepatic stellate cells) secrete some MMP9 (Fig. 1a, bottom row, j). In a previous report, cultured hepatic stellate cells have been shown to express MMP9, but the expression is weak (Li et al. 1999). Our data are in agreement with that of Li et al. (1999). We suggest that transplantation of MMP9-expressing BMCs might be important to improve liver fibrosis, and that FGF2 treatment enhances the expression of MMP9 in these transplanted BMCs.
We have also focused on the regulation of cell proliferation and apoptosis in this model system. The expression levels of Ki-67 and MMP9 are highest in the BMC + rFGF2 group, but the proportion and distribution of TUNEL-positive apoptotic cells are almost the same in all groups. Moreover, although co-expression of GFP and Ki-67 can be detected, co-localization of GFP-positive and TUNEL-positive cells in BMC + rFGF2 group has not been observed. These data indicate that the combined treatment accelerates the proliferation of bone-marrow-derived cells without causing apoptosis.
In conclusion, the administration of FGF2 in combination with BMT causes a statistically significant reduction in chemically induced liver fibrosis and a concurrent increase in the expression of MMP9. Thus, we are encouraged that this treatment has the potential of being an effective and efficient therapy for patients with severe liver disease, thereby improving liver function and alleviating liver fibrosis.
Abbreviations
- BMC:
-
bone marrow cell
- GFP:
-
green fluorescent protein
- CCl4 :
-
carbon tetrachloride
- BMT:
-
bone marrow transplantation
- FGF2:
-
fibroblast growth factor 2
- TNFα:
-
tumor necrosis factor α
- ECM:
-
extracellular matrix
- MMP:
-
matrix metalloproteinase
- NFκB:
-
nuclear factor κ B
- TIMP:
-
tissue inhibitor of metalloproteinase
- rFGF:
-
recombinant fibroblast growth factor
- αSMA:
-
alpha-smooth muscle actin
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling
- TdT:
-
terminal deoxynucleotidyl transferase
- dUDT:
-
deoxyuridine triphosphate
References
Bezerra JA, Bugge TH, Melin-Aldana H, Sabla G, Kombrinck KW, Witte DP, Degen JL (1999) Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver. Proc Natl Acad Sci USA 96:15143–15148
Emonard H, Hornebeck W (2000) Matrix metalloproteinases (MMPs). In: Bikfalvi A (ed) Encyclopedic reference of vascular biology and pathology. Springer, Berlin Heidelberg New York, pp 172–181
Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528–1530
Fleming TJ, Fleming ML, Malek TR (1993) Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol 151:2399–2408
Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M (2001) Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J Physiol Lung Cell Mol Physiol 281:L1444–L1452
Ishikawa T, Terai S, Urata Y, Marumoto Y, Aoyama K, Sakaida I, Murata T, Nishina H, Shinoda K, Uchimura S, Hamamoto Y, Okita K (2006) Fibroblast growth factor 2 facilitates the differentiation of transplanted bone marrow cells into hepatocytes. Cell Tissue Res 323:221–231
Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK (1997) Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology 26:896–904
Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A (2001) Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128:5181–5188
Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105:369–377
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6:1229–1234
Li YL, Sato M, Kojima N, Miura M, Senoo H (1999) Regulatory role of extracellular matrix components in expression of matrix metalloproteinases in cultured hepatic stellate cells. Cell Struct Funct 24:255–61
Liu JF, Crepin M, Liu JM, Barritault D, Ledoux D (2002) FGF-2 and TPA induce matrix metalloproteinase-9 secretion in MCF-7 cells through PKC activation of the Ras/ERK pathway. Biochem Biophys Res Commun 293:1174–1182
Mars WM, Kim TH, Stolz DB, Liu ML, Michalopoulos GK (1996) Presence of urokinase in serum-free primary rat hepatocyte cultures and its role in activating hepatocyte growth factor. Cancer Res 56:2837–2843
Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M (2002) Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med 8:1011–1017
Omori K, Terai S, Ishikawa T, Aoyama K, Sakaida I, Nishina H, Shinoda K, Uchimura S, Hamamoto Y, Okita K (2004) Molecular signature associated with plasticity of bone marrow cell under persistent liver damage by self-organizing-Map-based gene expression. FEBS Lett 578:10–20
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410:701–705
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP (1999) Bone marrow as a potential source of hepatic oval cells. Science 284:1168–1170
Sakaida I, Nagatomi A, Hironaka K, Uchida K, Okita K (1999) Quantitative analysis of liver fibrosis and stellate cell changes in patients with chronic hepatitis C after interferon therapy. Am J Gastroenterol 94:489–496
Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K (2004) Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 40:1304–1311
Serandour AL, Loyer P, Garnier D, Courselaud B, Theret N, Glaise D, Guguen-Guillouzo C, Corlu A (2005) TNFalpha-mediated extracellular matrix remodeling is required for multiple division cycles in rat hepatocytes. Hepatology 41:478–486
Shinoda K, Mori S, Ohtsuki T, Osawa Y (1992) An aromatase-associated cytoplasmic inclusion, the “stigmoid body,” in the rat brain. I. Distribution in the forebrain. J Comp Neurol 322:360–376
Springer T, Galfre G, Secher DS, Milstein C (1979) Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol 9:301–306
Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H, Okita K (2003) An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem (Tokyo) 134:551–558
Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS (2000) Liver from bone marrow in humans. Hepatology 32:11–16
Acknowledgements
We thank Dr. Masaru Okabe (Osaka University) for the gift of GFP transgenic mice and Mr. Jun Oba for valuable technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (nos. 16390211 and 16590597) and for translational research from the Ministry of Health, Labor and Welfare (H-trans-5 and H17-Special-015).
Rights and permissions
About this article
Cite this article
Ishikawa, T., Terai, S., Urata, Y. et al. Administration of fibroblast growth factor 2 in combination with bone marrow transplantation synergistically improves carbon-tetrachloride-induced liver fibrosis in mice. Cell Tissue Res 327, 463–470 (2007). https://doi.org/10.1007/s00441-006-0334-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0334-x