Abstract
Claudin-5 is a transmembrane protein reported to be primarily present in tight junctions of endothelia. Unexpectedly, we found expression of claudin-5 in HT-29/B6 cells, an epithelial cell line derived from human colon. Confocal microscopy showed colocalization of claudin-5 with occludin, indicating its presence in the tight junctions. By contrast, claudin-5 was absent in the human colonic cell line Caco-2 and in Madin–Darby canine kidney cells (MDCK sub-clones C7 and C11), an epithelial cell line derived from the collecting duct. To determine the contribution of claudin-5 to tight junctional permeability in cells of human origin, stable transfection of Caco-2 with FLAG-claudin-5 cDNA was performed. In addition, clone MDCK-C7 was transfected. Synthesis of the exogenous FLAG-claudin-5 was verified by Western blot analysis and confocal fluorescent imaging by employing FLAG-specific antibody. FLAG-claudin-5 was detected in transfected cells in colocalization with occludin, whereas cells transfected with the vector alone did not exhibit specific signals. Resistance measurements and mannitol fluxes after stable transfection with claudin-5 cDNA revealed a marked increase of barrier function in cells of low genuine transepithelial resistance (Caco-2). By contrast, no changes of barrier properties were detected in cells with a high transepithelial resistance (MDCK-C7) after stable transfection with claudin-5 cDNA. We conclude that claudin-5 is present in epithelial cells of colonic origin and that it contributes to some extent to the paracellular seal. Claudin-5 may thus be classified as a tight-junctional protein capable of contributing to the “sealing” of the tight junction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three types of transmembranal proteins located in epithelial and endothelial tight junctions have been identified: occludin (Furuse et al. 1993), the junctional adhesion molecule (first reported by Martin-Padura et al. 1998; for a review, see: Bazzoni 2003), and the claudin family (Furuse et al. 1998; Morita et al. 1999a,1999b). The largest group among them, the claudin family, currently comprises 24 known proteins, numbered from 1 to 24 (Tsukita et al. 2001). Recent research has demonstrated that tight junction assembly is a dynamic process (Sasaki et al. 2002; Matsuda et al. 2004). Furthermore, the significant physiological relevance of this family has been highlighted by the finding of hereditary diseases linked to mutations in claudin genes (first reported by Simon et al. 1999; for a review, see Sawada et al. 2003).
Claudin-1 is a major component of tight junctions of tight epithelia, e.g. of the epidermis (Tebbe et al. 2002; Langbein et al. 2002; Brandner et al. 2002). Its functional relevance has been recently demonstrated as mice lacking claudin-1 die within hours after birth because of dehydration (Furuse et al. 2002). Claudin-2 is responsible for the formation of a paracellular pore (Furuse et al. 2001; Amasheh et al. 2002). Claudin-3 appears to have minor influence on permeability properties (Furuse et al. 2001), but its functional relevance has been revealed in a report concerning the specific binding of Clostridium perfringens enterotoxin to claudin-3 and -4 (Sonoda et al. 1999). Claudin-4 decreases epithelial cation permeability (Van Itallie et al. 2001). The characterization of claudin-2, -4, and -15 by mutagenesis studies has shown that variations in the amino acids in the extracellular loop of claudins determine their permeability properties (Colegio et al. 2002, 2003).
Claudin-5 has been cloned in correlation with the identification of the hereditary disease velo–cardio–facial syndrome (Sirotkin et al. 1997). It constitutes tight junction strands in endothelial cells (Morita et al. 1999a,1999b; Kiuchi-Saishin et al. 2002; Morita et al. 2003) and is a component of the blood–brain barrier (Nitta et al. 2003) and the blood–testis barrier (Kamimura et al. 2002). Claudin-5 has been detected in tight junctions of pancreatic acinar cells (Rahner et al. 2001), in autotypic tight junctions of Schwann cells (Poliak et al. 2002), during the embryonic development of chick retinal pigment epithelium (Kojima et al. 2002), and in alveolar epithelial cells (Wang et al. 2003).
In claudin-5-deficient mice, the blood–brain barrier against small molecules (<800 Da), but not larger molecules, is perturbed (Nitta et al. 2003). Furthermore, serum-derived factors have been reported to decrease both transepithelial resistance (Rt) and the presence of claudin-5 molecules in tight junctions of cultured capillary endothelial cells from porcine brain (Nitz et al. 2003), supporting the notion of a “sealing” potential of claudin-5. By contrast, synthesis of claudin-5 in a human cystic fibrosis airway epithelia cell line, IB3.1, results in an increase of permeability (Coyne et al. 2003).
In the intestine, claudin-5 is reported to be strictly located in the junctions of both endothelial and epithelial cells without gradation along the crypt-to-villus surface axis in any part of the intestine (Rahner et al. 2001) and in biopsy specimens of the sigmoid colon, comprising epithelium and vascular endothelium (Bürgel et al. 2002). In parallel to the present study, the synthesis of murine claudin-5 in MDCK II cells has been demonstrated to increase transepithelial resistance, whereas paracellular flux of monosaccharides is not significantly changed when compared with controls (Wen et al. 2004). However, functional studies of the contribution of claudin-5 to the epithelial barrier are just beginning. In the present study, we have focused on the specific contribution of human claudin-5 to paracellular permeability by means of stable transfection with FLAG-claudin-5 cDNA in human colonic cell line (Caco-2) and a tight canine cell line (MDCK subtype C7).
Materials and methods
Cells and solutions
Monolayers of Madin-Darby canine kidney (MDCK) subtypes C7 and C11 (Gekle et al. 1994) and of the human colon carcinoma cell lines Caco-2 and HT-29 clone B6 (Kreusel et al. 1991) were grown in 25-cm2 culture flasks containing DMEM (Biochrom, Berlin, Germany) supplemented with 10% (v/v) fetal bovine serum, 100 mg/ml streptomycin, and 100 U/ml penicillin (Biochrom). Cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
Electrophysiology
For electrophysiological and molecular analyses, monolayers were grown on porous polycarbonate culture plate inserts (effective area: 0.6 cm2; Millicell-HA, Millipore, Bedford, Mass., USA). Transepithelial resistance referring to the tissue area (Rt, Ω cm2) was measured in Ussing chambers. The experimental setup was specially designed for the insertion of Millicell filter supports (Kreusel et al. 1991). Inserts were mounted in Ussing chambers, and water-jacketed gas lifts were filled with 10 ml circulating fluid on each side. Bathing Ringer solution contained: 113.6 mM NaCl, 2.4 mM Na2HPO4, 0.6 mM NaH2PO4, 21 mM NaHCO3, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM d(+)-glucose, 10 mM d(+)-mannose, 2.5 mM l-glutamine, and 0.5 mM β-hydroxybutyric acid. Ringer solution was gassed with 95% O2 and 5% CO2, to ensure a pH value of 7.4 at 37°C. Prior to each single experiment, the resistance of the bathing solution and the filter support was measured and used as the baseline.
Reverse transcription/polymerase chain reaction and molecular cloning of human claudin-5
Total RNA was obtained from MDCK subtypes C7 and C11 and from the human colon carcinoma cell lines Caco-2 and HT-29/B6 by using RNAzol B reagent (WAK Chemie, Bad Soden, Germany). First-strand cDNA was synthesized by the reverse transcription (RT) reaction (M-MLV; Gibco BRL, Bethesda, Md., USA) with oligo dT primer. Sense (5′ GCTGCCCATGTGGCAGGTGA 3′) and antisense (5′ GCCCAGCCGATGTACAGCG 3′) primers were selected according to the human claudin-5 sequence (Sirotkin et al. 1997) and the canine claudin-5 sequence (Canis familiaris: a BLAST nucleotide homology search in the dog genome dataset at http://pre.ensembl.org/ revealed the C. familiaris claudin-5 sequence on chromosome 26 at nucleotide positions 31,720,293–31,720,949 of the dog genome). The resulting polymerase chain reaction (PCR) product was analyzed on a 1% agarose gel and screened for the presence of a band of the expected size (430 bp).
For the cloning of the complete coding sequence of human claudin-5, sense (5′ CCTCTAGCCATGGGGTCCGCA 3′) and antisense (5′ CCAGCGCCCTCAGACGTAGTT 3′) primers were synthesized according to the human claudin-5 sequence (Sirotkin et al. 1997) and used for PCR (Metabion, Martinsried, Germany) by employing HT-29/B6 cDNA synthesized by the RT reaction. The resulting 675-bp PCR product encompassing the complete claudin-5 cDNA was cloned into pCR2.1-Topo (Promega, Madison, Wis., USA). The correctness of the cDNA was verified by sequencing, and the cDNA was then subcloned into the eukaryotic expression vector pFLAG-CMV-4 (Sigma Aldrich, St. Louis, Mo., USA), further referred to as pFLAG-Cld5. MDCK-C7 and Caco-2 cells were stably transfected with pFLAG-CLD5 by employing the Lipofectamine plus approach (Gibco BRL). G418-resistant cell clones were screened for claudin-5 synthesis by Western blot analysis. MDCK-C7 and Caco-2 cells transfected with an empty vector (pFLAG-CMV-4) served as controls. Synthesis rates of 60% were regarded as sufficient for further studies.
Western blot analysis
Western blot analysis was performed as reported recently (Amasheh et al. 2002). Cells were scraped from the permeable supports and homogenized in TRIS–buffer containing 20 mM TRIS, 5 mM MgCl2, 1 mM EDTA, 0.3 mM EGTA, and protease inhibitors (Complete; Boehringer, Mannheim, Germany). Protein was obtained by three freeze–thaw cycles and subsequent passage through a 26 G 1/2 needle. The membrane fraction was obtained by a 5-min centrifugation at 200g (4°C) and a subsequent 30-min centrifugation of the resulting supernatant at 43,000g (4°C). Pellets were resuspended in TRIS–buffer, and the protein content was determined by employing BCA protein assay reagents (Pierce, Rockford, Ill., USA) and quantification with a plate reader (Tecan, Austria). Aliquots of 2.5 μg protein were mixed with SDS buffer (Laemmli), loaded onto a 12.5% SDS polyacrylamide gel, and electrophoresed. Proteins were detected by immunoblotting with antibodies raised against human occludin, claudin-1, -2, -3, -5, and FLAG peptide. Chemiluminescence was induced with a Lumi-LightPLUS Western blotting kit (Roche, Mannheim, Germany), detected with an LAS-1000 imaging system (Fuji, Tokyo, Japan), and analyzed by quantification software (AIDA, Raytest, Straubenhardt, Germany). Testing of antibody specificity of rb-anti-claudin-5 was performed through competition with custom-synthesized immunization peptide provided by the company that produced the antibody (Zymed Laboratories, San Francisco, Calif., USA). Antibody (5 μg) was precipitated with 50×excess of immunization peptide prior to the standard immunoblotting procedure. Bands that disappeared were regarded as specific, whereas the remaining bands were regarded as the result of unspecific binding.
Immunofluorescence analysis
Immunofluorescence studies analysis and photography were performed as reported by Florian et al. (2003). Briefly, cells were grown on coverslips (18×18 mm; Menzel, Braunschweig, Germany). Confluent monolayers were rinsed with phosphate-buffered saline (PBS), fixed with methanol, and permeabilized with PBS containing 0.5% Triton X-100. The concentrations of the primary antibody were 20 μg/ml. Fluorescence images were obtained with a confocal microscope (Zeiss LSM 510 META) at excitation wavelengths of 543 and 488 nm. Details concerning the microscopical set-up are available upon request.
Flux measurements
Unidirectional tracer flux measurements were performed under short-circuit conditions with 25 kBq/ml [3H]-mannitol (Biotrend, Cologne, Germany) from the apical to the basolateral side as reported by Amasheh et al. (2002). The medium also contained non-labeled mannitol (10 mM). Four 15-min flux periods were analyzed. A 100-μl sample was taken from the donor (apical) side upon initiation and completion, and 900 μl Ringer’s solution and 4 ml Ultima Gold high flash-point liquid scintillation cocktail (Packard Bioscience, Groningen, The Netherlands) were added. Samples (1 ml) of the receiving (basolateral) side, replaced with fresh Ringer’s, were mixed with 4 ml liquid scintillation cocktail. Subsequently, all 5-ml samples were analyzed in a Tri-Carb 2100TR Liquid Scintillation counter (Packard, Meriden, Conn., USA).
Chemicals
All chemicals, unless otherwise noted, were purchased from Sigma Aldrich. Antibodies raised against claudin-1, -2, -3, -5, and occludin were purchased from Zymed Laboratories (San Francisco, Calif., USA). Secondary antibodies, viz., Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit (both used at concentrations of 2 μg/ml), were purchased from Molecular Probes (MoBiTec, Göttingen, Germany).
Statistical analysis
Data are expressed as means ± standard error of the mean. Statistical analysis was performed by using Student’s t test. P<0.05 was considered significant.
Results
Presence of claudin-5 in epithelial cells with different genuine Rt
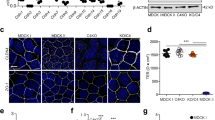
Confluent monolayers of the epithelial cell line (MDCK) subtypes C7 and C11 and the human colon carcinoma cell lines Caco-2 and HT29/B6 revealed general differences of transepithelial resistance (Rt). Among the cells studied, MDCK-C7 showed the highest Rt (1,396±67 Ω cm2, n=12, and MDCK-C11 revealed the lowest Rt value (58±1.3 Ω cm2, n=12). Transepithelial resistances of HT-29/B6 and Caco-2 cells were 603±6 and 251±3 Ω cm2, respectively (n=15 each, Fig. 1a).
Transepithelial resistance measurements and detection of tight junction proteins. a Rt of MDCK-C7, -C11, HT-29/B6, and Caco-2 cells revealed the highest values for MDCK-C7, representing a “tight” tight junction, and low values for MDCK-C11 and Caco-2 cells, representing relatively permeable tight junctions. b Membrane fractions of MDCK-C7, -C11, HT-29/B6, and Caco-2 revealed differential tight-junctional protein synthesis. Occludin and claudin-1 were detected in all cell types, whereas the pore-forming tight-junctional protein claudin-2 was not detectable in MDCK-C7 and Caco-2 cells. Claudin-5 showed no signals in Caco-2 cells and only marginal signals in MDCK-C7 cells. c Demonstration of antibody specificity through immuno-neutralization with the immunization peptide. After the blocking of a fraction of claudin-5 antibody with 50× excess of claudin-5 peptide supplied by the producer, the respective band of HT-29/B6 cells was not detectable, whereas MDCK signals remained, demonstrating unspecific binding. d Results of a PCR employing primers corresponding to both human and canine cDNA sequences, and the cDNA preparations of MDCK, HT-29/B6, and Caco-2 cells. The expected 430-bp fragment of the claudin-5 sequence could be detected in HT-29/B6 preparations, whereas no specific signals were detected in cDNA obtained from MDCK and Caco-2 cells (marker: low-DNA-mass ladder; Invitrogen, Carlsbad, Calif., USA)
To correlate functional and structural parameters, the presence of tight junction proteins occludin, and claudin-1, -2, -3, and -5 was analyzed as shown in Fig. 1b. Occludin, claudin-1, and claudin-3 were detectable in all cells, whereas the pore-forming protein claudin-2 was not detectable in MDCK-C7 and Caco-2 cells. Claudin-5 was not specifically detected in Caco-2. An antigen–antibody competition experiment employing 50×excess of blocking peptide is shown in Fig. 1c; the strong signal of HT-29/B6 was not detectable after blockage of the antibody with immunization peptide and thus was specific. In contrast, signals of MDCK-C7 and -C11, albeit weak, were still detectable after immuno-neutralization of the antibody, demonstrating unspecificity. In addition, a 430-bp fragment of the claudin-5 sequence could be detected by RT-PCR from HT-29/B6 preparations, whereas no specific signals were detected in MDCK and Caco-2 preparations (Fig. 1d).
The localization of endogenous claudin-5 was determined in immunofluorescence studies in Caco-2 and HT-29/B6 cells by employing laser scanning confocal microscopy (Fig. 2). Z-scans revealed tight-junctional presence in co-localization with occludin in HT-29/B6, whereas immunostainings revealed no specific claudin-5 signals in the tight junction of Caco-2 cells.
Immunofluorescent staining of HT-29/B6 and Caco-2 cells. Confluent monolayers grown on coverslips were stained with a monoclonal antibody against occludin (green) and a polyclonal antibody against claudin-5 (red). Claudin-5 was not specifically detectable in tight junctions of Caco-2, whereas in HT-29/B6, a colocalization with occludin was detected. We computed an xz-projection at the indicated green line (rectangular image above merge image), and a yz-projection at the red line(rectangular image right of merge image). The xy-scans shown were focused at the plane of the cells tight junctions. A magnification of single strand regions is shown extreme right
Stable transfection of MDCK-C7 and Caco-2 with FLAG-claudin-5 cDNA
In order to determine the contribution of claudin-5 to paracellular permeability, stable transfection of MDCK-C7 and Caco-2 with claudin-5 cDNA was performed. For specific determination of elevated claudin-5 synthesis, the 5′ terminal region of the claudin-5 open reading frame was linked to a DNA sequence encoding the FLAG epitope, resulting in a protein with a N-terminal FLAG peptide sequence.
Clones were screened for successful transfection by Western blotting with the anti-FLAG antibody. FLAG-specific signals were detected in stably transfected clones, whereas controls did not show FLAG-specific signals. Clones of MDCK-C7 and Caco-2 that showed the highest levels of FLAG-claudin-5 synthesis were selected for further characterization (C7-FLAG-Cld5 and Caco-2-FLAG-Cld5). In addition, levels of the endogenous claudins were analyzed after transfection. No change of the presence of claudin-1, -2, -3, and occludin was detectable in stably transfected cells (Fig. 3).
Western blots of transfected cells after stable transfection; the synthesis of FLAG-claudin-5 (FLAG Cld5) was detected by Western blotting in both MDCK-C7 and Caco-2 cells. Clones with the strongest FLAG Cld5 signals were selected for further comparative analyses (C7-FLAG-Cld5 and Caco-2-FLAG-Cld5, respectively). Controls were transfected with vector cDNA alone (Vec). No change of endogenous occludin, claudin-1, -2, and -3 was detectable
Immunolocalization of FLAG-Cld5
Confocal microscopy revealed the strongly focused presence of FLAG-claudin-5 in the tight junctions of the majority of both C7-FLAG-Cld5 and Caco-2-FLAG-Cld5 cells grown as monolayers. FLAG-claudin-5 was colocalized with occludin and remained stable during the first passages (Fig. 4).
Immunofluorescent staining with anti-occludin and anti-FLAG antibodies. Immunofluorescent staining with polyclonal or monoclonal antibodies against the occludin (red) and the FLAG epitope of FLAG-Cld5 (green) were detected in the tight junction region of both MDCK-C7-FLAG-Cld5 (C7-FLAG-Cld5) and Caco-2-FLAG-Cld5 (Caco-2-FLAG-Cld2) cells, whereas in mock-transfected cells (C7-Vec, Caco-2-Vec), only occludin was detectable. Extreme right Higher magnification of single strand regions
Electrophysiology and flux measurements
Functional analysis of the clones was performed by measurements of transepithelial resistance (Rt) and [3H]-mannitol flux analyses. No change of Rt was detected in transfected MDCK cells (1,426.7±90.1 Ω cm2 for C7-Vec; 1,208.1±95.4 Ω cm2 for C7-FLAG-Cld5; n=15 and 14 measurements, respectively), whereas a marked increase of resistance was detectable in transfected Caco-2 cells (242.1±8.4 Ω cm2 for Caco-2-Vec; 457.7±18.1 Ω cm2 for Caco-2-FLAG-Cld5); n=18 measurements each, P<0.001; Fig. 5a).
a Rt and b [3H]-mannitol flux measurements of transfected cells. Transfection of the tight epithelial cell clone MDCK-C7 revealed no significant alterations of transepithelial resistance or [3H]-mannitol fluxes. In contrast, Caco-2 cells exhibited a marked increase of Rt and a decrease of mannitol fluxes after stable transfection with claudin-2-cDNA (Caco-FLAG-Cld5)
Flux measurements employing [3H]-mannitol (182.2 Da) did not differ in MDCK C7 clones with or without FLAG-Cld5 (29.3±1.8 nmol h−1 cm−2 for C7-Vec vs. 28.0±1.8 nmol h−1 cm−2 for C7-FLAG-Cld5; n=9, respectively), whereas Caco-2 cells stably transfected with claudin-5 cDNA showed a decreased permeability for mannitol (181.0±25.8 nmol h−1 cm−2 for Caco-2-Vec; 89.8±14.4 nmol h−1 cm−2 for Caco-2-FLAG-Cld5; n=9 and 7, respectively P<0.001; Fig. 5b).
In an additional set of experiments, MDCK-C11 cells exhibiting low genuine resistance, because of the predominant presence of claudin-2, were stably transfected with claudin-5 cDNA. This led to no alterations of transepithelial resistance (not shown).
Discussion
A number of hereditary diseases have been identified as being linked to mutations of the genomic claudin-5 sequence. Expression of the claudin-5 gene is changed in glioblastoma multiforme (Liebner et al. 2000) and deletions within the genomic claudin-5 sequence have been associated with two related autosomal dominant genetic disorders, viz., velo–cardio–facial syndrome (VCFS) and DiGeorge syndrome (DGS). VCFS is characterized by craniofacial anomalies, learning disabilities, and conotruncal cardiac abnormalities (Shprintzen et al. 1978, 1981; Lipson et al. 1991; Wilson et al. 1992). The penetrance and expressivity of the phenotypes are highly variable, and more than 40 additional phenotypes have been associated with the syndrome, including skeletal muscle hypotonia, inguinal hernia, thrombocytopenia, mental retardation, psychiatric illness, and hypospadias (Goldberg et al. 1997). These findings demonstrate the physiological importance of claudin-5.
Tight-junctional proteins in epithelial cell lines of renal and intestinal origin
We have studied the presence of claudins in the human intestinal cell lines Caco-2 and HT-29/B6 and in MDCK subclones that bear properties of collecting duct cells. The MDCK-C7 clone has “tight” tight junctions, whereas the MDCK-C11 clone exhibits “leaky” tight junctions (Gitter et al. 1997). Occludin has been used as a reference for the location of tight junction strands, with the immunostaining of the claudins being compared with occludin detection.
Like occludin, claudin-1 and claudin-3 have been detected in all the cell types examined here. These claudins appear to belong to the basic pattern of epithelial tight junction proteins, as both are found in combination in many organs, such as liver, lung, kidney, colon (Morita et al. 1999a, 1999b), and skin (Tebbe et al. 2002). Whereas claudin-1 is known to be crucial for the “sealing” of the barrier (Furuse et al. 2002), claudin-3 appears not to have a direct effect on tight junction barrier properties (Furuse et al. 2001) or may be easily replaced.
Claudin-2 forms a cation-selective paracellular pore that is not present in the tight barrier of MDCK-C7 cells (Amasheh et al. 2002). As this study demonstrates, it is, however, also absent in Caco-2 cells, which means that the lack of claudin-2 does not necessarily lead to the tight sealing of the tight junction; an adequate presence of “tightening” proteins is thus necessary to obtain a definitive seal.
Presence of claudin-5 in epithelial cells
Morita et al. (1999a,1999b) have detected claudin-5 in endothelial cells in some segments of blood vessels, but not in epithelial cells of the native mouse intestine. By contrast, Rahner et al. (2001) have found claudin-5 in the epithelial tight junctions of stomach (surface and gastric glands) and throughout the intestine without gradations along the crypt-to-villus surface axis. Caco-2 cells, although of colonic origin, represent a model for the functions of small intestinal epithelium (Hidalgo et al. 1989). However, claudin-5 is absent in Caco-2 cells, whereas it is present in the native intestine (Rahner et al. 2001). We have nevertheless demonstrated that claudin-5 is present in HT-29/B6 cells, which originate from human colon (Kreusel et al. 1991) and exhibit features of differentiated epithelial crypt cells (Gitter et al. 2000).
Claudin-5 has not been detected in native epithelial cells of the kidney, despite its presence in kidney endothelia (Kiuchi-Saishin et al. 2002; Morita et al. 1999a, 1999b; Reyes et al. 2002). In our study, endogenous claudin-5 signals in MDCK cells seem not to be specific. Although synthesis in low-resistance MDCK cells leads to an increase of barrier properties (Wen et al. 2004), this effect is not universal. We have found, in our experiments, that claudin-5 synthesis in moderately high resistance Caco-2 cells (which lack claudin-2) increases their resistance, but that claudin-5 does not induce further “sealing” of the paracellular barrier in high resistance MDCK cells (subclone C7).
As tight junction strands can be regarded as a heteropolymer of various tight-junctional proteins that contribute different properties to the characteristics of the tight junction, the displacement of a critical number of stronger “tightening” tight junction proteins might lead to a decrease in the effectiveness of the paracellular barrier; the insertion of a “tightening” or “sealing” tight-junctional protein may reverse this effect. Whether these effects can be achieved in MDCK-C7 cells by higher levels of claudin-5 synthesis remains to be elucidated.
Thus, Caco-2 cells seem to be a suitable model for the characterization of claudin-5 by the transfection approach because of the lack of both claudin-2 and -5 and its low genuine transepithelial resistance.
Role of claudin-5 in epithelial tight junctions
This study has shown that claudin-5 is able to tighten the barrier to a certain extent. In MDCK-C7 cells, which contain genuine “tight” tight junctions, however, no contribution of claudin-5 to barrier properties has been detected.
Furthermore, if claudins are able to interact in a heterophilic manner in tight junction strands between cells, certain binding partners may be able to compensate for the elevation of a single molecule. This principle has been described recently for the combination of claudin-2 and claudin-8 (Yu et al. 2003), supporting a model whereby tissue-specific binding partners may play an important role for the final determination of paracellular permeability. Thus, the stable transfection of cell lines may lead to different, sometimes divergent, results depending on the genuine presence of potential binding partners. In our study, the presence of several other tight-junctional proteins is unchanged after stable transfection. Additional effects on any of the remaining tight-junctional proteins, however, cannot completely be ruled out.
The “sealing” capacity of claudin-5 has also been suggested by Nitta et al. (2003) in studies focusing on blood-brain barrier endothelia. In their study, the knockout of claudin-5 leads to a dramatic size-selective degradation of the blood-brain barrier function; the remaining barrier is permeable to molecules smaller than 800 Da. Moreover, claudin-5-deficient mice die within 10 h after birth. In accordance with these findings, our study shows that the permeability to mannitol is reduced by the synthesis of claudin-5 in Caco-2 cells. We have focused on permeability measurements of mannitol, as potential changes in ionic permeability may indicate the displacement of other claudins; no such changes have however been observed.
In the knockout experiments, lethal effects resulting from the perturbation of the epithelial barrier have not been ruled out (Nitta et al. 2003; Matter and Balda 2003). These results raise the question of whether claudin-5 directly exhibits barrier properties in epithelia. Our study demonstrates that claudin-5 is present in cells derived from colon epithelia. In addition, in the tight junction of Caco-2 cells, exogenous claudin-5 can be classified as a “sealing” tight junction protein as the presence of this protein induces a “tightening” of the paracellular barrier, whereas the synthesis of claudin-5 in cells with high endogenous transepithelial resistance does not add to barrier properties.
References
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976
Bazzoni G (2003) The JAM family of junctional adhesion molecules. Curr Opin Cell Biol 15:525–530
Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I (2002) Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol 81:253–263
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2002) Mechanisms of diarrhea in collagenous colitis. Gastroenterology 123:433–443
Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM (2002) Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol 283:C142–C147
Colegio OR, Van Itallie C, Rahner C, Anderson JM (2003) Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol 284:C1346–C1354
Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG (2003) Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285:L1166–L1178
Florian P, Amasheh S, Lessidrensky M, Todt I, Bloedow A, Ernst A, Fromm M, Gitter AH (2003) Claudins in the tight junctions of stria vascularis marginal cells. Biochem Biophys Res Commun 304:5–10
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141:1539–1550
Furuse M, Furuse K, Sasaki H, Tsukita S (2001) Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin–Darby canine kidney I cells. J Cell Biol 153:263–272
Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111
Gekle M, Wunsch S, Oberleithner H, Silbernagl S (1994) Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflügers Arch 428:157–162
Gitter AH, Bertog M, Schulzke JD, Fromm M (1997) Measurement of paracellular epithelial conductivity by conductance scanning. Pflügers Arch 434:830–840
Gitter AH, Bendfeldt K, Schulzke JD, Fromm M (2000) Trans-/paracellular, surface/crypt, and epithelial/subepithelial resistances of mammalian colonic epithelia. Pflügers Arch 439:477–482
Goldberg R, Motzkin B, Marion R, Scambler PJ, Shprintzen RJ (1997) Velo–cardio–facial syndrome: a review of 120 patients. Am J Med Genet 45:313–319
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Kamimura Y, Chiba H, Utsumi H, Gotoh T, Tobioka H, Sawada N (2002) Barrier function of microvessels and roles of glial cell line-derived neurotrophic factor in the rat testis. Med Electron Microsc 35:139–145
Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S (2002) Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13:875–886
Kojima S, Rahner C, Peng S, Rizzolo LJ (2002) Claudin 5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol 186:81–88
Kreusel KM, Fromm M, Schulzke JD, Hegel U (1991) Cl− secretion in epithelial monolayers of mucus forming human colon cells (HT-29/B6). Am J Physiol 261:C574–C582
Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW (2002) Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 81:419–435
Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H (2000) Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol (Berl) 100:323–331
Lipson A, Yuille D, Angel M, Thomson P, Vanderwoord J, Beckenham E (1991) Velo–cardio–facial syndrome: an important syndrome for dismorphologists to recognize. J Med Genet 28:133–137
Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142:117–127
Matsuda M, Kubo A, Furuse M, Tsukita S (2004) A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci 117:1247–1257
Matter K, Balda MS (2003) Holey barrier: claudins and the regulation of brain endothelial permeability. J Cell Biol 161:459–460
Morita K, Furuse M, Fujimoto K, Tsukita S (1999a) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96:511–516
Morita K, Sasaki H, Furuse M, Tsukita S (1999b) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194
Morita K, Sasaki H, Furuse K, Furuse M, Tsukita S, Miyachi Y (2003) Expression of claudin-5 in dermal vascular endothelia. Exp Dermatol 12:289–295
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660
Nitz T, Eisenblatter T, Psathaki K, Galla HJ (2003) Serum-derived factors weaken the barrier properties of cultured porcine brain capillary endothelial cells in vitro. Brain Res 981:30–40
Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E (2002) Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol 159:361–372
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Reyes JL, Lamas M, Martin D, Carmen Namorado M del, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L (2002) The renal segmental distribution of claudins changes with development. Kidney Int 62:476–487
Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S (2002) Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci USA 100:3971–3976
Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H (2003) Tight junctions and human diseases. Med Electron Microsc 36:147–156
Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D (1978) A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo–cardio–facial syndrome. Cleft Palate J 15:56–62
Shprintzen RJ, Goldberg RB, Young D, Wolford L (1981) The velo–cardio–facial syndrome: a clinical and genetic analysis. Pediatrics 67:167–172
Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106
Sirotkin H, Morrow B, Saint-Jore B, Puech A, Das Gupta R, Patanjali SR, Skoultchi A, Weissman SM, Kucherlapati R (1997) Identification, characterization, and precise mapping of a human gene encoding a novel membrane-spanning protein from the 22q11 region deleted in velo–cardio–facial syndrome. Genomics 42:245–251
Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S (1999) Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 147:195–204
Tebbe B, Mankertz J, Schwarz C, Amasheh S, Fromm M, Assaf C, Schultz-Ehrenburg U, Sanchez Ruderish H, Schulzke JD, Orfanos CE (2002) Tight junction proteins: a novel class of integral membrane proteins: expression in human epidermis and in HaCaT keratinocytes. Arch Dermatol Res 294:14–18
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Van Itallie C, Rahner C, Anderson JM (2001) Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107:1319–1327
Wang F, Daugherty B, Keise LL, Wie Z, Foley JP, Savani RC, Koval M (2003) Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29:62–70
Wen H, Watry DD, Marcondes MC, Fox HS (2004) Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol 24:8408–8417
Wilson DI, Cross IE, Goodship JA, Brown J, Scambler PJ, Bain HH, Taylor JF, Walsh K, Bankier A, Burn J, Wolstenholme J (1992) A prospective cytogenetic study of 36 cases of DiGeorge syndrome. Am J Hum Genet 51:957–963
Yu AS, Enck AH, Lencer WI, Schneeberger EE (2003) Claudin-8 expression in Madin–Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278:17350–17359
Author information
Authors and Affiliations
Corresponding author
Additional information
We thank Anja Fromm and Sieglinde Lüderitz for excellent technical assistance. The work was supported by the Deutsche Forschungsgemeinschaft (DFG Fr 652/4) and by funds of the Campus Benjamin Franklin, Charité-Universitary Medicine Berlin.
Rights and permissions
About this article
Cite this article
Amasheh, S., Schmidt, T., Mahn, M. et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res 321, 89–96 (2005). https://doi.org/10.1007/s00441-005-1101-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-1101-0