Abstract
In cubomedusae, the central nervous system (CNS) is found both in the bell (the ring nerve) and in the four eye-bearing sensory structures (the rhopalia). The ring nerve and the rhopalia are connected via the rhopalial stalks and examination of the structure of the rhopalial stalks therefore becomes important when trying to comprehend visual processing. In the present study, the rhopalial stalk of the cubomedusae Tripedalia cystophora has been examined by light microscopy, transmission electron microscopy, and electrophysiology. A major part of the ring nerve is shown to continue into the stalk and to contact the rhopalial neuropil directly. Ultrastructural analysis of synapse distribution in the rhopalial stalk has failed to show any clustering, which indicates that integration of the visual input is probably spread throughout the CNS. Together, the results indicate that cubomedusae have one coherent CNS including the rhopalia. Additionally, a novel gastrodermal nerve has been found in the stalk; this nerve is not involved in visual processing but is likely to be mechanosensory and part of a proprioceptory system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cubozoan jellyfish have a remarkable sensory system, as they are the only cnidarians known to possess image-forming camera-type eyes (Nilsson et al. 2005). The eyes are situated on sensory structures called rhopalia, which are attached by a stalk to the bell close to its margin. All known species of cubomedusae have four rhopalia, each equipped with a set of six eyes of four morphological types (Claus 1878; Berger 1898; Yamasu and Yoshida 1976; Laska and Hündgen 1982). The visual input is therefore conveyed by a total of no less than 24 eyes. This elaborate sensory system, together with the advanced behavior of cubomedusae (Kinsey 1986; Hartwick 1991; Buskey 2003; Lewis and Long 2005), suggests the presence of a central nervous system (CNS) housing a respectively advanced neural circuitry.
Morphological and electrophysiological studies have shown that a significant part of the CNS of cubomedusae is situated within the rhopalia (Satterlie and Spencer 1979; Satterlie 2002; Parkefelt et al. 2005). Each rhopalium hangs in a stalk that connects it to the rest of the animal, and extracellular electrophysiological recordings from the stalk have so far only revealed a pacemaker signal coming from the rhopalia, which directly controls swimming (Satterlie and Spencer 1979; Satterlie and Nolen 2001; Satterlie 2002). This indicates that visual information is processed and integrated within the rhopalia. Complex nerve cell populations in the rhopalia with commissures interconnecting some of the eyes and the two sides of the rhopalia (Parkefelt et al. 2005) may form the structural basis for the processing of visual information. If visual information is processed and integrated within the rhopalia, and if the rhopalia directly control behavior, then they qualify as parts of the CNS and may even be called brains.
In addition, several studies with immunocytochemistry, transmission electron microscopy, and electrophysiology have shown that, within Cnidaria, both cubozoans and hydrozoans possess a well-defined CNS in the shape of a ring nerve (Satterlie 1979; Grimmelikhuijzen and Spencer 1984; Grimmelikhuijzen 1985; Grimmelikhuijzen et al. 1986; Yi-Chan et al. 2001; Mackie 2004). This shape of the CNS is a consequence of the radial symmetry of the animals (Spencer and Arkett 1984; Satterlie and Nolen 2001; Mackie 2004). The ring nerve is found in both the polyp and the medusa stage of cubozoans and hydrozoans, but so far no candidates for a CNS have been found in the other two cnidarian classes, viz., the Anthozoa and Scyphozoa, although scyphomedusae have complex ganglia associated with their sensory structures (Anderson et al. 1992).
Little is known about the ring nerve of cubozoans, but in hydrozoans it has been extensively studied. Here, it has the highest complexity in the medusa stage, in which it is morphologically divided into an inner and an outer ring nerve (Mackie 2004). The outer part is the largest and displays 500–700 neurites in cross section. In the mesopelagic hydromedusa Aglantha digitale, the ring nerve can be divided into at least seven subsystems with separate physiological properties and functions (Mackie and Meech 1995a,b, 2000; Moroz et al. 2004; Mackie 2004). There is significant communication between the subsystems allowing for complex behavioral control. Cubomedusae have a single ring nerve morphologically similar to the outer ring nerve of hydromedusae, but nothing is known about possible subdivisions (Laska and Hündgen 1984; Satterlie 2002).
From the above, the CNS of cubomedusae appears to consist of two major components, viz., the ring nerve and the complex ganglia in the rhopalia. This has prompted detailed analysis of the way that the nervous systems of the rhopalia connect with the ring nerve. For experimental purposes, it is convenient that each rhopalium hangs by a stalk, since this limited tissue volume should contain all the information regarding the interactions that may occur between the ring nerve and the rhopalia.
Here, we present an anatomical description of the rhopalial stalk of the cubomedusae Tripedalia cystophora. We also demonstrate that the nerves in the stalks in this species are best interpreted as parts of the ring nerve, and that a major part of the ring nerve is concerned with communication between the four rhopalia. To seek possible integration/processing hot spots, we have mapped the distribution of the synapses in the stalk. We further show that the stalk holds a gastrodermal nerve (GN), and that this nerve is possibly mechanosensory. Finally, extracellular electrophysiological recordings indicate that the many neurons in the stalk carry much more information than merely a pacemaker signal.
Materials and methods
Adult Tripedalia cystophora were hand-collected in the mangrove area outside the marine station Isla Magueyes at La Parguera, Puerto Rico. The bell diameter varied between 7 and 12 mm. The medusae were brought back to the marine station where they were kept alive in a holding tank until they were fixed or used for electrophysiological experiments.
Light microscopy
Whole medusae or rhopalia including the stalk were fixed in 2.5% glutaraldehyde, 2% paraformaldehyde, and 3% sucrose in 0.15 M sodium cacodylate buffer. After 5–6 days in fixative, they were transferred to 0.15 M sodium cacodylate buffer. One batch (three specimens) was dehydrated in a series of ethanol, transferred to pure acetone, and embedded in Epon 812 resin. In this material, the stalk was sectioned longitudinally into 1-μm-thick sections and stained with methylene blue. Another batch (four specimens) was dehydrated in a series of ethanol and embedded in paraffin. Here, the stalk was sectioned transversely or longitudinally into 8-μm-thick sections and stained according to the protocols for a modified tetrachrome stain (KOLW: Chromotrope – Orange G – Lichtgrün – Weigert’s hematoxylin). An unstained animal was used for stereo-microscopical purposes.
Confocal microscopy
Whole medusae were fixed in 4% paraformaldehyde in 0.15 M sodium cacodylate buffer. After 5–6 days in fixative, they were transferred to 0.15 M sodium cacodylate buffer. The medusae were dissected such that square tissue samples were obtained containing the rhopalial niche and about 1/8 of the ring nerve. Two preparations were made including the rhopalia, but in another two preparations, the rhopalia were removed to attain a clear view of the stalk. These tissue samples were treated overnight with a mouse monoclonal antibody against detyrosinated tubulin (ID-5; Synaptic System, used at 1:250) and afterward were incubated for 1 h with goat-anti-mouse IgG conjugated to Alexa568 (Molecular Probes, used at 1:100). The tissue was prepared as whole-mounts in phosphate-buffered saline (pH 7.2) mixed with glycerin (1+9) and observed in a laser-scanning confocal microscope (Multiprobe 2001, Molecular Dynamics, Sunnyvale, Calif.). Confocal sections (frames 512×512 pixels) were obtained with 20×/0.75 and 40×/1.0 oil immersion planapochromat objectives, by using pixel (x/y) resolution/z increments of 1.3/1.3 μm and 0.6/0.6 μm, respectively. The image series were deconvolved by using a three-dimensional adaptive blind deconvolution algorithm in AutoDeblur 9.0. The deconvolved image series was then imported to ImageSpace 3.11 (Molecular Dynamics), and autofocus and extended focus projections of the neural elements were obtained. The final print resolution of confocal images was set in Adobe Photoshop CS.
Transmission electron microscopy
Rhopalial niches were fixed in 2.5% glutaraldehyde, 2% paraformaldehyde, and 3% sucrose in 0.15 M sodium cacodylate buffer. After 5–6 days in fixative, they were washed in 0.15 M sodium cacodylate buffer, post-fixed in 1% osmium tetraoxide for 1 h at room temperature, dehydrated in a series of ethanol, transferred to pure acetone, and embedded in Epon 812 resin. Ultrathin sections were cut on a Leica ultratome, placed on single-slot grids, and contrasted with 4% uranyl acetate for 20 min at room temperature and with 2% lead citrate for 5 min at 5°C. Cross sections of the stalk were obtained at five different levels evenly distributed along the long axis. Longitudinal serial sections were also taken but for only half of the stalk, since it is bilaterally symmetrical. Cross sections of the ring nerve were taken from just outside the rhopalial niche, from the roof of the niche, and from next to the base of the stalk. The material was observed by transmission electron microscopy (TEM) in a Jeol 1240 microscope equipped with a Gatan multiscan 751 camera.
Definition of neurites and the mapping of their synapses
A neurite count was made from the cross sections of the stalk at the TEM level. However, cnidarian neurites are not easily distinguishable from extensions of other cell types, even at this level. The approach taken here was therefore to include only neurites displaying efferent synapses to create a general morphological description including size-range, shape, mitochondrial content, and the presence of large electron-lucent vacuoles.
To find integration/processing hot-spots for information passing to and from the rhopalia, a synapse map was constructed from longitudinal serial sections of one half of the epidermal nerve (EN) found in the rhopalial stalk. Approximately every 15th section (~750 nm) was examined over a distance of 20 μm, and all synapses were categorized as unidirectional or bidirectional and according to the length of the synaptic cleft (<0.5 μm=small, 0.5–1 μm=medium, >1 μm=large).
Electrophysiology
A rhopalium from a fresh hand-collected T. cystophora was cut off approximately midway along the stalk by using a fine pair of scissors. The rhopalium was transferred to a small Petri dish in an electrophysiological setup containing sea water. Once in the setup, a glass suction electrode was applied to the cut end of the stalk in the area of the EN and GN (for electrode details, see Derby 1995). The preparation was left under room illumination for 15 min after which 1 min of spontaneous activity was recorded.
A Linos microbench system was used for light stimulation. Light from a 50 W halogen bulb was focused into a light guide of 250 μm in diameter. The light guide was arranged in the Petri dish to stimulate either the small lens eye together with the pit eyes or the large lens eye together with the slit eyes. Flashes of 50 ms were applied by using a mechanical shutter controlled by a function generator (TGP10, TTI, Fort Worth, Tex.). Mechanical stimuli were applied by tapping the stereomicroscope in the setup with a hand-held needle. Recordings were obtained from eight preparations.
The signals were amplified 1000 times (DAM 50 amplifier; World Precision Instruments, Sarasota, Fla.) and filtered through a 60-Hz filter (Hum Bug, Quest Scientific, Vancouver, Canada) and a low- and high-pass filter in the amplifier (1 and 300 Hz, respectively). The recordings were stored and analyzed on a laptop by using an A/D converter (12 bit USB module, Data Translation, Marlboro, Mass.; software: Scope version 2.2.0.30, Data Translation).
Results
Orbit of ring nerve
The ring nerve of T. cystophora lies in the subumbrellar epidermis and has a sinusoidal shape (Fig. 1a). At the bell margin, it contacts the base of the tentacles in the pedalia (Fig. 1c) after which it continues aborally where it passes the roof of an invagination of the exumbrellar epidermis, the rhopalial niche (Fig. 1c). The main sensory structures of the medusa, the rhopalia (Fig. 1b), hang from the roof of the rhopalial niches in 150–250 μm long stalks (Fig. 1c).
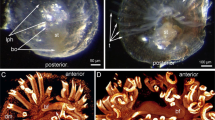
The CNS of the cubozoan jellyfish Tripedalia cystophora (LLE, P, PE, PG, Rh, SE and SLE). The major part of the CNS is constituted by an RN (a, c, d). The sinusoidal RN passes the roof of the RhN (d), and the basal part of the pedalium holding the tentacles (c). In these two areas, the RN connects to other major parts of the nervous system, viz., the tentacular nerve and the Rh. The rhopalia (b) are the main sensory structures of cubomedusae and carry six eyes together with a brain-like neuropil. The rhopalia hang from the roof of the rhopalial niche in a stalk (b, d). Bars indicate 1 mm (a), 100 μm (b–d). CNS indicates central nervous system; LLE, large lens eye; P, pedalium; PE, pit eye; PG, pedalial ganglion; Rh, rhopalium; SE, slit eye; SLE, small lens eye; RN, ring nerve; RhN, rhopalial niche
Rhopalial stalk
The rhopalial stalk is circular in cross section and has a monolayered epidermis varying in thickness from 8 to 20 μm, with the thicker side facing the rhopalium. The thickening of the epidermis is attributable to the presence of an epidermal nerve (EN) (Fig. 2b–d). The distal 3/4 of the stalk has myoepithelial cells forming a layer of longitudinal muscle fibrils encircling the mesoglea (Fig. 3b,d). Accordingly, motor neurites are found intermingled with the muscle fibrils (Fig. 3b,d, arrowhead). Inside the epidermis, a layer of mesoglea of 3–20 μm in thickness is found with the thinner area corresponding to the thickening of the epidermis. The gastrodermis in the stalk forms a tube that is 10–20 μm thick and homogeneous throughout the length of the stalk. On the central side, the gastrodermis contains a gastrodermal nerve (GN) bordering the mesoglea (Fig. 2b,d, arrowheads). The center of the stalk is a branch of the gastrovascular cavity (Fig. 2b–d).
Morphology of the stalk. The stalk attaches to the peripheral side of the rhopalium at the aboral end (a). In cross section, it displays three rings surrounding a branch of the GCV (b–d). The innermost ring is a mono-layered Gas, the middle ring is Mes, and the outermost ring is a mono-layered Epi. Both the Mes and Epi are of uneven thickness. The Epi is thickest on the central side where it retains the EN (b–d; asterisk, nerve cell body). In the same area, a GN (b–d) bulges out of the Gas (RC). Bars indicate 100 μm (a), 20 μm. GCV indicates gastrovascular cavity; Gas, gastrodermis; Mes, mesoglea; Epi, epidermis; EN, epidermal nerve; GN, gastrodermal nerve; RC, ring canal
The EN is about 40 μm wide and 5–10 μm thick (a). In the medial area, a furrow in the EN (arrowheads) houses the GN. In cross section, the Ne are irregular in shape and often contain large electron-lucent vesicles (b, asterisks). The EN has about 450 Ne in cross section, of which 5–10 have their soma in the stalk (c; see also Fig. 2d, asterisk). A large number of synapses are present throughout the length of the EN (b; see also Fig. 7). Many are bidirectional (b, arrows), but unidirectional synapses are the most abundant. Many of the unidirectional synapses are motor synapses (b, d; arrowheads) innervating the MF of the EC. Bars indicate 5 μm (a), 1 μm. EN indicates epidermal nerve; GN, gastrodermal nerve; Ne, neurites; MF, muscle fibrils; EC, epithelial cells; Gas, gastrodermis; Mes, mesoglea; NCB, nerve cell body; Nu, nucleus
Epidermal nerve
The EN of the stalk is a prominent nerve for which a conservative count yields approximately 450 neurites in cross section. It is situated on the central side of the stalk and is homogeneous throughout its entire length. EN is bi-lobed in cross section, the two parts being of equal size (Fig. 2d), indicating that the EN consists of two parallel nerve bundles. In total, 5–10 of the nerve cells have their cell body, which contains little cytoplasm and few organelles (Figs. 2d, 3c) in the EN. Their neurites are of a highly irregular shape and cannot be followed for more than a few µm. Therefore, no data are available on their synaptic contacts. No synapses have been seen at the cell bodies in the EN. All the neurites in EN are small, being 0.3–3 μm in diameter with the majority between 1 and 1.5 μm (Fig. 3b).
At the distal end, the EN merges with the neuropil of the rhopalium. In most cases, we have not been able to follow the neurites for long distances inside the rhopalium, but there seems to be a close connection with two clusters of nerve cells in the rhopalium, situated latero-aborally on both sides of the stalk base (Fig. 5a, arrowhead). About 20 irregular shaped cells occur in each of these clusters, and they have several unidirectional afferent synapses (Fig. 5b,d). These cells also have 2–3 neurites each, all of which can be followed for 10–15 μm before they “disappear” into the rhopalial neuropil. At least one of the neurites points toward the base of the stalk, and no synapses have been seen in these neurites.
The GN is prominent in the proximal part of the stalk (EN, Epi, Gas, MF) and contains approximately 125 neurites in cross section (a). In this part of the stalk, the GN bulges out from the rest of the Gas and is separated from the EN only by a thin layer of Mes. The GN tapers toward the rhopalium, and 20 µm proximal to the rhopalium, only about 20 neurites remain (c). In this area, the GN is separated from the rest of the Gas by a thin layer of Mes (c, arrows ). Structures resembling primary or sensory cilia (b, arrowheads) occur together with the neurites in the GN and are mostly found in the center of the proximal part of the nerve. The neurites display many dense-cored vescicles of about 100 nm in diameter (c, insert). No cell bodies are seen in GN, which splits up in the roof of the rhopalial niche. Each bundle has 10–20 neurites (d). Bar indicates 1 µm. GN indicates gastrodermal nerve; EN, epidermal nerve; Epi, epidermis; Gas, gastrodermis; MF, muscle fibrils; Mes, mesoglea
Tubulin immunohistochemistry clearly shows that the two lobes of EN split up completely in the roof of the rhopalial niche before they become parts of the ring nerve (Fig. 6a). They do not seem to merge directly with the rest of the ring nerve but rather run in parallel as a separate system (Fig. 6). In other words, a major part of the ring nerve bends down into the stalk and connects to the rhopalium directly. Thus, the EN can be seen as an extension of the ring nerve. This is not the entire ring nerve, since about half the neurites (as judged by the number of tubulin-immunoreactive fibers) continues straight across the roof of the rhopalial niche (Fig. 6b). The level of interaction between the two parts of the ring nerve beyond their junction is not known, but from our TEM observations, they seem to run as separate bundles at least for some distance (Fig. 6b). No synaptic interactions between the two bundles were found in the examined areas.
Neuronal connection between stalk and rhopalium (EN, Epi, Gas, GVC, Mes, NP, Nu). In KOLW-stained light-microscopical sections, a cluster of cells is seen in the rhopalium ventrally and aborally to the base of the stalk (a, arrowhead) They connect with the stalk through fibrous structures (a, arrow). When examined by electron microscopy, these cells are identified as nerve cells (b), with several incoming undirectional synapses (b, d, arrowheads) and two to three neurites (not shown). The somata of the nerve cells are extremely irregularly shaped and have a electron dense cytoplasm (b). The fibrous structures are identified as neurites by the large electron-lucent vesicles (c, asterisk) and the presence of both uni- and bidirectional synapses (c, arrowheads). Bars indicate 20 µm (a), 1 µm (b–d). EN indicates epidermal nerve; Epi, epidermis; Gas, gastrodermis; GVC, gastrovascular cavity; Mes, mesoglea; Np, neuropil; Nu, nucleus
Synapses in EN
To reveal whether the stalk represented an area in which two separate parts of the CNS were integrated, we created a map of the synapses in EN (Fig. 7). A total of 287 synapses were found in the examined half of the nerve. All three size classes (see Materials and methods for definitions) of both uni- and bilateral synapses were seen, with the small unidirectional synapses being the most numerous (n=128).
A persisting problem is that the stalk contains myoepithelium and associated motor neurons in the region of the EN. Of the small and medium unidirectional synapses, 25% and 35% respectively contact identifiable muscle fibrils. Two conservative assumptions can be made: (1) all small and medium unidirectional synapses are motor-synapses, and (2) all bidirectional and large unidirectional synapses are neuron/neuron synapses belonging to the neurites of the EN. Thus, 106 of the synapses belong to the EN and seem to have a random distribution throughout the nerve (Fig. 7c,e). A decline in the number of synapses is seen about 140 μm from the rhopalium, but this is attributable to the sections being slightly oblique. The 181 motor synapses are randomly distributed throughout the first 100 μm of the EN (Fig. 7d,f). After 100 μm, they rapidly decline coinciding with a decline in the number of muscle fibrils.
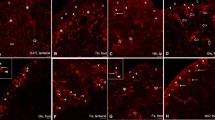
Neuronal connection between stalk and ring nerve (EE, Epi, Gas, Mes, RC). Confocal microscopic analysis of detyrosinated tubulin-immunoreactive elements (a, b) shows that the EN of the stalk is two separate parts of the RN, bending into the stalk (a). Another part of RN passes straight across the roof of the rhopalial niche (a, RN 1). At higher magnification, only about half of the RN is seen to bend down to the stalk (b). Transmission electron micrographs of the lateral part of the rhopalial niche show that here, the RN is divided into two distinct parts (RN 1, RN 2) running in parallel (c). In the middle part of the rhopalial niche, two separate nerves are found corresponding to the EN from the stalk (EN, RN in d). Bars indicate 50 µM (a), 20 µm (b–d). EE indicates external environment; Epi, epidermis; Gas, gastrodermis; Mes, mesoglea; RC, ring canal; EN, epidermal nerve; RN ring nerve
Gastrodermal nerve
The GN found in the stalk changes its appearance along the length of the stalk. In the proximal part of the stalk, it is prominent and comprises approximately 125 neurites in cross section (Fig. 4a). The neurite diameter ranges from 0.2 μm to 2 μm with the majority being between 0.5 μm and 1 μm. Many of the neurites display a number of dense-cored vesicles (Fig. 4c, insert). The GN gradually tapers distally (Fig. 4c) and ends just outside the rhopalium. In the distal part, the GN is separated from the rest of the gastrodermis by a thin layer of mesoglea (Fig. 4c, arrows). No obvious synapses have been found in the GN and its identification as a nerve is based on the presence of the dense-core vesicles and the general morphology of the neurites, which is similar to the morphology of the neurites found in the EN and in the ring nerve. Some of the neurons found in the GN appear to be ciliated, since the nerve contains structures similar to primary sensory cilia, where the 9×2+2 configuration disintegrates early in the cilia and is replaced by single-stranded microtubules (Fig. 4b). The cells giving rise to the cilia have not been found. At the exit in the proximal part of the stalk, the GN splits up into several smaller bundles of 10–20 neurites (Fig. 6d), which cannot be followed much further. The cell bodies of the GN have not been identified.
The gastrodermal nerve (GN) is prominent in the proximal part of the stalk (EN epidermal nerve, Epi epidermis, Gas gastrodermis, MF muscle fibrils) and contains approximately 125 neurites in cross section (a). In this part of the stalk, the GN bulges out from the rest of the gastrodermis and is separated from the EN only by a thin layer of mesoglea (Mes). The GN tapers toward the rhopalium, and by 20 μm proximal to the rhopalium, only about 20 neurites remain (c). In this area, the GN is separated from the rest of the gastrodermis by a thin layer of mesoglea (c, arrows). Structures resembling primary or sensory cilia (b, arrowheads) occur together with the neurites in the GN and are mostly found in the center of the proximal part of the nerve. The neurites display many dense-cored vesicles of about 100 nm in diameter (c, insert). No cell bodies are seen in GN, which splits up in the roof of the rhopalial niche. Each bundle has 10–20 neurites (d). Bar 1 μm
Electrophysiology of the stalk nerves
Electrophysiological recordings from the cut end of the rhopalial stalk revealed many active neurons (Fig. 8). In some cases, spontaneous activity took the form of large action-potential-like signals in the frequency range of 0.5 Hz to 1.5 Hz (Fig. 8a). In other cases, the spontaneous activity was complex, displaying many small arhythmic signals indicating the activity of a large number of small neurons (Fig. 8b). Stimulation of the rhopalium with 50-ms flashes of white light at 0.085 W/cm2 per second elicited two types of responses. One type was characterized as complex signals seemingly consisting of several co-occurring action potentials with latencies of 0.5–1 s (Fig. 8c). The other type consisted of graded potentials with much shorter latency periods (Fig. 8d). When attempts were made to place the electrode in the area of the gastrodermal nerve, little spontaneous activity was usually recorded, but the tapping of the setup rhythmically with a hand-held needle produced large signals in two cases (Fig. 8e). These signals adapted to a tapping of ~2 Hz and partly recovered following pauses of 3 s (Fig. 8e) suggesting the presence of mechanoreceptors in the GN.
Extracellular electrophysiological recordings from the cut end of the rhopalial stalk reveal that a wide variety of signals occurs in the stalk. The strongest signals are spontaneous and action-potential-like with a steady frequency between 0.5 Hz and 1.5 Hz (a). Other recordings show a much more diverse spontaneous electrical activity probably involving a number of cells (b). Visual information is also transmitted via structures the stalk (c-d; red lines stimulus). Stimulation of the large lens eye and the slit eyes with 50 ms flashes of white light produces action potentials in some of the stalk neurons, but with a long latency (c, arrows). The same stimuli applied to the small lens eye and pit eyes produce receptor potential-like responses in other stalk neurons (d, arrows). In some cases in which the suction electrode is aimed at the area of the gastrodermal nerve, possible mechanosensory signals are obtained (e). Tapping the setup with a needle gives large responses displaying typical adaptation when stimulated at 2 Hz. The response almost completely recovers after 3 s (e, arrow).
Discussion
In the present study, we have described the morphology of the rhopalial stalk of the cubomedusa Tripedalia cystophora by using light microscopy (LM), TEM, and extracellular electrophysiology. We have focused on the neural tissue in order to determine the way that the stalk connects the rhopalia with the ring nerve. Two separate nerves have been found, one in the gastrodermis and the other in the epidermis, the latter being the most prominent and displaying approximately 600 synapses. Immunostained whole-mount preparations and TEM examination have shown that the EN is a continuation of parts of the ring nerve that bend down into the stalk and connect with the rhopalia within the rhopalial neuropil. The electrophysiological data indicate that the information leaving the rhopalia is complex and more than merely a pacemaker signal.
Gastrodermal nerve
A GN has not previously been described in cubomedusae, although the structure was mentioned in one of the earliest studies of these animals (Claus 1878). Even though no direct evidence exists for the GN being a nerve, since we have found no clear synapses, the general appearance of its elements is neurite-like. The presence of the many dense-cored vesicles also strongly indicates that the cells are neurons, since similar vesicles are present in neurons of hydrozoans (Weber et al. 1982) and may represent synaptic vesicles. Cells containing such vesicles are often peptinergic, but immunohistological screening (including for FMRFamide) have failed to stain any cells in the GN (unpublished results).
The GN does not connect with the neuropil of the rhopalium and consequently cannot be involved in data transmission to and from these major sensory structures of the medusae. Other functions must therefore be ascribed to this nerve. Our TEM survey has revealed a number of structures within this nerve strongly resembling sensory cilia (Fig. 4b). The modality of these putative sensory cilia cannot be determined with certainty, since the outer segments of chemo- and mechanosensory cilia often have a similar appearance. The finding that they lie embedded in a nerve inside the animal favors mechanosensory rather than chemosensory functions. Our preliminary electrophysiological recordings support the presence of mechanoreceptors in the stalk, even though these results cannot be ascribed directly to the GN. The way that the mechanical stimulus is applied is also not completely reliable, since artifacts such as the shifting of the electrode tip may produce similar electrical signals. Nevertheless, the finding that the signal adapts and at least partly recovers is a strong argument for a biological origin. The oral end of the rhopalia contains a crystal (Fig. 2a) that acts as a weight ensuring that the rhopalia and thereby the eyes always have the same orientation relative to horizontal/vertical. This means that the stalk bends according to the orientation of the animal. If the putative sensory cells are indeed mechanoreceptors, then the GN could be a proprioceptor detecting the degree of bending of the stalk and might be part of a gravity-sensing device. The crystal might thus function both as a device to ensure a constant orientation of the eyes and as a statolith.
The target areas for the information from the GN are not known. The nerve splits up in the roof of the rhopalial niche and cannot be followed beyond the niche. It seems reasonable to assume that the information is passed on to the ring nerve, which controls the movements of the medusa (Satterlie 1979, 2002). This, however, would mean that the neurites have to cross the mesoglea at some point. None of our LM and TEM longitudinal series sections of the stalk has revealed such a crossing, but this may occur in the tissue beyond the stalk, i.e. the roof of the rhopalial niche.
Condensation of the gastrodermal nervous system has not been found in any other medusa and may represent a novel part of the CNS. The ring nerve of hydromedusae also encompasses two separate parts, but they are both epidermal (Mackie 2004). Cubopolyps have also been shown to possess a ring nerve consisting of two parallel rings, and here one is gastrodermal (Chapman 1978). The GN that we have observed in the stalk of T. cystophora might be the adult form of this nerve.
Epidermal nerve
We have shown that the EN of the rhopalial stalk is an extension of a major part of the ring nerve, which bends down into the stalk from both sides and forms two parallel nerve bundles running in the stalk. This has been demonstrated by tubulin immunohistochemistry in whole-mounts and is supported by the arrangement of the neurons in the EN. Although synapse counts have not been performed in other parts of the ring nerve, a similar density of synapses has been reported in the ring nerve of the hydromedusae Polyorchis penicillatus and Aglantha digitale (Singla 1978; Spencer 1979). The few nerve cell bodies compared with the number of neurites also conforms to expectations for the ring nerve if we assume that the somata are evenly distributed. The neurites of the ring nerve thus enter the rhopalia in which they form at least parts of the rhopalial neuropil. This interpretation is in contrast with the earlier suggestion that the stalk nerve represents neurites of ganglion cells in the rhopalium connecting to a ring nerve ganglion (Laska and Hündgen 1984).
Our results strongly suggest that the information from the rhopalia leave via the EN. Since the EN is part of the ring nerve, the CNS of T. cystophora can be regarded as a single coherent system with four specialized regions (the rhopalia). Each rhopalium has at least two cell clusters of about 20 nerve cells, each directly connected to its side of EN (Fig. 5). Unfortunately, the extremely irregular shape of the neurites and their small size (some as thin as 200 nm) makes them impossible to follow for more than 10–15 μm. We have therefore been unable to determine whether the nerve cells in the rhopalium send their neurites all the way up through the stalk or whether they synapse onto neurites in the stalk or even within the rhopalium. However, the 40 respective cells are unlikely to account for all the contacts with the 450 neurites in the EN, and the EN is therefore bound to have connections to other rhopalial nerve cells. These other connections have not been found, but we believe that they are distributed in the neuropil of the rhopalium. Even though documentation is scarce, a subpopulation of these cells might be RFamide-immunoreactive (Martin 2002, 2004).
The presence of eyes directly embedded in the CNS is not unlike the situation in vertebrates in which the retina ontogenetically is part of the CNS. In hydrozoans, the situation is different, since the ocelli appear to be part of the peripheral nervous system connecting to the ring nerve via interneurons (second order neurons; Toh et al. 1979; Yamamoto and Yoshida 1980; Weber 1981; Singla and Weber 1982a, 1982b). Interestingly, the optic nerve formed by these interneurons are two parallel bundles contacting the ring nerve separately (Mackie 1971; Singla and Weber 1982a, 1982b), not unlike the situation in T. cystophora. Scyphomedusae lack a ring nerve and thus do not have a clearly defined CNS, but they possess ganglia associated with the ocelli bearing rhopalia (Satterlie 2002).
An interesting problem is the location at which the signals from the rhopalium are integrated with the ring nerve. Since the synapses in the stalk are not aggregated into any clusters, there is no support for the presence of acute zones in this area. Nevertheless, the possibility remains that the EN synapses are involved in this process, which might therefore take place along the entire stalk. Another possible arrangement, which we find the more likely, is that the neurites of the ring nerve neurons extend into the rhopalium and receive their input within the rhopalial neuropil. Irrespective of where the integration takes place, the large number of neurites and synapses in the EN of the stalk strongly suggests that the visual information leaving the rhopalium has not been finally processed. This suggestion is confirmed by the preliminary electrophysiological recordings presented here. In contrast to the findings in Carybdea rastonii (Satterlie and Spencer 1979; Satterlie 1979, 2002), we have not found only a single pacemaker signal to be present in the stalk. There is also significant neuronal activity in the form of weaker signals, some of which are directly light-induced (Fig. 8a–d). The presence of light-induced receptor-like potentials in the stalk supports the idea that neuronal processing of visual signals takes place in the ring nerve and in the rhopalia.
Possible inter-rhopalial connections
The EN consists of two parallel nerves, which seem to receive information from separate sides in the rhopalia (Fig. 9a). After entering the bell, the two nerves run in opposite directions. Our TEM survey shows that, at least for some distance, these nerves stay separate from the remaining part of the ring nerve (Fig. 6d). A possible interpretation is that the nerve leaving the stalk to the right of one rhopalium continues to the left side of the neighboring rhopalium (Fig. 9b). This indicates an exchange of information between the left and the right halves of adjacent rhopalia, and possibly the integration of visual input from adjacent rhopalia. This would also mean that the ring nerve is not one uniform system but rather a congregation of several subsystems, as has been found for hydromedusae (Mackie 2004). A large number of subsystems in the CNS with complex interactions is also to be expected in cubomedusae, since they have a more elaborate sensory system than hydromedusae and display complex behavioral patterns.
Model of the CNS of T. cystophora. In the lateral and aboral part of the rhopalium, a cluster of nerve cells (NCB) connects with the EN of the stalk (EN in a). The EN runs as two parallel nerves in the stalk, and these separate in the roof of the rhopalial niche. Each branch of the EN joins the ring nerve (RN) along which they run in parallel for some distance (a). Whether the EN and RN fuse or stay separate in the area between the rhopalia (Rh) is not known (b, question mark) GN gastrodermal nerve
References
Anderson PAV, Moosler A, Grimmelikhuijzen CJP (1992) The presence and distribution of antho-RFamide-like material in scyphomedusae. Cell Tissue Res 267:67–74
Berger EW (1898) The histological structure of the eyes of cubomedusae. J Comp Neurol 8:223–230
Buskey EJ (2003) Behavioral adaptations of the cubozoan medusa Tripedalia cystophora for feeding on copepod (Dioithona oculata) swarms. Mar Biol 142:225–232
Chapman DM (1978) Microanatomy of the cubopolyp, Tripedalia cystophora (class Cubozoa). Helgoländer Wiss Meeresunters 31:128–168
Claus C (1878) Ueber Charybdea marsupialis. Arbeiten Zool Instit Universität Wien 1:1–56
Derby CD (1995) Single unit electrophysiological recordings from crustacean chemoreceptor neurons. In: Spielman AI, Brand JG (eds) Experimental cell biology of taste and olfaction. Current techniques and protocols. CRC Press, New York, pp 241–250
Grimmelikhuijzen CJP (1985) Antisera to the sequence Arg-Phe-amide visualize neuronal centralization in hydroid polyps. Cell Tissue Res 241:171–182
Grimmelikhuijzen CJP, Spencer AN (1984) FMRFamide immunoreactivity in the nervous system of the medusa Polyorchis penicillatus. J Comp Neurol 230:361–371
Grimmelikhuijzen CJP, Spencer AN, Carré D (1986) Organization of the nervous system of physonectid siphonophores. Cell Tissue Res 246:463–479
Hartwick RF (1991) Observations on the anatomy, behaviour, reproduction and life cycle of the cubozoan Carybdea sivickisi. Hydrobiologia 216/217:171–179
Kinsey B (1986) Barnes on box jellyfish. James Cook University, Townsville
Laska G, Hündgen M (1982) Morphologie und Ultrastruktur der Lichtsinnesorgane von Tripedalia cystophora Conant (Cnidaria, Cubozoa). Zool Jb Anat 108:107–123
Laska G, Hündgen M (1984) Die Ultrastructur des neuromuskuläre Systems der Medusen von Tripedalia cystophora und Carybdea marsupialis (Coelenterata, Cubozoa). Zoomorphology 104:163–170
Lewis C, Long TAF (2005) Courtship and reproduction in Carybdea sivickisi (Cnidaria: Cubozoa). Mar Biol 147:477–483
Mackie GO (1971) Neurological complexity in medusae: a report of central nervous organization in Sarsia. Actas del I Simposio International de Zoofilogenia. University of Salamanca, Salamanca, pp 269–280
Mackie GO (2004) Central neural circuitry in the jellyfish Aglantha: a model "simple nervous system”. Neuro-Signals 13:5–19
Mackie GO, Meech RW (1995a) Central circuitry in the jellyfish Aglantha digitale. I. The relay system. J Exp Biol 198:2261–2270
Mackie GO, Meech RW (1995b) Central circuitry in the jellyfish Aglantha digitale. II. The ring gigant and carrier systems. J Exp Biol 198:2271–2278
Mackie GO, Meech RW (2000) Central circuitry in the jellyfish Aglantha digitale. III. The rootlet and pacemaker systems. J Exp Biol 203:1797–1807
Martin VJ (2002) Photoreceptors of cnidarians. Can J Zool 80:1703–1722
Martin VJ (2004) Photoreceptors of cubozoan jellyfish. Hydrobiologia 530/531:135–144
Moroz L, Meech RW, Sweedler JV, Mackie GO (2004) Nitric oxide regulates swimming in the jellyfish Aglantha digitale. J Comp Neurol 471:26–36
Nilsson DE, Coates M, Gislén l, Skogh C, Garm A (2005) Advanced optics in a jellyfish eye. Nature 435:201–205
Parkefelt L, Skogh C, Nilsson DE, Ekström P (2005) Bilateral symmetric organization of neural elements in the visual system of a coelenterate, Tripedalia cystophora (Cubozoa).J Comp Neurol 492:251–262
Satterlie RA (1979) Central control of swimming in the cubomedusan jellyfish Carybdea rastonii. J Comp Physiol [A] 133:357–367
Satterlie RA (2002) Neural control of swimming in jellyfish: a comparative story. Can J Zool 80:1654–1669
Satterlie RA, Spencer AN (1979) Swimming control in a cubomedusan jellyfish. Nature 231:141–142
Satterlie RA, Nolen TG (2001) Why do cubomedusae have only four swim pacemakers? J Exp Biol 204:1413–1419
Singla CL (1978) Locomotion and neuromuscular system of Agantha digitale. Cell Tissue Res 188:317–327
Singla CL, Weber C (1982a) Fine structure of the ocelli of Polyorchis penicillatus (Hydrozoa: Anthomedusae) and their connection with the nerve ring. Zoomorphology 99:117–129
Singla CL, Weber C (1982b) Fine structure of the ocellus of Sarsia tubulosa (Hydrozoa, Anthomedusae). Zoomorphology 100:11–22
Spencer AN (1979) Neurobiology of Polyorchis. II. Structure of effector systems. J Neurobiol 10:95–117
Spencer AN, Arkett SA (1984) Radial symmetry and the organization of central neurones in a hydrozoan jellyfish. J Exp Biol 110:69–90
Toh Y, Yoshida M, Tateda H (1979) Fine structure of the ocellus of the hydromedusan, Spirocodon saltatrix. I. Receptor cells. J Ultrastruc Res 68:341–352
Weber C (1981) Structure, histochemistry, ontogenetic development, and regeneration of the ocellus of Cladonema radiatum Dujardin (Cnidaria, Hydrozoa, Anthomedusae). J Morphol 167:313–331
Weber C, Singla CL, Kerfoot PAH (1982) Microanatomy of the subumbrellar motor innervation in Aglantha digitale (Hydromedusae: Trachylina). Cell Tissue Res 223:305–312
Yamamoto M, Yoshida M (1980) Fine structure of ocelli of an anthomedusan, Nemiopsis dofleini, with special reference to synaptic organization. Zoomorphology 96:169–181
Yamasu T, Yoshida M (1976) Fine structure of complex ocelli of a cubomedusan, Tamoya bursaria Haeckel. Cell Tissue Res 170:325–339
Yi-Chan JL, Gallin WJ, Spencer AN (2001) The anatomy of the nervous system of the hydrozoan jellyfish, Polyorchis penicillatus, as revealed by a monoclonal antibody. Invertebr Neurosci 4:65–75
Acknowledgements
We greatly appreciate the excellent laboratory work performed by Rita Wallén and Carina Rasmussen. We also thank the staff at the marine station Isla Magueyes, especially Wilson Rovira, for the help that they provided. Fruitful discussions with Mattias Ekerholm, Lund University, are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants 621-2002-4873 from the Swedish Research Council to D.-E. Nilsson and 21-2204-04 from the Danish Research Council to A. Garm.
Rights and permissions
About this article
Cite this article
Garm, A., Ekström, P., Boudes, M. et al. Rhopalia are integrated parts of the central nervous system in box jellyfish. Cell Tissue Res 325, 333–343 (2006). https://doi.org/10.1007/s00441-005-0134-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0134-8