Abstract
Senescence marker protein-30 (SMP30) is an androgen-independent factor that decreases with aging. To elucidate the physiological functions of SMP30, we transfected human SMP30 cDNA into the human hepatoma cell line, Hep G2. These Hep G2/SMP30 transfectants, which stably expressed large amounts of SMP30, proliferated at a slower rate and synthesized less DNA than mock transfectants (Hep G2/pcDNA3 controls). Thus, enhanced expression of SMP30 retarded the growth of Hep G2/SMP30 cells. Ultrastructural studies by scanning electron microscopy revealed numerous microvilli covering the surfaces of Hep G2/SMP30 cells, whereas few microvilli appeared on control cells. Subsequently, transmission electron microscopy revealed that groups of Hep G2/SMP30 cells exhibited bile canaliculi and possessed specialized adhesion contacts, such as tight junctions and desmosomes, at interplasmic membranes. However, in controls, units of only two cells were seen, and these lacked specialized adhesion junctions. Moesin and ZO-1 are known to be concentrated in microvilli and at tight junctions, respectively. Double-immunostaining was performed to examine whether moesin and ZO-1 were expressed in bile canaliculi with microvilli at the apical regions of Hep G2/SMP30 cells. The intensity of moesin and ZO-1 staining in the contact regions of each cell was markedly higher in Hep G2/SMP30 than in control cells. Moreover, moesin stained more interior areas, which corresponded to the microvilli of bile canaliculi. Clearly, bile canaliculi with microvilli formed at the apical ends of Hep G2/SMP30 cells. These results indicate that SMP30 has an important physiological function as a participant in cell-to-cell interactions and imply that the down-regulation of SMP30 during the aging process contributes to the deterioration of cellular interactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By using proteomic analysis of age-associated changes in soluble proteins of the rat liver, we discovered a novel protein that we designated senescence marker protein-30 (SMP30; Fujita et al. 1992). The amount of SMP30 significantly decreases with aging in an androgen-independent manner (Fujita et al. 1996, 1992), and the amino acid sequence of this 34-kDa protein is highly conserved among vertebrates, i.e., 70%–90% (Fujita et al. 1995; Sato et al. 2000). SMP30 is expressed mainly in hepatocytes and renal tubular epithelia (Fujita et al. 1992). In cultured hepatocytes, SMP30 is distributed in both the cytoplasm and nucleus (Ishigami et al. 2003). According to a database search, SMP30 has a domain that resembles bacterial and yeast RNA polymerase (Ishigami et al. 2003). Since nuclei are a marked site of SMP30 localization, one can assume that SMP30 acts in the regulation of gene expression.
Recently, we have established SMP30-knockout mice to clarify the relationship between age-associated decreases of SMP30 and age-associated organ disorders (Ishigami et al. 2002a). These knockout animals are viable and fertile but lower in body weight and shorter in life span than the wild-type (Ishigami et al. 2004). Throughout our experiments in vitro and in vivo, we have found that the livers of SMP30-knockout mice are far more susceptible to apoptosis mediated by tumor-necrosis factor α and Fas than those from the wild-type (Ishigami et al. 2002a). Moreover, histological and biochemical analyses of livers from SMP30-knockout mice show abnormal accumulations of neutral lipids and phospholipids (Ishigami et al. 2004). This abnormal lipid metabolism must increase the susceptibility of the tissue to apoptosis. Such changes in SMP30 expression might thus account for the deterioration of cellular functions and the lowered resistance to harmful stimuli in aged tissues.
Parenchymal hepatocytes are highly differentiated epithelial cells whose unique cell-to-cell architecture assists in their performance of major exocrine and metabolic functions. Specifically, hepatocytes are three-dimensional polygonal cells involved in uptake and secretion at or near plasma membranes (Arterburn et al. 1995). The apical surfaces are distinctive areas in which membranes between adjacent cells are sealed by tight junctions and through which bile is secreted into the biliary system. In such cells, the expression of SMP30 is highly maintained throughout the maturing process and adulthood of several species (Fujita et al. 1996, 1992). To assess the function of this protein in liver cells, we transfected Hep G2 cells with human SMP30 cDNA (Hep G2/SMP30); this resulted in the cells stably and abundantly expressing SMP30. Our previous study indicated that SMP30 participates in Ca2+ efflux by activating the calmodulin-dependent Ca2+-pump in Hep G2 cells and by conferring, on these cells, resistance to injury caused by high intracellular Ca2+ concentrations (Fujita et al. 1998).
This paper reports the detailed morphological comparison of transfected cells and mock-transfected controls, performed by both scanning and transmission electron microscopy. The morphological differences between the two cell types indicate that SMP30 enhances cell-to-cell adhesions and increases the polarization and differentiation of hepatocytes.
Materials and methods
Establishment of stable transfectants expressing the human SMP30
A human hepatoma cell line, Hep G2 cells (Knowles et al. 1980), was transfected with human SMP30 cDNA (Fujita et al. 1995) or, as a control, with pcDNA3 (Invitrogen, Carlsbad, Calif., USA), which did not contain the human SMP30 cDNA insert. For this procedure, we used Lipofectamine Reagent (Invitrogen) according to the manufacturer’s instructions and selected transfected cells by using G418. A clone with a high level of human SMP30 expression was selected and designated as Hep G2/SMP30. Control cells mock-transfected with pcDNA3 vector only were designated as Hep G2/pcDNA3.
Cell cultures and DNA synthesis assay
Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. The cells (2×105) were grown in 35-mm plastic dishes for 5 days, counted, and assessed for protein concentration. The protein concentration was determined by the BCA protein assay (Pierce Biotechnology, Rockford, Ill., USA) with bovine serum albumin as a standard. DNA synthesis activity was determined by 5-bromo-2′-deoxy-uridine (BrdU) incorporation with the BrdU-labeling Detection kit III (Roche Diagnostics, Indianapolis, Ind., USA) according to the manufacturer’s instructions.
SDS-polyacrylamide gel electrophoresis and Western blot analysis
Cellular expression of SMP30 was appraised by Western blot analysis as described previously (Ishigami et al. 2002b). Briefly, equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on vertical slab gels containing 14% (w/v) acrylamide and 0.25% (w/v) N,N′-methylenebisacrylamide, by the method of Laemmli (1970). Proteins were then electrophoretically transferred from acrylamide gels onto a nitrocellulose membrane (BAS-85, Schleicher & Schuell, Dassel, Germany) by the method of Towbin et al. (1979). The membrane was incubated successively with anti-rat SMP30 antibody (Ishigami et al. 2002a) and horseradish-peroxidase-labeled goat anti-rabbit IgG (Bio-rad Laboratories, Richmond, Calif., USA). Chemiluminescence signals were detected on Kodak XAR films by using ECL Western Blotting Detection Reagents (Amersham Bioscience, Piscataway, N.J., USA).
Ultrastructural analysis
Cells were cultured on glass slides in plastic dishes for 5 days and then fixed in 2.5% glutaraldehyde buffered with sodium cacodylate (pH 7.4) overnight at 4°C and postfixed in 1% osmic acid for 1 h at 4°C. For scanning electron microscopy, fixed cells were dehydrated through a graded series of ethanol, dried in a t-buthyl alcohol freeze-dryer (Eiko ID-2, Ibaraki, Japan) and coated with platinum/palladium (Polaron E-5450, UK). Specimens were observed in a scanning electron microscope S-4500 (Hitachi, Ibaraki, Japan) at an accelerating voltage of 3 kV. For transmission electron microscopy, fixed cells were dehydrated and embedded in Epon-812. Thin sections were then cut, stained with uracyl acetate and lead nitrate, and examined in a TEM-600 (Hitachi, Ibaraki, Japan) at an accelerating voltage of 50 kV.
Double-immunostaining of moesin and ZO-1
For immunohistochemical staining, cells were cultured on Falcon culture slides, fixed for 15 min with 10% formalin in phosphate-buffered saline (PBS), and permeabilized for 3 min with 0.5% Triton X-100 in PBS. For double-immunostaining with ZO-1 and moesin, cells were stained with mouse anti-ZO-1 monoclonal antibody (mAb; T8-754; Itoh et al. 1991) and rat anti-moesin mAb (M22; Takeuchi et al. 1994). Primary antibodies were visualized with rhodamine-conjugated anti-mouse IgG and fluorescein isothiocyanate (FITC)-conjugated anti-rat IgG (Tago Immunologicals, Calif., USA). Specimens were analyzed by confocal microscopy (LSM-510 laser scanning microscope, Carl Zeiss, Oberkochen, Germany).
Statistical analysis
The results are expressed as the mean ± S.D. The probability of statistical differences between experimental groups was determined by an unpaired Student’s t-test. A statistical difference was considered significant at P<0.05.
Results
Slow proliferation rate of Hep G2/SMP30 cells
To clarify the physiological functions of SMP30, we transfected human SMP30 cDNA into the human hepatoma cell line, Hep G2, thereby yielding Hep G2/SMP30 cells with a stable and abundant expression of human SMP30. Figure 1a shows that the amount of SMP30 expressed by Hep G2/SMP30 cells was far greater than that by Hep G2/pcDNA3 mock-transfected control cells. When viewed by phase-contrast light microscopy, Hep G2/SMP30 cells underwent no significant morphological changes compared with the control cells after 5 days of culture (Fig. 1b, c).
To quantitate the proliferation rates and protein contents of cells, Hep G2/SMP30 and control cells were cultured in 35-mm culture dishes at a starting number of 2.0×105 cells. After 5 days of culture, the total number of control cells was 3.0×106, whereas Hep G2/SMP30 numbered only 2.3×106 cells (Table 1), i.e., Hep G2/SMP30 cells proliferated at a 0.76-fold slower rate than control cells. In contrast, the amount of protein in Hep G2/SMP30 cells was 1.20-fold greater than that in control cells (Table 1). Assessed individually, the protein amount per cell was 1.57-fold greater in Hep G2/SMP30 cells compared with control cells. Moreover, DNA synthesis activity in Hep G2/SMP30 cells measured by BrdU uptake was only half that in Hep G2/pcDNA3 cells (Fig. 2). These results indicated that the proliferation rate of Hep G2/SMP30 cells was suppressed by the enhanced expression of SMP30.
Numerous microvilli and bile canaliculi form on Hep G2/SMP30 cells
Because Hep G2/SMP30 cells were quite difficult to detach from culture dishes by trypsin treatment, their ultrastructural features were examined. Hep G2/SMP30 and control cells were sparsely cultured for 5 days (approximately 30%–40% confluence) and analyzed by scanning electron microscopy. Interestingly, numerous microvilli covered the surfaces of Hep G2/SMP30 cells (Fig. 3b), whereas few microvilli were apparent on control cells (Fig 3a). Transmission electron microscopy then revealed units of two Hep G2/SMP30 cells with bile canaliculi and interplasmic membranes acting as specialized adhesion contacts, such as tight junction and desmosomes (Fig. 4). However, the visible units of two control cells in contact lacked specialized adhesion junctions (data not shown). When cells contacted each other and made bile canaliculi formations, their nuclei appeared indented toward the cell membrane at the apical ends of the structures, reflecting the greater polarization of these cells (Fig. 4). The adhesive behaviour of the cell plasma membranes was classed into six main categories, viz., Types A–F, with Type E being divided into Types E and E′, as shown in Fig. 5. Numerous two-cell adhesions without bile canaliculi (Types A–C) were found in control cells (Fig. 6). In contrast, the formation of bile canaliculi (Types D, E, E′, and F) was abundant in Hep G2/SMP30 cells. The bile canaliculi were sealed by tight junctions and usually had microvilli on their surfaces (Fig. 5, Types D, E, E′, and F). Occasionally, the bile canaliculi were distended and filled with micelle-like material (Fig. 5, Type E′). Usually, the membranes of two cells formed these bile canaliculi, but sometimes three or more cells interacted to form a single bile canaliculus (Fig. 5, Type F). Type F formation had five cells with adjacent membranes sealed by tight junctions at the apical ends to form a single bile canaliculus with numerous microvilli at the cell surfaces. Bile-canalicular formation was more abundant in Hep G2/SMP30 cells (Types D, E, E′, and F) than in control cells (Fig. 6). In particular, Types E and E′ accounted for approximately 50% of the Hep G2/SMP30 cells.
Cell-to-cell adhesion via apical surfaces of Hep G2/SMP30 cells. Transmission electron microscopy shows adhesion structures, such as tight junctions and desmosomes. Adherent cells have indented nuclei against the cell membrane at the apical ends of the structures, reflecting the polarization of these cells (arrows adhesion junctions). Bar 5 μm
Classification of cell surface adhesion patterns. The adhesive behaviour of the cell plasma membranes, such as in Fig. 4, was classed into six categories. a Type A (Hep G2/pcDNA3 cells); no tight junctions or desmosomes, although two cells adhere to each other. b Type B (Hep G2/pcDNA3 cells); some desmosomes are located between the two cells. c Type C (Hep G2/pcDNA3 cells); pre-interdigitations of plasma membranes of two adherent cells sealed at each end by tight junctions. d Type D (Hep G2/SMP30 cells); distended form of Type C, presumably a bile canaliculus is forming. e Type E (Hep G2/SMP30); the formation of a bile canaliculus sealed at each end by tight junctions between the two cells. e′ Type E′ (Hep G2/SMP30); micelle-like material secreted into the canaliculi. f Type F (Hep G2/SMP30 cell); single bile canaliculus formed by more than three cells attached to each other by tight junctions. Numerous microvilli were found inside the canalculus surface. All cells were cultured for 5 days before transmission electron microscopy. Bars: 1 μm
Quantitation of adhesion types. Six cell-surface adhesion types were found in 45 Hep G2/pcDNA3 cells and 67 Hep G2/SMP30 cells. Representations under the bar graph illustrate the adhesion Types A–F. Type E′ is presented as the dark gray part of the bar above the black bar for Type E (light gray bars Hep G2/pcDNA3 cells, black and dark gray bars Hep G2/SMP30 cells).
Expression of moesin and ZO-1 in cell-to-cell contact region
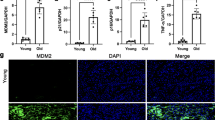
Moesin plays a crucial role in the formation of microvilli structures (Takeuchi et al. 1994). ZO-1 is concentrated in tight junctions and adherent junctions located at the apical end of epithelial cells (Ando-Akatsuka et al. 1999). Double-immunostaining were performed to examine whether moesin and ZO-1 were present in the bile canaliculi with microvilli at the apical end of Hep G2/SMP30 cells (Fig. 7). The intensity of moesin and ZO-1 staining at the contact regions of each cell was markedly greater in Hep G2/SMP30 cells than in controls (Fig. 7a, b, d, e). Moreover, moesin staining in the interior areas of Hep G2/SMP30 cells exceeded that in controls and corresponded to microvilli of bile canaliculi (Fig. 7c). Thus, the bile canaliculi with microvilli clearly formed at the apical ends of Hep G2/SMP30 cells.
Double-immunostaining of moesin and ZO-1 (arrowheads adhesion junctions). Hep G2/SMP30 cells (a–c) and Hep G2/pcDNA3 cells (d–f) were cultured on Falcon culture slides for 5 days and fixed with 10% formalin. Cells were stained with rat anti-moesin mAb (M22; a, d) and mouse anti-ZO-1 mAb (T8-754; b, e). Primary antibodies were visualized with FITC-conjugated anti-rat IgG (green) and rhodamine-conjugated anti-mouse IgG (red). c, f Merged views for a, b and d, e, respectively. Bars 10 μm
Discussion
Hep G2 cells transfected with human SMP30 cDNA to assess SMP30 function in liver cells were examined here by scanning electron microscopy and transmission electron microscopy. This ultrastructural analysis revealed that the number of microvilli and bile canaliculi in these SMP30 over-expressing cells far exceeded that in mock-transfected Hep G2/pcDNA3 cells used as controls. Moreover, double-immunostaining for moesin and ZO-1 confirmed these results and established that the microvilli and bile canaliculi formed at the apical ends of Hep G2/SMP30 cells. Normally, the Hep G2 cell line expresses only a small amount of SMP30, although hepatocytes isolated from adult rat liver tissues express large amount of SMP30 in vivo. Quantitative analysis has revealed that SMP30 constitutes 2% of the total soluble liver protein of adult rats (Fujita et al. 1992). In the present study, Hep G2/SMP30 transfected cells expressed a significantly higher level of SMP30 than control cells. Thus, transfection of Hep G2 cells with SMP30 cDNA enables these cells to express SMP30 at levels approaching those typically found in vivo, thereby approximating the physiological environment.
The formation of the numerous microvilli seen here indicates the increased potential of Hep G2/SMP30 cell-to-cell interactions. The association of cytosolic free Ca2+ ([Ca2+]i) with the cell shape and the dynamics of the actin cytoskeleton and with the formation and disassembly of cell-to-substrate adhesions is well known (Sjaastad and Nelson 1997). Ca2+ also serves as a second messenger in many biochemical signal-transduction events (Sjaastad and Nelson 1997). A Ca2+-dependent cell-to-cell adhesion molecule, the E-cadherin/catenin complex, provides a crucial function in adherent junctions. This complex also participates in the establishment and maintenance of normal epithelial morphology and differentiation (Jawhari et al. 1999). Despite the pivotal role of Ca2+ in cell adhesion, its regulatory mechanism is still unclear. Lee et al. (1999) have reported that Ca2+ arises transiently from the activation of stretch-activated Ca2+ channels, which triggers an influx of extracellular Ca2+. The subsequent increase in [Ca2+]i is involved in cell locomotion (Lee et al. 1999) and in the contraction of the bile canaliculi to yield the driving force for bile excretion in liver (Oshio and Phillips 1981). Our previous data have indicated that a transient [Ca2+]i increase in both Hep G2/SMP30 and Hep G2/pcDNA3 cells follows ATP stimulation. However, the peak value of [Ca2+]i is two-fold higher in Hep G2/SMP30 cells, and the rate of decrease after this [Ca2+]i peak is also enhanced two-fold in Hep G2/SMP30 cells compared with controls (Fujita et al. 1998). This outcome combined with our present results indicate that SMP30 may regulate [Ca2+]i for the maintenance of hepatocyte morphology and differentiation. We speculate that cell-to-cell and/or cell-to-matrix adhesion is increased by SMP30, since Hep G2/SMP30 cells are more adherent in cell-to-cell and/or cell-to-matrix interactions than control cells, as has been observed when cells are subcultured by trypsin treatment. The likelihood that SMP30 increases cell adhesion is also suggested by the following. Calreticulin is one of the major Ca2+-binding proteins resident in the endoplasmic reticulum (Krause and Michalak 1997). Additionally, calreticulin has been associated with the cytoplasmic domains of integrin α-subunits, and this interaction can influence integrin-mediated cell adhesion to matrices (Dedhar 1994). An integrin-induced transient elevation in [Ca2+]i resulting from an influx of extracellular Ca2+ has been observed in wild-type cells but not calreticulin-deficient cells (Coppolino et al. 1997). Moreover, calreticulin is an essential modulator of integrin-adhesive functions and of integrin-initiated signalling (Coppolino et al. 1997). Clearly, the relationship of SMP30 to cell adhesion and other biological functions deserves further exploration.
In a preliminary experiment, we loaded carboxyfluorescein dye into cells to compare the secretion ability between Hep G2/SMP30 and control cells by the method described in Kudo et al. (2004). The secretion ability of Hep G2/SMP30 cells was certainly higher than that of the controls, indicating the formation of functionally intact bile canaliculi.
In conclusion, over-expression of SMP30 in transfected Hep G2 cells induces the formation of numerous microvilli and bile canaliculi. The formation of microvilli and bile canaliculi is essential for the construction of liver tissues and maintains liver functions. Therefore, the down-regulation of SMP30 during the aging process may result in the accompanying deterioration of cell-to-cell interactions and increased susceptibility to harmful stimuli.
References
Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S (1999) Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol 179:115–125
Arterburn LM, Zurlo J, Yager JD, Overton RM, Heifetz AH (1995) A morphological study of differentiated hepatocytes in vitro. Hepatology 22:175–187
Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S (1997) Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature 386:843–847
Dedhar S (1994) Novel functions for calreticulin: interaction with integrins and modulation of gene expression. Trends Biochem Sci 19:269–271
Fujita T, Uchida K, Maruyama N (1992) Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta 1116:122–128
Fujita T, Mandel JL, Shirasawa T, Hino O, Shirai T, Maruyama N (1995) Isolation of cDNA clone encoding human homologue of senescence marker protein-30 (SMP30) and its location on the X chromosome. Biochim Biophys Acta 1263:249–252
Fujita T, Shirasawa T, Uchida K, Maruyama N (1996) Gene regulation of senescence marker protein-30 (SMP30): coordinated up-regulation with tissue maturation and gradual down-regulation with aging. Mech Ageing Dev 87:219–229
Fujita T, Inoue H, Kitamura T, Sato N, Shimosawa T, Maruyama N (1998) Senescence marker protein-30 (SMP30) rescues cell death by enhancing plasma membrane Ca2+-pumping activity in Hep G2 cells. Biochem Biophys Res Commun 250:374–380
Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, Enomoto N, Sato N, Shimosawa T, Maruyama N (2002a) Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-a- and Fas-mediated apoptosis. Am J Pathol 161:1273–1281
Ishigami A, Ohsawa T, Asaga H, Akiyama K, Kuramoto M, Maruyama N (2002b) Human peptidylarginine deiminase type II: molecular cloning, gene organization, and expression in human skin. Arch Biochem Biophys 407:25–31
Ishigami A, Handa S, Maruyama N, Supakar PC (2003) Nuclear localization of senescence marker protein-30, SMP30, in cultured mouse hepatocytes and its similarity to RNA polymerase. Biosci Biotechnol Biochem 67:158–160
Ishigami A, Kondo Y, Nanba R, Ohsawa T, Handa S, Kubo S, Akita M, Maruyama N (2004) SMP30 deficiency in mice causes an accumulation of neutral lipids and phospholipids in the liver and shortens the life span. Biochem Biophys Res Commun 315:575–580
Itoh M, Yonemura S, Nagafuchi A, Tsukita S (1991) A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol 115:1449–1462
Jawhari AU, Farthing MJ, Pignatelli M (1999) The E-cadherin/epidermal growth factor receptor interaction: a hypothesis of reciprocal and reversible control of intercellular adhesion and cell proliferation. J Pathol 187:155–157
Knowles BB, Howe CC, Aden DP (1980) Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209:497–499
Krause KH, Michalak M (1997) Calreticulin. Cell 88:439–443
Kudo A, Kashiwagi S, Kajimura M, Yoshimura Y, Uchida K, Arii S, Suematsu M (2004) Kupffer cells alter organic anion transport through multidrug resistance protein 2 in the post-cold ischemic rat liver. Hepatology 39:1099–1109
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K (1999) Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400:382–386
Oshio C, Phillips MJ (1981) Contractility of bile canaliculi: implications for liver function. Science 212:1041–1042
Sato A, Asashima M, Yokota T, Nishinakamura R (2000) Cloning and expression pattern of a Xenopus pronephros-specific gene, XSMP-30. Mech Dev 92:273–275
Sjaastad MD, Nelson WJ (1997) Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays 19:47–55
Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S (1994) Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol 125:1371–1384
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Acknowledgements
We thank Dr. K. Noda and Ms. S. Kanai for technical assistance. The excellent editorial assistance of Ms. P. Minick and Mr. Y. Fujita are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Akihito Ishigami and Toshiko Fujita contributed equally to this work.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan (to S.H., A.I., and N.M.), and a grant from the Health and Labour Sciences Research Grants for Comprehensive Research on Aging and Health and Research on Dementia and Fracture supported by the Ministry of Health, Labour, and Welfare, Japan (to A.I and N.M.), and a Grant-in-Aid for Smoking Research Foundation, Japan (to N.M.).
Rights and permissions
About this article
Cite this article
Ishigami, A., Fujita, T., Inoue, H. et al. Senescence marker protein-30 (SMP30) induces formation of microvilli and bile canaliculi in Hep G2 cells. Cell Tissue Res 320, 243–249 (2005). https://doi.org/10.1007/s00441-004-1073-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1073-5