Abstract
The spatial localisation of insulin-like growth-factor-binding protein-2 (IGFBP-2) and its mRNA was investigated during larval and post-larval developmental stages of the gilthead seabream (Sparus aurata) by immunohistochemistry and in situ hybridisation with specific antisera and riboprobes. During larval development, immunoreactivity was found in skin, muscle, gills, pharynx, intestine, liver and olfactory epithelium. After metamorphosis, immunoreactivity was found in the oesophageal epithelium (the strongest reaction) and in red skeletal muscle, heart muscle, the thymus and the epithelium of renal tubules. In the adult, immunostaining with IGFBP-2 antibody was also found in the saccus vasculosus, ovary and testis. IGFBP-2 mRNA was detected by in situ hybridisation mainly in the intestine, skeletal musculature and ovary. These results show that IGFBP-2 protein and mRNA are expressed in a variety of seabream tissues, suggesting that IGFBP-2 regulates the actions of IGFs on these tissues during development and growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The insulin-like growth factor (IGF) system includes IGFs (IGF-I and IGF-II), type-I and type-II IGF receptors (IGF-Rs) and IGF-binding proteins (IGFBPs). IGF-I and IGF-II, which are structurally similar to insulin, are expressed in many tissues and have potent metabolic and mitogenic actions that affect cell development and growth. The mitogenic effects of IGFs are mediated mainly through interactions with the type-I receptor, whereas the role of the type-II receptor in mediating IGF action is less well understood (Jones and Clemmons 1995; Hwa et al. 1999). In biological fluids, IGFs are normally bound to IGFBPs, which protect them from degradation and modulate their action (Jones and Clemmons 1995; Rajaram et al. 1997; Duan 1998; Kelley et al. 2002). In mammals, six IGFBPs have been characterised and have been designated IGFBP-1 to IGFBP-6 (Jones and Clemmons 1995; Hwa et al. 1999). IGFBPs bind to IGFs with affinities generally greater than that of the type-I IGF receptor itself. However, IGFBP immunoreactivity has also been shown in various mammalian tissues, including endometrium (Bryant-Greenwood et al. 1993), cartilage (Martin et al. 2000), kidney (Miyatake et al. 1998) and ileum (Gordon et al. 2002).
Recent studies suggest that IGFBPs are also part of the IGF system in fish, as shown by the presence of IGFBPs in fish circulation (Anderson et al. 1993; Niu and LeBail 1993; Siharath et al. 1996; Park et al. 2000; Kelley et al. 2002) and the secretion of IGFBPs from fish tissues in vitro (Fukazawa et al. 1995; Siharath et al. 1995). Subsequently, proteins homologous to mammalian IGFBPs have been cloned from fish: IGFBP-1 and IGFPB-2 from zebrafish (Danio rerio; Duan et al. 1999; Maures and Duan 2002), IGFBP-2 from the goby (Gillichthys mirabilis; Gracey et al. 2001) and IGFBP-2 from the gilthead seabream (Sparus aurata; Funkenstein et al. 2002). These studies have shown that fish IGFBP-1 and IGFBP-2 are 40%–50% homologous to their mammalian counterparts. In addition, IGFBP proteins have been purified from rainbow trout (Oncorhynchus mykiss; Bauchat et al. 2001) and chinook salmon (Oncorhynchus tshawyscha; Shimizu et al. 2003).
Despite this advance in IGFBP research in fish over the past few years and the growing evidence for the expression of IGFs and IGF-Rs in a various tissues in many fish species (Richardson et al. 1995; Reinecke et al. 1997; Perrot et al. 1999; Funkenstein et al. 2002; Radaelli et al. 2003a, 2003b), information regarding the role of IGFBPs in fish development, growth and reproduction is relatively scarce. Northern blot and reverse transcription/polymerase chain reaction (RT-PCR) analyses have demonstrated IGFBP-2 expression during the early developmental stages and in the developing gonad of seabream (Funkenstein et al. 2002). Similarly, IGFBP-1 is expressed during the early developmental stages of zebrafish (Maures and Duan 2002). Expression studies with RT-PCR of RNA from adult seabream tissues have revealed high levels of IGFBP-2 mRNA in the liver, skin, immature gonad and pyloric caeca and detectable levels in other tissues (Funkenstein et al. 2002).
Information regarding the cellular localisation of IGFBP-2 during the development and growth of teleost fish is currently limited to the fish species shi drum (Umbrina cirrosa), which has been studied by using heterologous antibodies (Radaelli et al. 2003a). The aim of this study has been to examine the cellular localisation of IGFBP-2 protein and mRNA during the development and growth of the gilthead seabream (S. aurata), a marine fish of great interest for aquaculture, by means of homologous antibodies and riboprobes specifically produced for this work.
Materials and methods
Fish samples and tissue processing
Larvae and fry of seabream were obtained from fish hatcheries at Pellestrina (VE, Italy) and killed by an overdose of MS222 (Sandoz, Italy) anaesthesia. Animals and tissues used for both immunohistochemistry and in situ hybridisation were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, 0.1 M, pH 7.4) at 4°C overnight. Small fish (hatching to juveniles) were fixed in toto and sectioned in transverse and longitudinal (frontal and sagittal) planes. Organ and tissue samples were dissected from large adult fish and processed separately. Gonads were collected and processed from mature males with a functional testis, from mature females showing various stages of follicular development and from bisexual fish in which gonads contained both ovarian and testicular parts. Other samples for in situ hybridisation on frozen sections were immersed in liquid nitrogen-cooled isopentane immediately post-mortem and kept at −70°C until sections were cut.
Immunohistochemistry
Tissue preparation
Larvae (3, 4, 10, 15, 18, 22, 36, 50 and 52 days), fry (77 and 95 days) and juveniles (100 and 110 days) and adult organ and tissue samples, fixed as given above, were washed in PBS, dehydrated through a graded series of ethanol and embedded in paraffin. Sections were cut serially at a thickness of 4 μm on a microtome.
Antisera
Polyclonal antibodies were raised in rabbits against a synthetic peptide spanning amino acid 152 to amino acid 164 (YTPKKTRLKGGPQ) in the variable domain of the mature seabream IGFBP-2 (Funkenstein et al. 2002), a region sharing little sequence identity with other IGFBP-2. Both the peptide and antibodies were produced for us by Sigma (Italy). The antibody was used at a dilution of 1/1,000.
Immunohistochemical procedure
Immunohistochemical reactions were performed with the Envision method (goat anti-rabbit immunoglobulins conjugated to peroxidase-labelled complex; Dako). Before application of the primary antibody, endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 in PBS and non-specific binding sites were blocked by incubation in 1:5 non-immune rabbit serum. The primary antiserum was applied overnight at 4°C in a humid chamber. After being rinsed in PBS, the sections were incubated for 30 min at room temperature in the Envision system and, following another rinse in PBS, the immunoreactive sites were visualised by using a freshly prepared solution of 10 mg 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma) in 15 ml 0.05 M TRIS buffer, pH 7.6, containing 1.5 ml 0.03% H2O2. In order to ascertain structural details, sections were counterstained with Mayer’s haematoxylin, dehydrated, mounted in Eukitt and examined under an Olympus BX50 photomicroscope.
Controls
The specificity of the immunostaining was verified: (1) by incubating sections with PBS instead of the specific primary antiserum; (2) by incubating sections with pre-immune serum instead of primary antiserum; (3) by incubating sections with PBS instead of secondary antibody; (4) by absorption of the antisera with excess of synthetic peptide prior to incubation with sections. In all cases, staining was abolished. In addition, a dot blot of increasing amounts of the synthetic peptide and similar amounts of bovine serum albumin (BSA) and with the antibody and two detection methods showed that the reaction was proportional to sample load of the peptide (data not shown).
Molecular cloning and synthesis of RNA probe
A 355-bp fragment of seabream IGFBP-2 cDNA was cloned by PCR by using reverse-transcribed liver RNA and Primers 1 and 2 (described previously by Funkenstein et al. 2002). The PCR fragment was gel-purified and cloned in PGEM-Teasy (Promega, Madison, Wis.) for generation of a riboprobe.
Linearised seabream IGFBP-2 cDNA was used as template to generate sense and anti-sense RNA probes with bacteriophage SP6 or T7 RNA polymerases according to a protocol modified from Nieto et al. (1996). The reagents were added at room temperature in the following order: 10 μl sterile distilled water, 4 μl 5× transcription buffer (Promega, Italy), 2 μl 0.1 M dithiothreitole (Promega), 2 μl digoxigenin (DIG) nucleotide mix pH 8.0 (Roche, Italy), 1 μl linearised IGF-II plasmid (1 μg/μl), 0.5 μl ribonuclease inhibitor (100 U/μl; Roche), 1 μl T7 or SP6 RNA polymerase (10 U/μl; Promega). After incubation at 37°C for 2 h, 2 μl ribonuclease-free DNase I (Roche) were added and the incubation was continued for an additional 15 min. Precipitation of the synthesised RNA was achieved by the addition of 100 μl TE (10 mM TRIS–HCl, 1 mM EDTA), 10 μl 4 M lithium chloride and 300 μl ethanol and storage of the tube at −20°C for 30 min. The RNA was then centrifuged at 13,000 rpm for 10 min and the pellet was washed with 70% ethanol and air-dried. The RNA was reconstituted in 50 μl TE and 5 μl aliquots were run on a 1% agarose/TRIS-borate-EDTA gel to assess its quality and concentration. The RNA probe was diluted to 0.1 μg/μl and stored at −80°C.
In situ hybridisation procedures
Whole-mount larvae aged 4, 10, 18, and 20 days, fixed as described above, were washed in PBS twice, dehydrated and stored in methanol at −20°C. Following hydration in graded methanol, larvae were processed as described previously (Joly et al. 1993) with slight modifications as follows. After incubation in 6% H2O2 for 30 min, larvae were permeabilised by treatment with 10 μg/ml proteinase K (Roche) in PBS containing 0.1% Tween 20 (PBT) for about 20 min (duration of treatment depending on the size of the sample), washed in PBT and fixed again in 4% paraformaldehyde plus 0.2% glutaraldehyde in PBT for 20 min before the hybridisation step. After being washed in PBT, larvae were rinsed with 1:1 PBT/hybridisation solution and then incubated with hybridisation solution, pre-warmed to 60°C, for 30 min. This was replaced with a fresh hybridisation solution containing 0.1 μg/μl RNA probe and incubation was continued overnight at 55–60°C. After post-hybridisation washes, transcripts were identified by using the DIG nucleic acid detection kit (Roche). The hybridised larvae were viewed on a stereomicroscope connected to a digital camera (Olympus). Following in situ hybridisation, larvae were kept in PBT containing 100 mM EDTA at 4°C. For histological examination, hybridised larvae were dehydrated and embedded in paraffin; 10-μm-thick serial sections (some counterstained with Mayer’s haematoxylin) were dehydrated, mounted in Eukitt and examined under an Olympus BX50 photomicroscope.
Frozen sections of larvae (4, 10, 18, and 20 days), fry (77 day), juveniles (110 day) and adult tissues were processed for in situ hybridisation experiments as described by Eizema et al. (2003) except for the following modifications. The riboprobes (500 ng/ml final concentration) were resuspended in the following hybridisation buffer: 50% (deionised) formamide, 1× SSC (saline–sodium citrate buffer), 10% dextran sulfate, 1× Denhardt’s solution, 0.67 M NaCl, 0.1 μg/μl yeast tRNA, and 0.1 μg/μl herring sperm DNA. Riboprobes were then heated at 80°C for 5 min before the hybridisation step. Approximately 50 μl hybridisation buffer containing the probe was used per slide, the sample being covered with a coverslip. Hybridisation was performed overnight at 45–50°C in a humidified incubator. Coverslips were removed by rinsing in 6× SSC, followed by two high-stringency steps at 50°C for 20 min in 0.5× SSC and 20% formamide and two rinses in 2× SSC at room temperature. Non-hybridised probe was digested with 2 μg/ml RNase A in 0.5 M NaCl, 10 mM TRIS–HCl pH 8.0, at 37°C for 30 min, followed by five washes in 2× SSC at room temperature and another high-stringency wash for 10 min. The sections were rinsed twice with 2× SSC and maleic buffer (0.1 M maleic acid, 0.15 M NaCl, pH 7.5) and blocked twice for 15 min and once for 60 min with 5% inactivated BSA (Sigma) in maleic buffer at room temperature. Methods for the detection of hybridised probes were adapted from the manufacture’s protocols by using a digoxigenin antibody conjugated with fluorescein (FITC; Roche). Images were obtained with a Leica TCS-SP2 confocal laser scanning microscope (CLSM).
Results
Immunohistochemistry
General
The results of immunohistochemical localisation of IGFBP-2 in various tissues are described below and summarised in Table 1.
Gut
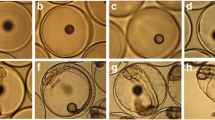
In larvae immediately after hatching, the epithelial cells of the developing gut showed anti-IGFBP-2 immunoreactivity (Table 1). Positive staining was present in the epithelium of the pharynx at all ages studied but was particularly intense around metamorphosis (about days 50–60; Fig. 1A, inset). During post-larval life, marked immunostaining was found in the oesophageal epithelium, whereas mucous cells were negative (Fig. 1B). In the intestinal epithelium, reactivity was evident even in post-hatching larvae aged 4 days (Fig. 1C). During mid-larval life, enterocytes were diffusely immunostained, whereas mucous cells were negative (Fig. 1D). At metamorphosis, immunostaining was diffuse in the enterocytes of the proximal intestine (data not shown) but was dense in the apical portion of enterocytes of the distal intestine (Fig. 1F). In the adult intestinal epithelium, moderate immunoreactivity was found in the apical border of the cytoplasm, whereas the brush border was negative (Fig. 1G).
Immunohistochemical localisation of IGFBP-2 in seabream gut (counterstained with haematoxylin). A Sagittal section of a 22-day-old larva. Immunostaining is present in the epithelium of the pharynx (asterisks). Immunoreaction is also found in the gills (arrows). Inset: Pharyngeal epithelium (asterisks) in a 52-day-old fry at higher magnification. B Oesophagus of a 77-day-old fry. Immunoreaction is found in the epithelium (asterisks). Mucous cells are negative (arrows). C Sagittal section of a 4-day-old larva IGFBP-2 immunoreactivity is found in the intestinal epithelium (I) and in the liver (L). D The enterocytes of the epithelium of the intestine show intense immunoreactivity in a 36-day-old larva. Mucous cells are negative (arrowhead). Inset: Non-reactive cytoplasm of mucous cells (arrow). E Sagittal section of a 52-day-old larva showing a marked reaction in the liver (L). F Transverse section of the distal intestine of a 52-day-old fry. IGFBP-2 immunostaining is present in the apical portion of enterocytes (arrows). G Adult proximal intestine showing immunoreaction in the apical border of the enterocytes (asterisks). Bars 10 μm (A, inset in A, B, C), 4 μm (D, E–G), 2 μm (inset in D)
In the liver, intense IGFBP-2 immunoreactivity was evident in 4-day larvae (Fig. 1C) but there was a steady decline towards metamorphosis (data not shown). In fry and juveniles, the liver showed intense reactivity (Fig. 1E), whereas in the adult, IGFBP-2 immunoreactivity was only seen in scattered hepatocytes (Table 1). Exocrine and endocrine cells of the pancreas were negative at all the stages examined.
Muscle
In skeletal muscle, IGFBP-2 immunoreactivity was found in all the stages examined (Table 1). In larvae immediately after hatching, moderate immunostaining was detected in all the fibres of the trunk musculature (Fig. 2A), whereas in a 15-day larvae, immunopositivity was limited to the fibres of the superficial monolayer (Fig. 2B). After metamorphosis, strong reactivity was found in the red fibres (Fig. 2C), whereas in adults, both red and pink muscle fibres were immunopositive (Table 1). Immunostaining was found in the heart at all the stages examined (Table 1, Fig. 2D).
Immunohistochemical localisation of IGFBP-2 in seabream muscle (counterstained with haematoxylin). A Transverse section of a 4-day-old larva. IGFBP-2 immunoreactivity is present in the trunk musculature (M). Stronger immunoreaction is found in the skin (arrows) and in the intestine (I). The spinal cord (SC) and notochord (N) are immunonegative. B Transverse section of a 15-day-old larva. IGFBP-2 immunoreactivity is found in the superficial monolayer fibres (arrows) located under the skin (asterisks immunoreaction in skin, M fast-white muscle fibres, SC spinal cord, N notochord). C Transverse section of a 110-day-old juvenile. IGFBP-2 immunostaining is located in the trunk musculature mainly at the level of red muscle fibres (R). Arrows indicate the immunoreaction in skin (P pink muscle fibres, W white muscle fibres). D Adult heart musculature exhibits IGFBP-2 immunoreactivity. Bars 10 μm (A, B, D), 100 μm (C)
Gonads
In the ovarian part of bisexual gonads, IGFBP-2 immunostaining was found in the perinuclear cytoplasm of previtellogenic oocytes (Fig. 3A). In functional females with a mature ovary, immunostaining was observed in the follicular cells of yolk-granule-stage follicles (Fig. 3B). No reactivity was observed in oocytes at the perinucleolus stage.
Immunohistochemical localisation of IGFBP-2 in adult seabream gonads (counterstained with haematoxylin). A Ovarian part of a bisexual gonad showing immunostaining in the cytoplasm of previtellogenic oocytes (arrows). B Mature ovary of a functional female. IGFBP-2 immunoreactivity is detected in the cytoplasm of follicular cells (asterisks). C, D Testicular parts of a bisexual gonad showing immunoreactivity in clusters of spermatogonia (arrows) and spermatids (asterisks) and in cells bordering the cysts (arrowheads). Bars 10 μm (A), 4 μm (B–D)
In the testicular part of bisexual gonads, IGFBP-2 immunoreactivity was found in the cytoplasm of spermatogonia and spermatids (Fig. 3C) and in cells bordering cysts (Fig. 3D). No reaction was observed in functional males with a mature testis.
Other organs and tissues
In the gills, IGFBP-2 immunoreactivity was found in all the stages examined (Table 1). During larval life, the immunostaining was faint (Table 1), whereas from metamorphosis, numerous intensely positive cells were detected in the epithelium at the level of primary and secondary lamellae (Fig. 4A). In the adult, intense reactivity was also found in cells located at the base of the secondary lamellae (Fig. 4B). From metamorphosis, moderate IGFBP-2 immunoreactivity was found in the epithelium surrounding the thymus and in some scattered cells of the thymic parenchyma (Fig. 4C). The kidney showed IGFBP-2 immunopositivity in both larval, post-larval and adult stages (Table 1). The reactivity was detected in the epithelium of proximal and distal tubules (Fig. 4D). In the proximal tubules, the brush border was also immunoreactive (Fig. 4D, inset). In the olfactory epithelium, faint immunoreactivity was evident even in young larva (Table 1). In post-larval stages, intense IGFBP-2 immunoreactivity was also detected in the olfactory bulb (Fig. 4E). Marked immunostaining was also found in the epithelium of the saccus vasculosus in adults (Fig. 4F). In skin epithelium, IGFBP-2 immunoreactivity was found in all the stages examined (Table 1).
Immunohistochemical localisation of IGFBP-2 in gills, thymus, kidney, olfactory bulb and the saccus vasculosus of seabream (counterstained with haematoxylin). A Gills of a 77-day-old fry. Immunreactivity is found in the epithelium of the gill filaments. Cartilage (C) is unreactive. B Gills of an adult. Immunoreactivity is detected in the epithelium of the gill filaments; several cells located at the base of the secondary lamellae are positive (arrows). C Thymus of a 77-day-old fry. IGFBP-2 immunoreactivity is found in the epithelium surrounding the thymus (arrowheads) and in some scattered cells of the parenchyma (arrows). D Kidney of a 110-day-old juvenile. Immunostaining is present in the epithelium of the renal tubules (asterisks). Inset: Tubular epithelial cells and their brush border (arrowhead). E Olfactory bulb (arrows) of a 52-day-old fry with an intense IGFBP-2 immunoreaction (asterisk the eye). F The epithelium of the adult saccus vasculosus (arrowheads) shows intense IGFBP-2 immunostaining (asterisks blood vessels). Inset: At higher magnification, immunoreactivity is visible in the cytoplasm of the epithelial cells of the saccus vasculosus. Bars 40 μm (A, B, E), 10 μm (C, D, F), 4 μm (inset in D, inset in F)
In situ hybridisation
A faint hybridisation signal was detected in the intestinal epithelium of adult seabream (data not shown). Skeletal muscle was positive at all stages examined (Fig. 5A–E). Expression of IGFBP-2 mRNA was detected even in muscle fibres of 4-day-old larva (Fig. 5A). At around 20-days post-hatching, the hybridisation signal was found in the apical regions of myomeres of the trunk (Fig. 5B, C) and at the level of the superficial monolayer (Fig. 5C). In juveniles, strong expression of IGFBP-2 mRNA was present in the red muscle fibres, whereas white fibres were negative (Fig. 5D). In adult, a strong signal was also found in numerous fibres of the pink muscle layer (Fig. 5E). In the ovarian part of bisexual gonads, IGFBP-2 mRNA was found in the cytoplasm of previtellogenic oocytes (Fig. 5F).
In situ hybridisation with IGFBP-2 mRNA in seabream. A Transverse section of a 4-day-old larva showing a positive fluorescent signal in the trunk musculature (M) and no signal in the spinal cord (SC) and notochord (N). B In a 20-day-old larva (whole-mount), a positive hybridisation signal is found in the apical regions of myomeres (arrows). C In a transverse section of the larva shown in B, a positive hybridisation signal is present at the level of the superficial monolayer (arrow) and in the apical part of the myomeres (arrowheads). The spinal cord (SC) exhibits no signal. D Transverse section of a 110-day-old juvenile. Fluorescent in situ hybridisation showing a positive signal in the trunk musculature at the level of red muscle fibres (R); white muscle fibres (W) are negative. Arrowheads indicate the skin. E Fluorescent in situ hybridisation of adult trunk musculature showing a positive signal in the pink fibres. Fast fibres (arrows) were negative. F In the ovarian part of an adult bisexual gonad, a fluorescent signal is found in the cytoplasm of previtellogenic oocytes (arrows). Bars 10 μm (A), 40 μm (B), 20 μm (C, E, F), 100 μm (D)
Discussion
In this study, we report the spatial localisation of IGFBP-2 protein and mRNA, identified by using homologous antibodies and riboprobes for immunohistochemistry and in situ hybridisation, from hatching to adult stages in the gilthead seabream, S. aurata.
Although the expression of IGFBP-2 gene has been reported in fish liver and in extra-hepatic tissues (Duan et al. 1999; Funkenstein et al. 2002), information regarding the cellular localisation of IGFBP-2 protein in fish is limited to a single study of the shi drum (U. cirrosa) in which antiserum raised against a synthetic peptide of mouse IGFBP-2 was employed (Radaelli et al. 2003a). The previous study gave rise to a slightly different localisation pattern compared with the current study.
IGFBP-2 protein and mRNA are widely distributed in various tissues and organs throughout the life cycle of the seabream, suggesting that IGFBP-2 expression early in fish development plays a role in introducing IGF-I (and probably also IGF-II) to target organs, as IGF bioavailability is modulated by IGFBPs (for a review, see Kelley et al. 1996). This idea is supported by the similar pattern, in general, of cellular localisation of IGFBP-2 (reported here) to that of IGF-I and IGF-II found in the seabream and shi drum (Funkenstein et al. 1997; Perrot et al. 1999; Radaelli et al. 2003a, 2003b). However, the use of immunohistochemistry and in situ hybridisation in the current study has enabled us to identify the pattern of expression in various cell types within individual tissues and organs. Thus, with regard to muscle during midlarval life, IGFBP-2 mRNA has been found in the superficial monolayer fibres and in the apical regions of the myomeres (areas of hyperplastic growth of white muscle fibres; Rowlerson et al. 1995) in which IGF-I and IGF-II mRNA are also expressed (Funkenstein et al. 1997; Radaelli et al. 2003b). The presence of IGFBP-2 in red muscle fibres of juvenile seabream also corresponds with the localisation of IGF-I and IGF-II in red muscle at these developmental stages (Perrot et al. 1999; Radaelli et al. 2003b). Similarly, we demonstrate that, in adult fish, IGFBP-2 is present in red and pink fibres but not in white fibres, a distribution similar to that of IGF-II (Radaelli et al. 2003b). The IGFBP-2 immunoreactivity found in heart musculature of fry and adults is also consistent with the presence of IGF-I and IGF-II in heart muscle (Radaelli et al. 2003a, 2003b) and the expression of IGFBP-2 mRNA in the heart of adult seabream (Funkenstein et al. 2002).
The intestinal tract also displays a similar pattern of IGFBP-2 localisation to that reported for IGF-I and IGF-II. Thus, an intense reaction has been found in the epithelial layers of the pharynx, oesophagus and distal intestine during larval and post-larval development, in accordance with the localisation of IGF-I and IGF-II and their receptor in these tissues (Perrot et al. 1999; Radaelli et al. 2003a, 2003b). Liver also displays high immunoreactivity for IGFBP-2, suggesting that the marked expression of IGFBP-2 mRNA noted in fish liver (Duan et al. 1999; Funkenstein et al. 2002) gives rise to high levels of IGFBP-2 protein. Surprisingly, no IGFBP-2 protein or mRNA have been detected in pancreatic tissue at any developmental stage, although IGF-I (but not IGF-II) has been detected in pancreatic tissue of shi drum and seabream (Funkenstein et al. 1997; Perrot et al. 1999; Radaelli et al. 2003a, 2003b).
Our immunohistochemical study of IGFBP-2 protein in the gonads of various developmental stages of S. aurata provides novel information on the cellular localisation of this IGF-binding protein in fish gonads. Both IGFBP-2 peptide and mRNA are present in the perinuclear cytoplasm of previtellogenic oocytes from immature bisexual gonads. A similar finding of IGFBP-2 mRNA in oocytes has been reported in bovine preantral follicles (Armstrong et al. 2002). IGFBP-2 immunoreactivity in granulosa cells of yolk-granule-stage follicles from mature ovaries, found in the current study, is consistent with a similar cellular localisation of IGF-I, IGF-II and IGF-IR (Perrot et al. 2000; Radaelli et al. 2003b) supporting a role for the IGF system in oocyte maturation. In the present report, IGFBP-2 immunoreactivity has also been detected in testicular part of bisexual gonads, at the level of the cytoplasm of spermatogonia, in spermatids and in cells bordering cysts. This localisation is similar to that of IGF-I and IGF-IR (Perrot et al. 2000), supporting a role for the IGF system in testicular development and function. No immunoreactivity for IGFBP-2 could be found in adult testis. Interestingly, higher levels of IGFBP-2 mRNA have been reported in young seabream gonads with a predominantly ovarian part, consisting mainly of previtellogenic oocytes, than in later stages. In contrast, the amount of detectable IGFBP-2 mRNA is low in testis (Funkenstein et al. 2002). The presence of IGF-I and IGF-II mRNAs and their peptides has also been shown in the gonads of tilapia by using in situ hybridisation and immunohistochemistry (Schmid et al. 1999).
IGFBP-2 immunoreactivity has also been found in several organs and tissues involved in osmoregulation in fish: skin, the epithelial cells of gill filaments, the epithelium of kidney tubules and the saccus vasculosus. These observations are consistent with the distribution of the immunoreactivity of IGF-I, IGF-II or IGF-IR and the expression of IGFBP-2 mRNA reported in these tissues in several fish species (Richardson et al. 1995; Reinecke et al. 1997; Perrot et al. 1999; Funkenstein et al. 2002; Radaelli et al. 2003a,2003b). Previous studies have also shown the in vitro production of IGFBP-2 by cultured kidney from striped bass (Siharath et al. 1995). Taken together, these observations support an osmoregulatory role for the IGF system in teleosts.
IGFBP-2 immunoreactivity is detectable in the thymus, an organ of the immune system, consistent with the presence of IGF-II peptide in this organ (Radaelli et al. 2003b). The human thymus also contains IGF-II and IGFBP-2 to IGFBP-6 transcripts, supporting a role for the IGF system in the development of vertebrate T cells (Kecha et al. 1999).
Another interesting finding in the current study is the presence of IGFBP-2 immunoreactivity in the olfactory bulb. A more widespread expression of IGFBP-2 has been found in the mammalian brain (Lee et al. 1993) but our result is consistent with previous studies of seabream brain, in which IGF-IR (Perrot et al. 1999; Radaelli et al. 2003a) and IGF-II (Radaelli et al. 2003b) expression has almost exclusively been found in the olfactory bulb and epithelium. It also agrees with the failure to detect IGFBP-2 transcripts in whole-brain RNA extracts from adult seabream, even with the sensitive method of RT-PCR (Funkenstein et al. 2002).
In conclusion, we have shown that the spatial localisation of IGFBP-2 in S. aurata is similar to that of other components of the IGF system reported in fish, suggesting that IGFBP-2 produced locally controls IGF availability and activity in several fish tissues, especially red muscle, liver, gonads and many epithelia.
References
Anderson TA, Bennette LR, Conlon MA, Owens PC (1993) Immunoreactive and receptor-active insulin-like growth factor-I and IGF-binding protein in blood plasma from the freshwater fish Macquaria ambigua (golden perch). J Endocrinol 136:191–198
Armstrong DG, Baxter G, Hogg CO, Woad KJ (2002) Insulin-like growth factor (IGF) system in the oocyte and somatic cells of bovine preantral follicles. Reproduction 123:789–797
Bauchat JR, Busby W, Garmany A, Moore J, Swanson P, Lin M, Duan C (2001) Biochemical and functional analysis of a conserved insulin-like growth factor binding protein (IGFBP) isolated from rainbow trout hepatoma. J Endocrinol 170:619–628
Bryant-Greenwood GD, Rutanen EM, Partanen S, Coelho TK, Yamamoto SY (1993) Sequential appearance of relaxin, prolactin and IGFBP-1 during growth and differentiation of the human endometrium. Mol Cell Endocrinol 95:23–29
Duan C (1998) Nutritional and developmental regulation of insulin-like growth factor in fish. J Nutr 128:306S–314S
Duan C, Ding J, Li Q, Tsai W, Pozios K (1999) Insulin-like growth factor binding protein 2 is a growth inhibitory protein conserved in zebrafish. Proc Natl Acad Sci USA 96:15274–15279
Eizema K, Burg M van den, Kiri A, Dingboom EG, Oudheusden H van, Goldspink G, Weijs WA (2003) Differential expression of equine myosin heavy-chain mRNA and protein isoforms in a limb muscle. J Histochem Cytochem 51:1207–1216
Fukazawa Y, Siharath K, Iguchi T, Bern HA (1995) In vitro secretion of insulin-like growth factor-binding proteins from liver of striped bass, Morone saxatilis. Gen Comp Endocrinol 99:239–247
Funkenstein B, Almuly R, Chan SJ (1997) Localization of IGF-I and IGF-I receptor mRNA in Sparus aurata larvae. Gen Comp Endocrinol 107:291–303
Funkenstein B, Tsai W, Maures T, Duan C (2002) Ontogeny, tissue distribution, and hormonal regulation of insulin-like growth factor binding protein-2 (IGFBP-2) in a marine fish, Sparus aurata. Gen Comp Endocrinol 128:112–122
Gordon PV, Moats-Staats BM, Stiles AD, Price WA (2002) Dexamethasone changes the composition of insulin-like growth factor binding proteins in the newborn mouse ileum. J Pediatr Gastroenterol Nutr 35:532–538
Gracey A, Troll J, Somero G (2001) Hypoxia-induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc Natl Acad Sci USA 98:1993–1998
Hwa V, Oh Y, Ronsenfeld RG (1999) The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 20:761–787
Joly JS, Joly C, Schulte-Merker S, Boulekbache H, Condamine H (1993) The ventral and posterior expression of the evenskipped homeobox gene eve 1 is perturbed in dorsalized and mutant embryos. Development 119:1261–1275
Jones JI, Clemmons DR (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34
Kecha O, Martens H, Franchimont N, Achour I, Hazee-Hagelstein MT, Charlet-Renard C, Geenen V, Winkler R (1999) Characterization of the insulin-like growth factor axis in the human thymus. Neuroendocrinology 11:435–440
Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG (1996) Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol 28:619–637
Kelley KM, Schmidt KE, Berg L, Sak K, Galima MM, Gillespie C, Balogh L, Hawayek A, Reyes JA, Jamison M (2002) Comparative endocrinology of the insulin-like growth factor-binding protein. J Endocrinol 175:3–18
Lee WH, Michels KM, Bondy CA (1993) Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: correlation with insulin-like growth factors I and II. Neuroscience 53:251–265
Martin JA, Scherb MB, Lembke LA, Buckwalter JA (2000) Dexamethasone changes the composition of insulin-like growth factor binding proteins in the newborn mouse ileum. Iowa Orthop J 20:1–10
Maures T, Duan C (2002) Structure, developmental expression, and physiological regulation of zebrafish insulin-like growth factor binding protein-1. Endocrinology 143:2722–2731
Miyatake N, Shikata K, Wada J, Sugimoto H, Takahashi S, Makino H (1999) Differential distribution of insulin-like growth factor-1 and insulin-like growth factor binding proteins in experimental diabetic rat kidney. Nephron 81:317–323
Nieto MA, Patel K, Wilkinson DG (1996) In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol 51:219–235
Niu PD, LeBail PY (1993) Presence of insulin-like growth factor binding protein (IGF-BP) in rainbow trout (Oncorhynchus mykiss) serum. J Exp Zool 265:627–636
Park R, Shepherd BS, Nishioka RS, Grau EG, Bern HA (2000) Effects of homologous pituitary hormone treatment on serum insulin-like growth-factor-binding proteins (IGFBPs) in hypophysectomized tilapia, Oreochromis mossambicus, with special reference to a novel 20-kDa IGFBP. Gen Comp Endocrinol 117:404–412
Perrot V, Moiseeva EB, Gozes Y, Chan SJ, Ingleton P, Funkenstein B (1999) Ontogeny of the insulin-like growth factor system (IGF-I, IGF-II, and IGF-IR) in gilthead seabream (Sparus aurata): expression and cellular localization. Gen Comp Endocrinol 116:445–460
Perrot V, Moiseeva EB, Gozes Y, Chan SJ, Funkenstein B (2000) Insulin-like growth factor receptors and their ligands in gonads of a hermaphroditic species, the gilthead seabream (Sparus aurata): expression and cellular localization. Biol Reprod 63:229–241
Radaelli G, Domeneghini C, Arrighi S, Bosi G, Patruno M, Funkenstein B (2003a) Localization of IGF-I, IGF-I receptor and IGFBP-2 in developing Umbrina cirrosa (Pisces: Osteichthyes). Gen Comp Endocrinol 130:232–244
Radaelli G, Patruno M, Maccatrozzo L, Funkenstein B (2003b) Expression and cellular localization of insulin-like growth factor-II protein and mRNA in Sparus aurata during development. J Endocrinol 178:285–299
Rajaram S, Baylink DJ, Mohan S (1997) Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 18:801–831
Reinecke M, Schmid A, Ermatinger R, Loffin-Cueni D (1997) Insulin-like growth factor I in the teleost Oreochromis mossambicus, the tilapia: gene sequence, tissue expression, and cellular localization. Endocrinology 138:3613–3619
Richardson NA, Anderson AJ, Rimmer MA, Sara VR (1995) Localization of insulin-like growth factor-I immunoreactivity in larval and juvenile barramundi (Lates calcarifer). Gen Comp Endocrinol 100:282–292
Rowlerson A, Mascarello F, Radaelli G, Veggetti A (1995) Differentiation and growth of muscle in the fish Sparus aurata (L), II: hyperplastic and hypertrophic growth of lateral muscle from hatching to adult. J Muscle Res Cell Motil 16:223–236
Schmid AC, Naf E, Kloas W, Reinecke M (1999) Insulin-like growth factor-I and -II in the ovary of a bony fish, Oreochromis mossambicus, the tilapia: in situ hybridisation, immunohistochemical localisation, Northern blot and cDNA sequences. Mol Cell Endocrinol 156:141–149
Shimizu M, Swanson P, Hara A, Dickhoff W (2003) Purification of a 41-kDa insulin-like growth factor binding protein from serum of chinook salmon, Oncorhynchus tshawytscha. Gen Comp Endocrinol 132:103–111
Siharath K, Nishioka RS, Bern HA (1995) In vitro production of IGF-binding proteins (IGFBP) by various organs of the striped bass, Morone saxatilis. Aquaculture 135:195–202
Siharath K, Kelley KM, Bern HA (1996) A low-molecular-weight (25-kDa) IGF-binding protein is increased with growth inhibition in the fasting striped bass, Morone saxatilis. Gen Comp Endocrinol 102:307–316
Acknowledgements
The authors thank Mr. G. Caporale for his skilful technical work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the University of Padua (Progetto di Ateneo, 2001) and by the US–Israel Binational Agricultural Research and Development Fund (BARD, Project IS-2769-96R)
Rights and permissions
About this article
Cite this article
Radaelli, G., Patruno, M., Rowlerson, A. et al. Cellular localisation of insulin-like growth factor binding protein-2 (IGFBP-2) during development of the marine fish, Sparus aurata. Cell Tissue Res 319, 121–131 (2005). https://doi.org/10.1007/s00441-004-0952-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-0952-0