Abstract
The mammary gland reaches a fully differentiated phenotype at lactation, a stage characterized by the abundant expression of β-casein. We have investigated the expression and regulation of gap junction proteins (connexins, Cx) during the various developmental stages of mouse mammary gland. Immunohistochemical analysis, with specific antibodies, reveals that Cx26 and Cx32 are expressed and confined to the cell borders of luminal epithelial cells in all developmental stages of the gland. Cx26 and Cx32 expression, at the mRNA and protein levels, increases in pregnancy and peaks in lactation. Whereas Cx43 mRNA decreases in pregnancy and lactation, the functional activity of Cx43 protein, which has been localized to myoepithelial cells, is regulated (through phosphorylation) during pregnancy and peaks during lactation. Cx30 mRNA and proteins have, for the first time, been detected in mammary gland epithelia. Using reverse transcription/polymerase chain reaction and sequencing techniques, we show that Cx30 is abundant in pregnant and lactating mammary gland. Cx30 protein levels have not been detected in the mammary gland prior to day 15 of pregnancy, whereas maximum expression occurs at the onset of lactation. In mouse mammary cells in culture, Cx30 is epithelial-cell-specific and is induced by lactogenic hormones. These data identify a novel player in mammary differentiation and suggest a potential role for Cx30 in the fully differentiated gland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gap junctional intracellular communication (GJIC) is critical in diverse cell and tissue functions (White and Paul 1999). Gap junctions are perceived as “modulators of cellular differentiation” in several systems (Pitts et al. 1988; Paul et al. 1995; Bruzzone et al. 1996; Kumar and Gilula 1996) and recent studies have revealed a role for connexins (Cxs) in the stratification and differentiation of human epidermal cells (Wiszniewski et al. 2000) and lung alveolar epithelial cells (Alford and Rannels 2001) and in bone homeostasis, promoting osteoblast differentiation (Gramsch et al. 2001; Romanello et al. 2001; Schiller et al. 2001). The basic structural component of gap junctions is Cx. Cxs constitute a family of more than 20 homologous proteins that are temporally and spatially distributed throughout the body (for reviews, see Goodenough et al. 1996; Kumar and Gilula 1996; Kidder and Mhawi 2002; Sohl and Willecke 2003). Gap junction channels allow the direct exchange of small molecules (up to 1.5 kDa), such as ions, metabolites and second messenger molecules, between adjacent cells via passive diffusion (Goodenough et al. 1996). They also allow the rapid propagation of electrical currents that are believed to underlie tissue homeostasis in excitable tissues, such as the heart and uterus (Bennett et al. 1991). Characterization of the Cx family has only recently been extended to the functional level, with the inactivation of specific Cx genes and the observation of different phenotypic events produced in knockout animals (for a review, see Sohl and Willecke 2003).
During the development of the mammary gland, duct-lining and alveolar epithelial cells progress through a programme of expansive proliferation followed by a terminal differentiation that allows the biosynthesis and secretion of milk during lactation. Although the role of intercellular communication via gap junctions in the development, cellular differentiation and function of this secretory epithelium in the rodent and human mammary gland have been addressed, contradictory reports have been presented regarding the temporal expression pattern of these proteins within the mammary epithelium (for reviews, see Locke 1998; El-Sabban et al. 2003a). The cloning of individual Cx isoforms and the development of specific anti-Cx antibodies have enabled the work on the identification of the different Cxs present in the mammary gland to be extended and clarified without misinterpretation attributable to antibody non-specificity.
Studies have suggested that GJIC plays a critical role in the coordinated changes through the development, differentiation, maintenance and involution of the mammary gland (Monaghan et al. 1994, 1996; Pozzi et al. 1995; Yamanaka et al. 1997; Locke et al. 2000; Yamanaka et al. 2001). However, a direct correlation between functional GJIC and mammary epithelial differentiation, either in vivo (Perez-Armendariz et al. 1995; Pozzi et al. 1995; Monaghan and Moss 1996; Locke et al. 2000) or in vitro (Lee et al. 1991, 1992; Tomasetto et al. 1993; Hirschi et al. 1996; Sia et al. 1999) has not been established. Recently, we have demonstrated, in mammary CID-9 cells, that proper cell/extracellular matrix (ECM) interaction favours GJIC. Our studies suggest CID-9 cells are capable of differentiating and expressing β-casein in the absence of an exogenous basement membrane in a β1-integrin-independent pathway, provided that the cells are coupled via functional gap junctions (El-Sabban et al. 2003b).
Mouse Cx30, identified and cloned by Dahl et al. (1996), is strongly expressed in adult mouse brain and skin and is less abundant in uterus, lung and eye tissue and, to a lesser extent, in testis and sciatic nerve. Sequence comparisons of nucleotides and amino acids have revealed that mouse Cx30 shares 77% homology with mouse Cx26. In this report, we demonstrate the expression of Cx30, study its distribution (hitherto not reported in the mammary gland) and discuss its implications for mammary differentiation, at different stages of development, in the context of the expression pattern and regulation of other Cxs. This has provided further evidence of a role for gap junctions in modulating mammary gland development and differentiation.
Materials and methods
Materials
Anti-smooth muscle α-actin fluorescein isothiocyanate (FITC)-conjugated antibody was purchased from Sigma (St Louis, Mo.). Rabbit anti-Cx26, -Cx30, -Cx32 and -Cx43 were obtained from Zymed (San Francisco, Calif.). Polyclonal rabbit anti-mouse milk antiserum was provided by Dr. Mina Bissell (Lawrence Berkeley National Laboratory, Berkeley, Calif.). Mouse anti-D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Biogenesis, England. Secondary goat anti-rabbit IgG (H + L) conjugated to FITC, propidium iodide, and Prolong Antifade kit were purchased from Molecular Probes, Netherlands. Protease inhibitors (Complete) were obtained from Boehringer Mannheim, Germany. The Biorad protein assay reagent was obtained from BioRad (Calif.). Immobilon-P, polyvinylidene fluoride (PVDF) membranes were obtained from Millipore (Bedford, Mass.). Horseradish-peroxidase-conjugated anti-rabbit IgG, tetramethylbenzidine (TMB) and the enhanced chemiluminescence (ECL) system were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). cDNA inserts for Cx43 was amplified by using specific primers from skin cDNA. cDNA inserts for β-casein and whey acidic protein (WAP) were kindly provided by Dr. M.J. Bissell (Lawrence Berkeley National Laboratory). Cx degenerate primers, Cx26-, Cx30- and Cx43-specific primers and β-actin and β-casein primers were from Invitrogen (USA) and ThermoHybaid (Germany). Amersham Hybond-N membrane, α-32PdCTP and the Rediprime nick translation kit were supplied by Amersham Pharmacia Biotech (Uppsala, Sweden). All other materials used were of molecular biology grade.
Animals
One-month-old BALB/c female mice were used. Day 1 of pregnancy was assessed following the detection of a vaginal plug indicating that coitus has occurred the previous day. On various days of pregnancy, mice were sacrificed by cervical dislocation and the mammary glands removed for further processing.
For immunostaining, the glands were either fixed in 4% formaldehyde in phosphate-buffered saline (PBS) and embedded in paraffin or fixed in OCT blocks and frozen at −70°C. For Western blotting, mammary glands (3–5 animals per developmental stage) were pooled, homogenized and processed for electrophoresis as described below.
Polymerase chain reaction analysis of Cx and β-casein expression
Degenerate primers able to amplify all known mouse Cxs (Urban et al. 1999) were used. Mouse mammary gland and heart cDNAs were obtained from OriGene (OriGene Technologies, Rockville, Md.). Polymerase chain reaction (PCR) was carried out for 33 cycles with the following protocol: 93°C for 30 s, 55°C for 45 s and 72°C for 45 s with Taq DNA polymerase (Gibco BRL Life Technologies, Paisley, Scotland, UK). The cycle number was selected to be within the linear phase of PCR with respect to mammary cDNA template concentration. For amplification of the β-actin control, the template was diluted ten-fold and the cycle number was 33. To identify the amplified products, mammary PCRs were pooled and cloned into pGEM-T (Promega). Ten clones were analysed by DNA sequencing. Actin was used to assess the loading and quality of RNA. Cx-specific and degenerate primers were designed based on published sequences:
| Sense | Anti-sense |
|---|---|---|
Universal | ggctgtravaaygtctgctaygac | tgggvckggavabgaagcagt |
Cx26 | gaatgtatgctacgaccacca | ctttcctgagcaatacctaacg |
Cx30 | gggtaccacctaccctgggtac | tgcattctggccactatctgag |
Cx32 | aatgctacggcttgaggggcatg | gcctgctcaccggcataggag |
Cx43 | gttcagcctgagtgcggtctac | ggctctgctggaaggtcgctgatc |
β-casein | gtggcccttgctcttgcaag | agtctgaggaaaagcctgaac |
GAPDH | acgaccccttcattgacctc | ctttccagaggggccatccac |
β-actin | cgcctgcgcctggtcgtcgaca; | gtcacgcaccgatttcccgct |
Total cellular RNA was extracted from cultured cells (SCp2, SCg6 cells) on day 5 of culture by using the RNeasy minikit. mRNA was reverse-transcribed into cDNA by using the reverse transcription/PCR (RT-PCR) kit, Reddy Mix Version (Abgene, Promega). The PCR conditions were 1 cycle at 47°C for 30 min, 1 cycle at 94°C for 2 min, 1 cycle at 94°C for 20 s, 40 cycles of 30 s each at 50–65°C for annealing, followed by 1 min at 72°C for extension and a final cycle of 5 min at 72°C for final extension. In each experiment, a control sample of the kit, a sample with water as template and a sample without the RT mix were included. All samples were analysed by gel electrophoresis (2% agarose, 0.5 μg/ml ethidium bromide in 100 ml 1× TBE; 1× TBE = 0.09 M TRIS-borate. 0.002 M EDTA, pH 8.3).
RNA extraction and Northern blot analysis
Total cellular RNA was extracted from the mammary glands according to Chomczynski and Sacchi (1987). RNA (20 μg) was subjected to electrophoresis through 1% agarose/formaldehyde gel, blotted overnight onto Amersham Hybond-N membrane in 10× SSC (1× SSC = 150 mM NaCl, 15 mM sodium citrate, pH 7.0) and UV-crosslinked for subsequent hybridization. Cx, β-casein and WAP c-DNA were 32PdCTP-labelled by using the Rediprime kit (Amersham) and hybridization was performed overnight at 42°C in a shaker-incubator. The blots were then washed at high stringency (0.1% SDS, 68°C) and signals were detected by autoradiography.
Protein extraction and Western blot analysis
Proteins from cellular lysates of mammary gland homogenates were mixed with 2× sample buffer at a 1:1 ratio (v/v). Samples were resolved on a 12% polyacrylamide gel and transferred to Immobilin-P PVDF membranes in transfer buffer (40 mM glycine, 50 mM TRIS base, 0.04% SDS, 20% methanol). Membranes were blocked for 1 h in a wash buffer (Dulbecco’s PBS, 0.1% Tween 20) with 3% skimmed milk and incubated for 2 h at room temperature with the corresponding polyclonal rabbit anti-Cx antibody. The blots were then washed three times. Bound antibody was detected by addition of horseradish-peroxidase-conjugated anti-rabbit IgG followed by TMB for Cx26 immunoblots or ECL detection for Cx32 and Cx43 immunoblots. All washes and incubations were performed at room temperature.
Immunoprecipitation
For Cx30 Western blots, extracted proteins were immunoprecipitated with Cx30 antibody and processed by using the Boehringer kit (Boehringer Mannheim, Mannheim, Germany).
Immunostaining
Indirect immunostaining
Mammary gland cryo-sections (10-μm sections on gelatin-coated coverslips) were blocked with 3% normal goat serum for 1 h at room temperature, followed by a 2-h incubation at room temperature with rabbit anti-Cx26, -Cx30, -Cx32 or -Cx43. The concentrations of the antibodies were as recommended by the supplier. Sections were then reacted with goat anti-rabbit IgG (H + L) conjugated to FITC, for 1 h, followed by propidium iodide at 5 μg/ml as a nuclear stain. Sections were mounted in AntiFade. Cx26 and Cx32 immunolocalization in the liver was used as a positive control. For Cx26, a mammary glad at pregnancy day 10 (P10) and, for Cx32, a mammary gland at lactation day 9 (L9) without primary antibody were used as negative controls. Cx43 immunolocalization in the heart was used as a positive control. For Cx43, an L9 mammary gland without primary antibody was used as a negative control. Cx30 immunolocalization in the brain was used as a positive control. For Cx30, a mammary gland at involution day 2 (I2) without primary antibody was used as a negative control. Samples were then observed by fluorescence microscopy (Zeiss LSM 410).
Direct immunostaining
Sections of fixed paraffin-embedded mammary gland were deparaffinized and rehydrated in an ethanol gradient, washed and blocked as previously described and then incubated with FITC-conjugated anti-smooth muscle α-actin. Nuclei were counterstained with propidium iodide and mounted with Antifade.
Cell culture
A low-passage-number mouse CID-9 cell strain (obtained from Mina Bissell Lawrence Berkeley National Laboratory, Berkeley, CA) was grown in “growth medium” (all constituents from Sigma) consisting of Dulbecco’s modified Eagle’s medium nutrient mixture F12 Ham (DMEM/F12) with 5% fetal bovine serum (FBS), insulin (5 μg/ml), and gentamycin (50 μg/ml) and then switched to “differentiation medium” (all constituents from Sigma) consisting of DMEM/F12 containing insulin (5 μg/ml), hydrocortisone (1 μg/ml) and supplemented with ovine prolactin (3 μg/ml) as described by El-Sabban et al. (2003b) and Desprez et al. (1993). HC11 mouse mammary epithelial cells (obtained from Nancy Hynes, Friedrich Miescher Institute, Basel, Switzerland) were cultured in complete medium (CM) consisting of RPMI/10% FBS/insulin (5 μg/ml)/epithelial growth factor (10 ng/ml). For prolactin induction, cells in 10-cm dishes were maintained at confluency for 4 days in CM and then primed by incubation in RPMI/2%FBS/insulin for 2 days before induction with ovine prolactin (5 μg/ml) and dexamethasone (1 μg/ml). During this time, dark grey alveolar structures developed that had been shown to secrete β-casein. RNA samples were harvested immediately before induction with prolactin and at 2 and 4 days thereafter. The medium in other dishes of cells treated with prolactin and dexamethasone was replaced on day 4 with RPMI without supplements and cells were harvested after a further 2 days.
Sub-clones of the mouse CID-9 cell strain, viz. epithelial (SCp2) and myoepithelial-like (SCg6) cells (provided by P.Y. Desprez, Geraldine Brush Cancer Research Institute, San Fransisco, Calif.), were grown in culture plates in “growth medium” (see above) in a humidified incubator (95% air, 5% CO2) at 37°C (Forma Scientific, Ohio). SCp2 were transferred, on the first day after plating, to either “differentiation medium” (see above) or “non-differentiating medium”, which was equivalent to “differentiation medium” lacking prolactin.
Culture substrata
Cells were plated on various substrata as indicated for each experiment. SCp2 and SCg6 were seeded at 5.0×105 cells/ml on tissue culture plastic. Growth-factor-reduced Matrigel (1.5% vol/vol in media; Collaborative Biomedical Products, Bedford, Mass.) was dripped onto cells plated on plastic dishes 24 h after plating (Streuli et al. 1995). Cells 24 h after plating were washed with PBS and “growth medium” was replaced with “differentiation medium”.
Results
Expression of multiple Cx transcripts in the developing mammary gland
Total RNA samples (1 μg) from virgin (V), pregnant (P), lactating (L) or involuting (I) mammary gland were screened under high stringency with universal primers known to amplify most known mouse Cxs (Urban et al. 1999). Under these conditions, a single band representing the β-Cxs was evident (Fig. 1A). Predominance of Cx26 and Cx30 expression over other Cxs tested (Cx43 and Cx32; data not shown) in the four major developmental stages of mammary glands was confirmed by use of Cx-specific primers (Fig. 1B). Since commercially available RNA samples are pooled from different animals presumably at different intervals within each developmental stage, we decided to determine accurately the specific turning points in the process of mammary cell differentiation in context of Cx expression.
Differential expression of Cxs during mammary development. A RT-PCR analysis with degenerate primers amplifying all known mouse Cxs was used to determine the differential pattern of Cx expression in virgin (V), pregnant (P), lactating (L) and involuting (I) mammary gland. Heart (H) and skin (S) cDNA were amplified as controls for mobility to reveal the various Cx transcripts (α, β, β′). Actin was amplified as an internal standard. PCR was limited to 33 cycles. B Predominance of Cx30 over other Cxs in developing mammary gland. RT-PCR analysis with Cx-specific primers for Cx26 and Cx30 to determine Cx-specific pattern of expression in V, P, L and I mammary glands. The cycle number was selected to be within the linear phase of PCR with respect to mammary cDNA template concentration
Cellular distribution and expression of Cx26, Cx32 and Cx43 during mammary gland development
The use of timed pregnancies to establish the exact gestation age enabled us to pool mammary tissues (3–5 animals) at specific time points of pregnancy. Nine distinct time points (representing V, P3, L3 and I2) were established to determine accurately the temporal expression of the known Cxs (Cx26, Cx32 and Cx43). Mammary tissue was removed and processed either for cellular localization, or for level of expression and regulation of gap junction proteins and other differentiation markers.
Indirect fluorescence immunohistochemistry was performed for Cx26 and Cx32 in mouse mammary glands at various stages of development: V, P10, P15, L0, L6 and I9 showed that both Cx26 and Cx32 were expressed in the mouse mammary gland at all stages of development (Fig. 2A). Labelling was intense throughout pregnancy and lactation and decreased after weaning (involution). The pattern of Cx26 and Cx32 distribution in the involuted gland (I9) was similar to that of mammary gland from virgin mouse (Fig. 2A). Immunolocalization of smooth muscle actin in L0 and L6 mammary glands (Fig. 3B) revealed the pattern of myoepithelial cell distribution. Comparative analysis of Cx26 and Cx32 stain distribution with that of smooth muscle actin suggested that the majority of Cx26 and Cx32 labelling was confined to the cell border of the luminal epithelial cell population and not to the myoepithelial cell population. Moreover, punctate labelling of Cx26 and Cx32 was evident in the ductular epithelium of V and P15 glands (Fig. 2A, see also Fig. 2A inset D in Cx32). Western blot analysis showed that Cx26 protein was undetectable in the virgin mammary gland, and up-regulated in pregnancy and during the lactating state (Fig. 2b). Cx26 protein was undetectable in the involuted mammary gland. Cx32 protein was detected during pregnancy and lactation. However up-regulation of Cx32 proteins was observed mainly during lactation (Fig. 2B).
Developmental regulation of Cx26 and Cx32 expression in the mammary gland. A Cx26 and Cx32 immunolocalization in frozen sections of mammary gland from V, P10, P15, L0, L6 and I9. Cx26 labelling was confined to the cell border of the luminal epithelial cell population (arrows in P10, P15, L0 and L6). Cx32 labelling was confined to the cell border of the luminal epithelial cell population (arrows in L6); note also the staining of the ductal epithelial cells (Insert D in V, arrowhead in P15). Bar 50 μm. B Western blot analysis of Cx26 (arrowhead) and Cx32 proteins at various stages of mammary development: V, P5, P10, P15, L0, L6, L9 and I9
Developmental regulation of Cx43 expression in the mammary gland. A Cx43 mRNA expression at various stages of mammary gland development: V, P10, P15, L0, L6, L9 and I9. Bottom panel Ethidium bromide staining of total RNA; 18 s and 28 s are indicated. B Cx43 immunolocalization in frozen sections of mammary gland from V, P10, P15, L0, L6 and I9). Cx43 was localized to the myoepithelial cell population (arrows in P10, L0 and L6). Immunolocalization of smooth muscle α-actin in L0, L6 and I9 mammary gland (arrows) revealed the pattern of myoepithelial cell distribution. Bar 50 μm. C Western blot analysis of Cx43 proteins at various stages of mammary development: V, P5, P10, P15, L0, L6, L9 and I9. P2, P1 and P0 indicate different phosphorylation states of Cx43
Northern blot analysis was used to assess the transcriptional regulation of Cx43. Levels of Cx43 mRNA were constant in the mammary gland of virgin animals to animals up to 10 days of pregnancy. However, Cx43 mRNA was down-regulated in mid-pregnancy (P15) and reached low levels during lactation. mRNA levels were restored to virgin-like levels by day 9 post-weaning (I9; Fig. 3A). Immunolocalization studies showed that Cx43 was detected during all stages of development (Fig. 3B). The levels of Cx43 immunoreactivity during alveolar development increased from pregnancy to lactation and declined in the involuting mammary gland to a level similar to that of a gland from virgin animals. Comparative analysis of smooth muscle α-actin distribution (Fig. 3B) and Cx43 immunolabelling suggested that Cx43 was localized to the myoepithelial-epithelial cell contact regions (Fig. 3B, see P10, L0 and L6, arrowheads). Finally, the pattern of Cx43 protein expression revealed that different Cx43 phosphorylation isoforms (P0, P1 or P2) were expressed at different stages of mammary gland development. In the virgin and pregnant mammary gland, the predominant Cx43 isoform was the P0 hypo-phosphorylated form. In the lactating gland, the P1 and P2 phosphorylated forms were evident and became the predominant isoforms. This pattern was reversed during involution (I9) and resembled the virgin stages (Fig. 3C).
The relative sensitivity and detection limits of the various tools used to analyse the expression Cxs naturally play a significant role in the elaboration of our conclusions. The possible differential interaction of antibodies with cryo-sections compared with their interaction with denatured proteins in Western blots may be responsible for the variable sensitivity observed. Immunohistochemistry is the best indicator of the expression of Cxs, since it provides a local and, to a lesser extent, a quasi-quantitative assessment of expression.
Cx30 is expressed and strictly regulated during mammary gland development
The regulation of mRNA expression of the novel Cx30 was evaluated by semi-quantitative RT-PCR analysis of mammary gland samples from all stages of mammary gland development (Fig. 4A). Cx30 transcripts were barely detectable in the virgin gland and increased with time, peaking at day 15 of pregnancy. Cx30 mRNA remained high into lactation, declined during involution (I2) and became undetectable by I9. This pattern was mirrored by Northern blot analysis (data not shown).
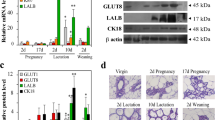
Developmental regulation of Cx30 expression in mammary gland. A RT-PCR of Cx30 mRNA expression at various stages of mammary gland development: V, P5, P10, P15, L0, L6, L9, I2 and I9. Bottom panel RT-PCR for β-actin mRNA to demonstrate equal loading. B Cx30 immunolocalization in frozen sections of mammary gland from V, P10, P15, L0, L6 and I9. Cx30 labelling was punctate and localized to the epithelial cell population (arrows in P15 and L0). Bar 50 μm. C Western blot analysis of Cx30 proteins at various stages of mammary development: V, P5, P10, P15, L0, L6, L9 and I2. D Northern blot analysis of β-casein and WAP transcripts at various gestation times (P6–P18)
Immunolocalization studies of Cx30 showed a distinct pattern of expression from other studied Cxs. Cx30 immunoreactivity was not detected in mouse mammary gland (Fig. 4B) prior to P15. Maximum staining was noted at the onset of lactation L0 (Fig. 4B) and was localized to the epithelial cell population. Staining was absent in the involuting mammary gland.
The temporal expression of Cx30 protein was assessed by Western blot analysis. The expression of Cx30 protein was in agreement with the immunohistochemical staining described above. Cx30 protein levels increased at day 15 of pregnancy and peaked by L0. A decline in expression was evident by day 6 of lactation (Fig. 4C). The apparent restricted expression of Cx30 was coincidental with the onset of β-casein and WAP mRNA expression (Fig. 4D), both markers for differentiated mammary gland.
Induction of Cx30 expression by lactogenic hormones
The expression pattern of this newly described Cx (Cx30) in mammary gland revealed its potential important function in mammary development. We explored the expression of Cx30 in a mouse cell line known to undergo differentiation in vitro in response to lactogenic hormones. Confluent monolayers of HC11 cells were treated with lactogenic hormones for 2 and 4 days, after which time the hormones were withdrawn and incubation continued for 2 days. β-Casein was amplified as a control for the induction of differentiation and GAPDH as a control for cDNA amount. Cx30 levels 2 days post-induction increased significantly over untreated cells. The transcript levels were maintained until the withdrawal of lactogenic hormones (Fig. 5A). The pattern of β-casein expression paralleled that of Cx30 and was dependent on lactogenic hormones. In addition, the isolated epithelial sub-clone SCp2 and myoepithelial sub-clone SCg6 from CID-9 mouse mammary strain cells confirmed the restricted distribution of Cx30. RT-PCR and Western blotting showed that Cx30 transcripts (Fig. 5B) and proteins (Fig. 5C) were uniquely present in SCp2 cells. The induction of differentiation in these cells, as assessed by β-casein production, was mirrored by an up-regulation in Cx30 expression. In contrast, SCg6 cells did not express any Cx30.
Expression and regulation of Cx30 in mammary epithelial cells. A RT-PCR of Cx30 and β-casein mRNA at 0, 2 and 4 days after induction of differentiation by treatment with the lactogenic hormones prolactin (5 μg/ml) and dexamethasone (1 μg/ml). On day 4 after induction, prolactin and dexamethasone were withdrawn, and RNA was extracted on day 6 (wd). Cx30 was amplified over 33 cycles. For β-casein and GAPDH, the template was diluted ten-fold and amplification was carried out over 25 or 34 cycles, respectively. B Cx30, β-casein and β-actin mRNA expression by SCg6 mammary myoepithelial cells and SCp2 mammary epithelial cells. RT-PCR for Cx30 (37 cycles), β-casein and β-actin (35 cycles each) mRNA isolated from SCg6 mammary myoepithelial cells plated on plastic and SCp2 mammary epithelial cells plated on EHS-drip and supplemented with non-differentiation medium and differentiation medium for 5 days. C Cx30 protein expression by SCg6 and SCp2 mammary cells in culture. Western blots for Cx30 in SCg6 cells plated on plastic and SCp2 cells plated on EHS-drip and supplemented with non-differentiation medium for 5 days and differentiation medium for 5 days. GAPDH demonstrates equal loading
Discussion
During the development of the mammary gland, alveolar and duct-lining cells undergo a process of proliferation and differentiation that ultimately leads to milk synthesis and its secretion into the alveolar lumina. The function of gap junctions as modulators of mammary gland development, whether in vivo (Monaghan and Moss 1996; Pozzi et al. 1995; Perez-Armendariz et al. 1995; Locke et al. 2000) or in vitro (Lee et al. 1992; Tomasetto et al. 1993), is yet to be established. Pitelka et al. (1973) were the first to describe gap junctions between epithelial cell populations in all the developmental stages of the mouse mammary gland. Later, gap junctional complexes were also reported between adjacent myoepithelial cells (Strum et al. 1983). Subsequently, Berga (1984) reported functional gap junctions in the lobules of the lactating gland.
Previous studies of Cx expression in mouse mammary glands by using available antibodies or hybridization probes have reported the expression of Cx26, Cx32 and Cx43. The surge in the discovery of new members of the Cx family of proteins has prompted us to re-examine this question by employing universal primers for RT-PCR of mammary cDNA and then sequencing the products. In this study, we have taken a comprehensive approach to establish the differential pattern of expression, cellular distribution and modulation of the various Cxs present in the normal mouse mammary gland representing developmental time points from virgin, pregnant, lactating and involuting gland.
Our studies demonstrate that Cx26 and Cx32 expression is modulated during the various stages of mammary gland development. Both Cxs can be detected in the virgin gland at low levels, presumably in ductular cells, increasing throughout the progression of the mammary gland into pregnancy and peaking at lactation. In involution, Cx26 and Cx32 expression decreases to levels comparable with that of virgin gland. These data are consistent with reported immunostaining studies on the expression of Cx26 in the pregnant and lactating mammary gland (Monaghan et al. 1994; Pozzi et al. 1995; Locke et al. 2000). Cx26 seems to be the major Cx localized to the duct and luminal epithelium, whereas Cx43, Cx40 and Cx32 have not been detected at any point during mammary gland development (Monaghan et al. 1994). From the undetectable levels in the virgin mammary gland, increasing levels of Cx26 have been seen during progression of mammary gland development through pregnancy, with maximum labelling being reached during lactation. Our results thus suggest that junctions made primarily of Cx26 are physiologically important in the synchronous secretion of milk. The importance of Cx26 is verified in knock out animals where its deficiency is embryonic-lethal. However, its role in normal physiology and development has been highlighted by the work of Cohen-Salmon et al. (2002) who have shown that the targeted ablation of Cx26 causes hearing impairment and cell death. The undetectable levels of Cx26 and others (i.e. Cx43, Cx40, Cx32) in virgin mice contrasts with reports showing that gap junctions are present in the mouse mammary gland at all stages of development (Pitelka et al. 1973). Pozzi et al. (1995) and Perez-Armendariz et al. (1995) have identified not only Cx26, but also Cx32 and Cx43 in the rodent mammary gland. However, their data describing Cx26 and Cx32 are contradictory. On one hand, the first study reports Cx26 reactivity only in the lactating state of both mouse and rat mammary gland, although Cx26 mRNA is present in the virgin mouse. Cx32 follows the same pattern of expression, whereby it has only been detected during lactation in the luminal epithelium of the mammary glands of BALB/c mouse and Sprague Dawley rat (Pozzi et al. 1995). On the other hand, Perez-Armendariz et al. (1995) have detected Cx26 and Cx32 transcripts and proteins in the virgin gland and during all stages of development of the mammary gland of mice and rats. It is possible that these differences in Cx expression are species-specific. Studies performed by Locke et al. (2000) have also identified Cx26 and Cx32 expression in the mammary gland; with regard to Cx26, protein and mRNA levels increase throughout pregnancy and peak during lactation (Locke et al. 2000). Our Northern and Western blotting and immunocytochemistry results indicate that Cx32 distribution is restricted to the lactating mammary gland, as previously reported by Pozzi et al. (1995). However, our finding of Cx32 expression in the virgin mammary gland is inconsistent with previous studies by Pozzi et al. (1995) and Monaghan et al. (1994) who have not detected Cx32 in the virgin gland or at any stage of mammary gland development. Locke et al. (2000) have detected Cx32 mRNA only in the lactating, but not in the virgin, pregnant or involuting gland. Similar results have been shown previously by Perez-Armendariz et al. (1995) who have detected Cx32 at all stages of development of the mammary gland of CD1 mice and Wistar rats. The continued expression of Cx32 and its localization to epithelial cells, during all stages of development of the mammary gland suggest that these Cxs play a role in maintaining basal GJIC in the mammary gland and specifically in the epithelial cell population. The up-regulation of Cx26 and Cx32 in pregnancy and lactation in the luminal cells suggests that they have a cooperative role in the induction of milk secretion (Locke et al. 2000). Evidence for functional heterotypic gap junctions formed from Cx26 and Cx32 has been provided by studies of Xenopus oocytes by Barrio et al. (1991) and in hepatocytes by Stauffer (1995). More recently, Locke et al. (2000) have demonstrated that Cx26 and Cx32 form both homomeric and heteromeric connexons in the epithelial cells of mouse mammary gland, suggesting that the acquisition of a secretory phenotye requires a general increase in the number and size of gap junctions and possibly in changes in the existing association between Cxs. Moreover, the work of Cohen-Salmon et al. (2002) and Teubner et al. (2003) has demonstrated that Cx26 and Cx30 colocalize in the inner ear.
Cx43 proteins are expressed at all the developmental stages of the mammary gland. However, Western blot analysis has revealed that the active phosphorylated form of Cx43 proteins is specifically up-regulated in lactation. Moreover, immunolocalization studies have shown that Cx43, which is localized to myoepithelial cells, can be detected in the virgin mouse mammary gland at low levels. Staining levels increase with the progression of the mammary gland from pregnancy to lactation and then decline in involution. These results are consistent with those of Pozzi et al. (1995) and Perez-Armendariz et al. (1995). Interestingly, no Cx43 have been detected in the mouse mammary gland in studies by Monaghan et al. (1994). Cx43 appears to be the major Cx in the gap junctions of myocytes (Green and Serves 1993) and the myometrium (Risek et al. 1990), which further emphasizes that cells with contractile activity communicate via gap junctions that are mainly composed of Cx43. The function of Cx43 in the non-lactating gland is more complex, especially as the fate of the myoepithelial cells in the virgin and involuting mammary gland is still not well established. Localization of Cx43 to the myoepithelial cell population suggests that gap junctions composed of Cx43 may exist either among the myoepithelial cells or between myoepithelial cells and secretory epithelial cells. Heterocellular communication between epithelial and myoepithelial cells has only recently been explored. A study by Radice et al. (1997) indicates that myoepithelial and epithelial cells are able to communicate. Interestingly, Cx43 mRNA expression decreases, whereas the active phosphorylated form of the protein increases in the fully differentiated stage of the mammary gland in lactation. This suggests that the regulation of Cx43 is at the post-translational level. Rosenberg et al. (1996) have reported that, as hepatic cells differentiate, Cx26 and Cx43 mRNA levels decrease whereas that of Cx32 increases, suggesting that Cx gene expression can be used as a marker for hepatic cell differentiation. A similar mechanism for Cx26 and Cx43 expression can be proposed for the mammary gland. Yamanaka et al. (1997) have reported increased phosphorylation of Cx43 in myoepithelial cells in contrast to the down-regulation of Cx43 mRNA levels at the onset of lactation. This is in agreement with studies from our laboratory that have demonstrated that CID-9 cells cultured on Matrigel-matrix under optimal differentiation conditions down-regulate their Cx43 mRNA expression and increase Cx43 phosphorylation (El-Sabban et al. 2003b).
Sequence analysis of amplification products from RT-PCR of mammary mRNA, employing Cx universal primers, has revealed multiple clones encoding Cx30, previously reported only in brain and skin (Lautermann et al. 1998; Nagy et al. 1999). This is the first report of Cx30 expression in the mouse mammary gland. The expression of Cx30 follows a different pattern than that of Cx26, Cx32 and Cx43. Indeed, as detected by immunohistochemistry, the maximal expression of Cx30 has been noted in the last trimester of pregnancy and at the onset of lactation, suggesting that its expression may be associated with a specific developmental shift of the mammary gland. At this developmental stage, the transcription of the WAP is turned on during pregnancy and its expression is maintained into lactation (Aggeler et al. 1991). Thus, GJIC, via Cx30 junctional complexes either alone or with Cx26 heterotypic junctions (Ahmad et al. 2003), may induce the expression of milk proteins such as WAP. The correlation between Cx30 expression and differentiation is further supported by our in vitro studies in which the reversible induction of β-casein expression is concomitant with Cx30 expression. Correlation between Cx expression and tissue function has been derived from null mutation experiments. Cx30 knock out homozygous mutants (Cx30(−/−)) are fertile and have not been reported to exhibit any feeding or lactational abnormalities. However, such mice exhibit a severe constitutive hearing impairment (Teubner et al. 2003). Moreover, Cx30-deficient mice have recently been demonstrated to show increased emotionality and decreased rearing activity and neurochemical changes (Dere et al. 2003). The Cx30 protein had been previously detected in the brain, skin, lung, kidney and uterus by specific antibodies (Lautermann et al. 1998; Willecke et al. 2002; Nagy et al. 1999). However, no obvious histological abnormalities have been noted in these organs of Cx30-deficient mice. Cx30-deficient mice are currently being analysed in further detail, especially since Cx30 missense mutations have been reported to cause a skin disease in man, namely hidrotic ectodermal dysplasia (Clouston syndrome; Lamartine et al. 2000; Smith et al. 2002). Thus, in light of our findings, an analysis of mammary gland development and the lactational phenotype of Cx30(−/−) mice would be worthwhile.
Having established Cx30 expression in the mammary gland and as Cx30 exhibits 77% amino acid identity to Cx26, the role attributed to Cx26 in mammary gland development and its putative role as a tumour suppressor protein (class II; Lee et al. 1991) requires reconsideration, especially with the availability of discriminating Cx antibodies specific to Cx30 and Cx26.
In conclusion, the differential temporal and spatial expression of mammary Cxs may be important for the transduction of specific molecular signals influencing the onset and/or maintenance of the secretory phenotype of alveolar epithelial cells. Knowledge of the mechanisms underlying the modulation of Cx expression and regulation in the mammary gland is essential to our understanding of basic developmental processes.
References
Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ (1991) Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci 99:407–417
Ahmad S, Chen S, Sun J, Lin X (2003) Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun 307:362–368
Alford AI, Rannels DE (2001) Extracellular matrix fibronectin alters connexin43 expression by alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 280:L680–L688
Barrio LC, Suchyna T, Bargiello TA, Xian Hu L, Rognski R, Bennett MVL, Nicholson B (1991) Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci USA 88:8410–8414
Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC (1991) Gap junctions: new tools, new answers, new questions. Neuron 6:305–320
Berga SE (1984) Electrical potentials and cell-to-cell dye movement in mouse mammary gland during lactation. Am J Physiol 247:C20–C25
Bruzzone R, White TW, Paul DL (1996) Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 238:1–27
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C (2002) Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol 12:1106–1111
Dahl E, Manthey D, Chen Y, Schwarz H-J, Chang YS, Lalley PA, Nicholson BJ, Willecke K (1996) Molecular cloning and functional expression of connexin-30, a gap junction gene highly expressed in adult brain and skin. J Biol Chem 271:17903–17910
Dere E, De Souza-Silva MA, Frisch C, Teubner B, Sohl G, Willecke K, Huston JP (2003) Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur J Neurosci 18:629–638
Desprez PY, Roskelley C, Judith C, Bissell MJ (1993) Isolation of functional cell lines from a mouse mammary epithelial cell strain: the importance of basement membrane and cell–cell interaction. Mol Cell Differ 1:99–110
El-Sabban M, Abi-Mosleh L, Talhouk R (2003a) Developmental regulation of gap junctions and their role in mammary epithelial cell differentiation. Adhesion systems in the control of mammary gland morphogenesis and function. J Mammary Gland Biol Neoplasia 8:463–474
El-Sabban M, Sfeir A, Daher M, Kalaany N, Bassam R, Talhouk R (2003b) Gap junctional communication induces β-casein expression in mammary epithelial cells in the absence of cell/ECM interaction. J Cell Sci 116:3531–3541
Goodenough DA, Goliger JA, Paul DL (1996) Connexins, connexons, and intercellular communication. Annu Rev Biochem 65:475–502
Gramsch B, Gabriel HD, Wiemann M, Grummer R, Winterhager E, Bingmann D, Schirrmacher K (2001) Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res 264:397–407
Green CR, Serves NJ (1993) Distribution and role of gap junctions in normal myocardium and human ischaemic heart disease. Histochemistry 99:105–120
Hirschi KK, Xu CE, Tsukamoto T, Sager R (1996) Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ 7:861–870
Kidder GM, Mhawi AA (2002) Gap junctions and ovarian folliculogenesis. Reproduction 123:613–620
Kumar NM, Gilula N (1996) The gap junction communication channel. Cell 84:383–388
Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaitre G, Hand C, Hayflick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G (2000) Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet 26:142–144
Lautermann J, Ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E (1998) Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 294:415–420
Lee SW, Tomasetto C, Sager R (1991) Positive selection of candidate tumor suppressor genes by subtractive hybridization. Proc Natl Acad Sci USA 88:2825–2829
Lee SW, Tomasetto C, Paul D, Keyomarsi K, Sager R (1992) Transcriptional down regulation of gap junction proteins blocks junctional communication in human mammary tumor cell lines. J Cell Biol 118:1213–1221
Locke D (1998) Gap junctions in normal and neoplastic mammary gland. J Pathol 186:343–349
Locke D, Perusinghe N, Newman T, Jayatilake H, Evans WH, Monaghan P (2000) Developmental expression and assembly of connexins into homomeric and heteromeric gap junction hemichannels in the mouse mammary gland. J Cell Physiol 183:228–237
Monaghan P, Moss D (1996) Connexin expression and gap junctions in the mammary gland. Cell Biol Int 20:121–125
Monaghan P, Perusinghe N, Carlile G, Evans WH (1994) Rapid modulation of gap junction expression in mouse mammary gland during pregnancy, lactation, and involution. J Histochem Cytochem 42:931–938
Monaghan P, Clarke C, Perusinghe NP, Moss DW, Chen XY, Evans WH (1996) Gap junction distribution and connexin expression in human breast. Exp Cell Res 223:29–38
Nagy JI, Patel D, Ochalski PA, Stelmack GL (1999) Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88:447–468
Paul DL, Bruzzone R, Gimlich R, Goodenough D (1995) Expression of a dominant negative inhibitor of intercellular communication in early Xenopus embryo causes delamination and extrusion of cells. Development 121:371–381
Perez-Armendariz EM, Luna J, Aceves C, Tapia D (1995) Connexins 26, 32 and 43 are expressed in virgin, pregnant and lactating mammary gland. Dev Growth Differ 37:421–431
Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK (1973) Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol 56:797–818
Pitts JD, Finbow M, Kam E (1988) Junctional communication and cellular differentiation. Br J Cancer 58:52–57
Pozzi A, Risek B, Kiang DT, Gilula NB, Kumar NM (1995) Analysis of multiple gap junction gene products in the rodent and human mammary gland. Exp Cell Res 220:212–219
Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO (1997) Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol 139:1025–1032
Risek B, Guthrie S, Kumar N, Gilula NB (1990) Modulation of gap junction transcript and protein expression during pregnancy in the rat. J Cell Biol 110:269–282
Romanello M, Moro L, Pirulli D, Crovella S, D’Andrea P (2001) Effects of cAMP on intercellular coupling and osteoblast differentiation. Biochem Biophys Res Commun 282:1138–1144
Rosenberg E, Fans RA, Spray DC, Monfils B, Abreu S, Danishefsky I, Reid LM (1996) Correlation of expression of connexin rnRNA isoforms with degree of cellular differentiation. Cell Adhes Commun 4:223–235
Schiller PC, D’Ippolito G, Balkan W, Roos BA, Howard GA (2001) Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone 28:362–369
Sia MA, Woodward TL, Turner JD, Laird DW (1999) Quiescent mammary epithelial cells have reduced connexin43 but maintain a high level of gap junction intercellular communication. Dev Genet 24:111–122
Smith FJ, Morley SM, McLean WH (2002) A novel connexin 30 mutation in Clouston syndrome. J Invest Dermatol 118:530–532
Sohl G, Willecke K (2003) An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes 10:173–180
Stauffer KA (1995) The gap junction proteins beta(1)-connexin (connexin32) and beta(2)-connexin (connexin 26) can form heteromeric hemichannels. J Biol Chem 270:6768–6772
Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ (1995) Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol 129:591–603
Strum JM, Phelps PC, McAtee MM (1983) Resting human female breast tissue produces iodinated proteins. J Ultrastruct Res 84:130–139
Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K (2003) Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet 12:13–21
Tomasetto C, Neveu MJ, Daley J, Horan PK, Sager R (1993) Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol 122:157–167
Urban M, Rozental R, Spray DC (1999) A simple RT-PCR-based strategy for screening connexin identity. Braz J Med Biol Res 32:1029–1037
White TW, Paul DL (1999) Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol 61:283–310
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 283:725–737
Wiszniewski L, Limat A, Saurat J, Meda P, Salomon D (2000) Differential expression of connexins during stratification of human keratinocytes. Invest Dermatol 115:278–285
Yamanaka I, Kuraoka A, Inai T, Jshibashi T, Shibata Y (1997) Changes in the phosphorylation states of connexin43 in myoepithelial cells of lactating rat mammary glands. Eur J Cell Biol 72:166–173
Yamanaka I, Kuraoka A, Inai T, Ishibashi T, Shibata Y (2001) Differential expression of major gap junction proteins, connexins 26 and 32, in rat mammary glands during pregnancy and lactation. Histochem Cell Biol 115:277–284
Acknowledgements
The authors are grateful to Dr. Fadia Homeidan for critical reading of the manuscript and to Mr. Wissam Mehio for assisting in its preparation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the University Research Board and Lebanese National Council for Scientific Research (R.S.T. and M.E.S.), the Medical Practice Plan, Diana Tamari Sabbagh Research Fund, Terry Fox Cancer Research fund (M.E.S.), Third world Academy of Science (R.S.T.) and United States Army Breast Cancer Research Fund grant DAMD17-00-1-0219 (R.C.E.)
R.S. Talhouk and R.C. Elble contributed equally to this article
Rights and permissions
About this article
Cite this article
Talhouk, R.S., Elble, R.C., Bassam, R. et al. Developmental expression patterns and regulation of connexins in the mouse mammary gland: expression of connexin30 in lactogenesis. Cell Tissue Res 319, 49–59 (2005). https://doi.org/10.1007/s00441-004-0915-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-0915-5