Abstract
Exposure to estrogenic substances during a time window, the so-called “critical period,” in perinatal life causes an irregular development of the genital tract that leads to ovary-independent proliferation and cornification in the vaginal epithelium in mice. We have previously demonstrated that retinol inhibits the irreversible effects of estrogen on the vagina. Here, mice kept in a vitamin-A-deficient condition during perinatal life were shown to be more sensitive to the harmful effects of estrogen. In addition, expression of mRNA for retinol binding protein type 2 (CRBP2), a “small intestine-specific” cytosolic protein that captures intracellular retinal and retinol, was detected in the vaginal epithelium. Induction of increased expression of CRBP2 mRNA by estrogen was also evident in the uterus and epididymis. Both estradiol-17β and diethylstilbestrol markedly increased the tissue content of CRBP2 mRNA in the vagina and uterus during the neonatal “critical period” but not after 15 days of age. These results taken together imply that estrogen disrupts the local vitamin A balance by an induction of CRBP2 gene expression in the epithelium in the developing mouse genital tract, and that retinoid imbalance may contribute to the genesis of irreversible effects of estrogen on the vagina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure of animals to estrogenic substances during their perinatal life causes endocrine disruption that leads to impaired development of the genital tract, infertility and cancer (Takasugi et al. 1962; Takasugi 1979; McLachlan 1979; Bern and Talamantes 1981; Mori 1986; Colborn et al. 1993; Iguchi et al. 1995; Cunha et al. 2001). Human health is also affected by estrogenic endocrine disrupters, and an exposure to a synthetic estrogen, diethylstilbestrol (DES), in utero results in a high incidence of abnormalities of the reproductive organs such as adenocarcinoma in the vagina and prostatic hyperplasia (Herbst et al. 1971; Golden et al. 1998; Santti et al. 1999). Studies of endocrine disruption by exogenous estrogens were carried out firstly by Takasugi et al. in the 1960s (Takasugi et al. 1962; Takasugi 1963; Takasugi and Bern 1964), and demonstrated that both ovary-dependent and -independent stratification and cornification of the vaginal epithelium were observed in adult mice treated neonatally with estrogen. Normally, in response to estrogen secreted periodically from the ovary, vaginal epithelial cells proliferate to constitute stratified epithelium, which consists of several layers of epithelial cells with a keratinized cell layer on the luminal side. In the absence of the ovary, the vaginal epithelium stays in a degenerated state with one to three layers of epithelial cells. In mice that are exposed to estrogen during the neonatal period, the vaginal epithelium proliferates to form a tissue architecture very similar to that observed in the estrogen-stimulated vagina even after ovariectomy at adulthood. The ovary-independent persistent vaginal changes are irreversible and are caused by a direct action of estrogen on the developing genital tract, while the ovary-dependent vaginal changes are reversible and are caused by the disturbed estrogen secretion that is ascribable mostly to an estrogen effect on the developing brain (Dalterio et al. 1985). We have previously demonstrated that the occurrence of the former type of irreversible vaginal change is blocked by treatment of neonatal mice with vitamin A given simultaneously with estrogen (Mori 1968, 1969). The molecular mechanism by which vitamin A inhibits the toxic effects of estrogens, however, is not yet well understood. Vitamin A does not affect the temporary estrogen-induced proliferation and cornification in the vagina in either neonatal or adult mice, as long as the dose of vitamin A is not extraordinarily high.
Vaginal expression of estrogen receptors does not appear to be affected by vitamin A in estrogen-treated neonatal mice. Although vitamin A given simultaneously with estrogen in neonatal mice inhibits the downregulation of estrogen receptors (ER) in the adult mouse vagina induced by neonatal estrogen treatment, these changes in ER expression seem to be the result of estrogen-independent vaginal changes rather than the cause of them (Masui et al. 2001). The accumulation of negative data concerning the inhibitory effects of vitamin A on the temporary estrogen signaling has led to the idea that estrogens may disturb vitamin A signaling in the genital tract in neonatal mice.
Vitamin A plays essential roles in the physiology of vertebrates not only as a component of the cell membrane but also as an important signal-mediating factor involved in cell growth and differentiation, embryonic development, and vision (Conlon 1995; Evans and Kaye 1999). The transport and distribution of vitamin A is mediated by a group of specific binding proteins. These include retinol binding protein, which transports retinol from the liver to various target cells via the circulation; cellular retinol binding proteins (CRBP) and cellular retinoic acid binding proteins (CRABP), both of which contribute not only to prevention of the absorption of vitamin A into the cell membrane but also to specific delivery of vitamin A within the cell by affecting the availability of retinoids to retinoid-metabolizing enzymes; and several tissue-specific vitamin A binding proteins in the visual tissue and epididymis (Noy 2000). Cellular retinol binding protein type 2 (CRBP2) is a small monomeric cytoplasmic protein of approximately 16 kDa that is expressed exclusively in the absorptive epithelial cells in the small intestine in both rats and humans, except that the liver and kidney in fetus express rather lower levels of the gene (Schaefer et al. 1989; Crow and Ong 1985; Folli et al. 2001).
Here we report on the effect of estrogens on the expression of vitamin-A-interacting proteins examined in the female genital tract in neonatal mice, especially on the estrogen-induced expression of CRBP2 mRNA in the developing vagina and uterus.
Materials and methods
Animals and hormone treatments
Congenic SHN strain mice derived from Swiss albino mice (Nagasawa et al. 1976) were maintained under light- and temperature-controlled conditions as described elsewhere (Matsuda et al. 1994). The mice were fed a normal or low vitamin A diet (Clea Japan Inc., Tokyo, Japan). The low vitamin A condition was produced in newborn mice by feeding the mothers with the low vitamin A diet from 3–4 days before gestation until postnatal day 6. All procedures performed on the mice were described in detail in a protocol that was approved by the Animal Care and Use Committee of the Graduate School of Science, University of Tokyo.
Estradiol-17β (E2) and DES were dissolved in a minimal volume of ethanol to make concentrated stocks. E2 and DES were diluted in sesame oil either at various concentrations (0.0005–5) or at 0.01 µg/µl, respectively. The initial vehicle was removed by evaporation in a vacuum chamber. Vitamin A acetate (retinol acetate; RolA) was dissolved in a minimal amount of dimethylsulfoxide and finally diluted in sesame oil at 5 IU/µl. Mice were given a subcutaneous injection of estrogen and/or vitamin A in approximately 20 µl of sesame oil daily. All the chemicals were purchased from Sigma-Aldrich Japan K.K. (Tokyo, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR)
Immediately after decapitation and pithing of mice, the genital tissues were excised and frozen in a plastic tube in liquid nitrogen. The vaginal tissue sample contained the cervix, and the uterine sample mainly consisted of the uterine horn. The tissues from three individual mice were pooled and treated as one sample. Genomic DNA-free total RNA was extracted from the frozen tissue, and then RT-PCR was performed basically as described previously (Matsuda and Mori 1997). Briefly, the first-strand cDNA was made by using Superscript II reverse transcriptase (Invitrogen K.K., Tokyo, Japan) with random DNA hexamers (Takara Shuzo K.K., Tokyo, Japan) as primer, and the cDNA was amplified by PCR with Hot-start ExTaq DNA polymerase (Takara) and a set of specific PCR primers. The sequence information of the PCR primers and the conditions of PCR are listed in Table 1. The first-strand cDNA obtained from 80 ng of total RNA was subjected to PCR in a 24-µl volume. PCR was stopped before it reached a plateau. The products were subjected to electrophoresis on a 1.5% agarose gel in TAE buffer and stained with ethidium bromide. The fluorescence of DNA-bound ethidium bromide was visualized under 254 nm UV light with an image analyzer (BioImage, Nihon Bioimage Ltd., Tokyo, Japan). The intensity of the fluorescence was finally analyzed with NIH image software (National Institutes of Health, USA).
“Virtual” Northern blotting
“Virtual” Northern blotting is an alternative method to conventional Northern blotting, in which full-length cDNA is used instead of RNA (Franz et al. 1999), and it is useful especially when the amount of tissue sample is as small as that in the case of the developing genital tract. The procedure was basically the same as previously described (Imaoka et al. 2002). The alkaline phosphatase-labeled DNA probes for CRBP1, CRBP2, cytokeratin 18 (K18), lecithin:retinol acyltransferase (LRAT), β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were made from a plasmid insert that contained an RT-PCR product of each gene. The PCR primer sequences are listed in Table 1. The length of exposure time to chemiluminescence film was 1 h for RBPs and LRAT, and 10 min for K18, β-actin and GAPDH.
Histology and in situ hybridization
Formalin-fixed paraffin sections of tissues were made and stained with Mayer’s hematoxylin and eosin for the ordinary histological examination. In situ detection of CRBP2 mRNA was performed on sections of frozen tissue with the use of a 35S-labeled complementary RNA probe, basically following a protocol made for the detection of prolactin receptor mRNA in paraffin-embedded uterine tissue (Yamashita et al. 1999). Briefly, tissue was frozen in liquid nitrogen and cut with a cryostat at 10 µm thickness. The sections were transferred onto a silane-coated slide glass, fixed in 4% paraformaldehyde-containing phosphate-buffered saline (PBS), pH 7.4, at 25°C for 20 min, and then treated with 0.2 N HCl and 0.3% Triton X100-containing PBS for 15 min each. After equilibration with 50% formamide (FA), 4 × SSC buffer, the section was reacted with 5×104 cpm/µl of 35S-labeled cRNA probe in a hybridization buffer for 16 h at 48°C. The probe was made with T7 RNA polymerase (Takara) from the template DNA, which was amplified by PCR with Pfu DNA polymerase (Promega K.K., Tokyo, Japan) and an M13 primer set (see Table 1) from pGEM T-Easy plasmid (Promega) that contained 398 bp CRBP2 PCR product described above as the insert. After hybridization, excess probe was removed from the tissue section by successive washing steps: 5 mM dithiothreitol (DTT), 2 × SSC at 50°C, twice; 50% FA, 5 mM DTT, 2 × SSC at 50°C, twice; 10 µg/ml of ribonuclease A, 2 × SSC at 37°C; 1 × SSC at 42°C, twice; 0.5 × SSC at 42°C, twice, each for 20 min. The sections were dried and covered with a microautoradiography emulsion (NR-2; Konica Co., Tokyo, Japan). They were developed according to the manufacturer’s instructions and counterstained with hematoxylin. The silver grains were observed with a dark field condenser and a light microscope (BH2; Olympus Optical Co., Ltd., Tokyo, Japan).
Results
Effect of vitamin A treatment and vitamin A deficiency on the incidence of neonatal estrogen-induced ovary-independent proliferation of the vaginal epithelium
Neonatal mice were injected with 0.01–100 µg of E2 daily for 5 days, and the vaginal tissue was histologically examined at adulthood (45-day-olds) 10 days after ovariectomy. In the non-estrogenized control mice, the vaginal epithelium stayed in a degenerated state with one to three layers of the epithelial cells. High-dose (10 and 100 µg, daily) E2 exposure during the neonatal period led the vagina to having a thick epithelium that consisted of several layers of stratified epithelial cells and keratinized cells even without hormonal stimulation from the ovary at adulthood, while low-dose (0.01, 0.1 and 1 µg, daily) E2 did not induce such an irreversible change in the vagina (Fig. 1C). In the group of mice neonatally treated with retinol acetate together with E2, on the other hand, the ovary-independent hypertrophy of the vaginal epithelium was not observed regardless of the dose of E2. These results are in agreement with our previous observation (Mori 1968, 1969; Masui et al. 2001). In contrast, vitamin-A-deficient (VAD) mice produced by feeding their mothers a VAD diet were more sensitive to neonatal E2 than those whose mothers were fed a normal diet. A daily injection of 1 µg of E2 induced ovary-independent hypertrophy of the vaginal epithelium in some mice kept under low vitamin A conditions during perinatal life but never in the mice kept in normal or high vitamin A conditions. Thus, the estrogen action that leads to irreversible change in the developing vagina was downregulated by vitamin A and upregulated by depletion of vitamin A.
Neonatal estrogenization-induced ovary-independent hyperplasia in the vaginal epithelium, and modification of the estrogen effect by vitamin A level (A). One to three layers of epithelial cells were observed in the vagina in an ovariectomized adult mouse without neonatal hormone treatments. B Hyperplastic epithelium was observed in ovariectomized adult mice neonatally treated with E2 (10 µg, daily for 5 days). C Incidence of ovary-independent hyperplasia of the vaginal epithelium in the neonatally E2-treated mice kept in low (column 1), normal (column 2) and high (column 3) vitamin A conditions during the neonatal period. The low vitamin A condition was made by feeding the mothers with a vitamin-A-deficient diet, and the high vitamin A condition by injections of retinol acetate to the pups. Solid and clear areas in the columns indicate the percentage of mice with and without hyperplastic vaginal epithelium, respectively. Notice that the high vitamin A conditions completely inhibited the induction of ovary-independent hyperplasia of the vaginal epithelium even with a neonatal exposure to high dose of E2, while daily injections with as low as 1 µg of E2 were effective in inducing the irreversible vaginal changes in some of the mice kept under the low vitamin A conditions (asterisk a significant difference in the incidence was observed when compared to that of the normal group of mice treated with the same dose of E2, as shown by the χ2-test (p<0.05). Bar 50 µm
Induction of CRBP2 mRNA expression by E2 in the genital tracts of neonatal mice
The expression levels of some retinoid binding protein mRNAs were examined by RT-PCR in the vagina (including the uterine cervix), uterine horn and epididymis in neonatal mice treated with 20 µg/day of E2 or the vehicle only for 5 days after birth. Twenty micrograms per day of E2 was employed since the dose effectively leads to developmental estrogenization syndrome of the vagina in almost all SHN mice (Mori 1968, 1969; Masui et al. 2001), while 10 µg/day was not effective in all cases as shown in Fig. 1C. The genital tissues were excised 24 h after the last injection. Of the several gene mRNAs examined, CRBP2 mRNA was markedly more abundant in the hormone-treated genital tracts than in the tracts from the vehicle-treated controls (Fig. 2A). On the other hand, the expression levels of the other gene products, including CRBP1 mRNA, were not remarkably influenced by the E2 treatment, except that signals from RXRβ mRNA appeared weaker in the estrogenized genital tracts and that from RARγ more intense in the estrogenized uterus. The other organs, including gonads and liver, generated no detectable PCR products for CRBP2 under the present RT-PCR conditions (not all data shown). Figure 2B shows the relative amount of CRBP2 mRNA estimated from the fluorescence intensity of the PCR products in an ethidium-bromide-stained agarose gel. The values were standardized by the amounts of β-actin product. The hormone-nonstimulated developing genital tracts expressed only a trace level of CRBP2 mRNA, and E2 significantly increased the expression of the gene product such that an increase in fluorescence intensity of more than tenfold was observed in the Müllerian ducts and an increase of approximately fourfold in the epididymis.
Effect of E2 on the expression of mRNA for retinoid-binding proteins in the genital tracts in neonatal mice (A). Duplicate pooled RNA samples of the vagina (Vg), uterus (Ut), epididymis (Epd) and testis (Tes) were subjected to RT-PCR to examine gene expression changes induced by E2. Mice were injected daily with E2 or the vehicle (oil) for 5 days, and sacrificed at day 6 after birth. CRBP2 gene expression appeared to be induced by E2 in the genital tract, while no visible PCR products were obtained from the testis. B The expression level of CRBP2 mRNA was estimated from the fluorescence intensity of RT-PCR products after normalization with the β-actin level in the genital tract. Clear and solid columns and vertical bars indicate the means ± standard errors of the relative CRBP2 mRNA level from four to five pooled RNA samples in oil-treated control and E2-treated mice, respectively. The genital tract in neonatal mice expressed a low to moderate level of basal CRBP2 mRNA in the oil-treated control group, and the expression was significantly increased by the E2 treatment (*p<0.05 by Student’s t-test)
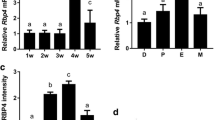
The amount of CRBP2 mRNA was also estimated by RT-PCR further in the uterine cervix-containing vagina and uterine horn at 24 h after a single injection of E2 (10 µg/1 g of body weight) or the vehicle (Fig. 3A–C). In the vehicle-treated control vagina and uterus, the tissue content of CRBP2 mRNA gradually increased after birth until it reached a plateau in about 10 days. The plateau was not caused by an artifact made during PCR, such as exhaustion of substrate, primers or polymerase (data not shown). Estradiol markedly increased the Müllerian CRBP2 mRNA level. Interestingly, the stimulatory effect of E2 on the CRBP2 gene expression was observed only during several days after birth, the so-called “critical period” in which the vagina has the potential to enter an ovary-independent proliferative state in response to exogenous estrogen. The time “windows” when the tissue was sensitive to the estrogen effect on CRBP2 expression seemed to be different between the vagina and uterus. Concerning the stimulatory effect of estrogen on CRBP2 expression, the uterus was less sensitive to estrogen than the vagina just after birth, while it was still responsible at day 10 when the vagina had become almost insensitive to the hormone.
“Critical period” specificity and dose dependency of the induction of CRBP2 mRNA expression by E2 in the mouse Müllerian duct (A–C). The expression level of CRBP2 mRNA relative to β-actin mRNA was examined by RT-PCR in the vagina and uterus at 24 h after a single injection of E2 (solid circles) or oil (clear circles) at various days after birth. A A representative image of RT-PCR products for CRBP2 and β-actin in the vagina (Vg) and uterus (Ut) in the mice of various ages. B, C Relative fluorescence intensity of CRBP2 cDNA (arbitrary units) in the vagina (B) and uterus (C). Solid and open circles indicate the means of data from four to six pooled RNA samples in E2- and oil-treated mice, respectively, and the vertical bars indicate the standard errors of means. The Müllerian tissue content of CRBP2 mRNA gradually increased as the pups grew in the oil-treated normal group, and was increased by E2 during several postnatal days, the period known as the “critical period” (asterisk there was a significant difference in the CRBP2 expression level between the E2-treated group and the age-matched oil-treated control by Student’s t-test; p<0.05). D Effect of DES and various doses of E2 on the Müllerian expression of CRBP2 mRNA in neonatal mice. The expression levels of CRBP2 and β-actin mRNAs were examined by RT-PCR in the vagina and uterus at 24 h after a single injection of 0.01–100 µg of E2 (E0.01–E100), 1 µg of DES, or oil (Cont) at the age of 3 days. The panels show representative images of RT-PCR products. E2 induced CRBP2 mRNA expression dose dependently in the vagina and uterus. DES also induced Müllerian CRBP2 expression, and was effective at a lower dose than E2
The effect of a single injection of DES and various doses of E2 on the Müllerian expression of CRBP2 mRNA was examined in the cervico-vagina and uterine horn at the age of 3 days (Fig. 3D). E2 induced CRBP2 mRNA expression at 24 h after the hormone treatment in a dose-dependent manner. A low dose of E2 (0.01–0.1 µg) did not alter the gene expression, 1 and 10 µg of E2 moderately to strongly increased the expression, and 100 µg of E2 induced the most intense expression of CRBP2 gene in the Müllerian duct. A synthetic non-steroidal estrogen, DES (0.1 µg), was also a potent inducer of CRBP2 gene in the developing genital duct.
Distribution of CRBP2 mRNA in the genital tract
In situ hybridization was performed to determine the tissue distribution of CRBP2 mRNA in the vagina of E2-treated mice and the vehicle-treated control. Antisense probe specifically bound to the epithelium of E2-treated vagina, especially to stratified but not keratinized cells (Fig. 4). This suggests that the majority of CRBP2 mRNA originated from the epithelial cells in the vagina.
Distribution of CRBP2 mRNA in the E2-treated vagina examined by in situ hybridization. A tissue section of E2-treated (A, B and C, D) and vehicle-treated control (E, F) vagina that was hybridized with 35S-labeled antisense (A, B and E, F) and sense (C, D) probes was observed by dark- (A, C, E) and bright- (B, D, F) field microscopy. Silver grains in the emulsion were observed as fine white dots in a dark field. Arrowheads indicate an area of the stratified but not keratinized epithelium. Bar 50 µm
Effect of vitamin A on the E2-induced expression of the vaginal CRBP2 gene in neonatal mice
The expression level of vaginal CRBP1 and CRBP2 mRNAs was examined by “virtual” Northern blotting in the mice neonatally treated with 20 µg of E2 and/or 100 IU of RolA for 5 days. Single bands of approximately 0.8- and 0.9-kb products were detected with the probes specific for CRBP1 and CRBP2, respectively, in the vagina of neonatal mice (Fig. 5). The induction of CRBP2 gene expression by E2 was confirmed with this method, while expression of CRBP1, LRAT, GAPDH and β-actin mRNAs was not affected by E2. The expression of mRNA for keratin 18, a molecular marker of the epithelium, was not greatly affected by E2 either. Thus, the increase in CRBP2 mRNA in the estrogenized vagina was not caused simply by an increase in the percentage of proliferated epithelium-derived mRNA in the whole vaginal RNA sample, but rather mostly by a specific induction of the gene expression within each epithelial cell.
Effect of vitamin A on the estrogen-induced expression of CRBP2 mRNA in the neonatal mouse vagina examined by “virtual” Northern blotting. Full-length-rich vaginal cDNA derived from oil-, RolA-, E2-, and E2- and RolA-treated mice was transferred onto a membrane and hybridized with gene-specific probes. Note that the intensity of the band corresponding to CRBP2 gene expression was increased by E2 but not by RolA
RolA injection into neonatal mice did not alter the basal and E2-induced expression of CRBP2 mRNA in the neonatal mouse vagina.
Discussion
This study demonstrated that a VAD condition sensitized neonatal mice to the estrogen effect that leads to ovary-independent hyperplasia of the vaginal epithelium. It has also revealed that CRBP2 is expressed not exclusively in the small intestine but also in the genital tracts of both female and male mice and that the CRBP2 gene expression is induced by estrogen in these organs during a “critical period” in neonatal mice. The distribution of CRBP2 mRNA in the mouse genital tract is restricted to the epithelium, and thus is strikingly different from that of CRBP1, which is expressed mostly in the smooth muscle cells (Wardlaw et al. 1997; Zheng and Ong 1998).
CRBP2 associates with retinol and retinal with similar affinity, and modifies the catalytic activity of vitamin A metabolism-related enzymes mostly by inhibiting accessibility of the substrate to some enzymes but not to the others. For example, acyl-CoA:retinol acyltransferase (EC 2.3.1.76) is incapable of catalyzing esterification of CRBP2-bound retinol, while LRAT (EC 2.3.1.135) processes CRBP-bound retinal (Herr and Ong 1992). Oxidation of retinol to retinal is blocked by CRBP2, while microsomal retinal reductase readily produces retinol when presented with CRBP2-bound retinal (Kakkad and Ong 1988). Overall, it is proposed that CRBP2 contributes to the transfer of dietary retinol (from retinylester) and retinal (from β-carotene) into chyromicron in the form of lecithin retinol ester in addition to protecting the cell membrane from the lipolytic effect of free retinoids in the small intestinal mucosal epithelium (Harrison and Hussain 2001). Retinol is stored mostly in the liver in the esterified form until it is released into the circulation bound to retinal binding protein. Vitamin A is taken up by peripheral tissues such as the genital tracts in the form of free retinol in a passive diffusion manner depending on the concentration gradient between the blood and cytosol. In the target cells, retinol is re-esterified for the storage or oxidized to retinal and subsequently retinoic acids which modify gene transcription by interacting with specific receptors in the nucleus, in addition to being used as a component of cell membrane. Upregulation of CRBP2 expression in the genital tract epithelium is expected to result in a shift of the equilibrium of retinoids, i.e., in a decrease in the intracellular level of retinoids, especially retinal and possibly its oxidative product, retinoic acid, consequently modifying the transcription of genes. In CRBP2-upregulated cells, the intracellular level of free retinol will be restored to a level equal to that in blood by retinol influx from the circulation, and to some extent the intracellular level of free retinal will be restored, but the restoration will be delayed, especially when the animal is kept under VAD conditions. In our present study, estrogen induced vaginal CRBP2 expression and VAD lowered the threshold of the dose of estrogen that caused irreversible changes in the vagina. These results taken together imply that a decrease in the intracellular level of retinoids caused by upregulated CRBP2 contributed to the occurrence of estrogen-induced irreversible change in the vagina in neonatal mice. This upregulation of CRBP2 by estrogen appeared to be important for estrogen to induce ovary-independent hyperplasia in the vagina but may not be sufficient for it, as the VAD condition alone did not result in the vaginal change.
Ovary-independent hyperplasia of the vaginal epithelium induced by neonatal exposure to estrogen is due to an irreversible change in the nature of the stroma (Cunha et al. 1977). This estrogen effect is considered to be mediated by several steps of cross talk between the epithelium and stroma. In the developing genital tract, estrogen is likely to act on the stroma through ERα at first (Couse et al. 2001; Prins et al. 2001); subsequently, keratinocyte growth factor (KGF) mediates the estrogen signal to the epithelium through an epithelium-specific receptor, KGF-receptor, in the vagina (Hom et al. 1998; Masui et al. 2003). Then an unknown factor from the epithelium is conjectured to induce an alteration in the development of the stroma toward a persistent upregulation of epithelium-proliferating factor(s), which causes ovary-independent hyperplasia of the epithelium. Development of the Wolffian duct in male mice is also impaired by perinatal exposure to estrogen (Cunha et al. 2001; Atanassova et al. 2001). A decrease in the number of sperm and an impaired expression pattern of ERα are probably the result of direct effects of estrogenic substances on the Wolffian duct. Interestingly, these estrogen effects are also inhibited by vitamin A administered simultaneously with estrogen during the perinatal period (Nakahashi et al. 2001 and unpublished data), suggesting that the estrogen effects on the male and female genital tracts share an identical molecular mechanism in which vitamin A signal is involved. In the present study, upregulation of CRBP2 gene expression was observed in both neonatal male and female genital tracts, and thus the CRBP2 gene may mediate the estrogen effects in those tissues. Furthermore, the basal level of CRBP2 expression appeared to be much higher in the vagina than in the uterine horn, and the expression levels were increased by estrogen in neonatal mice, suggesting that estrogen induced anterization of CRBP2 expression in those tissues in contrast to the expression pattern of Hox genes that are posterized by perinatal exposure to estrogen (Block et al. 2000). This brings to mind the fact that retinoic acid is a novel anterization factor in the formation of the anterior-posterior axis in the earlier stages of the fetal development and induces posterization of Hox genes (Green 1990). A decrease in the intracellular level of retinoids caused by CRBP2 upregulation could result in posterization of expression of Hox genes in the developing genital tract.
References
Atanassova N, McKinnell C, Williams K, Turner KJ, Fisher JS, Saunders PT, Millar MR, Sharpe RM (2001) Age-, cell- and region-specific immunoexpression of estrogen receptor alpha (but not estrogen receptor beta) during postnatal development of the epididymis and vas deferens of the rat and disruption of this pattern by neonatal treatment with diethylstilbestrol. Endocrinology 142:874–886
Bern HA, Talamantes F (1981) Neonatal mouse models and their relation to disease in the human female. In: Herbst AL, Bern HA (eds) Developmental effects of diethylstilbestrol (DES) in pregnancy. Thieme Stratton, New York, pp 129–147
Block K, Kardana A, Igarashi P, Taylor HS (2000) In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J 14:1101–1108
Colborn T, von Saal FS, Soto AM (1993) Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378–384
Conlon RA (1995) Retinoic acid and pattern formation in vertebrates. Trends Genet 11:314–319
Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS (2001) Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol 238:224–238
Crow JA, Ong DE (1985) Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc Natl Acad Sci U S A 82:4707–4711
Cunha GR, Lung B, Kato K (1977) Role of the epithelial-stromal interaction during the development and expression of ovary-independent vaginal hyperplasia. Dev Biol 56:52–67
Cunha GR, Wang YZ, Hayward SW, Risbridger GP (2001) Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev 13:285–296
Dalterio S, Bartke A, Steger R, Mayfield D (1985) Neonatal exposure to DES in BALB/c male mice: effects on pituitary-gonadal function. Pharmacol Biochem Behav 22:1019–1024
Evans TR, Kaye SB (1999) Retinoids: present role and future potential. Br J Cancer 80:1–8
Folli C, Calderone V, Ottonello S, Bolchi A, Zanotti G, Stoppini M, Berni R (2001) Identification, retinoid binding, and x-ray analysis of a human retinol-binding protein. Proc Natl Acad Sci U S A 98:3710–3715
Franz O, Bruchhaus II, Roeder T (1999) Verification of differential gene transcription using virtual northern blotting. Nucleic Acids Res 27:e3
Golden RJ, Noller KL, Titus-Ernstoff L, Kaufman RH, Mittendorf R, Stillman R, Reese EA (1998) Environmental endocrine modulators and human health: an assessment of the biological evidence. Crit Rev Toxicol 28:109–227
Green JB (1990) Retinoic acid: the morphogen of the main body axis? Bioessays 12:437–439
Harrison EH, Hussain MM (2001) Mechanisms involved in the intestinal digestion and absorption of dietary vitamin A. J Nutr 131:1405–1408
Herbst AL, Ulfelder H, Poskanzer DC (1971) Adenocarcinoma of the vagina. Association of maternal stilboestrol therapy with tumor appearance in young women. N Engl J Med 284:878–881
Herr FM, Ong DE (1992) Differential interaction of lecithin-retinol acyltransferase with cellular retinol binding proteins. Biochemistry 31:6748–6755
Hom YK, Young P, Thomson AA, Cunha GR (1998) Keratinocyte growth factor injected into female mouse neonates stimulates uterine and vaginal epithelial growth. Endocrinology 139:3772–3779
Iguchi T, Fukazawa Y, Bern HA (1995) Effects of sex hormones on oncogene expression in the vagina and on development of sexual dimorphism of the pelvis and anococcygeus muscle in the mouse. Environ Health Perspect 103 (Suppl 7):79–82
Imaoka T, Horseman ND, Lockefeer JA, Mori T, Matsuda M (2002) Cortactin-binding protein 90 (CBP90) expression in the mouse mammary glands during prolactin-induced lobuloalveolar development. Zool Sci 19:443–448
Kakkad BP, Ong DE (1988) Reduction of retinaldehyde bound to cellular retinol-binding protein (type II) by microsomes from rat small intestine. J Biol Chem 263:12916–12919
Masui F, Matsuda M, Akazome Y, Imaoka T, Mori T (2001) Prevention of neonatal estrogen imprinting by vitamin A as indicated by estrogen receptor expression in the mouse vagina. Cell Tissue Res 306:441–447
Masui F, Matsuda M, Mori T (2003) Inhibition of KGF-induced ovary-independent cornification of vaginal epithelium by vitamin A in neonatally estrogenized mice. Cell Tissue Res 311:251–258
Matsuda M, Mori T (1997) Effect of hormones on expression of prolactin receptor messenger ribonucleic acids in pancreatic islets of adult female mice in vitro. Zool Sci 14:159–165
Matsuda M, Mori T, Park MK, Yanaihara N, Kawashima S (1994) Enhanced cell proliferation by hyperprolactinemia in both exocrine and endocrine pancreas in mice. Eur J Endocrinol 130:187–194
McLachlan JA (1979) Transplacental effects of diethylstilbestrol in mice. Natl Cancer Inst Monogr 51:67–72
Mori T (1968) Effects of neonatal injections of estrogen in combination with vitamin A on the vaginal epithelium of adult mice. Annot Zool Jpn 41:113–118
Mori T (1969) Further studies on the inhibitory effect of vitamin A on the development of ovary-independent vaginal cornification in neonatally estrogenized mice. Proc Jpn Acad 45:115–120
Mori T (1986) Abnormalities in the reproductive system of aged mice after neonatal estradiol exposure. J Endocrinol Invest 9:397–402
Nagasawa H, Yanai R, Taniguchi H, Tokuzen R, Nakahara W (1976) Two-way selection of a stock of Swiss albino mice for mammary tumorigenesis: establishment of two new strains (SHN and SLN). J Natl Cancer Inst 57:425–430
Nakahashi K, Matsuda M, Mori T (2001) Vitamin A insufficiency accelerates the decrease in the number of sperm induced by an environmental disruptor, bisphenol A, in neonatal mice. Zool Sci 18:819–821
Noy N (2000) Retinoid-binding proteins: mediators of retinoid action. Biochem J 348:481–495
Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS (2001) Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res 61:6089–6097
Santti R, Makela S, Strauss L, Korkman J, Kostian ML (1999) Phytoestrogens: potential endocrine disruptors in males. Toxicol Ind Health 14:223–237
Schaefer WH, Kakkad B, Crow JA, Blair IA, Ong DE (1989) Purification, primary structure characterization, and cellular distribution of two forms of cellular retinol-binding protein, type II from adult rat small intestine. J Biol Chem 264:4212–4221
Takasugi N (1963) Vaginal cornification in persistent-estrous mice. Endocrinology 72:607–619
Takasugi N (1979) Development of permanently proliferated and cornified vaginal epithelium in mice treated with steroid hormones and the implication in tumorigenesis. Natl Cancer Inst Monogr 51:57–66
Takasugi N, Bern HA (1964) Tissue changes in mice with persistent vaginal cornification induced by early postnatal treatment with estrogen. J Natl Cancer Inst 33:855–865
Takasugi N, Bern HA, DeOme KB (1962) Persistent vaginal cornification in mice. Science 138:438–439
Wardlaw SA, Bucco RA, Zheng WL, Ong DE (1997) Variable expression of cellular retinol- and cellular retinoic acid-binding proteins in the rat uterus and ovary during the estrous cycle. Biol Reprod 56:125–132
Yamashita M, Matsuda M, Mori T (1999) In situ detection of prolactin receptor mRNA and apoptotic cell death in mouse uterine tissues with adenomyosis. In Vivo 13:57–60
Zheng WL, Ong DE (1998) Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy. Biol Reprod 58:963–970
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Grants-in-Aid for Priority Area B (to T.M. and M.M.) and for Young Scientists (to M.M.) from the Ministry of Culture, Sports, Science and Technology, Japan
Rights and permissions
About this article
Cite this article
Matsuda, M., Masui, F. & Mori, T. Neonatal estrogenization leads to increased expression of cellular retinol binding protein 2 in the mouse reproductive tract. Cell Tissue Res 316, 131–139 (2004). https://doi.org/10.1007/s00441-004-0852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-0852-3