Abstract
Immunoreactivity to antibodies (ABs) against FMRFamide, CARP, and three FMRFamide gene encoded peptides, i.e., EFLRIamide, the non-FMRFamide peptide "SEEPLY," and the 35-amino-acid "acidic peptide," were investigated in developing embryos and juveniles of Lymnaea stagnalis. Five early transient embryonic neurons revealed immunoreactivity to EFLRIamide. One of the early neurons, the central posterior, also expressed SEEPLY and CARP immunoreactivity. Two neurons in the anlage of the left and right parietal ganglia coexpressed immunoreactivity to EFLRIamide (type 1 transcript) and acidic peptide (type 2 transcript). Within the developing ganglia altogether 30 neurons expressed the type 1 transcript, and three expressed the type 2 transcript. No peripheral cells immunoreactive to SEEPLY or acidic peptide ABs were found, whereas bipolar EFLRIamide- and CARP-immunoreactive cells were abundant in the lip, mantle and foot. After hatching, the number of immunoreactive neurons in ganglia increased up to 223 and the neurons expressing tetrapeptides were dominant (91%). No neurons coexpressing type 1 transcript and type 2 transcript could be detected in juveniles and adults. At this time, an extensive innervation is developed in the periphery, including foot, mantle, buccal mass, salivary glands and alimentary tract, established mainly by EFLRIamide-immunoreactive cells and varicose fibers of extrinsic and intrinsic origin. It is suggested that both sensory and regulatory function can be attributed to the FMRFamide gene encoded tetrapeptides throughout embryonic and juvenile development in Lymnaea, whereas heptapeptides are presumed to play a modulatory role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropeptides are known to be involved in both interneuronal signaling and peripheral regulation of a variety of physiological processes. One of the most significant groups of such peptides includes FMRFamide and structurally related peptides (FMRFa-related peptides, FMRFa-RPs). This peptide family has a ubiquitous occurrence throughout the animal kingdom and its members exhibit powerful neuromodulatory actions on both central neurons and peripheral targets (reviewed by Greenberg and Price 1992; Walker 1992; Brownlee et al. 1996).
Cloning of the gene encoding FMRFa and FMRFa-RPs has been successfully performed in different invertebrate species (for references, see Santama et al. 1993, 1995a). In the pond snail Lymnaea stagnalis, the FMRFa gene consists of five exons which are alternatively spliced: exon I to exon II (tetrapeptides), and exon I to exons III, IV and V (heptapeptides) (Kellett et al. 1994). Two different transcripts (type 1 and type 2 mRNA, respectively) are expressed in mutually exclusive neurons within the adult CNS (Bright et al. 1993). Distinct sets of neuropeptides appear as a result of post-translational processing (Benjamin and Burke 1994). Antisera specifically recognizing different members of the FMRFa family have been successfully raised. Those antibodies allow specific labeling of the neurons containing pentapeptide EFLRIamide (EFLRIa, Santama et al. 1995b), the non-FMRFamide-like 22-amino-acid peptide SEQPDVDDYLRDVVLQSEELY ("SEEPLY," Santama et al. 1993), and the 35-amino-acid SDPFFRFGKQQVATDDSGELDDEILSRVSDDDKNI ("acidic peptide," Santama et al. 1996). The presence and distribution of these peptides in the CNS of adult Lymnaea has been described in detail. EFLRIa and SEEPLY are expressed in the same cells where exon II is transcribed (type 1 mRNA) and acidic peptide is produced in all cells that transcribe type 2 mRNA (Bright et al. 1993; Santama et al. 1993, 1995a, 1995b, 1996).
During the embryogenesis of the molluscs Aplysia, Lymnaea, Helisoma, Crepidula and Ischnochiton, FMRFa-immunoreactive (IR) cells appear to be the first elements of the nervous system, and their pioneering role in the final organization of the CNS has been suggested (Croll and Voronezhskaya 1995, 1996; Dickinson et al. 1999, 2000; Voronezhskaya et al. 2002a). Preliminary immunocytochemical analysis of the ontogeny of FMRFa-IR cells has revealed two specific subsets of neurons, one showing transient and another sustaining expression of FMRFa immunoreactivity (Voronezhskaya and Elekes 1996).
The limited number of neurons in embryos and juveniles of Lymnaea stagnalis offers a unique opportunity to follow the development of identified cells from the first appearance to the final position in the adult brain. We examined when the different FMRFa-RPs encoded by one gene start and cease to be expressed. More specifically, we wanted to answer the following questions: (a) Do the neurons express one and the same peptide phenotype during their whole life or does the phenotype change? (b) Does the mechanism of alternative splicing start to function from the very beginning of the neural differentiation? (c) Is there any distinct peptide content of the subpopulation of the transient neurons? In addition, the distribution of these peptides in various peripheral tissues has been described to address the question of their possible sensory and/or regulatory functions.
Materials and methods
Animals and staging
Egg masses and juvenile specimens were collected from laboratory-bred populations of snails Lymnaea stagnalis (Mollusca, Gastropoda, Pulmonata). The embryonic development was staged on the basis of a specific set of morphological, morphometric, and behavioral features and stages were expressed as a percentage of total embryonic development as described previously (E0–E100, Voronezhskaya et al. 1999). Following eclosion, juvenile snails normally spend another 1–3 days inside the egg mass jelly, and we refer to such specimens as hatchlings. After emerging from the egg mass, the animals were categorized according to shell size (P1–P6, Croll and Chiasson 1989). A minimum of 50 animals were examined at each stage of development from E20 to P1, 30 at stages P2–P4, and 25 animals at P5.
Immunocytochemistry
Embryos were removed from shells and processed as whole-mounts. Juvenile snails were anesthetized by either 10–20 min incubation (veliger–P2) or injection (P3–P6) of cold 50 µM MgCl2. Central (cerebral, buccal, pedal, pleural, parietal and visceral) ganglia together with an attached portion of the esophagus were dissected from juvenile snails. The central ganglia and esophagus from larger (P5 and P6) specimens were incubated in 0.5% protease (type XIV, Sigma, USA) for 5 min prior to fixation. All tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.2). After 4 h of fixation at 4°C, the tissues were washed for 12 h in phosphate-buffered saline (PBS, pH 7.2) containing 4% Triton X-100 (TX) and then processed for immunohistochemistry.
The tissues were incubated in polyclonal antisera raised against the FMRFa gene encoded peptides: the pentapeptide EFLRIa, the 22-amino-acid peptide SEEPLY, and acidic peptide (all antibodies were donated by Prof. Paul R. Benjamin, University of Sussex, Brighton, UK), or in anti-FMRFa antiserum (rabbit polyclonal, #20091, DiaSorin, Stillwater, USA) and CARP (rabbit polyclonal, donated by Dr. M. Ohtani, Hiroshima University, Higashi-Hiroshima, Japan). All antibodies, except SEEPLY, were diluted 1:1,000 in PBS containing 1.0% normal goat serum (NGS) and 4.0% TX. SEEPLY antibody was diluted 1:200 in supermix (50 mM TRIS base, 150 mM NaCl, pH 7.6, containing 0.25% w/v gelatine and 1% TX) (Santama et al. 1993). All incubations were performed at 4°C for 24–48 h. Then the tissues were rinsed with PBS (3×15 min) and incubated for 12 h in goat anti-rabbit antiserum conjugated to fluorescein or rhodamine (DAKO, Denmark) diluted 1:50 in PBS. Following several rinses in PBS, the tissues were mounted in a 3:1 mixture of glycerol and PBS and then viewed and photographed in a Zeiss Axioplan compound microscope equipped with the appropriate filter sets.
Method control experiments involved the application of the identical procedure as above, except that the primary antiserum was replaced by either 1% normal serum or the serum dilutant (PBS-TX). No staining was observed after any of these control experiments. The specificity of the primary antisera raised against EFLRIa, SEEPLY and acidic peptide has previously been proven in the adult Lymnaea CNS (Santama et al. 1993, 1995b, 1996). The specificity of the anti-FMRFa antiserum has been tested previously on Lymnaea embryos and juveniles (Croll and Voronezhskaya 1996; Voronezhskaya and Elekes 1996). The specificity of the anti-CARP has also been proven in adult gastropods (Fujiwara-Sakata et al. 1991; Hernádi et al. 1995). In Lymnaea embryos, no staining was observed following the preincubation with anti-CARP or anti-EFLRIa antisera diluted to 1:1,000 containing 100 μg/ml synthetic CARP or EFLRIa.
Results
Embryos
Trochophore/early veliger stages
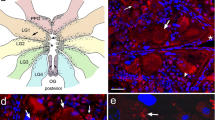
The first positive immunoreactivity appears at the late trochophore/early veliger stage (E28–E32). Both anti-EFLRIa and anti-FMRFa antibodies label the perikarya of three posterior and two anterior cells (diameter: 25–30 μm) and their processes. The morphology of the three posterior cells is not identical. One bipolar cell is located centrally at the caudal extreme, underneath the developing shell (central posterior, c in Fig. 1A, B). Two other cells are multipolar and are found at the right and left sides of the middle part of the embryonic body (right and left posterior, r and l in Fig. 1A, B). These three cells send their processes anteriorly to the region of the cephalic plates, where fibers separate into two bundles. One branch turns ventrally and terminates in the region of the rudiment of the ipsilateral pedal ganglion (Fig. 1B); the other branch crosses the midline of the embryonic body (Fig. 1A, B). The perikarya of two anterior cells (a in Fig. 1A) are located dorsolaterally to the mouth opening. Their short processes penetrate the epithelium and the long processes extensively ramify underneath the apical plate (Fig. 1A, D). The perikarya of all five early cells are located outside the area of the anlage of the central ganglia. Only one of the five early cells, the central posterior one, shows SEEPLY immunoreactivity (Fig. 2A, Table 1). No acidic peptide or CARP immunoreactivity can be demonstrated at this stage of development.
First embryonic neurons. A EFLRIa-IR, right-side view of an E30 embryo. Central (c), left and right (l, r) posterior cells issue anteriorly directed fibers. Long processes from two anterior cells (a) form a plexus beneath the apical plate (arrows) (mth mouth opening, sh developing shell, ft foot). B FMRFa-IR, the same stage as in A. Only three posterior cells and their processes are visible. C High magnification of right posterior EFLRIa-IR neuron (r). D Pair of anterior (a) EFLRIa-IR neurons with a rich network of processes at the E40 stage. Pair of anterior cells (a) (E) and right posterior cell (r) (F) in a E95 prehatching embryo. Scale bars 100 μm (A, B), 20 μm (C–F)
A SEEPLY-IR elements in E35 embryo. Only the central posterior cell (c) and its processes express immunoreactivity. Note the absence of both SEEPLY and CARP immunoreactivities in the anterior as well as in the right and left posterior cells. B–D CARP immunoreactivity. B E40 stage. The central posterior cell is faintly stained (arrow), two cells adjoining their processes (arrowheads). C E45 stage. Two cells are added in the region of the developing cerebral ganglion (double arrowheads). D E55 stage. Only the four neurons in the rudiments of the ganglia maintain CARP immunoreactivity (arrowheads). Distribution pattern of their developing process is identical to that of EFLRIa-IR and FMRFa-IR cells. Fronto-sagittal view. Scale bars 100 μm
During the veliger to adult-like form stages (E30–E90), the right and left posterior cells emanate numerous filopodia-like processes, and the pair of anterior EFLRIa-IR cells establishes a rich network of varicose processes (Fig. 1C, D). In pre-hatching snails (E95), the processes of these cells as well as the processes of the central posterior EFLRIa/SEEPLY/CARP-IR cell look as if they started to degenerate (Fig. 1E, F). In posthatching and juvenile snails (P1–P3), neither the perikarya nor the processes of the three posterior and two anterior cells are visible.
At stages E35–E40, CARP immunoreactivity appears in the perikarya and processes of three cells. One is faintly stained and is possibly identical to the early central posterior EFLRIa /FMRFa-IR neuron (c) according to its morphology and location. The process of this cell is visible in the cerebral commissures and cerebropedal connectives. Two other cells display intensive immunostaining. They are located at the left and right sides of the body adjoining the fiber from the central posterior cell. Each cell sends an axon process in the anterior direction, which follows the fiber originating from the central posterior (Fig. 2B). The position and morphology of these two CARP-IR neurons are certainly different from the left and right early posterior EFLRIa-IR cells (see Fig. 1A). Two additional cells appear in the regions of developing cerebral ganglia (CG) (Fig. 2C). At E55, the central posterior cell (c) ceases to express CARP immunoreactivity, while the other neurons maintain their immunoreaction to this antibody (Fig. 2D).
Metamorphosis
The first EFLRIa-IR and FMRFa-IR neurons appear in the CNS at the beginning of metamorphosis (E50). Both antibodies visualize three cells in the developing right parietal ganglion (RPaG), and two cells in the developing visceral ganglion (VG) (Fig. 3A, C). In each ganglion, one cell also reveals acidic peptide immunoreactivity (EFLRIa/acidic peptide) (Fig. 3B, D, Table 1), and the remaining cells (two neurons in RPaG, and one in VG) also reveal SEEPLY immunoreactivity (EFLRIa /SEEPLY) (Fig. 8, Table 1). All these neurons have an irregular shape and their axon process follows the pathway established by the early cells (arrow in Fig. 3C, D). The individual fibers originating from the EFLRIa/SEEPLY-IR cells cannot be recognized among the other EFLRIa-IR fibers within the developing connectives. In contrast, the axons of the two EFLRIa/acidic peptide-IR neurons are the only acidic peptide-IR elements before hatching and thus can be easily traced through all the embryonic stages. Each acidic peptide-IR neuron gives rise to two processes (Figs. 3B, 8). The longer process originating from the neuron located in the RPaG projects through the ganglionic ring via the RPlG to the RPeG, where it turns abruptly, runs into the RCG, and then continues its way through the cerebral commissure into the LCG. The longer fiber from the EFLRIa/acidic peptide-IR neuron located in the VG follows a symmetrical course through the left part of the ganglionic ring. The shorter process of the neuron located in the RPaG projects to the VG, whereas that of the neuron located in the VG runs to the RPaG. Such a pathway of the processes of these EFLRIa/acidic peptide-IR cells is very distinct and undoubtedly differs from that formed by the CARP-IR cells located in the same ganglion (see Fig. 2B–D). These two EFLRIa/acidic peptide-IR neurons and their exclusively centrally located processes are the only acidic peptide-IR elements detected during the entire period of embryogenesis.
Immunolabeled elements in E50 stage embryos. A EFLRIa-IR peripheral cells (a, c, l, r) and labeled neurons (arrows) within rudiments of the central ganglia (RPa, V) can be seen. B Acidic peptide immunoreactivity is displayed only by two neurons, located in the RPa and V ganglia, respectively, both possessing exclusively centrally located processes. C, D Double immunostaining for EFLRIa (C) and acidic peptide (D). Both EFLRIa and acidic peptide immunoreactivities are expressed in one (double arrowhead in D) of the three EFLRI-IR neurons (arrowheads in C) located in RPa ganglion. Arrow indicates the axon process of the double-labeled neuron. a anterior, c, l, r central, left and right peripheral early neurons, cc cerebral commissure, RC right cerebral ganglion, RPa right parietal ganglion, RPe, LPe right and left pedal ganglia, V visceral ganglion. Scale bars 100 μm (A, B), 20 μm (C, D)
The EFLRIa-IR cells in the RPaG and the VG are located symmetrically on the right and left sides of the embryo in the premetamorphic veliger (E45) (Fig. 4A). During metamorphosis the group in the anlage of the VG shifts ventrally and the group in the anlage of the RPaG moves right and dorsally, forming a figure-of-eight pattern. Thus the crossing of the rudiment of the visceral connectives, the so-called chiastoneury, can be observed at the postmetamorphic, adult-like form stage (E75) of Lymnaea embryogenesis (Fig. 4B). In the course of further development, all ganglia of the visceral loop move rostrodorsally, undergo partial detorsion and will finally be located around the esophagus. Thus, the anatomical arrangement of the CNS resembles its adult organization only by hatching.
A The early EFLRIa-IR elements mark the bilaterally symmetric organization of the developing CNS. E45 embryo. B EFLRIa-IR elements delineate the figure-of-eight pattern of the developing CNS. E75 embryo. C EFLRIa-IR and D FMRFa-IR elements in the foot of E65 stage embryos. The brush-like structures of central origin (arrows in C) are only visible after the application of anti-EFLRIa antiserum, whereas the FMRFa-IR processes show the usual appearance of varicose axons (arrowheads in D). A–D Frontal views (LC left cerebral ganglion, Pe pedal ganglion, ra radula, other labeling as in Fig. 3). Scale bars 50 μm
Also at the beginning of metamorphosis (E50), two EFLRIa-IR neurons appear symmetrically on the left and right sides of the caudal portion of the foot. The neurons are bipolar, their short processes penetrate the epithelium, whereas their long processes run into the neuropil of the respective PeG (Fig. 5E). No CARP-IR elements are detected in the foot at that stage. Immunopositive neurons within the PeG appear by the end of metamorphosis (E65). Two cells occur in each ganglion, one of them expressing EFLRIa-IR and the other SEEPLY-IR (Figs. 5B, 8).
A CARP-IR bipolar neurons at the anterior margin of a foot of an E95 embryo (arrowheads). B–E Peripheral EFLRIa-IR elements in the foot at E95 (B), E65 (C), and E50 (D, E) stages of development, respectively. B, C The varicose innervation of the foot (asterisks, B; double arrowhead, C) established by neurons located in the pedal ganglia (Pe). Bipolar neurons (large arrowhead, B; small arrowheads, C) with apical dendrites in the epithelium (arrow, C). D, E Peripheral bipolar neurons (arrowhead) at the posterior portion of the foot. Their short, dendritic process (arrow) penetrates the epithelium, while the long processes (double arrowheads, parallel arrows) run towards the developing pedal ganglia (Pe). Scale bars 50 μm (A, B), 20 μm (C–E)
Postmetamorphic, adult-like stages
Thus, by the early postmetamorphic stage E65, altogether nine cells expressing FMRFa-gene-encoded peptides are present in the VG, RPaG and PeG, whereas all other ganglia contain only immunoreactive fibers in the neuropil (Fig. 8). All these neurons are FMRFa-IR, of which two cells located in the PeG also reveal EFLRIa immunoreactivity, whereas two other cells are SEEPLY-IR. Two neurons located in the RPaG and VG express both EFLRIa and acidic peptide immunoreactivity, and three neurons located in the RPaG and VG display colocalizing EFLRIa and SEEPLY immunoreactivities. Colocalization of SEEPLY immunoreactivity and acidic peptide immunoreactivity cannot be observed in any neurons. In addition, four cells (one in each CG, one in RPaG and one in VG) display CARP immunoreactivity (Table 1).
This limited number of centrally located neurons gives rise to numerous fibers running to the periphery. At the E63–E68 stage, over 10–12 h, EFLRIa-IR brush-like endings appear in the foot, tentacles and body wall (Fig. 4C), and varicose fibers are present in the mantle (Fig. 6A). Later, EFLRIa-IR fibers with varicosities but no brush-like endings can be seen in the peripheral tissues. Anti-FMRFa antiserum fails to reveal the brush-like endings at the same stage of development, although the general pattern of the distribution of peripheral processes is identical for both types of immunostaining (Fig. 4D). Some of the fibers projecting to the mantle and foot, but not to the tentacles and body wall, reveal SEEPLY immunoreactivity.
Immunoreactive elements in the mantle and alimentary tract of hatchlings. A EFLRIa-IR peripheral cells (arrows) and varicose innervation (arrowheads) of central origin in the frontal margin of the mantle. B EFLRIa-IR neurons (arrowheads) and the network of their processes in the alimentary tract of a hatchling. Insert: Higher magnification view of a EFLRIa-IR cell (double arrowhead) with its varicose processes (arrowheads). C CARP-IR bipolar neurons (arrowheads) and varicose fibers (arrows) in the frontal region of the mantle. D CARP immunoreactivity is not present in the alimentary tract (asterisk), whereas the fibers in the connective tissue surrounding the visceral mass express immunoreactivity (arrow). Scale bars 100 μm (A, C), 50 μm (B, D), 20 μm (B, insert)
During the last two days before hatching (E75–E100), a limited number of EFLRIa-IR and SEEPLY-IR neurons, but no acidic peptide-IR cells, are added to the already labeled population in the CNS (Fig. 8, lower schemes). A group of neurons on the dorsal surface of each CG near the cerebral commissure express both EFLRIa and SEEPLY immunoreactivity. In this group, three cells are seen from E75 to hatching, and a further three to five cells appear at the P1–P5 stages. None of these neurons is present at stage P6. Thus, these cells show a transient expression of FMRFa-RP content. The solitary neuron on the ventral surface of the LPaG between the root of the viscero-parietal connective and the left pallial nerve exhibits only SEEPLY immunoreactivity. Two groups, both containing three EFLRIa-IR neurons, each occupy the dorsal surface of each BG near the buccal commissure (Fig. 9). The following neurons are found in each PeG: two SEEPLY-IR neurons in the anterioventral region, one EFLRIa-IR neuron on the dorsal surface between the pedal commissures, and two pairs of EFLRIa-IR cells at the anterior extreme region. No neurons in the pedal ganglia express more than one type of immunoreactivity (Table 1).
By prehatching (E90–E95), more neurons and an extensive innervation appear at the periphery, and this process also continues in hatchlings and P1 juveniles. The innervation partly originates from the central EFLRIa/FMRFa-IR and CARP-IR neurons, and is partly due to the appearance of additional neurons at the periphery. Numerous bipolar EFLRIa-IR and CARP-IR neurons appear in the foot (Fig. 5A, B), tentacles, and the anterior margin of the mantle (Fig. 6A, C). The short process of each cell penetrates the epithelium and the long process runs to the corresponding, ipsilateral, PeG (Fig. 5D, C). In the anterior region of the foot, the cells are distributed more densely, and are located both underneath the epithelium and along the pedal nerves. No peripheral SEEPLY-IR or acidic peptide-IR cells can be found.
Extensive innervation by varicose EFLRIa-IR fibers can be found in the buccal mass, salivary glands, alimentary tract, and connective tissue surrounding the visceral complex (Figs. 6B, 7C, D, 9). The processes of the buccal neurons run via the buccal nerves and ramify in the buccal mass (Fig. 9). A pair of cell bodies of irregular shape are found on both the right and left sides of the dorsal part of the buccal mass, outside the BG. Each cell sends one fiber to the respective salivary gland, where it forms a network of varicose fibers (Figs. 7C, 8, 9). A series of EFLRIa-IR cells are located on the right and left sides along the entire length of the alimentary tract, except the stomach (Fig. 6B). No CARP-IR elements can be seen in the alimentary tract, whereas the CARP-IR fibers are present in the connective tissue surrounding the visceral mass (Fig. 6D).
Acidic peptide-IR fibers (arrows) in the frontal region of the mantle of a hatchling (A) and a P1 juvenile snail (B). Note the lack of labeled cell bodies. C EFLRIa-IR varicose processes (arrowheads) in the salivary gland of a hatchling (asterisks gland cells). D EFLRIa-IR varicose fibers (arrowheads) in the connective tissue surrounding the visceral mass in a hatchling. Scale bars 100 μm (A, B), 20 μm (C), 50 μm (D)
Schematic representation of the distribution of EFLRIa-IR, SEEPLY-IR and acidic peptide (ACP) IR cells in postmetamorphic (E65, upper row) and adult-like form (E90, lower row) embryos of Lymnaea. Frontal views. Ganglia: B buccal, LPa left parietal, RPa right parietal, Pe pedal, V visceral, a anterior, c central posterior, l left posterior, r right posterior early peripheral neurons, cc cerebral commissure, EFLRI EFLRIamide, ACP acidic peptide
Schematic representation of the distribution of the EFLRIa-IR elements in the buccal mass (bm), buccal ganglia (B), salivary gland and esophagus in hatchlings. Three EFLRIa-IR neurons located in the B, innervating the buccal mass and the radula, and a pair of neurons of irregular shape (dotted) located outside the B, innervating the salivary gland. The esophagus has a rich EFLRIa-IR innervation of intrinsic origin
Hatchlings
At this stage some of the existing neurons partly change their initial immunoreactivity, and a few cells are added to already existing populations (Fig. 11). Two neurons, one in VG and one in RPaG, which during the intracapsular stages of development expressed both EFLRIa and acidic peptide immunoreactivity, reveal exclusively acidic peptide immunostaining. A solitary SEEPLY-IR neuron in the LPaG starts to display EFLRIa immunostaining as well. This cell is likely to be the LP1 neuron, according to its relatively large size (diameter 15–18 μm at the time of appearance), previously described morphology and location (Benjamin and Ings 1972; Winlow and Benjamin 1976). Indeed, this neuron expresses type I (exon I) transcript in adult Lymnaea (Bright et al. 1993).
One more acidic peptide-IR neuron (diameter 20–22 μm at the time of appearance) appears in the VG. In some preparations, we can trace a distinctive bifurcating axon of this newly appearing cell; hence, we identify it as the VW1 neuron (Benjamin and Winlow 1981; Santama et al. 1996). In hatchlings, acidic peptide-IR varicose fibers are present in all ganglia, and few acidic peptide-IR fibers appear in the mantle (Fig. 7A).
Thus, altogether 33 immunoreactive cells are present in the CNS of hatchlings, among which 16 EFLRIa-IR, 10 EFLRIa/SEEPLY-IR, two SEEPLY-IR, and three acidic peptide-IR can be distinguished (Figs. 10A, 11, upper schemes).
Acidic peptide-IR neurons and their processes in a hatchling (A) and a P1 stage juvenile snail (B). The labeled neurons are located in the right parietal (RPa) and visceral (V) ganglia, while their processes innervate other ganglia, such as the pedal (Pe), pleural (RPl), and left parietal (LPa). In the hatchling (A), only three labeled cells are present (arrowheads). These neurons (arrowheads) do not join any of the cell groups (Bgp, Egp, Fgp) appearing at the P1 stage (B). Scale bars 100 μm
Mapping of the distribution of neurons expressing EFLRIa-IR, SEEPLY-IR, and acidic peptide (ACP) IR neurons in the CNS of hatchlings (H, upper row) and P1 stage juveniles (P1, lower row) of Lymnaea. Solid symbols cells located on the dorsal surface, open symbols cells located on the ventral surface; arrows in the upper row neurons colocalizing EFLRIa and acidic peptide immunoreactivities during intracapsular (embryonic) stages of development, which reveal acidic peptide immunolabeling only after hatching; arrows in the lower row identified cells and cell groups (Winlow and Benjamin 1976; Benjamin and Winlow 1981): Bgp B group, Egp E group, Fgp F group, LP1 left parietal 1, VW1 visceral white interneuron. Ganglia: B buccal, C cerebral, LPa left parietal, RPa right parietal, Pl pleural, Pe pedal, V visceral
Juveniles
At the P1 stage, numerous new groups of immunopositive cells appear and are added to the already existing groups. Altogether up to 223 immunolabeled neurons occur; among them 171 neurons express EFLRIa immunoreactivity, 17 both EFLRIa and SEEPLY immunoreactivity, 12 are SEEPLY-IR, and 23 acidic peptide-IR. The clusters of identified neurons are summarized in Fig. 11 (lower schemes). Three groups, each consisting of three neurons, are located on the dorsal surface of each BG. Nine groups (three to six neurons in each) are found in the CG, six groups on the dorsal and three on the ventral surface of each. Three groups are located on the dorsal and four on the ventral surface of the PeG, each group containing three to six cells. A single pair of cells, identified by Santama et al. (1993) in the buccal ganglia of adults, starts to express SEEPLY immunoreactivity. Three SEEPLY-IR neurons appear on the dorsal surface of the PeG and two on the ventral surface.
Three groups of cells within the VG and RPaG, which can unequivocally be identified as the Egp, Fgp and Bgp cells (Winlow and Benjamin 1976; Benjamin and Winlow 1981; Bright et al. 1993; Benjamin and Burke 1994), become immunoreactive at the P1 juvenile stage. Each group contains different neurons displaying any of the three types of immunoreactivity. EFLRIa and SEEPLY-IR cells mainly occupy the dorsal surface of the ganglia, whereas the acidic peptide-IR cells are located ventrally. Within the Bgp group, 12 neurons are EFLRIa-IR, three of them are also immunoreactive to the anti-SEEPLY antibody, and nine neurons are exclusively acidic peptide-IR. Within the Egp group, six neurons exhibit both EFLRIa and SEEPLY immunoreactivity, and one is acidic peptide-IR. Within the Fgp, seven neurons are EFLRIa-IR, two of them are also SEEPLY-IR, and nine neurons are acidic peptide-IR. Thus 50% of the EFLRIa-IR cells are also immunopositive against the anti-SEEPLY antibody, whereas acidic peptide immunoreactivity is never co-localized with EFLRIa and SEEPLY immunoreactivities. The number of cells within each group (Bgp, Egp, Fgp) expressing different types of immunoreactivity in the course of the juvenile development is listed in Table 2.
Discussion
An important step in understanding the function of the FMRFa gene has been taken by Santama et al. (1993, 1995, 1996) and Bright et al. (1993), describing the organization of the FMRFa locus, the processes of splicing and post-translational processing of FMRFa gene transcripts, and the detailed distribution of final multiple neuropeptides in the CNS of adult Lymnaea. Another question concerns the initial steps of the function of this complex cascade of events during neuronal differentiation and development. At the embryonic stages, Lymnaea has a small number of immunoreactive cells, and each has a well-defined location, morphology and arborization pattern of the processes. This allows for the identification of individual cells from preparation to preparation at different developmental stages, and an analysis of the developmental dynamics of different FMRFa-RPs expression within identified neurons.
Characterization of the peptide antisera used
We used five polyclonal antisera in this study. Four were raised against different peptides encoded by the FMRFa gene. This gene in Lymnaea encodes two main groups of peptides: tetrapeptides (exons I–II; type 1 transcript) and heptapeptides (exons I, III–V; type 2 transcript). The first antibody was raised against FMRFa and reacts with all members of the FMRFa family of peptides. The second and third antisera against SEEPLY and acidic peptide, respectively, were raised to a unique region of the two alternatively spliced forms of the FMRFamide precursor (Santama et al. 1993, 1996). The anti-SEEPLY antiserum is immunoreactive to the 22-amino-acid peptide, SEQPDVDDYPRDVVLQSEEPLY, which is a non-FMRFa-like peptide processed from the tetrapeptide protein precursor and it is produced in most neurons that express the exon II of the FMRFa locus. Anti-SEEPLY antibody is a valid indirect indicator of FMRFa expression, assuming that all such neurons fully process the exon II protein precursor to produce the entire set of peptides (Santama et al. 1993). The acidic peptide antibody reacts with the 35-amino-acid SDPFFRFGKQQVATDDSGELDDEILSRVSDDDKNI, which is a final post-translational product of the type 2 protein precursor and has been shown to be synthesized in all cells that transcribe type 2 FMRFa mRNA (Santama et al. 1996). Thus, the SEEPLY and acidic peptide antibodies are really discriminatory for one transcript or the other.
The fourth antiserum was also raised by Santama et al. (1995b) to a short, amidated peptide, EFLRIamide. The authors characterized it as an antiserum reactive to EFLRIamide-like peptides, which detects all the three related peptides of this type: EFLRIamide, pQFYRIamide and pQFLRIamide. The EFLRIamide antiserum (used at 1:500 dilution) was not immunoreactive to other exon II- and exon III-encoded peptides, although it appears to exhibit some cross-reactivity to FMRFamide. It was 16 times more selective for EFLRIamide than for FMRFamide/GDPFLRFamide. Although it was considered to be specific against EFLRIamide-like peptides, it may cross-react with myomodulin-like peptides that also occur in Lymnaea (Santama et al. 1995b). Thus, the fifth antibody, CARP, which is known to visualize myomodulin-like peptides in gastropods (Fujiwara-Sakata et al. 1991; Hernádi et al. 1995), was used as a positive control to discriminate between the FMRFamide-like and myomodulin-like family of peptides. Based on it, it is concluded that at least in the embryonic Lymnaea EFLRIamide antibodies visualize FMRFa-RPs.
Early embryonic cells
Five of the seven immunoreactive neurons detected at premetamorphic stages (E28–E45) correspond to the cells described as the early transient FMRFa-ir neurons in pulmonate snails (Croll and Voronezhskaya 1995, 1996; Voronezhskaya and Elekes 1996). Two neurons are located at the anterior extreme, and three behind the prototroch. Anterior cells are identical with the transient anterior catecholaminergic neurons (TAC) believed to be the remnants of the apical organ (Voronezhskaya et al. 1999). EFLRIa and FMRFa immunoreactivities were expressed in these neurons during intracapsular stages of development only. Among the posterior neurons, only one cell, the central posterior, was immunoreactive to three antisera: EFLRIa, SEEPLY and CARP, whereas four other neurons displayed only EFLRIa immunoreactivity. In addition, two neurons in the anlage of the RPaG and LPaG were CARP-IR. Such a distribution of EFLRIa-IR and CARP-IR elements indicates that: (a) EFLRIamide antibodies visualize peptides related to FMRFa family and not to myomodulin family at least in embryonic tissues, and (b), during premetamorphic stages, FMRFa-RPs are possibly coexpressed with myomodulin-like peptides in the central posterior neuron. The two anterior and central posterior but not the left and right posterior cells also exhibit immunoreactivity against neurocalcin, the member of the superfamily of calcium-binding proteins (Dickinson and Croll 2001). Such differences in both morphology and transmitter content suggest different functional roles of the central, and the left and right posterior, cells. Early cells immunoreactive to FMRFamide were found in a variety of molluscan species and have been suggested to outline the framework of the adult CNS (Croll and Voronezhskaya 1995, 1996; Dickinson et al. 1999, 2000; Voronezhskaya et al. 2002). Our observation of torsion, chiastoneury and detorsion, marked by EFLRIa-IR and FMRFa-IR elements during Lymnaea development, supports this hypothesis.
Thus, our results demonstrate that the early peripheral transient neurons of Lymnaea express immunoreactivity exclusively to the tetrapeptide group. We suggest that among this group EFLRIamide-like peptides play the key role during the processes of early morpho- and embryogenesis.
Neurons in the CNS
Altogether 32 labeled neurons can be detected in prehatching snails. Interestingly, two neurons among them appear to express both EFLRIa and acidic peptide immunoreactivity, whereas these cells do not reveal either SEEPLY or CARP immunolabeling. Thus, the cross-reactivity of EFLRIa antibody with some myomodulin-like peptide seems to be unlikely. It is more probable that two transcripts of the FMRFamide gene, one encoding primarily tetrapeptides and the other encoding primarily heptapeptides, are coexpressed in these neurons during intracapsular stages of development. After hatching these cells maintain their immunoreactivity only to the anti-acidic peptide antibody. Consequently, in juveniles and adults, the neurons express mutually exclusive type 1 or type 2 transcripts as has been previously shown in adult Lymnaea (Bright et al. 1993). Two EFLRIa/acidic peptide-IR neurons can only be identified within the CNS throughout the entire embryonic development. Therefore these neurons may provide a useful system to study the commitment of a cell to expressing one spliced RNA form while excluding another one. On the other hand, the following aspects also need to be taken into consideration. First, SEEPLY may often stain rather weakly; hence the possiblility of colocalization with other immunoreactivities cannot be completely excluded (Santama et al. 1993). Second, myomodulin immunolabeling was also demonstrated in a number of FMRFa-IR neurons in adult Lymnaea (Santama et al. 1994). Consequently, the cross-reactivity between the anti-EFLRI and anti-CARP antibodies in the developing Lymnaea is still a possibility, in addition to the breaking down of the alternative splicing 'rule'.
The number of cells expressing FMRFa-RPs phenotype shows a dramatic, by about sevenfold, increase after hatching. Numerous cells as well as new cell groups appear at the first juvenile stages, and neurons are continuously added to the CNS during further postembryonic development. This is in contrast to the ontogeny of other transmitter systems described in Lymnaea. It has been demonstrated that almost all identified groups of serotonin-, dopamine- and octopamine-containing neurons are already present by the time of hatching, and new cells appear mainly in the already existing cell clusters (Croll and Chiasson 1989; Elekes et al. 1996; Voronezhskaya et al. 1999; Croll et al. 1999).
Peripheral neural elements
Peripheral neurons detected in this study exhibited FMRFa, EFLRIa or CARP immunoreactivity. Neither acidic peptide-IR nor SEEPLY-IR cells could be found. It was not possible to discriminate whether different antibodies visualize the same or different sets of cells in the frontal margin of the mantle and the foot. All neurons were bipolar, probably sensory cells, sending their axons into the CNS. The local network established by neurons located in the alimentary tract shows exclusively EFLRIa-IR and FMRFa-IR. The fibers that project to the periphery from the neurons differentiated within the ganglia display mostly EFLRIa immunoreactivity, some of them also expressing SEEPLY immunolabeling. In contrast, acidic peptide-IR elements were first restricted to the ganglionic ring, and start to appear in peripheral tissues only after hatching. It seems that the different peptides encoded by the FMRFamide gene in both sensory and peripheral regulatory processes are differentiated spatially and temporally.
At stage E65, EFLRIa-IR neurons innervate the foot, forming there characteristic brush-like structures, which are probably growth cones. This suggests that these peptidergic cells acquire their transmitter phenotype after they have found their final location within the ganglia but before their peripheral projections reach the final targets. Indeed, electron-microscopic investigations have shown that the first muscle fibers in the embryonic Lymnaea can be observed by metamorphosis, whereas the appearance of the first neuromuscular contacts is connected to the late stages (from E75) of embryogenesis (Nagy and Elekes 2002), when the immunolabeled brush-like structures are replaced by "regular" varicose arborizations in the foot.
The possible functional role of FMRFa-gene peptides
The first cells appear at the periphery and express immunoreactivity exclusively to the tetrapeptide family of FMRFa-RPs. Three early posterior cells represent a transitional larval system to which a guiding role has been attributed (Croll and Voronezhskaya 1995, 1996). According to our data, the neurons within the developing ganglia start to express immunoreactivity and thus complete their differentiation only after the processes originating from the early posterior cells terminated within the neuropil of the respective ganglia. Although only a temporal correlation between these developmental processes was observed, the suggestion that tetrapeptides (most probably EFLRIa) may induce neuronal differentiation cannot be excluded.
The presence of EFLRIa immunoreactivity in numerous peripheral bipolar cells and presumably sensory anterior cells indicates the participation of this pentapeptide in transmission of sensory stimuli. Involvement of these neurons in the induction of metamorphosis has been suggested (Hadfield 1978; Voronezhskaya et al. 1992; Croll et al. 1997; Kemp et al. 1997; Hadfield et al. 2000). The role of FMRFa-RPs in this developmental process, however, has not been tested at all.
During the intracapsular developmental stages, only a limited number of cells within the CNS express the peptide products of the FMRFa gene (Figs. 8, 11, upper schemes). Among the important neuronal networks known to express the FMRFamide gene products are those responsible for cardiac and respiratory functions, feeding, whole body withdrawal responses, and penis motor control (Bright et al. 1993; Benjamin and Burke 1994). The FMRFa-gene encoded peptide content of cardiorespiratory motoneurons, like Ehe and VW1, was specifically analyzed and demonstrated by MALDI-ToF mass spectrometry in the adult Lymnaea CNS (Worster et al. 1998). With the exception of sexual maturity, this entire behavioral repertoire already exists in postmetamorphic snails, starting from stage E60 (Mescheriakov 1990; Voronezhskaya et al. 1999). However, the key peptidergic neurons of these networks, such as VW1 and LP1 cells, can only be identified at a very late embryonic stage (E90), and the Egp, Bgp and Fgp groups appear even later, at the first posthatching stage, P1. It suggests that either most of the FMRFa-RPs containing neurons playing a modulatory role in the networks, underlying different behavioral programs or the motor programs, are not completely mature by the beginning of their functioning. The preliminary analysis of the cardiac system supports the second possibility: the frequency of the heart beat is very stable and only slightly increases during the embryonic period in Lymnaea, whereas its rate decreases by about fourfold and becomes very variable during the first 5 days after hatching (M.Yu. Khabarova, personal communication).
The transient subpopulation of FMRFa-IR cells in the cerebral and pedal ganglia described previously (Voronezhskaya and Elekes 1996) expresses the full set of peptides encoded by exon II and ceases to exhibit positive immunoreaction by the time of sexual maturation. Hence, tetra- and pentapeptides but not heptapetides of the FMRFa family are produced in juvenile transient cells. In the sexual organs of Lymnaea, numerous catecholaminergic cells were found projecting into the neuropil of the central ganglia by the time of sexual maturation (P6 stage) (Croll et al. 1999). The onset of functioning of these peripheral neurons may result in the increase in the dopamine level within the brain, which may affect the subpopulations of certain peptidergic neurons, which, in response, cease to express their peptide content. It can be supposed that these peptidergic neurons can fullfil the relay function in switching from a juvenile to a sexually mature type of behavior. The data showing that the number of FMRFa-IR neurons in mature CNS of the snail, Achatina, decreases as a response to incubation in a dopamine-containing solution (Takayanagi and Takeda 1988) support our hypothesis.
Conclusion
Our findings demonstrate that the majority of the FMRFamide-RPergic neurons express one and the same transmitter phenotype from the beginning of differentiation. However, some solitary cells acquire their final contents only 2–3 days after appearance. No changeover from the type 1 transcript to the type 2 transcript expression (or vice versa) was found.
On the basis of the different spatiotemporal and cellular pattern of appearance, multiple roles can be attributed to FMRFa-RPs during the ontogenesis of Lymnaea: (a) regulatory and sensory functions at the periphery, and (b) participation in mainly modulatory processes in the CNS. The members of the EFLRIa group appear to be the key peptides participating in the early processes of morpho- and embryogenesis, whereas the heptapeptide group seems to have a more specific role, primarily in central signaling, during the juvenile stages and maturation.
References
Benjamin PR, Burke JF (1994) Alternative mRNA splicing of the FMRFamide gene and its role in neuropeptidergic signalling in a defined neural network. Bioassays 16:335–342
Benjamin PR, Ings C (1972) Golgi-Cox studies on the central nervous system of a gastropod mollusc. Z Zellforsch 128:564–582
Benjamin PR, Winlow W (1981) The distribution of three wide-acting synaptic inputs to identified neurons in the isolated brain of Lymnaea stagnalis (L.). Comp Biochem Physiol 70A:293–337
Bright K, Kellett E, Saunders SE, Brierley M, Burke JF, Benjamin PR (1993) Mutually exclusive expression of alternatively spliced FMRFamide transcripts in identified neuronal systems of the snail Lymnaea. J Neurosci 13:2719–2729
Brownlee DJA, Fairweather I, Holden-Dye L (1996) Nematode neuropeptides: localization, isolation and functions. Parasitol Today 12:343–351
Croll RP, Chiasson BJ (1989) Postembryonic development of serotoninlike immunoreactivity in the central nervous system of the snail, Lymnaea stagnalis. J Comp Neurol 280:122–142
Croll RP, Voronezhskaya EE (1995) Early FMRFamide-like immunoreactive cells in gastropod neurogenesis. Acta Biol Hung 46:295–303
Croll RP, Voronezhskaya EE (1996) Early elements in gastropod neurogenesis. Dev Biol 173:344–347
Croll RP, Jackson DL, Voronezhskaya EE (1997) Catecholamine-containing cells in larval and postlarval bivalve molluscs. Biol Bull 193:116–124
Croll RP, Voronezhskaya EE, Hiripi L, Elekes K (1999) Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: II. Postembryonic development of central and peripheral cells. J Comp Neurol 404:297–307
Dickinson AJG, Croll RP (2001) Neurocalcin-like immunoreactivity in embryonic stages of the gastropod molluscs Aplysia californica and Lymnaea stagnalis. Invert Biol 120:206–216
Dickinson AJG, Nason J, Croll RP (1999) Histochemical localization of FMRFamide, serotonin and catecholamines in embryonic Crepidula fornicata (Gastropoda, Prosobranchia). Zoomorphology 119:49–62
Dickinson AJG, Croll RP, Voronezhskaya EE (2000) Development of embryonic cells containing serotonin, catecholamines and FMRFamide-related peptides in Aplysia californica. Biol Bull 199:305–315
Elekes K, Voronezhskaya EE, Hiripi L, Eckert M, Rapus J (1996) Octopamine in the developing nervous system of the pond snail, Lymnaea stagnalis. Acta Biol Hung 47:73–87
Fujiwara-Sakata M, Muneoka Y, Kobayashi M (1991) Action and immunoreactivity of neuropeptides in the buccal neuromuscular system of a prosobranch mollusc, Rapana thomasiana. Cell Tissue Res 264:57–62
Greenberg MJ, Price DA (1992) Relationships among the FMRFamide-like peptides. In: Joosse J, Buis RM, Tilders FJH (eds) The peptidergic neuron. Progress in brain research. Elsevier, Cambridge, pp 25–37
Hadfield MG (1978) Metamorphosis in marine molluscan larvae: an analysis of stimulus and response. In: Chia FS, Rice M (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier, North-Holland, New York, pp 165–175
Hadfield MG, Meleshkevitch EA, Boudko DY (2000) The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol Bull 198:67–76
Hernádi L, Terano Y, Muneoka Y, Kiss T (1995) Distribution of catch-relaxing peptide (CARP)-like immunoreactive neurons in the central and peripheral nervous system of Helix pomatia. Cell Tissue Res 280:335–348
Kellett E, Saunders SE, Li KW, Staddon JW, Benjamin PR, Burke JF (1994) Genomic organization of the FMRFamide gene in Lymnaea: multiple exons encoding novel neuropeptides. J Neurosci 14:6564–6570
Kempf SC, Page LR, Pires A (1997) Development of serotonin-like immunoreactivity in the embryos and larvae of nudibranch mollusks with emphasis on the structure and possible function of the apical sensory organ. J Comp Neurol 386:507–528
Marois R, Croll RP (1992) Development of serotonergic cells within the embryonic central nervous system of the pond snail, Lymnaea stagnalis. J Comp Neurol 322:255–265
Mescheriakov VN (1990) The common pond snail Lymnaea stagnalis L. In: Dettlaff DA, Vassetzky SG (eds) Animal species for developmental studies. Plenum, New York, pp 69–132
Nagy T, Elekes K (2002) Ultrastructure of neuromuscular contacts in the embryonic pond snail, Lymnaea stagnalis L. Acta Biol Hung 53:125–139
Santama N, Li KW, Bright K, Yeoman M, Geraerts WPM, Benjamin PR, Burke JF (1993) Processing of the FMRFamide precursor protein in the snail Lymnaea stagnalis: characterization and neuronal localization of a novel peptide, "SEEPLY". Eur J Neurosci 5:1003–1006
Santama N, Wheeler CH, Burke JF, Benjamin PR (1994) Neuropeptides myomodulin, small cardioactive peptides, and buccalin in the central nervous system of Lymnaea stagnalis: purification, immunoreactivity, and artifacts. J Comp Neurol 342:335–351
Santama N, Benjamin PR, Burke JF (1995a) Alternative RNA splicing generates diversity of neuropeptide expression in the brain of the snail Lymnaea: in situ analysis of mutually exclusive transcripts of the FMRFamide gene. Eur J Neurosci 7:65–76
Santama N, Wheeler CH, Skingsley DR, Yeoman MS, Bright K, Kaye I, Burke JF, Benjamin PR (1995b) Identification, distribution and physiological activity of three novel neuropeptides of Lymnaea: EFLRIamide and pQFYRIamide encoded by the FMRFamide gene, and a related peptide. Eur J Neurosci 7:234–246
Santama N, Li KW, Geraerts WPM, Benjamin PR, Burke JF (1996) Post-translational processing of the alternative neuropeptide precursor encoded by the FMRFamide gene in the pulmonate snail Lymnaea stagnalis. Eur J Neurosci 8:968–977
Syed NI, Lukowiak K, Bulloch AGM (1990) In vitro reconstruction of the central pattern generator of the mollusk Lymnaea. Science 250:282–285
Takayanagi H, Takeda N (1988) Dynamics of FMRFamide immunoreactivity in response to physiologically active substances in the central nervous system of the snail, Achatina fulica. Comp Biochem Physiol 91A:609–612
Voronezhskaya EE, Elekes K (1996) Transient and sustained expression of FMRFamide-like immunoreactivity in the developing nervous system of Lymnaea stagnalis (Mollusca, Pulmonata). Cell Mol Neurobiol 16:661–676
Voronezhskaya EE, Pavlova GA, Sakharov DA (1992) Possible control of molluscan embryogenesis by neuronal catecholamines. Russ J Dev Biol 23:295
Voronezhskaya EE, Hiripi L, Elekes K, Croll RP (1999) Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: I. Embryonic development of dopamine-containing neurons and dopamine-dependent behaviors. J Comp Neurol 404:297–307
Voronezhskaya EE, Tyurin SA, Nezlin LP (2002) Neuronal development in larval chiton Ischnochiton hakodadensis. J Comp Neurol 444:25–38
Walker RJ (1992) Neuroactive peptides with an RFamide or Famide carboxyl terminal. Comp Biochem Physiol 102C:213–222
Winlow W, Benjamin PR (1976) Neuronal mapping in the brain of the pond snail Lymnaea stagnalis (L.) In: Salánki J (ed) Neurobiology of invertebrates. Gastropoda brain. Akadémiai Kiadó, Budapest, pp 41–59
Worster BM, Yeoman MS, Benjamin PR (1998) Matrix-assisted laser desorption/ionization time flight mass spectrometric analysis of the pattern of peptide expression in single neurons resulting from alternative mRNA splicing of the FMRFamide gene. Eur J Neurosci 10:3498–3507
Acknowledgements
The authors' thanks are due to Professor Paul Benjamin, University of Sussex, for reading and commenting on the paper. The skillful technical assistance of Ms. Éva Karácsonyi and the photographic work of Mr. Boldizsár Balázs are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA), Nos. 6284, 23472 and 34106 to K.E., from the Hungarian and Russian Academies of Sciences, exchange program No. 33 to E.E.V. and K.E., and from the Russian Foundation for Basic Research (RFBR), grant 99-04-418 to E.E.V.
Rights and permissions
About this article
Cite this article
Voronezhskaya, E.E., Elekes, K. Expression of FMRFamide gene encoded peptides by identified neurons in embryos and juveniles of the pulmonate snail Lymnaea stagnalis . Cell Tissue Res 314, 297–313 (2003). https://doi.org/10.1007/s00441-003-0800-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0800-7