Abstract
Nasal chondromesenchymal hamartoma (NCMH) is a rare nasal tumor that typically presents in young children. We previously reported on NCMH occurrence in children with pleuropulmonary blastoma (PPB), a rare pulmonary dysembryonic sarcoma that is the hallmark neoplasm in the PPB-associated DICER1 tumor predisposition disorder. Original pathologic materials from individuals with a PPB, PPB-associated tumor and/or a DICER1 mutation were centrally reviewed by the International PPB Registry. Paraffin-embedded NCMH tumor tissue was available in three cases. Laser-capture microdissection was used to isolate mesenchymal spindle cells and cartilage in one case for Sanger sequencing of DICER1. Nine patients (5F/4M) had PPB and NCMH. NCMH was diagnosed at a median age of 10 years (range 6–21 years). NCMH developed 4.5–13 years after PPB. Presenting NCMH symptoms included chronic sinusitis and nasal congestion. Five patients had bilateral tumors. Local NCMH recurrences required several surgical resections in two patients, but all nine patients were alive at 0–16 years of follow-up. Pathogenic germline DICER1 mutations were found in 6/8 NCMH patients tested. In 2 of the patients with germline DICER1 mutations, somatic DICER1 missense mutations were also identified in their NCMH (E1813D; n = 2). Three additional PPB patients developed other nasal lesions seen in the general population (a Schneiderian papilloma, chronic sinusitis with cysts, and allergic nasal polyps with eosinophils). Two of these patients had germline DICER1 mutations. Pathogenic germline and somatic mutations of DICER1 in NCMH establishes that the genetic etiology of NCMH is similar to PPB, despite the disparate biological potential of these neoplasms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasal chondromesenchymal hamartoma (NCMH) was first described in 1998 as a rare nasal tumor that typically presents as a unilateral mass in children (McDermott et al. 1998). A mixed mesenchymal pattern of chondroid and/or osseous islands of tissue with a variably cellular stroma characterized the morphologic features. The lesion may be expansile and locally destructive, with extension into the intracranial space (Kim et al. 2004). In that initial report, there was a child with NCMH and pleuropulmonary blastoma (PPB, “case 7”). The co-existence of these two very rare tumors prompted the authors of that first report to speculate about a possible etiologic association (McDermott et al. 1998). A 15-year-old girl with NCMH and PPB (as well as a “testosterone-secreting left ovarian stromal tumor”) was reported in 2007 (Johnson et al. 2007). The report of four additional children with both NCMH and PPB strengthened this putative association (Priest et al. 2010).
Pleuropulmonary blastoma, a rare pediatric dysembryonic sarcoma of the lung and pleura (Manivel et al. 1988), is the hallmark tumor in the PPB-associated DICER1 tumor predisposition disorder (OMIM 601200). Approximately, 65 % of patients with this incompletely penetrant syndrome harbor mutations in DICER1 (Hill et al. 2009). DICER1 is an RNase endoribonuclease that catalyzes the cleavage of double-stranded RNA to produce mature microRNAs (miRNAs) and short interfering RNAs (siRNAs) which post-transcriptionally modulate gene expression. Individuals with germline DICER1 mutations are at increased risk of developing a variety of tumors, including PPB (Hill et al. 2009). Since the initial report of seven cases of NCMH, there are approximately 25 cases reported relative to various aspects of this tumor with its predilection for children 12 months of age or less (Mattos and Early 2011; Greci et al. 2011; Yao-Lee et al. 2011; Uzomefuna et al. 2012; Cho et al. 2013; Li et al. 2013a, b). None of these reports made reference to DICER1 status. Another report described an 11-year-old boy with a history of PPB at age 3 years, who presented with nasal obstruction and whose NCMH had a somatic, balanced t(12;17) (q24;q21) translocation (Behery et al. 2012).
In this paper, we report the results of germline and tumor DICER1 mutation testing in nine patients with NCMH from the International PPB Registry: we include the four previously reported patients (Priest et al. 2010) with NCMH, and describe five new patients with NCMH and PPB. We also describe three PPB patients who developed other nasal lesions, indicating that in PPB patients nasal polyps are not invariably NCMH.
Methods
The International PPB Registry (IPPBR), founded in 1988, is a collaboration of the Department of Pediatric Hematology and Oncology at Children’s Hospitals and Clinics of Minnesota (Minneapolis, MN), the Department of Surgical Pathology at Children’s National Medical Center (Washington, DC) and the Department of Pathology and Immunology at Washington University Medical Center (St. Louis, MO). Additional patients were evaluated at the Clinical Center of the National Institutes of Health (Bethesda, MD) through the “DICER1-related PPB Cancer Predisposition Syndrome Natural History Study” [National Cancer Institute (NCI) Protocol 11-C-0034; NCT-01247597] at the NCI. The Institutional Review Board at each institution approved this study; all patients (and/or parents) gave written consent to participate.
Individuals referred to the IPPBR and/or NCI study with a PPB, a PPB-associated tumor and/or a DICER1 mutation is accessioned consecutively. Select patients are evaluated at the NCI and receive a history and physical, imaging, lab work and sub-specialty consultation. Data on clinical presentation, imaging, pathologic materials, family history, treatment and long-term follow-up are collected on IPPBR and NCI enrollees. All pathologic materials submitted to the IPPBR, including the NCMH and non-NCMH nasal lesions in this report, were centrally reviewed by IPPBR pathologists (DAH and LPD).
DNA was extracted from peripheral blood leukocytes for assessment of germline DICER1 mutations. Formalin-fixed, paraffin-embedded blocks (FFPE) of NCMH were available in three cases. From each of these blocks, four 10-micron scrolls were prepared. Following paraffin removal, DNA was extracted using the Maxwell 16 Formalin-Fixed Paraffin-Embedded Tissue LEV DNA Purification Kit (Promega, Madison, WI) on the Maxwell 16 Instrument (Promega). DNA quality and quantity was assessed using a Nanodrop (ThermoFisher, Wilmington, DE) and Qubit (Qubit 2.0, Life Technologies), respectively. We performed laser-capture microdissection to selectively separate cystic epithelium and underlying mesenchyme and cartilage on one of the NCMH tumors (Table 1, case 6), (mmiCellCut Plus, Molecular Machines and Industries, Inc., Haslett, MI). A minimum of 100 cells was captured from epithelium and mesenchyme/cartilage, respectively. DNA extractions were performed using QIamp DNA FFPE kit (Qiagen, Valencia, CA).
For germline DICER1 sequencing, the coding region of DICER1 was sequenced and analyzed for variants as described previously (Hill et al. 2009). For tumors, DNA was sequenced using primer pairs for small amplicons containing the codons for amino acids 1,705, 1,709, and 1,809, 1,810, 1,813, and 1,814, each covering a somatic missense hotspot seen in PPB (Pugh et al. 2014): primer pair 1: forward: 5′-tggggatcagttgctatgtg-3′ and reverse: 5′-CGGGTCTTCATAAAGGTGCT-3′; primer pair 2: forward: 5′-tggactgcctgtaaaagtgg-3′ and reverse: 5′-ATGTAAATGGCACCAGCAAG-3′. The sequence traces were assembled and the chromatograms were scanned for variants using Sequencher version 5.1.
Results
Nine patients (five females and four males) with PPB were diagnosed with NCMH at a median age of 10 years (range 6–21 years). Presenting NCMH symptoms included chronic sinusitis and nasal congestion; five patients had bilateral tumors (Table 1). Three patients had cystic PPB (Type I and a lung cyst that was not examined microscopically); six patients had solid PPB (Type II, II/III and III). NCMH developed 4.5–13 years after PPB diagnosis. Two patients (#1 and #9) had local NCMH recurrence (4 and 1 recurrences) requiring additional surgical resections. All nine NCMH patients were alive at 0–16 years of follow-up. Seven patients with PPB Types I, II or III received chemotherapy before developing NCMH. One patient with Type I PPB and the patient with the lung cyst received no chemotherapy. Patient #7 received radiation therapy to the chest.
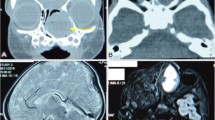
The pathological features of NCMH cases in this report were similar. Each showed a polypoid mass or masses containing variable-sized cysts lined by respiratory epithelium. Nodules of immature or mature cartilage were surrounded by spindle-cell mesenchyme. The polyp matrix contains a loose myxoid ground substance containing inflammatory cells, small vessels and variable amounts of fibrosis. Interestingly, the NCMH from patient #9 in this report lacked cartilage nodules (Fig. 1).
Patients #10, #11 and #12 had other nasal pathology including, respectively, a Schneiderian papilloma, chronic sinusitis with cysts, and allergic nasal polyps with eosinophils (Tables 1, 2). Tissue from the Schneiderian papilloma was available for DICER1 sequencing; no somatic mutations were found. Tissue from the other two cases was not available for DICER1 sequencing. All three had a history of Type Ir PPB (Hill et al. 2008). None were treated with chemotherapy or radiation.
Germline mutations in DICER1 were observed in 6/8 (75 %) NCMH patients tested and in 2/3 of the patients with other nasal lesions (Table 2).
DICER1 somatic mutations were seen in all 3 NCMH tissues tested (Table 2); adequate materials for somatic DICER1 sequencing were not available from the non-tested NCMH. The DICER1 somatic mutation NM_177438.2:c.5439G>T, p.Glu1813Asp (E1813D) was identified in laser-dissected mesenchymal spindle cells and cartilage but not epithelium from patient #6, indicating that there is a clonal population of tumor cells in mesenchyme, similar to the early pathogenesis of PPB. The same exact base substitution was seen in the NCMH from patient #9 and the same amino acid was affected in patient #2, c.5437G>A; p.Glu1813Lys (E1813K). Patient-specific germline DICER1 mutations were detected in tumor tissue in patients #6 and #9.
Discussion
In this paper, we expand on our prior report of four PPB patients to nine who have developed NCMH and provide evidence that the genetic pathogenesis of this benign tumor mirrors that of PPB (Priest et al. 2010).
Out of 405 central pathology-confirmed PPB registered by the IPPBR we are aware of 8 (2 %) who developed NCMH. Since NCMH appears to occur at a later age than PPB, the prevalence of NCMH in patients with PPB enrolled in the IPPBR may be underestimated; long-term follow-up is required to permit for accurate estimation of this parameter. At this time, there are no formal screening recommendations for NCMH in DICER1 carriers. In patients of all ages with a DICER1 mutation (or with a DICER1-associated tumor), a review of systems pertaining to nasal masses is appropriate: are there respiratory or feeding difficulties, rhinorrhea, epistaxis, nasal obstruction, snoring, mouth breathing, hyponasal speech, visual disturbances, headache or otitis media? On examination, evidence of orbital involvement of the tumor (e.g., ptosis, hypotropia, ophthalmoplegia) should be noted. In our experience, nasal endoscopy in the clinic (with topical anesthesia) and/or maxillofacial computed tomography (CT) are both effective means of NCMH detection. All of the NCMH cases seen to date in the PPB syndrome have followed a benign course. Surgical removal is curative, although local recurrences can be seen as was the case in two of nine patients discussed here. This appears to be similar to the historical experience with DICER1-associated cystic nephroma (CN) and in contrast to the natural history of lung cysts where there is a known risk for progression to high-grade sarcoma (Priest et al. 1997, 2006). As a potential note of caution, sarcomatous transformation in CN has been recently described in DICER1 mutation carriers (Doros et al. 2014) and a recent report documents one adult patient with malignant transformation in an NCMH (Li et al. 2013b).
Not all nasal lesions in children with PPB and/or DICER1 mutations are NCMH. Patient 10 in our cohort was diagnosed with a ~2-cm Schneiderian papilloma, inverted type, with its origin from the lateral nasal wall. These lesions are benign epithelial neoplasms arising from the Schneiderian mucosa (an ectodermally derived respiratory mucosa) of the sinonasal tract. Chronic sinusitis appears to be a risk factor for Schneiderian papilloma. Tumor tissue for DICER1 sequencing for this case was not available. We are not aware of any other published associations between Schneiderian papillomas and PPB or DICER1 mutations. Patient 10 had a long history of allergic sinusitis prior to the diagnosis of the Schneiderian papilloma. The other two patients with PPB and non-NCMH nasal masses had chronic sinusitis with polyps, one of which had cysts. The pathologic differentiation of NCMH from inflammatory polyps may be difficult since fewer than 20 cases have been reported in the literature and the full spectrum of morphologic change in NCMH may be unknown. This is demonstrated by one case which was previously diagnosed as “respiratory epithelial adenomatoid polyp” only later to be confirmed as NCMH that lacked cartilage. Schneiderian papillomas, allergic sinusitis and chronic sinusitis with polyps occur in the general population; our data are insufficient to suggest that patients with a DICER1 mutation have a higher incidence of non-NCMH pathology.
PPB has a purely cystic form: Type I, and Type I regressed (Ir) PPB (Hill et al. 2008); a solid/cystic form (Type II), and a completely solid form (Type III). The outcome of cystic form Type I or Ir is better than the outcome for Types II and III (Messinger et al. 2012; Williams et al. 2012). The list of other DICER1-associated tumors continues to grow and includes CN (Bahubeshi et al. 2010), familial multinodular goiter with and without Sertoli–Leydig cell tumors (SLCT) (Rio Frio et al. 2011), Wilms tumor (Foulkes et al. 2011), ciliary body medulloepithelioma (Slade et al. 2011), botryoid-type embryonal rhabdomyosarcoma (ERMS) of the uterine cervix (Doros et al. 2012), and pineoblastoma (Sabbaghian et al. 2012). However, phased (determination of cis or trans) biallelic DICER1 mutations or loss of heterozygosity of DICER1 has been established only in Wilms tumor (Wu et al. 2013), pineoblastoma (Sabbaghian et al. 2012), SLCT (Heravi-Moussavi et al. 2012), primitive germ-cell tumor of the yolk-sac type (Heravi-Moussavi et al. 2012) and PPB (Pugh et al. 2014). Somatic missense mutations of certain “hotspot” residues (E1705, D1709, G1809, D1810 and E1813) have been detected in CN (Doros et al. 2014), SLCT, nonseminomatous testicular germ-cell tumors, ERMS and rare epithelial ovarian and endometrial carcinomas (Heravi-Moussavi et al. 2012) and PPB (de Kock et al. 2013; Pugh et al. 2014). The hotspot mutations affect amino acids in the DICER1 RNase IIIb domain, the protein site that is critical for miRNA interaction and cleavage of mature miRNA from the 5′ arm of the precursor miRNA hairpin.
Germline mutations in DICER1 were identified in 6/8 (75 %) NCMH patients evaluated. Somatic mutations involving glutamine at hotspot position 1,813 in the RNase IIIb domain of DICER1 were detected in all three NCMH tumors tested. We did not specifically test if the position E1813 mutations are on the wild-type chromosome in NCMH (in trans) with the known germline DICER1 mutation in the patients. However, other hotspot residues have been shown to be in trans with a germline DICER1 mutation; in SLCT and primitive germ-cell tumor of the yolk-sac type (Heravi-Moussavi et al. 2012) and PPB (Pugh et al. 2014), phased biallelic mutations in DICER1 have been found. In these tumor types, the investigators established that the hotspot residue D1709 was somatically mutated.
Somatic mutations of DICER1 E1813 have been described previously in DICER1-familial PPB tumor predisposition syndrome tumors. The E1813 amino acid residue is invariably conserved, including in S. pombe, G. intestinalis, T. maritime and E. coli (Takeshita et al. 2007). Three SLCT with somatic mutations (only) affecting DICER1 residue E1813 (E1813Q, E1813G, E1813K) and one SLCT with a germline DICER1 mutation and a somatic DICER1 E1813 K mutation have been reported (Heravi-Moussavi et al. 2012). In a 3-year-old child with a Type II PPB, a pathogenic DICER1 germline mutation and a somatic DICER1 E1813G mutation were found, although phase was not determined (de Kock et al. 2013). Tumor sequencing in 51 PPBs found two somatic DICER1 E1813D, E1813G, E1813 K, and E1813Q in 4, 10, 2 and 2 % of PPBs, respectively (Pugh et al. 2014). Sanger sequencing of the RNase IIIa and IIIb domains in DICER1 in a collection of 154 gonadal tumors uncovered somatic E1813K, E1813Q and E1813D mutations in three different SLCT (Witkowski et al. 2013). In the online Catalogue of Somatic Mutations in Cancer (COSMIC, accessed 10/10/13), there were two E1813Q variants in a colorectal carcinoma, one E1813G variant in a endometrial carcinoma and one E1813A variant in a endometrial carcinoma.
RNase III enzymes like DICER1 cut miRNAs and require divalent metal ions, with Mg2+ as the preferred species; in the RNase IIIb domain, amino acid residue E1813 serves as a magnesium-binding site and is invariably conserved (Takeshita et al. 2007). In PPB, the pathogenic mutations in DICER1 hot-spot residues E1705, D1709, G1809, D1810 and E1813 have been shown to disrupt the cleavage of the 5p miRNA from the precursor miRNA hairpin (Pugh et al. 2014). The miRNA sequence shows normal mature 3p miRNA but the 5p miRNA retains the hairpin sequence (Pugh et al. 2014). Presumably these 5p miRNAs with retained hairpin sequence are non-functional. Coupled with a loss-of-function mutation on the other DICER1 allele (germline mutation in many cases), these specific somatic mutations contribute to defective repression of genes regulated by 5p miRNAs.
In summary, we found germline DICER1 mutations in 6/8 evaluated patients with NCMH. In the NCMH of two of the six patients with DICER1 germline mutations, we found somatic DICER1 E1813D mutations, a known pathogenic missense variant. Another known somatic DICER1 missense variant, E1813 K, was also detected in the NCMH of a third patient without a known germline DICER1 mutation. Detecting pathogenic somatic and germline mutations in DICER1 establishes genetic proof of a tumor association with the gene. NCMH should be considered as part of the DICER1 tumor spectrum.
Web resources
Catalogue of somatic variants in cancer (COSMIC): http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
References
Bahubeshi A, Bal N, Rio Frio T, Hamel N, Pouchet C, Yilmaz A, Bouron-Dal Soglio D, Williams GM, Tischkowitz M, Priest JR, Foulkes WD (2010) Germline DICER1 mutations and familial cystic nephroma. J Med Genet 47(12):863–866. doi:10.1136/jmg.2010.081216
Behery RE, Bedrnicek J, Lazenby A, Nelson M, Grove J, Huang D, Smith R, Bridge JA (2012) Translocation t(12;17)(q24.1;q21) as the sole anomaly in a nasal chondromesenchymal hamartoma arising in a patient with pleuropulmonary blastoma. Pediatr Dev Pathol 15(3):249–253. doi:10.2350/11-11-1121-CR.1
Cho YC, Sung IY, Son JH, Ord R (2013) Nasal chondromesenchymal hamartoma: report of a case presenting with intraoral signs. J Oral Maxillofac Surg 71(1):72–76. doi:10.1016/j.joms.2012.03.020
de Kock L, Plourde F, Carter MT, Hamel N, Srivastava A, Meyn MS, Arseneau J, Soglio DB, Foulkes WD (2013) Germ-line and somatic DICER1 mutations in a pleuropulmonary blastoma. Pediatr Blood Cancer. doi:10.1002/pbc.24692
Doros L, Yang J, Dehner L, Rossi CT, Skiver K, Jarzembowski JA, Messinger Y, Schultz KA, Williams G, Andre N, Hill DA (2012) DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr Blood Cancer 59(3):558–560. doi:10.1002/pbc.24020
Doros L, Rossi C, Yang J, Field A, Williams G, Messinger Y, Schultz KA, Cajaiba M, Perlman E, Cathro H, LeGallo R, LaFortune K, Chikwava K, Faria P, Geller J, Dome J, Mullen E, Gratias E, Dehner LP, Hill DA (2014) DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol. doi:10.1038/modpathol.2013.242 (Epub ahead of print)
Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, Choong CS, Charles A, Frieder RP, Dishop MK, Graf N, Ekim M, Bouron-Dal Soglio D, Arseneau J, Young RH, Sabbaghian N, Srivastava A, Tischkowitz MD, Priest JR (2011) Extending the phenotypes associated with DICER1 mutations. Hum Mutat 32(12):1381–1384. doi:10.1002/humu.21600
Greci V, Mortellaro CM, Olivero D, Cocci A, Hawkins EC (2011) Inflammatory polyps of the nasal turbinates of cats: an argument for designation as feline mesenchymal nasal hamartoma. J Feline Med Surg 13(4):213–219. doi:10.1016/j.jfms.2010.07.009
Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L, Fejes AP, Chow C, Tone A, Kalloger SE, Hamel N, Roth A, Ha G, Wan AN, Maines-Bandiera S, Salamanca C, Pasini B, Clarke BA, Lee AF, Lee CH, Zhao C, Young RH, Aparicio SA, Sorensen PH, Woo MM, Boyd N, Jones SJ, Hirst M, Marra MA, Gilks B, Shah SP, Foulkes WD, Morin GB, Huntsman DG (2012) Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med 366(3):234–242. doi:10.1056/NEJMoa1102903
Hill DA, Jarzembowski JA, Priest JR, Williams G, Schoettler P, Dehner LP (2008) Type I pleuropulmonary blastoma: pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol 32(2):282–295. doi:10.1097/PAS.0b013e3181484165
Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, Williams G, Bracamontes D, Messinger Y, Goodfellow PJ (2009) DICER1 mutations in familial pleuropulmonary blastoma. Science 325(5943):965. doi:10.1126/science.1174334
Johnson C, Nagaraj U, Esguerra J, Wasdahl D, Wurzbach D (2007) Nasal chondromesenchymal hamartoma: radiographic and histopathologic analysis of a rare pediatric tumor. Pediatr Radiol 37(1):101–104. doi:10.1007/s00247-006-0352-6
Kim B, Park SH, Min HS, Rhee JS, Wang KC (2004) Nasal chondromesenchymal hamartoma of infancy clinically mimicking meningoencephalocele. Pediatr Neurosurg 40(3):136–140. doi:10.1159/000079857
Li GY, Fan B, Jiao YY (2013a) Endonasal endoscopy for removing nasal chondromesenchymal hamartoma extending from the lacrimal sac region. Can J Ophthalmol 48(2):e22–e23. doi:10.1016/j.jcjo.2012.10.007
Li Y, Yang QX, Tian XT, Li B, Li Z (2013b) Malignant transformation of nasal chondromesenchymal hamartoma in adult: a case report and review of the literature. Histol Histopathol 28(3):337–344
Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR, Dehner LP (1988) Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer 62(8):1516–1526
Mattos JL, Early SV (2011) Nasal chondromesenchymal hamartoma: a case report and literature review. Int J Pediatr Otorhinolaryngol Extra 6:215–219
McDermott MB, Ponder TB, Dehner LP (1998) Nasal chondromesenchymal hamartoma: an upper respiratory tract analogue of the chest wall mesenchymal hamartoma. Am J Surg Pathol 22(4):425–433
Messinger YH, Williams GM, Priest JR, Harris A, Doros LA, Schultz KAP, Dehner LP, Hill DA (2012) Outcome of 116 cases of pleuropulmonary blastoma type I and type Ir (regressed): a report from the International PPB Registry (IPPBR). J Clin Oncol 30S:9522
Priest JR, McDermott MB, Bhatia S, Watterson J, Manivel JC, Dehner LP (1997) Pleuropulmonary blastoma: a clinicopathologic study of 50 cases. Cancer 80(1):147–161
Priest JR, Hill DA, Williams GM, Moertel CL, Messinger Y, Finkelstein MJ, Dehner LP, International Pleuropulmonary Blastoma R (2006) Type I pleuropulmonary blastoma: a report from the International Pleuropulmonary Blastoma Registry. J Clin Oncol 24(27):4492–4498. doi:10.1200/JCO.2005.05.3595
Priest JR, Williams GM, Mize WA, Dehner LP, McDermott MB (2010) Nasal chondromesenchymal hamartoma in children with pleuropulmonary blastoma—a report from the International Pleuropulmonary Blastoma Registry. Int J Pediatr Otorhinolaryngol 74(11):1240–1244. doi:10.1016/j.ijporl.2010.07.022
Pugh TJ, Yu W, Yang J, Field AL, Ambrogio L, Carter SL, Cibulskis K, Giannikopoulos P, Kiezun A, Kim J, McKenna A, Nickerson E, Getz G, Hoffher S, Messinger YH, Dehner LP, Roberts CW, Rodriguez-Galindo C, Williams GM, Rossi CT, Meyerson M, Hill DA (2014) Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene. doi:10.1038/onc.2014.150
Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, Pouchet C, Gilbert L, O’Brien PK, Serfas K, Broderick P, Houlston RS, Lesueur F, Bonora E, Muljo S, Schimke RN, Bouron-Dal Soglio D, Arseneau J, Schultz KA, Priest JR, Nguyen VH, Harach HR, Livingston DM, Foulkes WD, Tischkowitz M (2011) DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli–Leydig cell tumors. JAMA 305(1):68–77. doi:10.1001/jama.2010.1910
Sabbaghian N, Hamel N, Srivastava A, Albrecht S, Priest JR, Foulkes WD (2012) Germline DICER1 mutation and associated loss of heterozygosity in a pineoblastoma. J Med Genet 49(7):417–419. doi:10.1136/jmedgenet-2012-100898
Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, Jenkinson H, King D, Morland B, Pizer B, Prescott K, Saggar A, Side L, Traunecker H, Vaidya S, Ward P, Futreal PA, Vujanic G, Nicholson AG, Sebire N, Turnbull C, Priest JR, Pritchard-Jones K, Houlston R, Stiller C, Stratton MR, Douglas J, Rahman N (2011) DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet 48(4):273–278. doi:10.1136/jmg.2010.083790
Takeshita D, Zenno S, Lee WC, Nagata K, Saigo K, Tanokura M (2007) Homodimeric structure and double-stranded RNA cleavage activity of the C-terminal RNase III domain of human Dicer. J Mol Biol 374(1):106–120. doi:10.1016/j.jmb.2007.08.069
Uzomefuna V, Glynn F, Russell J, McDermott M (2012) Nasal chondromesenchymal hamartoma with no nasal symptoms. BMJ Case Rep. doi:10.1136/bcr.11.2011.5148
Williams GM, Priest JR, Finkelstein MJ, Harris A, Doros LA, Kratz C, Schultz KAP, Hill DA, Dehner LP, Messinger YM (2012) Effect of radiation on outcome of types II and III PPB: a report from the International Pleuropulmonary Blastoma Registry. J Clin Oncol 30S:9521
Witkowski L, Mattina J, Schonberger S, Murray MJ, Huntsman DG, Reis-Filho JS, McCluggage WG, Nicholson JC, Coleman N, Calaminus G, Schneider DT, Arseneau J, Stewart CJ, Foulkes WD (2013) DICER1 hotspot mutations in non-epithelial gonadal tumours. Br J Cancer 109(10):2744–2750. doi:10.1038/bjc.2013.637
Wu MK, Sabbaghian N, Xu B, Addidou-Kalucki S, Bernard C, Zou D, Reeve AE, Eccles MR, Cole C, Choong CS, Charles A, Tan TY, Iglesias DM, Goodyer PR, Foulkes WD (2013) Biallelic DICER1 mutations occur in Wilms tumours. J Pathol 230(2):154–164. doi:10.1002/path.4196
Yao-Lee A, Ryan M, Rajaram V (2011) Nasal chondromesenchymal hamartoma: correlation of typical MR. CT and pathological findings. Pediatr Radiol 41(5):675–677. doi:10.1007/s00247-011-2034-2
Acknowledgments
This work was supported by the Division of Cancer Epidemiology and Genetics (DCEG) of the National Cancer Institute’s Intramural Research Program. This research was supported by the Intramural Research Program of the NIH and the National Cancer Institute. D.A.H. is supported by National Cancer Institute R01CA143167.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments reported in this manuscript comply with the current laws of the United States.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, D.R., Messinger, Y., Williams, G.M. et al. Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder. Hum Genet 133, 1443–1450 (2014). https://doi.org/10.1007/s00439-014-1474-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-014-1474-9