Abstract
The complement system plays multiple roles in host defense against infection and is supposed to confer genetic susceptibility to leprosy. We aimed to examine whether genetic variants of the Ficolin-2 (FCN2), Mannose-binding lectin (MBL2) and Complement factor H (CFH) genes, which are involved in activation and regulation of the complement system, are associated with leprosy in Han Chinese from Southwest China. 527 leprosy patients and 583 matched controls were recruited from Yunnan Province, China, and were analyzed in this study. We sequenced the promoter region of the FCN2 and MBL2 genes and exon 8 of the FCN2 gene and genotyped three tag SNPs of the CFH gene. Association analysis was performed to discern potential effect of these three genes with leprosy and its subtypes. Luciferase assay was used to characterize the role of different promoter alleles of the FCN2 and MBL2 genes. Genetic variants of FCN2 (rs3811140 and rs7851696), MBL2 (rs11003125, rs7100749, rs11003124 and rs7096206) and CFH (rs1065489 and rs3753395) were significantly associated with leprosy and its subtypes. Haplotypes/genotypes representing low FCN2 and MBL2 transcriptional activity conferred risk to paucibacillary leprosy. Our data confirmed the expected positive association of complement genes with leprosy susceptibility and clinical outcomes in Han Chinese.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leprosy is a chronic endemic infectious disease caused by intracellular pathogen Mycobacterium leprae that affects skin and nerves (Britton and Lockwood 2004). Its clinical form can be classified along a spectrum of five subtypes [tuberculoid (TT), borderline-tuberculoid (BT), mid-borderline leprosy (BB), borderline-lepromatous (BL), and lepromatous (LL)] from tuberculoid leprosy characterized by a strong Th1 cellular immunity to lepromatous leprosy characterized by a strong Th2 humoral immunity, or can be simply classified into two subtypes [paucibacillary (PB; includes TT and BT) and multibacillary (MB; includes BB, BL and LL)] according to the latest WHO case definition (Britton and Lockwood 2004). The causal agent M. leprae, which cannot be cultured in vitro, is conserved between strains and has an eroded genome (Cole et al. 2001). Susceptibility and clinical outcomes of leprosy are highly determined by host genetic background and immune status (Misch et al. 2010). Genetic polymorphisms of many genes such as toll-like receptors (Bochud et al. 2008; Wong et al. 2010), tumor necrosis factor-alpha (TNFα), mannose-binding lectin (MBL2), vitamin D receptor (VDR) (Sapkota et al. 2010) and NOD2 (Zhang et al. 2009), to name a few, were reported to be associated with susceptibility to leprosy. For a more complete list of leprosy susceptible genes, one may refer to the Leprosy Susceptible Human Gene Database (LSHGD) (George Priya Doss et al. 2012). Therefore, leprosy is a good model for studying the genetic structure and etiopathogenesis of those infectious, inflammatory and autoimmune diseases (Misch et al. 2010).
The complement system plays multiple roles in host defense against infection. Activation of complement system results in opsonization of pathogens and immune complexes, recruitment of leukocytes, inflammation, and cell lysis (Ricklin et al. 2010). With up to 30 plasma and cell surface proteins, the complement system can be activated through three pathways. The classical pathway (CP) is initiated by antigen–antibody complexes followed by complement C1 activation (Cooper 1985). The lectin pathway (LP) is activated through recognition of certain pathogen surface by mannose-binding lectin (MBL) and ficolins (FCNs) (Endo et al. 2006). The alternative pathway is activated by C3 binding to microbial surfaces and to antibodies (Zipfel et al. 2007). These three activation pathways converge into a final common pathway when C3 convertase cleaves C3 into C3a and C3b, leading to the generation of opsonins, inflammatory peptides, and formation of the membrane attack complex (Ricklin et al. 2010). The involvement of complement system in leprosy has been noticed several decades ago (Saha and Chakraborty 1977; Saha et al. 1983). However, there are some controversies regarding the exact involvement and roles of the three complement pathways in leprosy (de Messias-Reason et al. 2007, 2009; Dornelles et al. 2006; Gomes et al. 2008; Saha and Chakraborty 1977; Saha et al. 1983).
The Ficolin-2 (FCN2) gene and Mannose-binding lectin (MBL2) gene encode soluble pattern recognition molecules that bind to different pathogen-associated molecular patterns, such as carbohydrates and lipoteichoic acid, leading to pathogen phagocytosis and activation of complement through the lectin pathway (Garred et al. 2009, 2010). The Complement factor H (CFH) gene encodes a soluble protein with 20 short consensus repeats (SCR, Sushi domain) and has an essential role in the regulation of complement activation (Rodríguez de Córdoba et al. 2004). It functions as a cofactor in the inactivation of C3b by factor I, as well as increases the rate of dissociation of C3 and C5 convertases in the alternative pathway (Zipfel et al. 2007). In this study, we analyzed genetic variants of the FCN2, MBL2 and CFH genes in 527 Han Chinese with leprosy and 583 matched controls from Yunnan, Southwest China, to investigate whether these two pathways are involved in leprosy. We showed evidence that these genes of both the lectin and alternative pathways confer genetic susceptibility to leprosy in Han Chinese.
Materials and methods
Subjects
Subjects recruited in this study were described in our recent studies (Li et al. 2011; Wang et al. 2012a). In brief, a total of 527 Han Chinese with leprosy (mean onset age 24.7 ± 12.3 years, containing 279 MB [109 LL, 145 BL and 25 BB] and 248 PB [175 TT and 73 BT]) and 583 healthy individuals (mean age 36.0 ± 15.5 years) were collected from Yunnan Province, Southwest China. Based on the comparison of matrilineal structure of the case and control populations, our samples were well matched and had no population stratification (Wang et al. 2012b). Informed consents conforming to the tenets of the Declaration of Helsinki were obtained from all participants prior to this study. The institutional review board of the Kunming Institute of Zoology approved this study.
Genotyping
Genomic DNA was extracted from whole blood by using the AxyPrep™ Blood Genomic DNA Miniprep Kit (Axygen, USA) following the manufacturer’s instructions. We used both sequencing and PCR-RFLP to genotype potentially functional variants in the promoter region of the FCN2 and MBL2 gene, and the exon 8 of the FCN2 gene, as well as three tag SNPs (rs3753394, rs3753395 and rs1065489) in the CFH gene. Purified PCR fragments were sequenced on ABI PRISM TM 3730xl DNA analyzer (Life Tech, Co. Ltd) using the BigDye® v3.1 dye terminator. For PCR-RFLP assay, PCR products were digested at 37 °C overnight with 1 U of EcoRV (for SNP rs3753394) and DdeI (for SNP rs1065489) (Fermentas, Thermo). Detailed information regarding the genotyped SNPs was listed in Table S1. Primers for genotyping were shown in Table S2. Quality control (QC) of our genotyping was performed by re-sequencing 5 % of total samples using both forward and reverse primers. Results of PCR-RFLP were verified by direct sequencing of 5 % of genotyped samples.
Haplotype analysis
Linkage disequilibrium (LD) plot of the three genes was constructed by Haploview 4.2 (Barrett et al. 2005). Haplotype reconstruction was conducted by PHASE 2.1 (Stephens et al. 2001). We further constructed the network of the MBL2 haplotypes by program NETWORK 4.6.1.0 (Bandelt et al. 1999).
Luciferase reporter assay
We constructed luciferase reporter plasmids by cloning promoter regions of the FCN2 and MBL2 genes containing different alleles/haplotypes into pGL3-Basic vector (Promega, Madison City, WI, USA). All inserts were confirmed by direct sequencing. The primers and enzymes used for plasmid construction were shown in Table S2.
HEK293T and HeLa cells were used for transient transfection to discern the potential effect of the FCN2 and MBL2 promoter regions with different haplotypes and/or alleles. Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad City, CA, USA) supplemented with 10 % fetal bovine serum (FBS) was used in cell culture. Cells were seeded in a 24-well plate at a density of 1 × 105 per well 12 h before transfection. After overnight culture, cells were co-transfected with 0.5 μg of each reporter vector and 0.05 μg of Renilla luciferase pRL-TK plasmid (Promega) using the X-tremeGENE HP transfection reagent (Roche, Indianapolis City, IN, USA). All transfection assays were performed in triplicate wells. After 24 h, cells were harvested and detected for luciferase activity on GloMax 96 Luminometer (Promega) following the Dual-Luciferase Reporter Assay System technical manual (Promega).
Statistical analysis
Deviation from the Hardy–Weinberg equilibrium (HWE) was assessed for each variant by using the Chi-square test (1 df). Cases and controls were compared for difference of allele, genotype and haplotype frequencies. Potential association between certain variant(s) and leprosy (including subtypes) was estimated by using the unconditional logistic regression model, with an adjustment of gender. The global difference in haplotype distribution between the cases and controls was estimated by the Chi-square test. Individual haplotype comparison was conducted by the Fisher’s exact test. All analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL). Power calculations were performed by using the Quanto software (Gauderman 2002). Luciferase activity was compared by one-way ANOVA test with Tukey’s post hoc tests using Prism 5 (GraphPad Software, San Diego, CA).
Results
Statistical power of the test
None of the genotyped SNPs showed any deviation from HWE in our control samples (a P value < 0.001 was regarded as a deviation from the HWE). We calculated the statistical power for all variants that presented positive associations in this study. The power of these variants given their specific odd ratios was expected to be above 85 % (excluding rs11003124 with a value of 76 %) (Table S1).
Association of the FCN2 variants with leprosy
Three SNPs (rs3124952, −986G/A; rs3124953, −602G/A; rs3811140, −557A/G) in the promoter region and two missense SNPs (rs17549193, c.707C>T, p.T236M; rs7851696, c.772G>T, p.A258S) in exon 8 of the FCN2 gene were identified (Table S1). Polymorphisms of rs3124952, rs3124953 and rs17549193 presented no association with leprosy. However, genotype GG of rs3811140 (OR = 2.227, 95 % CI = 1.083–4.587, P = 0.029) and genotype TT of rs7851696 (OR = 2.342, 95 % CI = 1.151–4.762, P = 0.019) were significantly associated with PB (Tables 1 and S3).
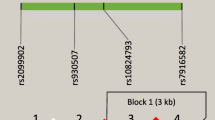
None of the five FCN2 SNPs were tightly linked according to the linkage disequilibrium (LD) structure (Fig. 1). Six haplotypes were reconstructed for the FCN2 promoter SNPs, which presented a significant global difference between leprosy patients and controls (P = 0.027). Major haplotype GGA and rare haplotype GAA showed a protective effect but the P values were marginally significant (Table 2). When the two nonsynonymous SNPs in exon 8 of the FCN2 gene were considered, haplotype GGACG (which has the highest frequency) conferred a protective effect against leprosy (OR = 0.789, 95 % CI = 0.661–0.943, P = 0.010) and PB subtype (OR = 0.746, 95 % CI = 0.598–0.932, P = 0.011). Note that these positive associations disappeared after Bonferroni correction (Tables 2 and S4).
The linkage disequilibrium (LD) structures of the FCN2 gene (a), the CFH gene (b) and the MBL2 gene (c) in leprosy patients and healthy controls. Results were performed based on the data obtained in this study. r 2 was used for the LD color scheme. Black squares represent high LD as measured by r 2, gradually coloring down to white squares of low LD. The individual square showed the 100 × r 2 value for each SNP pair. For the MBL2 gene, complete linkage disequilibrium was observed for block 1 containing rs11003123 and rs7095891 and block 2 containing rs11003124, rs7084554, rs36014597 and rs45560739. The five SNPs highlighted by dashed frame were chosen for subsequent analysis
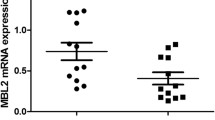
We constructed luciferase reporter vectors containing four major FCN2 promoter haplotypes (GGA, AGA, AAA and GGG) and transfected HEK293T and HeLa cells. Compared with haplotype GGA which has the highest frequency (0.74 in control population), haplotypes GGG and AGA had significantly lower transcriptional activity, whereas AAA had the highest activity, suggesting that these promoter haplotypes/alleles affected FCN2 expression (Fig. 2).
Luciferase assay for promoter fragments with different haplotypes and/or alleles of the FCN2 gene (a) and the MBL2 gene (b). HEK293T and HeLa cells were used for transfection. All assays were performed in triplicates for HEK293T. Shown are representative of eight (for the FCN2 gene) and four (for the MBL2 gene) independent experiments. Replication of the results on HeLa cells were performed in quadruplicate. Shown are representative of two independent experiments. One-way ANOVA test with Tukey’s post hoc tests were used for comparison. *P < 0.05, ***P < 0.0001, ns not significant
Association of the MBL2 variants with leprosy
We identified nine genetic variants (rs11003125, −619G/C; rs7100749, −504G/A; rs11003124, −496A/C; rs7084554, −418A/G; rs36014597, −405A/G; rs45560739, −391delAGAGAA; rs7096206, −290G/C; rs11003123, −139C/T; rs7095891, −66C/T) in promoter region of the MBL2 gene (Table S1). Complete linkage disequilibrium was observed at two regions: rs11003124 is linked with rs7084554, rs36014597 and rs45560739; and rs11003123 is linked with rs7095891 (Fig. 1). Therefore, only five SNPs (rs11003125, rs7100749, rs11003124, rs7096206 and rs7095891) were chosen for subsequent comparison and haplotype reconstruction.
All variants except for rs7095891 showed positive associations with leprosy or its subtypes (Tables 1 and S3). Genotype GC of rs11003125 showed a significant protective effect on MB patients (OR = 0.687, 95 % CI = 0.489–0.966, P = 0.031), while genotype GC of rs7096206 conferred a risk to PB (OR = 1.416, 95 % CI = 1.020–1.969, P = 0.038). Genotypes AA and GA of rs7100749 had different effects on leprosy subtypes [AA conferred a risk to MB (OR = 9.091, 95 % CI = 1.056–76.923, P = 0.044), GA had a protective effect against PB (OR = 0.458, 95 % CI = 0.268–0.782, P = 0.004)]. Minor allele C of rs11003124 conferred a risk to both leprosy per se (P = 0.007) and MB (P = 0.01) (Table 1).
Previous reports showed that circulating level of MBL was associated with genetic variants in exon 1 [rs5030737 (p.R52C), rs1800450 (p.G54D) and rs1800451 (p.G57E), known as D, B and C alleles, respectively] and the promoter region [rs11003125 (−619G/C), rs7096206 (−290G/C) and rs7095891 (−66C/T), known as H/L, Y/X and P/Q variants, respectively] (Madsen et al. 1994, 1995). Those SNPs formed the MBL “secretor” region (Garred et al. 2006; Madsen et al. 1998). We reconstructed the haplotypes based on the three promoter “secretor” SNPs. Distribution of the eight “secretor” haplotypes was significantly different between patients and controls (for leprosy per se, global P value = 0.025; for PB, global P value = 0.012) (Table 2). Haplotype CCC (LXP) showed a risk (OR = 1.482, 95 % CI = 1.135–1.936, P = 0.004) while CGC (LYP) conferred a protective effect (OR = 0.764, 95 % CI = 0.593–0.984, P = 0.039) in PB patients compared with controls. Comparison between MB and PB patients showed that haplotype CCC (LXP, 0.21 in PB and 0.12 in MB) was over-presented in PB (P < 0.001), whereas haplotype CGC (LYP, 0.20 in PB and 0.27 in MB) was over-presented in MB patients (P = 0.011). Distribution of haplotypes defined by all five MBL2 promoter SNPs was significantly different in patients compared with controls (global P value <0.001) (Table S4).
We constructed luciferase reporter vectors containing four major MBL2 “secretor” haplotypes GGC (HYP), CGC (LYP), CCC (LXP) and CCT (LXQ) and measured their relative promoter activity in HEK293T and HeLa cells. We found that truncated promoter fragment (without exon 1) had no transcriptional activity. The prevalent haplotype GGC (HYP) had the highest transcriptional activity, whereas CGC (LYP) and CCT (LXQ) had a medium transcriptional activity. The PB risk haplotype CCC (LXP) had a significantly lower promoter activity than the other three haplotypes, indicating potential MBL deficiency (Fig. 2).
We made a network for all “secretor” haplotypes to infer their evolutionary relationship. Ancestral haplotype LYP (CGC) was inferred based on the MBL2 gene sequences of Chimpanzee, Gorilla, Macaque and Gibbon retrieved from Ensembl (Fig. 3). These “secretor” haplotypes with higher or lower promoter activity were evolved from LYP with a medium activity. The derived haplotype HYP (GGC; with a high promoter activity and the highest frequency) might have an advantageous effect. Haplotype LXP (CCC; with a low activity) had a deleterious effect, as it was significantly over-presented in PB patients (0.211) versus controls (0.153). Distribution of the “secretor” haplotypes in leprosy per se or MB patients showed a similar profile compared with controls (Fig. 3).
Network analysis of promoter “secretor” haplotypes of the MBL2 gene in patients with leprosy and healthy controls. The numbers inside or outside the circles referred to the frequency of the haplotype in case and control populations. High, Medium and Low indicate the promoter activity of the four major determined haplotypes based on the luciferase assay. The “secretor” haplotypes were defined by rs11003125 (−619G/C, −550H/L), rs7096206 (−290G/C, −221Y/X) and rs7095891 (−66C/T, +4P/Q) and were named according to conventional nomenclature (HYP/GGC, LYP/CGC, LXP/CCC, LYQ/CGT, LXQ/CCT, HXP/GCC, HXQ/GCT, HYQ/GGT; see Table S1). Numbers on the branches “+4”, “−221”, “−550” indicate the SNPs +4P/Q, −221Y/X and −550H/L, respectively. Haplotype LXP in PB patients was the positively associated haplotype and was marked with asterisk. Ancestral haplotype LYP (ENSG00000165471) was inferred by comparison to chimpanzee (CTC; ENSPTRG00000002512), gorilla (CGC/LYP; ENSGGOG00000009057), macaque (CGC/LYP; ENSMMUG00000019110) and gibbon (CGC/LYP; ENSNLEG00000012005) from Ensembl (http://asia.ensembl.org/index.html)
Association of CFH Tag SNPs with leprosy
Among the analyzed tag SNPs (rs3753394, near gene-5′; rs3753395 in intron 12; rs1065489, c.2808G>T, p.E936D) of the CFH gene, two SNPs (rs1065489 and rs3753395) showed positive associations with leprosy (Tables 1 and S3). Genotype GT of rs1065489 was significantly associated with leprosy per se (P = 0.016) and PB (P = 0.014). Allele T of rs1065489 conferred a protective effect (OR = 0.834, 95 % CI = 0.703–0.988, P = 0.036) against leprosy. Genotype AT of rs3753395 was significantly associated with leprosy (P = 0.002) and its subtypes. Minor allele T of rs3753395 conferred a protective effect against leprosy (OR = 0.822, 95 % CI = 0.688–0.982, P = 0.031) and MB (OR = 0.787, 95 % CI = 0.634–0.976, P = 0.030) (Tables 1 and S3).
LD structure showed that these CFH SNPs were in weak linkage (Fig. 1). Distribution of haplotypes (rs3753394–rs3753395–rs1065489) was significantly different between patients and controls (for leprosy per se, global P value = 0.009; for MB, global P value = 0.014). Haplotype CAG harboring both major alleles of rs3753395 and rs1065489 showed a strong risk to leprosy (for leprosy per se, OR = 1.499, 95 % CI = 1.188–1.891, P = 0.0006; for MB, OR = 1.527, 95 % CI = 1.161–2.008, P = 0.002; for PB, OR = 1.468, 95 % CI = 1.102–1.957, P = 0.008), while haplotype CTT conferred a weak protective effect on leprosy per se (P = 0.032) and MB (P = 0.016) (Table 2).
Discussion
Leprosy is considered as a genetic disease (Alter et al. 2011) and can be used as a good model to study the innate immune response to intracellular infection (Modlin 2010). The M. leprae cell surface is rich in 6-lipoarabinomannan (LAM), a mannose-containing carbohydrate (Sato and Imi 1968), thus had a binding potential to ficolin-2 and MBL. It has been shown that normal ficolin-2 level confers protection against leprosy and low MBL level confers a protective effect against LL in Brazilian patients (de Messias-Reason et al. 2007, 2009), while Dornelles et al. (2006) reported that deficiency of MBL had a protective effect on MB. Serum level of ficolin-2 was associated with genetic variants in the promoter and exon 8 of this gene (Hummelshoj et al. 2005). Genetic variants in the promoter and exon 1 of the MBL2 gene were also reported to have a significant effect on its circulating level (Garred et al. 2006). Functional genetic variants of these two genes have also been identified as the cause of many common immunodeficiency and infectious diseases (Dommett et al. 2006; Faik et al. 2011). The CFH gene has an essential role in regulation of the alternative pathway (Rodríguez de Córdoba et al. 2004). Genetic variants in the CFH gene have been reported to be associated with many diseases (Caprioli et al. 2003; Thakkinstian et al. 2006). Recently, genome-wide association study showed that CFH gene variants were associated with host susceptibility to meningococcal disease (Davila et al. 2010).
It was reported that the complement system assists the immune escape of intracellular mycobacteria, such as M. tuberculosis and Mycobacterium bovis BCG, by binding of C3 to the mycobacteria through the classical, alternative or lectin pathway (Carroll et al. 2009; Ferguson et al. 2004; Rooijakkers and van Strijp 2007). However, whether all three complement pathways were involved in leprosy remains controversial. In this study, we genotyped functional and/or tag SNPs of the FCN2, MBL2 and CFH genes in Han Chinese patients with leprosy from Yunnan, China, followed by experimental validation. We identified positive associations of genetic variants in these three genes with leprosy and its subtypes (Tables 1, 2), providing evidence for an active involvement of both lectin pathway and alternative pathway in leprosy.
Both genotypes GG of rs3811140 (representing a low serum ficolin-2 level) and TT of rs7851696 in the FCN2 gene showed a significant risk to PB in our study. Munthe-Fog et al. (2007) reported that genotype TT of rs7851696 was related to a significantly lower level of ficolin-2. Compared with allele G, allele T of rs7851696 had a remarkable increase in binding capacity towards N-acetylglucosamine (Hummelshoj et al. 2005). These reports indicated that individuals with low serum level and high binding capacity of ficolin-2 might be more sensitive to M. leprae invasion and easily developed PB leprosy. Indeed, normal ficolin-2 level was shown to protect leprosy in Brazilians (de Messias-Reason et al. 2009). We found that the most frequent FCN2 haplotype GGA, which has a medium promoter activity, showed a trend of protective effect against leprosy patients in Han Chinese (Table 2; Fig. 2), adding further support to those reported results. None of the five FCN2 variants analyzed in this study showed any signal as expression quantitative trait loci (eQTLs, which are genomic loci that regulate gene expression) by searching the eQTL database Genevar (http://www.sanger.ac.uk/resources/software/genevar/). However, rs3124952 (−986G/A) and rs7851696 [+6424G/T (p.A258S)] of the FCN2 gene showed positive signals as eQTL for nearby genes in HapMap CHB population (Fig. S3). eQTLs for FCN2 were mainly located in the COL5A1 gene which is upstream of FCN2, but there is no report for an interaction between FCN2 and its nearby genes so far. Although we did not link any of the analyzed FCN2 SNPs with eQTLs, we found that the FCN2 promoter variants affected transcriptional activity.
Previous studies on MBL2 and leprosy had conflicting results. Fitness et al. (2004) found no association of MBL2 variants with PB patients from Africa. In Brazilian patients, de Messias-Reason et al. (2007) reported a positive association of MBL2 high-expression haplotype with leprosy and a protective effect of low-expression haplotypes/genotypes on LL, but this result was not confirmed by Vasconcelos et al. (2011). In this study, we found that the MBL2 promoter polymorphisms were strongly associated with leprosy in Han Chinese. Similar to FCN2, our results showed that low level of MBL2 transcriptional activity conferred a risk to PB, while a medium level of MBL2 transcriptional activity had a protective effect against PB (Table 2). Compared with PB patients, low level of MBL2 transcriptional activity had a protective effect for MB (Table 2). None of the nine MBL2 promoter variants analyzed in this study were reported as eQTLs. However, we found that SNPs rs11595039, rs2384188, rs2173519 and rs12411335 in the upstream region of the MBL2 gene were eQTLs for this gene. Intriguingly, we found that truncated promoter region without exon 1 of the MBL2 gene had no promoter activity in luciferase assay, indicating an essential role of exon 1 for transcription. The exon 1 region contains three MBL deficiency-related variants p.R52C, p.G54D and p.G57E, which were reported to affect MBL oligomerization (Garred et al. 2006). We speculate that exon 1 altered both function and serum level of MBL, as demonstrated by the luciferase assay (Fig. 2).
We used online tool AliBaba (http://www.gene-regulation.com/pub/programs/alibaba2/index.html) to predict potential transcription factor binding sites in the promoter region of the FCN and MBL2 genes. Some of genotyped promoter SNPs were located within regions enriched for transcription factor binding sites. As we did not perform any experiments to confirm this prediction, we could only deduce the role of specific SNPs responsible for the difference in luciferase activity by comparing the sequence of promoter haplotype. Based on the results in Fig. 2, it seemed that the three promoter SNPs of FCN2 and two SNPs (H/L and X/Y) of MBL2 were causal variants responsible for the observed difference of the promoter activity.
We further compared the distribution pattern of major FCN2 and MBL2 promoter haplotypes in all five leprosy clinical subtypes (Figs. S1, S2). There was no statistically significant difference among all seven groups (Control, TT, BT, BB, BL, LL and leprosy per se). When we pooled these FCN2 and MBL2 haplotypes with similar promoter activity (medium group and low group), we found that FCN2 haplotype frequencies were similar in all subtypes except for BB (Fig. S2a). Note that such a pattern should be received with caution as the sample size for BB was too small. Nonetheless, the apparent distinctness emerging for BB needs attention. The distribution of MBL2 haplotypes presented significant difference in TT leprosy relative to other subtypes (Fig. S2b), which was inconsistent with previous studies that reported a prevalent association of MBL2 variants with LL (Table S7). Note that positive associations were mainly observed in subtypes rather than in leprosy per se. As the clinical features between the two leprosy subtypes PB and MB are very different, we would expect that these associations might reflect different molecular mechanisms or genetic bases between disease susceptibility and process. We speculated that the positively associated lectins may be involved in pathogen clearance as opsonin, thus affect the bacteria load in PB and MB. Collectively, our results for the MBL2 and FCN2 genes in Han Chinese indicated that the lectin pathway contributed to activation of complement system in leprosy, especially for PB patients.
The complement factor H inactivates the alternative complement pathway and is often used to mediate immune evasion by pathogens (Rooijakkers and van Strijp 2007; Vogl et al. 2008). Genetic variants of this gene were also reported to be associated with age-related macular degeneration and pneumococcal meningitis (Davila et al. 2010; Thakkinstian et al. 2006). We found that rs1065489 and rs3753395 of CFH were positively associated with leprosy at allele, genotype and haplotype levels, suggesting an active role of this gene in leprosy. Three SNPs (rs10733086, rs1410997 and rs1831282; Fig. S3) that were located in the CFH gene showed a signal as eQTL for CFH, but these SNPs were not linked with rs3753395 and rs1065489. Thus, the positive association of rs3753395 and rs1065489 with leprosy may not reflect potential difference in CFH expression between the case and control groups. We also identified several SNPs that were located in nearby genes as eQTLs for the CFH gene (Fig. S3). We searched dbSNP, HapMap and related references for potential functional variants in the CFH gene that were tagged by rs3753395 and rs1065489 (Table S5). None was found to be potentially causal excluding rs1065489. However, no chemical property or structural change that would affect regulation of the alternative pathway was reported for this variant (Boon et al. 2008; Caprioli et al. 2003).
The current study has several limitations. First, we lacked further experiments to characterize the specific role of those SNPs showing an association with leprosy per se and/or its subtypes. Second, heterozygous genotypes of MBL2 SNPs (rs11003125, rs7100749, rs11003124 and rs7096206) presented an association with leprosy subtype, but we had no available data to explain the possible heterozygous effect. Third, although we observed functional effect of different haplotypes of FCN2 and MBL2 genes in our luciferase assay, the exact role of these haplotypes in leprosy needs further elucidation. Finally, we only analyzed three tag SNPs for the CFH gene, which had limited capacity to cover the entire gene. Considering the important role of the CFH gene in different diseases (Davila et al. 2010; Thakkinstian et al. 2006), it is worthwhile to genotype more tag SNPs in the future.
In summary, we found that genetic variants of the FCN2, MBL2 and CFH genes of the lectin and alternative pathways confer genetic susceptibility to leprosy in Han Chinese. Combined with available knowledge from literatures (Table S6), we suggested that all three complement pathways were involved in leprosy. Further independent replication analyses and functional assays are needed to validate our findings.
References
Alter A, Grant A, Abel L, Alcais A, Schurr E (2011) Leprosy as a genetic disease. Mamm Genome 22:19–31
Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265
Bochud PY, Hawn TR, Siddiqui MR, Saunderson P, Britton S, Abraham I, Argaw AT, Janer M, Zhao LP, Kaplan G, Aderem A (2008) Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J Infect Dis 197:253–261
Boon CJ, Klevering BJ, Hoyng CB, Zonneveld-Vrieling MN, Nabuurs SB, Blokland E, Cremers FP, den Hollander AI (2008) Basal laminar drusen caused by compound heterozygous variants in the CFH gene. Am J Hum Genet 82:516–523
Britton WJ, Lockwood DN (2004) Leprosy. Lancet 363:1209–1219
Caprioli J, Castelletti F, Bucchioni S, Bettinaglio P, Bresin E, Pianetti G, Gamba S, Brioschi S, Daina E, Remuzzi G, Noris M (2003) Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet 12:3385–3395
Carroll MV, Lack N, Sim E, Krarup A, Sim RB (2009) Multiple routes of complement activation by Mycobacterium bovis BCG. Mol Immunol 46:3367–3378
Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG (2001) Massive gene decay in the leprosy bacillus. Nature 409:1007–1011
Cooper NR (1985) The classical complement pathway: activation and regulation of the first complement component. Adv Immunol 37:151–216
Davila S, Wright VJ, Khor CC, Sim KS, Binder A, Breunis WB, Inwald D, Nadel S, Betts H, Carrol ED, de Groot R, Hermans PW, Hazelzet J, Emonts M, Lim CC, Kuijpers TW, Martinon-Torres F, Salas A, Zenz W, Levin M, Hibberd ML (2010) Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet 42:772–776
de Messias-Reason IJ, Boldt AB, MoraesBraga AC, von Rosen Seeling Stahlke E, Dornelles L, Pereira-Ferrari L, Kremsner PG, Kun JF (2007) The association between mannan-binding lectin gene polymorphism and clinical leprosy: new insight into an old paradigm. J Infect Dis 196:1379–1385
de Messias-Reason I, Kremsner PG, Kun JF (2009) Functional haplotypes that produce normal ficolin-2 levels protect against clinical leprosy. J Infect Dis 199:801–804
Dommett RM, Klein N, Turner MW (2006) Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens 68:193–209
Dornelles LN, Pereira-Ferrari L, Messias-Reason I (2006) Mannan-binding lectin plasma levels in leprosy: deficiency confers protection against the lepromatous but not the tuberculoid forms. Clin Exp Immunol 145:463–468
Endo Y, Takahashi M, Fujita T (2006) Lectin complement system and pattern recognition. Immunobiology 211:283–293
Faik I, Oyedeji SI, Idris Z, de Messias-Reason IJ, Lell B, Kremsner PG, Kun JF (2011) Ficolin-2 levels and genetic polymorphisms of FCN2 in malaria. Hum Immunol 72:74–79
Ferguson JS, Weis JJ, Martin JL, Schlesinger LS (2004) Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun 72:2564–2573
Fitness J, Floyd S, Warndorff DK, Sichali L, Mwaungulu L, Crampin AC, Fine PE, Hill AV (2004) Large-scale candidate gene study of leprosy susceptibility in the Karonga district of northern Malawi. Am J Trop Med Hyg 71:330–340
Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO (2006) Mannose-binding lectin and its genetic variants. Genes Immun 7:85–94
Garred P, Honoré C, Ma YJ, Munthe-Fog L, Hummelshøj T (2009) MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement. Mol Immunol 46:2737–2744
Garred P, Honoré C, Ma YJ, Rorvig S, Cowland J, Borregaard N, Hummelshøj T (2010) The genetics of ficolins. J Innate Immun 2:3–16
Gauderman WJ (2002) Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med 21:35–50
George Priya Doss C, Nagasundaram N, Srajan J, Chiranjib C (2012) LSHGD: a database for human leprosy susceptible genes. Genomics 100:162–166
Gomes GI, Nahn EP Jr, Santos RK, Da Silva WD, Kipnis TL (2008) The functional state of the complement system in leprosy. Am J Trop Med Hyg 78:605–610
Hummelshoj T, Munthe-Fog L, Madsen HO, Fujita T, Matsushita M, Garred P (2005) Polymorphisms in the FCN2 gene determine serum variation and function of ficolin-2. Hum Mol Genet 14:1651–1658
Li Y–Y, Li X-A, He L, Wang D, Chen W-Y, Chen L, Lu JB, Yao Y-G (2011) Trends in new leprosy case detection over 57 years (1952–2008) in Yuxi, Yunnan Province of Southwest China. Lepr Rev 82:6–16
Madsen HO, Garred P, Kurtzhals JA, Lamm LU, Ryder LP, Thiel S, Svejgaard A (1994) A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics 40:37–44
Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, Svejgaard A (1995) Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol 155:3013–3020
Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P (1998) Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol 161:3169–3175
Misch EA, Berrington WR, Vary JC Jr, Hawn TR (2010) Leprosy and the human genome. Microbiol Mol Biol Rev 74:589–620
Modlin RL (2010) The innate immune response in leprosy. Curr Opin Immunol 22:48–54
Munthe-Fog L, Hummelshøj T, Hansen BE, Koch C, Madsen HO, Skjødt K, Garred P (2007) The impact of FCN2 polymorphisms and haplotypes on the ficolin-2 serum levels. Scand J Immunol 65:383–392
Ricklin D, Hajishengallis G, Yang K, Lambris JD (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797
Rodríguez de Córdoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sánchez-Corral P (2004) The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol 41:355–367
Rooijakkers SH, van Strijp JA (2007) Bacterial complement evasion. Mol Immunol 44:23–32
Saha K, Chakraborty AK (1977) Serum complement profile in human leprosy and its comparison with immune complex diseases. Int J Lepr Mycobact Dis 45:327–337
Saha K, Sharma V, Chakrabarty AK, Sehgal VN (1983) Breakdown product of factor B as an index of complement activation in lepromatous leprosy and its relation with bacillary load. Scand J Immunol 17:37–43
Sapkota BR, Macdonald M, Berrington WR, Misch EA, Ranjit C, Siddiqui MR, Kaplan G, Hawn TR (2010) Association of TNF, MBL, and VDR polymorphisms with leprosy phenotypes. Hum Immunol 71:992–998
Sato S, Imi M (1968) The surface structure of M. leprae. Int J Lepr Mycobact Dis 36:303–308
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Thakkinstian A, Han P, McEvoy M, Smith W, Hoh J, Magnusson K, Zhang K, Attia J (2006) Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet 15:2784–2790
Vasconcelos LR, Fonseca JP, do Carmo RF, de Mendonca TF, Pereira VR, Lucena-Silva N, Pereira LM, Moura P, Cavalcanti Mdo S (2011) Mannose-binding lectin serum levels in patients with leprosy are influenced by age and MBL2 genotypes. Int J Infect Dis 15: e551–557
Vogl G, Lesiak I, Jensen DB, Perkhofer S, Eck R, Speth C, Lass-Flörl C, Zipfel PF, Blom AM, Dierich MP, Würzner R (2008) Immune evasion by acquisition of complement inhibitors: the mould Aspergillus binds both factor H and C4b binding protein. Mol Immunol 45:1485–1493
Wang D, Feng J-Q, Li Y–Y, Zhang D-F, Li X-A, Li Q-W, Yao Y-G (2012a) Genetic variants of the MRC1 gene and the IFNG gene are associated with leprosy in Han Chinese from Southwest China. Hum Genet 131:1251–1260
Wang D, Su L-Y, Zhang A-M, Li Y–Y, Li X-A, Chen L–L, Long H, Yao Y-G (2012b) Mitochondrial DNA copy number, but not haplogroup, confers a genetic susceptibility to leprosy in Han Chinese from Southwest China. PLoS ONE 7:e38848
Wong SH, Gochhait S, Malhotra D, Pettersson FH, Teo YY, Khor CC, Rautanen A, Chapman SJ, Mills TC, Srivastava A, Rudko A, Freidin MB, Puzyrev VP, Ali S, Aggarwal S, Chopra R, Reddy BS, Garg VK, Roy S, Meisner S, Hazra SK, Saha B, Floyd S, Keating BJ, Kim C, Fairfax BP, Knight JC, Hill PC, Adegbola RA, Hakonarson H, Fine PE, Pitchappan RM, Bamezai RN, Hill AV, Vannberg FO (2010) Leprosy and the adaptation of human toll-like receptor 1. PLoS Pathog 6:e1000979
Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Cheng H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LG, Yin G, Jiang ZX, Wang XD, Yu JP, Hu ZH, Gong CH, Liu YQ, Liu RY, Wang DM, Wei D, Liu JX, Cao WK, Cao HZ, Li YP, Yan WG, Wei SY, Wang KJ, Hibberd ML, Yang S, Zhang XJ, Liu JJ (2009) Genomewide association study of leprosy. N Engl J Med 361:2609–2618
Zipfel PF, Mihlan M, Skerka C (2007) The alternative pathway of complement: a pattern recognition system. Adv Exp Med Biol 598:80–92
Acknowledgments
We thank all the participants in this study and Mr. Mei-Sheng Xiao and Miss Ling Xu for assistance with luciferase assay. This study was supported by the National Natural Science Foundation of China (31271346, 30925021 and 81260237), Top Talents Program of Yunnan Province (2009CI119) and the Chinese Academy of Sciences.
Conflict of interest
All authors declare no potential conflicts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, DF., Huang, XQ., Wang, D. et al. Genetic variants of complement genes Ficolin-2, Mannose-binding lectin and Complement factor H are associated with leprosy in Han Chinese from Southwest China. Hum Genet 132, 629–640 (2013). https://doi.org/10.1007/s00439-013-1273-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-013-1273-8