Abstract
We report a consanguineous Pakistani family with seven affected individuals showing a syndromic form of congenital microcephaly. Clinical features of affected individuals include congenital microcephaly with sharply slopping forehead, moderate to severe mental retardation, anonychia congenita, and digital malformations. By screening human genome with microsatellite markers, this autosomal recessive condition was mapped to a 25.2 cM interval between markers D18S1150 and D18S1100 on chromosome 18p11.22–q12.3. However, the region of continuous homozygosity between markers D18S1150 and D18S997 spanning 15.33 cM, probably define the most likely candidate region for this condition. This region encompasses a physical distance of 12.03 Mb. The highest two-point LOD score of 3.03 was obtained with a marker D18S1104 and multipoint score reached a maximum of 3.43 with several markers. Six candidate genes, CEP76, ESCO1, SEH1L, TUBB6, ZNF519, and PTPN2 were sequenced, and were found to be negative for functional sequence variants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microcephaly is a clinical term defined as reduced head circumference of at least three standard deviations below the age- and sex-related means, and reflects an underlying reduction in brain volume (Woods et al. 2005). Primary microcephaly (MCPH, MIM-251200) solely refers to reduced cerebral cortex along with non-progressive mental retardation and without other developmental abnormalities or neurological deficits. MCPH brains exhibit normal architecture suggesting that microcephaly is a consequence of reduced cell number either as a result of reduced neuro-progenitor division or increased apoptosis during neurogenesis (O’Driscoll et al. 2006).
Syndromic forms of congenital anomalies including prenatal or postnatal microcephaly as a cardinal feature have been well documented. Clinical manifestations other than microcephaly differentiate one syndrome from the other; sometimes a particular feature marks a specific syndrome. Seckel syndrome (MIM-210600) and Filippi syndrome (MIM-272440) are two entities in which microcephaly, mental retardation, short stature and digital malformations are common. A ‘bird-headed’ facial appearance is an important feature for diagnosis of Seckel syndrome while digital anomalies and severe mental retardation are distinctive marks in Filippi syndrome (Filippi 1985; Woods et al. 1992; Goodship et al. 2000; Borglum et al. 2001; Kilinç et al. 2003; Sharif and Donnai 2004). Genetic mapping techniques identified three loci for Seckel syndrome: ATR-SKL1 at chromosome 3q22–q24 (Goodship et al. 2000), SKL2 at chromosome 18p11.31–q11.2 (Borglum et al. 2001), SKL3 at chromosome 14q21–q22 (Kilinç et al. 2003); however, no results have been reported about association of a chromosomal region with Filippi syndrome. Mutations in a gene encoding ataxia-telangiectasia and RAD3-related protein (ATR) were found responsible for ATR-SKL1 (O’Driscoll et al. 2003).
In the present study, we have ascertained a consanguineous Pakistani family with four males and three females, affected with congenital microcephaly associated with several other deformities. Further by genome scan using microsatellite markers, we have established linkage of this condition on chromosome 18p11.22–q11.2.
Materials and methods
Family history
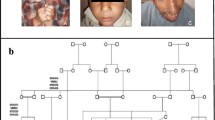
Before the start of the study, approval was obtained from the Quaid-I-Azam University Institutional Review Board. Informed consent was obtained from all family members who agreed to participate in the study. This family belongs to a remote mountainous village of Northwest Frontier Province of Pakistan. The family pedigree shows four generations with seven affected and thirteen normal individuals (Fig. 1). The pedigree of the family provided convincing evidence of autosomal recessive mode of inheritance. DNA from nine individuals including five affected was available for genotyping. The affected individuals were examined by medical physicians at the local hospital.
Clinical findings
Ages of the affected individuals vary between 14 and 28 years at the time of the study. There was recognizable learning deficiency in all affected individuals; they were unable to read or write but have acquired certain self-help skills. All affected individuals have striking microcephaly at birth with sharply sloping forehead (Fig. 2a). On examination head circumferences were 5–7 standard deviations below the age- and sex-related means. Moderate to severe, non-progressive mental retardation was observed in all the affected individuals. There was no sex difference in degree of microcephaly or mental retardation. Total absence of fingernails and toenails (anonychia congenita) were observed in all the affected individuals (Fig. 2b–d). Digital malformations of variable degree (Fig. 2c, d) including polydactyly of fingers and toes and syndactyly of second and third toe (synpolydactyly) were present in the patients. Few white spots were present on the skin of hands and feet in all affected individuals (Fig. 2b).
To exclude possibility of chromosomal aberrations as cause of the syndrome, G-banding chromosomal analysis was carried out in two affected individuals of the family.
Extraction of genomic DNA and genotyping
Genomic DNA was extracted from venous blood samples, collected from a total of nine individuals, including five affected (IV-2, IV-3, IV-5, IV-6, IV-7). Genome scan was carried out using a total of 430 microsatellite markers spaced on an average distance of 7 cM on the nuclear genome. PCR was performed according to standard procedure in a total volume of 25 μl with 40 ng of genomic DNA, 240 nM of each primer, 200 μM of each dNTPs, 1.5–2 mM MgCl2, 1 unit of Taq DNA polymerase and 1× PCR buffer (MBI Fermentas, Sunderland, UK). PCR was carried out for 35 cycles: 95°C for 1 min, 57°C for 1 min and 72°C for 1 min in a T3000 thermal cycler (Biometra, Germany). PCR products were resolved on 8% non-denaturing polyacrylamide gel, stained with ethidium bromide and the genotypes were assigned by visual inspection.
Linkage analysis
The order of genome scan and fine mapping markers were determined based on the National Center for Biotechnology Information (NCBI) Build 36.1 sequence-based physical map (International Human Genome Sequence Consortium 2001). Genetic map distances were then derived from the Rutgers combined linkage-physical map of the human genome (Kong et al. 2004). PEDCHECK (O’Connell and Weeks 1998) was used to identify Mendelian inconsistencies while the MERLIN (Abecasis et al. 2002) program was utilized to detect potential genotyping errors. Haplotypes were constructed using SIMWALK2 (Weeks et al. 1995; Sobel and Lange 1996). Two-point linkage analysis was carried out on fine mapping markers using the MLINK program of the FASTLINK computer package (Cottingham et al. 1993). Multipoint linkage analysis was performed using ALLEGRO (Gudbjartsson et al. 2005). An autosomal recessive mode of inheritance with complete penetrance and a disease allele frequency of 0.001 were used for the analysis. Equal marker allele frequencies were used for the fine mapping markers, because it was not possible to estimate allele frequencies from the founders, since these markers were only genotyped in this family.
Candidate gene sequencing
Using Primer3 software (Rozen and Skaletsky 2000), primers were designed for amplification of the exons and splice junction sites of the following candidate genes: CEP76 (NM_024899.2), ESCO1 (NM_052911), SEH1L (NM_001013437), TUBB6 (NM_0325325.1), ZNF519 (NM_145287.1), and PTPN2 (NM_080422). DNA from two affected and an unaffected individual were diluted to 5 ng/μl, amplified by PCR under standard conditions, and purified with Rapid PCR Amplification System (Marligen Biosciences Ijamsville, MD, USA). Sequencing was performed using a Big Dye Terminator v3.1 Cycle Sequencing Kit, together with an ABI-Prism Automated DNA Sequencer (Applera Corporation, Cleveland, OH, USA).
Results
To identify the gene underlying the syndromic form of congenital microcephaly (Jawad syndrome), we followed a classical linkage analysis approach. Prior to embarking on genome-wide scan, we performed co-segregation and homozygosity analysis with microsatellite markers corresponding to candidate regions involved in the related phenotypes. Exclusion mapping was performed for six known autosomal recessive primary microcephaly loci (MCPH1–MCPH6) as described previously (Gul et al. 2006a, b). Examination of the haplotypes showed that the affected individuals were heterozygous for different combinations of the parental alleles and thus linkage based upon homozygosity to the candidate loci was excluded.
After exclusion of the known primary microcephaly loci, a genome scan was carried out using DNA samples initially from five affected individuals (IV-2, IV-3, IV-5, IV-6, and IV-7) of the family (Fig. 1). In the course of screening 430 highly polymorphic microsatellite markers spaced approximately 7 cM apart, from Rutgers Combined Linkage-Physical map (Kong et al. 2004), 4 markers including D3S3053 (3q26.31), D11S1304 (11q25), D13S800 (13q22.1), and D18S1104 (18q11.2) were found to be homozygous in all five affected family subjects. Upon testing rest of the family members, linkage to three of these regions was excluded. All affected members were homozygous and unaffected members were heterozygous at marker D18S1104, located at 47.2 cM on chromosome 18q11.2. This region was saturated further by typing 33 additional markers selected from the Rutgers Combined Linkage-Physical map (Kong et al. 2004). Fifteen markers (D18S1149, D18S45, D18S40, D18S37, D18S73, D18S1114, D18S453, D18S71, D18S482, D18S1116, D18S542, D18S1150, D18S1153, D18S464, D18S843) were proximal to genome scan marker D18S1104, while 18 markers (D18S869, D18S480, D18S1108, D18S1107, D18S866, D18S997, D18S478, D18S1151, D18S877, D18S463, D18S36, D18S456, D18S1133, D18S1100, D18S1128, D18S1130, D18S1123, D18S1143) lie distal to D18S1104.
After genotyping all the family members with these markers, the data were analyzed using two-point and multipoint linkage analysis. Seventeen of the markers (D18S1149, D18S45, D18S40, D18S37, D18S73, D18S1114, D18S482, D18S1116, D18S1153, D18S869, D18S1107, D18S866, D18S478, D18S1151, D18S463, D18S456, D18S1128) were un-informative and, therefore, not included in the analysis. Analysis of the marker genotypes within this region with PEDCHECK (O’Connell and Weeks 1998) did not elucidate any genotyping errors.
Table 1 summarizes the two-point and multipoint LOD scores obtained for a total of 17 markers. The maximum two-point LOD score of 3.03 (θ = 0.00) was obtained with marker D18S1104 and the maximum multi-point LOD score of more than 3 was obtained with several markers, including D18S542, D18S71, D18S453, D18S1104, D18S480 and D18S1108, which support linkage to this region.
Haplotypes using SIMWALK2 (Weeks et al. 1995; Sobel and Lange 1996) were constructed to determine the linkage interval. A recombination event between markers D18S1150 and D18S542 defined the centromeric boundary of this interval, and it was observed in the two affected individuals (IV-3 and IV-7). The telomeric boundary of the interval was defined by a historic recombination event that occurred between markers D18S1108 and D18S997 in affected individuals IV-5, IV-6, and IV-7. Two markers D18S36 and D18S1133 distal to D18S997 showed homozygous pattern in all the affected individuals, which is probably due to lack of information in the parent (III-5). Therefore, the region of continuous homozygosity spanning between markers D18S1150 and D18S997 is 15.33 cM according to Rutgers combined linkage-physical map of the human genome (Kong et al. 2004). This region corresponds to 12.03 Mb according to the sequence-based physical map Build 36 of the human genome (International Human Genome Sequence Consortium 2001).
Within this homozyogosity region of 12.03 Mb at chromosome 18p11.22–q11.2, 111 genes are present (National Centre for Biotechnology Information http://www.ncbi.nlm.nih.gov/). Sequencing of six candidate genes including CEP76, ESCO1 (MIM-609674), SEH1L (MIM-609263), TUBB6, ZNF519 and PTPN2 (MIM-176887) failed to reveal the functional sequence variant.
Discussion
In the present investigation, we have studied a four-generation Pakistani family segregating a condition (Jawad syndrome) with clinical features sharing with previously described Filippi syndrome (MIM-272440) and Seckel syndrome (MIM-210600). However, there are clinical features including total absence of finger and toe nails (anonychia), observed in the affected individuals of our family that were not reported in the other two cases. In addition, the affected individuals of our family are not short-statured nor do they have any vision defect as reported in individuals with Seckel syndrome. We believe that the combination of clinical features observed in the affected individuals of our family is unique and therefore the name “Jawad Syndrome” has been assigned to this condition.
The disorder, presented here, demonstrated linkage to a 25.2 cM trans-centromeric region on chromosome 18p11.22–q12.3, between markers D18S1150 (37.42 cM) and D18S1100 (62.62 cM) according to Rutgers Combined Linkage-Physical map (Kong et al. 2004). However, the region of continuous homozygosity in the affected individuals between markers D18S1150 and D18S997 spanning 15.33 cM, probably defines the most likely candidate region for this condition.
The linkage analysis data clearly show that in our family, the candidate region overlaps with Seckel syndrome locus (SKL2, MIM-606744) mapped to a 32.30 cM interval between markers D18S78 (19.89 cM) and D18S866 (52.19 cM) on chromosome 18p11.31–q11.2 (Borglum et al. 2001). These two conditions share 14.77 cM (11.38 Mb) region on chromosome 18. The mapping of these two conditions to the same chromosomal region does not justify however that affected individuals of our family have Seckel syndrome with variable expression.
Of the genes that lie in the 12.03 Mb-region of Jawad syndrome, six candidate genes CEP76, ESCO1, SEH1L, TUBB6, ZNF519, and PTPN2 were selected for sequencing in our family. The CEP76 (centrosomal protein of 76 KD) is an ortholog of murin Cep76, which is a centrosomal protein and is thought to be involved in cell shape, polarity, motility and division (Andersen et al. 2003; Lim et al. 2006). The ESCO (establishment of cohesion 1) is an acetyltransferase required for the establishment of sister chromatid cohesion and couples the processes of cohesion and DNA replication (Bellows et al. 2003; Hou and Zou 2005). The SEH1L (Sec13-like protein isoform 1 like) encodes a protein, which is part of a nuclear pore complex, Nup107–160. This protein contains WD repeats and shares 34% amino acid identity with yeast Seh1 and 30% identity with yeast Sec13. It specifically localizes to kinetochores in mitosis (Cronshaw et al. 2002). Involvement of Tubulin beta-6 (TUBB6) in neurogenesis makes it a strong candidate for neuro-developmental disorders (Campbell et al. 2004). The ZNF519 belongs to the krueppel C2H2-type zinc-finger protein family and it contains 10 C2H2-type zinc fingers and 1 KRAB domain and may be involved in transcriptional regulation, which makes it a candidate for Jawad syndrome. Protein encoded by protein tyrosine phosphatase, non-receptor type (PTPN2) is a member of the protein tyrosine phosphatase (PTP) family. Members of the PTP family share a highly conserved catalytic motif, which is essential for the catalytic activity. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. Epidermal growth factor receptor and the adaptor protein Shc were reported to be substrates of this PTP, which suggested the roles in growth factor mediated cell signaling (Johnson et al. 1993).
DNA sequencing of the six candidate genes revealed no sequence variants. Because only the exonic regions were sequenced, the possibility of a functional variant in the regulatory regions cannot be ruled out. On the other hand there are a number of other genes and expressed sequence tags contained in the candidate interval. Further fine mapping and sequencing work are required in order to identify the gene for Jawad syndrome.
References
Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin: rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426:570–574
Bellows AM, Kenna MA, Cassimeris L, Skibbens RV (2003) Human EFO1p exhibits acetyltransferase activity and is a unique combination of linker histone and Ctf7p/Eco1p chromatid cohesion establishment domains. Nucleic Acids Res 31:6334–6343
Borglum AD, Balslev T, Haagerup A, Birkebaek N, Binderup H, Kruse TA, Hertz JM (2001) A new locus for Seckel syndrome on chromosome 18p11.31-q11.2. Eur J Hum Genet 9:753–757
Campbell GR, Pasquier E, Watkins J, Bourgarel-Rey V, Peyrot V, Esquieu D, Barbier P, de Mareuil J, Braguer D, Kaleebu P, Yirrell DL, Loret EP (2004) The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis. J Biol Chem 279:48197–48204
Cottingham R, Indury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263
Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ (2002) Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 158:915–927
Filippi G (1985) Unusual facial appearance, microcephaly, growth and mental retardation, and syndactyly. A new syndrome? Am J Med Genet 22:821–824
Goodship J, Gill H, Carter J, Jackson A, Splitt M, Wright M (2000) Autozygosity mapping of a seckel syndrome locus to chromosome 3q22. 1-q24. Am J Hum Genet 67:498–503
Gudbjartsson DF, Thorvaldsson T, Kong A, Gunnarsson G, Ingolfsdottir A (2005) Allegro version 2. Nat Genet 37:1015–1016
Gul A, Hassan MJ, Mahmood S, Chen W, Rahmani S, Naseer MI, Dellefave L, Muhammad N, Rafiq MA, Ansar M, Chishti MS, Ali G, Siddique T, Ahmad W (2006a) Genetic studies of autosomal recessive primary microcephaly in 33 Pakistani families: novel sequence variants in ASPM gene. Neurogenetics 7:105–110
Gul A, Hassan MJ, Hussain S, Raza SI, Chishti MS, Ahmad W (2006b) A novel deletion mutation in CENPJ gene in a Pakistani family with autosomal recessive primary microcephaly. J Hum Genet 51:760–764
Hou F, Zou H (2005) Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell 16:3908–3918
International Human Genome Sequence Consortium (2001) Initial sequence and analysis of the human genome. Nature 409:860–921
Johnson CV, Cool DE, Glaccum MB, Green N, Fischer EH, Bruskin A, Hill DE, Lawrence JB (1993) Isolation and mapping of human T-cell protein tyrosine phosphatase sequences: localization of genes and pseudogenes discriminated using fluorescence hybridization with genomic versus cDNA probes. Genomics 16:619–629
Kilinç MO, Ninis VN, Ugur SA, Tüysüz B, Seven M, Balci S, Goodship J, Tolun A (2003) Is the novel SCKL3 at 14q23 the predominant Seckel locus? Eur J Hum Genet 11:851–857
Kong X, Murphy K, Raj T, He C, White PS, Matise TC (2004) A combined linkage-physical map of the human genome. Am J Hum Genet 75:1143–1148
Lim J, Hao T, Shaw C, Patel AJ, Szabó G, Rual JF, Fisk CJ, Li N, Smolyar A, Hill DE, Barabási AL, Vidal M, Zoghbi HY (2006) A protein–protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell 125:801–814
O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266
O’Driscoll M, Jackson AP, Jeggo PA (2006) A causal link between impaired damage response and microcephaly. Cell cycle 5:2339–2344
O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33:497–501
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, NJ, pp 365–386
Sharif S, Donnai D (2004) Filippi syndrome: two cases with ectodermal features, expanding the phenotype. Clin Dysmorphol 13:221–226
Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet 58:1323–1337
Weeks DE, Sobel E, O’Connell JR, Lange K (1995) Computer programs for multilocus haplotyping of general pedigrees. Am J Hum Genet 56:1506–1507
Woods CG, Bond J, Enard W (2005) Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet 76:717–728
Woods CG, Crouchman M, Huson SM (1992) Three sibs with phalangeal anomalies, microcephaly, severe mental retardation, and neurological abnormalities. J Med Genet 29:500–502
Acknowledgments
We wish to thank the members of the family for their cooperation. The work presented was funded by the Higher Education Commission (HEC), Islamabad, Pakistan. Muhammad Jawad Hassan was supported by an indigenous PhD fellowship from HEC, Islamabad, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, M.J., Chishti, M.S., Jamal, S.M. et al. A syndromic form of autosomal recessive congenital microcephaly (Jawad syndrome) maps to chromosome 18p11.22–q11.2. Hum Genet 123, 77–82 (2008). https://doi.org/10.1007/s00439-007-0452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-007-0452-x