Abstract
The t(9;22) BCR/ABL fusion is associated with over 90% of chronic myelogenous and 25% of acute lymphocytic leukemia. Chromosome 11q23 translocations in acute myeloid and lymphoid leukemia cells demonstrate myeloid lymphoid leukemia (MLL) fusions with over 40 gene partners, like AF9 and AF4 on chromosomes 9 and 4, respectively. Therapy-related leukemia is associated with the above gene rearrangements following the treatment with topoisomerase II (topo II) inhibitors. BCR, ABL, MLL, AF9 and AF4 have defined patient breakpoint cluster regions. Chromatin structural elements including topo II and DNase I cleavage sites and scaffold attachment sites have previously been shown to closely associate with the MLL and AF9 breakpoint cluster regions, implicating these elements in non-homologous recombination (NHR). In this report, using cell lines and primary cells, chromatin structural elements were analyzed in BCR, ABL and AF4 and, for comparison, in MLL2, which is a homolog to MLL, but not associated with chromosome translocations. Topo II and DNase I cleavage sites associated with all breakpoint cluster regions, whereas SARs associated with ABL and AF4, but not with BCR. No close breakpoint clustering with the topo II/DNase I sites were observed; however, a statistically significant 5′ or 3′ distribution of patient breakpoints to the topo II DNase I sites was found, implicating DNA repair and exonucleases. Although MLL2 was expressed in all cell lines tested, except for the presence of one DNAse I site in the promoter, no other structural elements were found in MLL2. A NHR model presented demonstrates the importance of chromatin structure in chromosome translocations involved with leukemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukemia and chromosome translocations
Chromosome abnormalities, especially reciprocal chromosome translocations involving specific genes, are a hallmark of hematopoetic malignancies often associated with particular histological or immunological subtypes of leukemia or lymphoma. In over 90 and 25% of tumor cells from chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) patients, respectively, and more rarely in chronic neutrophilic leukemia the translocation t(9;22)(q34;q11) is detected, where the derivative 22q chromosome, also called the Philadelphia (Ph) chromosome, represents a 5′ to 3′ gene fusion between BCR (chromosome 22) and ABL (chromosome 9) (Advani and Pendergast 2002). The t(9;22) has also been characterized as a rare translocation found in tumor cells of <10% of all therapy-related leukemia groups with balanced translocations and is associated, following the treatment with topo II inhibitor drugs, with or without radiation therapy (Pedersen-Bjergaard 1997; Rowley and Olney 2002). In addition to the t(9;22), in <10% of leukemia cells from myelodysplastic/myeloproliferative diseases (MDS /MPD), other translocations have been observed, for example, the t(9;12)(q34;p13) which fuses ETV6 (TEL) to ABL (Papadopoulos et al. 1995), the t(8;22)(p11;q11) fusing BCR to FGFR1 (Demiroglu et al. 2001) and the t(4;22)(q12;q11), fusing BCR to PDGFRA (Baxter et al. 2002) (Table 1).
Over 45 chromosome translocations involving the myeloid lymphoid leukemia gene [MLL, also called ALL-1, Htrx or HRX] at 11q23 are found in approximately 15% of patients with AML and ALL (Mitelman 1994; Rowley and Olney 2002). The most common 11q23 translocations are the t(9;11)(p22;q23), t(6;11)(q21;q23) and t(11;19)(q23;p13.1) detected in AML, and the t(4;11) (q21;q23) and t(11;19)(q23;p13.3) primarily found in ALL patients, where the t(4;11) is noted in 10% of childhood and adult de novo ALL (Rowley 1999) (Table 1). The partner gene of MLL involved in the t(9;11) is AF9 [also called LTG9 or MLLT3] (Nakamura et al. 1993; Strissel et al. 2000) and in the t(4;11) AF4, also called FEL or LTG4 (Nakamura et al. 1993; Gu et al. 1992). Interestingly, two other genes of the AF4 gene family, AF5q31 and LAF4, have also been shown to fuse with MLL resulting in chromosome translocations found in acute leukemia (Taki et al. 1999; von Bergh et al. 2002). MLL translocations are also found in therapy-related leukemia following topo II inhibitor drug treatment with or without radiation therapy, with the t(9;11) detected in 48% of t-AML and the t(4;11) in 9% of t-ALL (Rowley and Olney 2002; Bloomfield et el. 2002). Over 80% of infant ALL and AML less than 1 year of age show MLL translocations (Greaves 1996). These cases involve the t(4;11) representing 50–60% of pro-B or rarely mixed lymphoblastic/monocytic leukemia and the remaining 40–50% involve the t(9;11), t(6;11) and more rarely t(1;11)(q21;q23) translocations with AML.
Chromatin structural elements, topo II inhibitors, DNA breakpoints and leukemia
The primary chromosomal scaffold proteins topo II and scaffold protein II are essential for chromosome condensation, bind preferentially at scaffold-associated regions (SARs) and are regulated by calcium (Strick and Laemmli 1995: Strick et al. 2001). Topo II is a key cellular protein with enzymatic and structural functions, responding to torsional stress of DNA. In addition, topo II is important during transcription, replication and also plays a role in apoptosis (Watt and Hickson 1994; Durrieu et al. 2000; Mondal and Parvin 2001). Multiple in vitro and some in vivo topo II chromatin cleavage sites locate within SARs and represent regions of open chromatin as defined by DNase I hypersensitive sites (HS) (Bode et al. 2000). SARs are AT-rich DNA of variable size located at chromosomal bases of interphase and metaphase chromatin loops (Strick and Laemmli 1995). It has been proposed that SARs have DNA unwinding properties, which not only facilitate the entry of transcription, replication or chromosome condensation protein factors, but also represent regions of DNA fragility (Bode et al. 2000). Topo II was first implicated in illegitimate recombination events at the mouse immunoglobulin kappa (Igκ) gene intronic SAR associated with an enhancer element (Sperry et al. 1989). Many DNase I HS sites are associated with transcriptional regulatory DNA elements and SARs at gene boundaries, but are also found within genes co-localizing with SARs and/or topo II sites at breakpoint cluster regions in IFN, MLL, AF9, AML1 and ETO (Aplan et al. 1996; Strissel et al. 1996a, b, 1998a, b, 2000; Strick et al. 2000; Bode et al. 2000; Zhang et al. 2002, Iarovala et al. 2004) (Table 1).
Topo II inhibitor compounds, including the chemotherapeutic agents [e.g. VP16 and Doxorubicin (Dox)] as well as natural bioflavonoids found in certain foods and dietary supplements, trap topo II in a DNA-cleavable complex and results in DNA double strand breaks (DSBs) (Liu 1989; Aplan et al. 1996; Strick et al. 2000). These compounds have been implicated in the mechanism of MLL and AML1 translocations leading to therapy-related leukemia following topo II inhibitor drugs and in de novo leukemia, especially in infants (Greaves 1996; Aplan et al. 1996; Strissel et al. 1996b, 2000; Reilling et al. 1998; Strick et al. 2000; Bloomfield et al. 2002; Zhang et al. 2002). In the 135 kb BCR gene the majority of CML patient breakpoints and some ALL patient breakpoints locate in a 5.8 kb region called the major breakpoint cluster region (MBCR), whereas ALL breakpoints mainly locate in intron 1 in a minor breakpoint region (mBCR) (Rowley 1999). In the 171 kb ABL gene three breakpoint cluster regions have been described (Jiang et al. 1990). Although the MLL gene spans approximately 87.5 kb (Wiedeman et al. 1999), so far all MLL patient translocation breakpoints occur in the 8.3 kb breakpoint cluster region (Rowley 1999). We previously defined two breakpoint cluster regions in the 140 kb AF9 gene where region 1 locates within intron 4 (15 kb), and region 2 spans intron 7 and 8 (7.3 kb) (Strissel et al. 2000). In the 128 kb AF4 gene, patient breakpoints are clustered between exons 3 and 7 in a 50 kb region (Reichel et al. 2001). DNA polymerase pause sites, the chi-element, heptamer or nonamer signal sequences, topo II in vitro binding consensus sequences, transilin sequences and GC-rich DNA have all been described and appear overrepresented at cloned patient breakpoint fusion junctions (Gu et al. 1992; Negrini et al. 1993; Domer et al. 1995; Abeysinghe et al. 2003). In contrast, Alu sequences, AT-rich and alternating purine–pyrimidine sequences appear enriched at deletion breakpoint junctions (Abeysinghe et al. 2003). Alu family members also mediate homologous recombination resulting in MLL duplications in AML (Schichman et al. 1994). In addition, large clusters of Alu sequences border breakpoint regions in BCR and ABL where they have been implicated in recombination (Schichman et al. 1994; Chissoe et al. 1995; Jeffs et al. 2001). Presently, there is no consistent DNA sequence motif found at breakpoint junctions which can explain a common recombination mechanism between non-homologous chromosomes. In this report, BCR and ABL, and AF4 were analyzed for common chromatin structural elements to validate the influence of these elements in chromosome recombination, which could explain a common mechanism between spontaneous and therapy-related AML, ALL and CML and the rare MDS and MPD. In addition, a homolog of MLL, the MLL2 gene at chromosome 19q13.1 (Fitzgerald et al. 1999; Huntsman et al. 1999) which presently is not associated with chromosome translocations and leukemia was analyzed for comparison. A proposed non-homologous recombination (NHR) model demonstrates the involvement of these chromatin structural elements in common translocations.
Materials and methods
Cell lines and primary cell culture
A variety of cell types including both lymphoid and myeloid were used for structural analysis as previously described (Strissel et al. 1998b, 2000; Strick et al. 2000; Zhang et al. 2002) (Table 2). Two Ph+ CML tumor cell lines were analyzed, BV173 T/B progenitor stem cells, and K562 cells. In addition, the Ph+ ALL SupB13 progenitor B-cell line was studied. The MM6 monocytic cell line derived from an acute monocytic leukemia patient with a t(9;11)(p22;q23), the ML2 myeloid cell line derived from an AML patient with a t(6;11)(q27;q23), the UoC-M1 cell line derived from a megakaryocytic leukemia patient with four copies of the MLL gene and the Jurkat T-cell line derived from an ALL patient were also investigated. Epstein Bar B-lymphoblastoid cell lines included LCL-9020 from a patient with t-AML and a t(9;11) and LCL-IB4 from normal cord blood (gift from Dr. R. Larson, University of Chicago). Normal human lung fibroblasts (ATCC) were also studied. Cell lines were maintained in RPMI 1640, or DMEM, with fetal calf serum 10–15% (Life Technologies) and then used for structural studies. Expansion of primary cells in culture from CD34 cells derived from cord and peripheral blood was as previously described (Strick et al. 2000).
Topo II cleavage of chromatin
The topo II chromatin cleavage assay was according to Strissel et al. (1998b). We previously demonstrated using low concentrations of both VP16 and Dox in cell culture experiments that topo II chromatin cleavage sites are reversible. However, over longer time periods the initial topo II chromatin cleavage sites become non-reversible most likely due to apoptotic nucleases (Strissel et al. 2000; Strick et al. 2000; Zhang et al. 2002). Therefore, in this investigation, we treated exponentially grown cells for 6 and 16 h with a range of concentrations using the non-intercalating topo II inhibitor etoposide (VP16) (Sigma) (5–100 μM) to produce endogenous topo II-cleaved DNA complexes. The intercalating drug doxorubicin (Sigma) (1–5 μM) was also studied. In addition, cells were treated for 6 and 16 h with specific bioflavonoids including Genistein, Quercetin and Fisetin (Sigma) 10–200 mM). The non-topo II inhibitor DNA damaging agent, N-methyl-formamide (NMF) (Sigma) (0.5 M) was also incubated with cell cultures. All cell cultures were assessed for viability using trypan blue staining.
Isolation of nuclei used for cleavage reactions with DNAse I and for SAR assays
For each cell line, nuclei were isolated according to Strissel et al. (2000). Nuclei were frozen in a solution containing 50% glycerol at −20°C for up to 4 months or at −70°C for up to 8 months. For DNase I treatment of nuclei, the method by Strissel et al. (1998b) was used. Methods for the isolation of SAR and non-SAR fractions were used according to Strissel et al. (1996a, 1998a).
DNA purification, Southern blot, DNA clones and DNA probe hybridizations
For all assays described above, the DNA was extracted, purified, digested with restriction enzyme and electroblotted. For all gene clones or PCR derived DNA-fragments were random-primed using 32P-dCTP (Amersham). Hybridization of DNA probes to Southern blots for detection of topo II/DNase I HS DNA fragments, or SAR and non-SAR DNA fragments was performed as previously described (Strick et al. 2000). All previously isolated DNAs (Strick et al. 2000), using CD34+ expanded cell cultures, were used in this study for analysis of topo II chromatin cleavage sites.
For BCR hybridizations and analysis, two DNA fragments from the 5.8 kb breakpoint cluster region; the 5′ BCR (0.6 kb HindIII/BamHI) and the 3′ BCR (1.4 kb HindIII/ EcoRI) were used (a kind gift from Dr. T. McKeithan, University of Chicago). In addition, the 0.7 kb PstI cDNA fragment consisting of exon 2–7 (a kind gift from Dr. C. Denny, UCLA) was used. For ABL hybridization and analysis, the p1.4 (1.4 kb HindIII/EcoRI) (a kind gift from Dr. D. Leibowitz, University of Indiana) and seven PCR fragments derived from normal genomic DNA were used, (NCBI accession number NT−035014, ABL-1 110,292–111,013; ABL-2 120,664–121,531; ABL-3 121,643–122,172; ABL-4, 136,863–137,279; ABL-5 exon 2 139,425–140,136; ABL-6 143,257–143,857; and ABL-7 158,547–159,031).
For AF4 hybridizations and analysis the 4AE3X1 (2.4 kb Eco/XhoI) DNA fragment (gift from Dr. J. Kersey, University of Minnesota) was used. In addition, the P1 cosmid clone (Domer et al. 1995) (gift from Dr. P. Domer, Northwestern University, Chicago, IL.) representing a large genomic region including the entire AF4 gene was used as a template [sequence attained from (Reichel et al. 1999)] to generate the PCR DNA probes, AF4–1-7, AF4-4A and AF4-6A. The P1 cosmid nucleotide positions of DNA probes are indicated as follows: AF4-1 2964-3732; AF4-2 3764-4264; AF4-3 6645-7172; AF4-4 11671-12109; AF4-4A 16,890-17,499; AF4-5 22,744-23,262; AF4-6 38,606-39,089; AF4-6A 40,694-40,717; and AF47 46,192-46,597.
The MLL2 1506 bp cDNA clone from exons 11–23, including the zinc finger region (Fitzgerald et al. 1999, kind gift from Dr. M. Diaz, Loyola University, Chicago, IL) was used for hybridizations to Southern blots. The entire MLL2 gene encompasses ~20,860 bp where the cDNA probe covers 83% of the gene when restricted with BamHI and HindIII. In addition, this probe also hybridized to over 2 kb of the 5′ region of MLL2 including the promoter. For MLL2 gene expression, RNA from six cell lines (BV173, Jurkat, MM6, K562, SupB13 and lung fibroblasts) and PHA-stimulated (3 days) normal peripheral blood T-lymphocytes as a positive control were isolated using Trizol (Invitrogen). For RT-PCR analysis of MLL2 500 ng of RNA was used according to instructions (Super-Script one step RT-PCR for long templates, Invitrogen). MLL2 primers were MLL2-TF 5′ GTGATTTAGAGAACGTGTGGCTGATGG 3′ and MLL2-BR 5′ CTGGCAGAAGACTTTCTTGTCATCCTG 3′.
Statistical analysis of breakpoints relative to topo II/DNAse I sites
The statistical analysis and strategy used in this report was based on Abeysinghe et al. (2003). Our criteria was to analyze the patient breakpoint distribution in increments of 5 kb in both the 5′ and 3′ directions for a total of 35 kb to single co-localizing topo II/DNAse I sites. Significant values were set at P<0.05 using the t test.
Results
Topo II, DNase I chromatin cleavage sites and SAR analysis in BCR
In this report 55 kb of the BCR gene from exon 2–16 was analyzed for topo II chromatin cleavage sites, DNase I HS sites and SARs in the BV173 CML cell line. This includes the MBCR from exon 12–15 where CML and ALL patient breakpoints locate. Figure 1a–c shows one strong co-localizing topo II and DNase I HS site in intron 13 and a second strong DNase I HS site in intron 8, 8.5 kb 5′ from the topo II/DNase I HS in the MBCR (data not shown). The intron 8 DNase I HS site is identical to the DNase I HS site detected in K562 cells by Schaefer-Rego et al. (1990). In addition, no topo II cleavage was detected at this DNase I HS site demonstrating different chromatin structure requirements for cleavage.
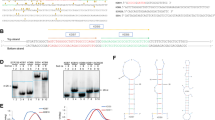
a BCR chromatin cleavage sites locate in the major breakpoint cluster region. The total 135 kb BCR gene depicted above shows exon 1–23 and includes both ALL and CML breakpoint cluster regions (mBCR and the MBCR). Also shown is the 55 kb region from exon 2–16 (thick black line on map) analyzed in this report for chromatin cleavage sites and SARs. The bottom map depicts the BCR region from 116,265–139,142 including exon 9–16 (vertical black bars), the major breakpoint cluster region (MBCR), and the topo II and DNase I HS (arrows above map). Restriction enzyme sites [BamHI (B), EcoRI (E), BglII (Bg)] are indicated above and on the map as thin black lines; HindIII restriction sites are indicated as thin black lines below and on the map. Below the Topo II/DNase I HS arrow, 909 patient breakpoints from the literature, which have been identified by Southern blot analysis, are demonstrated in brackets according to restriction site DNA fragments (P. L. Strissel and R. Strick, review in preparation). Thirty-seven cloned patient breakpoints are identified as short black lines (CML and a single breakpoint in intron 12 for a patient with MPD) and long black lines (ALL) below the MBCR (and summarized in the location below in brackets) including and between exon 13 at position 123,595 and exon 15 at position 126,716 (Chissoe et al. 1995 and references within; Jeffs et al. 1998 and references within; Baxter et al. 2002; P. L. Strissel and R. Strick, review in preparation). The probes used for hybridization were the 0.7 kb PstI cDNA (Timmons et al. 1989), and the 5′ BCR and 3′ BCR DNA fragments (shown below the bottom map). New DNA fragments were observed following hybridization of the 3′ BCR probe to BamHI, EcoRI or BglII restricted DNA isolated from topo II inhibitor-treated cells or DNase I-treated nuclei. For example, hybridizing with the 3( BCR probe a 3.0 kb (BamHI), a 2.6 kb (EcoRI), and a 2.0 kb (BglII) (below the map) all identified a topo II and co-localizing DNase I HS site in the middle of intron 13 where the t(9;22) patient breakpoints map. Using the same Southern blots and hybridizing with the 5′ BCR probe, the 5′ intron 8 DNase I site was identified. Full length Alu family members (arrow heads in 5′–3′) are indicated below the map. b A Topo II cleavage site locates in the BCR major breakpoint cluster region. Southern blot hybridized with the 3( BCR probe to BamHI restricted DNA isolated from primary myeloid progenitor cells cultured from cord blood CD34 cells (CB) and then treated in the presence or absence of topo II inhibitor drugs. Indicated are the germline 3.8 kb fragment and a new 3.0 kb topo II DNA-cleaved fragment following the treatment of cells with VP16 (V), Genistein (G), Quercetin (Q) and Fisetin (F); 25 and 50 μM amounts are noted above each lane. No DNA cleavage was observed using DMSO (D) or Ascorbic acid (A) at 200 μM. c A DNase I HS site co-localizes with a topo II/apoptotic cleavage site in the BCR major breakpoint cluster region. Southern blot hybridized with the 3( BCR probe to BamHI restricted BV173 DNA isolated from whole nuclei treated with DNase I (left), or cells treated with topo II inhibitors or a DNA damaging agent (right). In addition, to both the 8.0 kb derivative chromosome 9 ABL/BCR fusion gene fragment and the 3.8 kb BCR germline fragment, a new strong 3.0 kb DNase I HS was observed with increasing DNase I units (5.0 and 10.0 U, left panel). This same DNase I HS site also co-localized with a topo II-cleaved 3.0 kb DNA fragment induced with VP16 (V), Doxorubicin (D) and the non-topo II inhibitor NMF (N) (500 mM) (right panel), which induces apoptosis. Concentrations in μM are noted above each lane
To determine the incidence of the BCR topo II/DNase I HS sites in different cell types a total of seven leukemia cell lines, two B-lymphoblastoid cell lines, one normal-lung fibroblast and three primary cell cultures expanded from CD34 cells were analyzed (Table 2). Except for K562, 9020 and IB4, which have previously demonstrated a more resistant phenotype to drug treatments (Strissel et al. 1998b; Strick et al. 2000; Echlin-Bell et al. 2003), all chromatin cleavage sites were identified in all cell types, thus demonstrating no cell type specificity (Table 2). For example, Fig. 1b shows the MBCR topo II chromatin cleavage site in CD34 expanded myeloid/monocytic cells treated with VP16 and bioflavonoids. Figure 1c shows the topo II/DNase I HS sites in BV173 CML cells treated with VP16, Dox, or NMF. We observed a 3.8 kb germline BamHI fragment and a new 3.0 kb fragment following the drug treatment of cells. For BV173 cells an additional 8 kb BamHI germline fragment derived from the der9 chromosome was also observed. The MBCR topo II/DNase I HS cleavage sites were also confirmed with additional restriction enzymes Bgl II or EcoRI and hybridizing with the 3′ BCR probe (Fig. 1a, and data not shown). Interestingly, the non-topo II inhibitor drug NMF induced cleavage at the same site as topo II/DNase I in the MBCR; therefore, this cleavage site is also favored by apoptotic nucleases (Fig. 1c). We did not identify any high affinity SARs throughout the entire BCR gene, but low affinity binding SARs were previously identified in the first intron (Fig. 2c and data not shown).
a Topo II and DNase I HS sites in the ABL BCR C. The total ABL genomic map from nucleotide positions 1–170,360 is depicted above and includes the previously described BCRs A, B and C (Jiang et al. 1990). In the case of BCR C we have redefined this breakpoint cluster region to include all patient breakpoints mapping 3′ from exon 1a to exon 3. The map below represents 51 kb from nucleotides 109,263–166,339 analyzed for topo II and DNase I HS sites (arrows above map). The double black lines in BCR C (31.7 kb region and thick black line below map) indicates that 12.7 kb of the 5′ portion of BCR C was not included and mapped for chromatin elements. SAR1 is indicated below the BCR C region. Exons 1a, 2–10 (vertical black bars) and BamHI (B) restriction enzyme sites are indicated above and on the map as thin black lines. Alu family members (arrow heads in 5′–3′) and specific DNA ABL probes are indicated below the map. Hybridizing with the ABL-4 and ABL-6 probes to BamHI restricted DNA isolated from cells or nuclei treated with topo II inhibitors or DNase I new DNA fragments, 3.8, 8.0, 10.0 and 14.0 kb (below the map) identified four topo II and co-localizing DNase I HS sites. Indicated below are cloned patient breakpoints represented as short black lines (CML and one breakpoint in intron 2 involving a patient with MPD), and long lines (ALL) (Chissoe et al. 1995, Jeffs et al. 1998 and references within, Papadopoulos et al. 1995, P. L. Strissel and R. Strick, review in preparation). b A topo II cleavage site locates in BCR C in the ABL gene. Southern blot hybridized with the ABL-4 probe to BamHI restricted BV173 DNA isolated from cells treated with topo II inhibitor drugs. In addition to the 15.7 kb germline fragment a new 3.8 kb DNA topo II-cleaved fragment was observed with increasing concentrations of VP16 (V) and Doxorubicin (D). Control lane is indicated to the left (c), and concentrations in μM are noted above each lane. c Non-SAR and SAR distribution in the BCR, ABL and AF4 gene breakpoint cluster regions. Southern blot hybridized with different gene probes to BV173 restricted DNA following the isolation of LIS supernatant (S) and pellet (P) DNA fractions. Specific non-SAR and SAR fragments are shown: in BCR two non-SAR S-enriched DNA fragments, 5.6 and 4.0 kb, BglII hybridized with the 5′ BCR probe in triplicate experiments (left panel); in ABL a weak SAR was detected on a 4.8 kb HindIII fragment which hybridized with the p1.4 ABL probe in duplicate experiments (middle panel); and in AF4 a high affinity SAR is shown with >80% P-enrichment as identified on a 3.6 kb EcoRI fragment which hybridized with AF4- 7 in duplicate (right panel)
Chromatin structure analysis in ABL
Figure 2a shows 51 kb of the ABL gene extending from the last portion of intron 1b telomeric to exon 10. This region includes the breakpoint cluster region C (BCR C) where many published CML and ALL patient breakpoints locate (Jiang et al. 1990). Due to the finding that several additional patient breakpoints cluster 3′ to BCR C, we have expanded the breakpoint region to 44 kb to include all 23 CML and ALL patient breakpoints from the literature. Topo II chromatin cleavage sites and DNase I HS sites were analyzed using the CML BV173 cell line, the myeloid ML2 and JR cell lines and primary myeloid cells. SARs were mapped in the CML BV173 cell line. Co-localizing topo II/DNase I HS sites and a single DNase I site clustered in intron 1a as identified with the ABL-4, and p1.4 DNA probes (Fig. 2b and data not shown). Both DNase I HS sites in intron 1a were previously demonstrated in K562 cells (Schaefer-Rego et al. 1990). A low affinity 4.8 kb SAR in intron 1a was identified (Fig. 2c) co-localizing with the single DNAse I HS site, whereas the topo II/DNase I HS site located <1 kb telomeric to the SAR. In addition, three co-localizing topo II and DNase I HS sites were found in intron 4 (Fig. 2a and data not shown).
Localization of Topo II, DNase I HS and SARs in AF4
Figure 3a represents the AF4 gene including exon 2–10 (73 kb) where all published ALL patient breakpoints locate (Reichel et al. 2001). In the CML BV173 cell line topo II/DNase I HS sites, as well as SARs were analyzed. Using VP16-, Dox- and DNase-I-treated cells and nuclei, a strong co-localizing topo II/DNase I HS sites were identified just 5′ to exon 4 in intron 3 within a strong 6.0 kb SAR (Figs. 2c, 3a and data not shown). A VP16 time kinetic experiment, bioflavonoid treatment of cells and a DNase I experiment resulted in a total of four chromatin cleavage sites: two strong co-localizing topo II DNase I cleavage sites in intron 6 and two weak topo II cleavage sites not co-localizing DNase I in intron 5 and 6 (Fig. 3a–d). For example, the AF4-7 probe identified all the four cleavage sites on DNA fragments of 3.2, 2.5, 2.3 and 1.6 kb (Fig. 3b, c). All four topo II chromatin cleavage sites were confirmed in three additional hematopoietic cell types from RS4;11, SUPB13 and MM6 (data not shown). The AF4-7 probe also identified an 800 bp HaeIII scaffold-enriched DNA fragment in intron 5. This SAR co-localized with the two weaker topo II sites, whereas the two stronger intron 6 topo II/DNase I sites located approximately 2.4 kb telomeric (Fig. 3a–d). Throughout the remaining AF4 gene region no other chromatin cleavage sites or SARs were found.
a AF4 gene and topo II and DNase I HS sites in the breakpoint cluster region. The total AF4 133,722 kb gene depicted above shows exon 1–20 (vertical black bars) as well as the breakpoint cluster region (BCR, thick black line below top map) analyzed for topo II and DNase I HS. BamHI (B) restriction enzyme sites are shown below the map as short thin black lines. The bottom map represents 72,650 kb including exon 2–10 and all cloned ALL patient breakpoints (short black lines) and infant leukemia breakpoints (long lines) (Reichel et al. 2001). Chromatin topo II and DNase I HS sites (arrows above map) locate within and 3′ to breakpoint region. BamHI sites are located as lines below map. Specific DNA AF4 probes are indicated as black boxes below. Hybridizing the AF4–7 probe to BamHI restricted DNA isolated from topo II inhibitor-treated cells or DNase I-treated nuclei, new DNA fragments were observed. For example, 3.2, 2.5, 2.3 and 1.6 kb DNA fragments (below the map) identified two strong and two weak topo II and co-localizing DNase I HS sites 3′ in the t(4;11) patient breakpoint cluster region. SARs 1 and 2 are shown below the map. b VP16-induced Topo II DNA cleavage sites locate in the AF4 breakpoint region. Southern blot hybridized with the AF4-7 probe to BV173 restricted BamHI DNA isolated from cells treated with VP16. In addition to the germline 26 kb DNA fragment four new topo II DNA cleavage fragments, 3.2, 2.5, 2.3 and a 1.6 kb were observed at specific time periods from 0.5–8 h following VP16 drug treatment. Control is designated in the first lane (-). c Bioflavonoid induction of topo II DNA cleavage sites locate in the AF4 breakpoint cluster region. Southern blot hybridized with the AF4-7 probe to BV173 BamHI restricted DNA isolated from cells treated with topo II inhibitor bioflavonoids. In addition to the germline 26 kb DNA fragment, four new topo II DNA cleavage fragments were observed, 3.2, 2.5, 2.3 and 1.6 kb with the bioflavonoids Genistein (G), Quercetin (Q) and Fisetin (F). Note that these bioflavonoid-induced topo II cleavage sites are the same as those seen after treatment with VP16. All μM concentrations are noted directly above the lane. Control is indicated in the left lane (0). d DNase I HS sites co-localize with strong topo II sites in the AF4 BCR. BV173 Southern blot hybridized with the AF4-7 probe to BamHI restricted DNA isolated from nuclei treated with increasing concentrations [0, 5, 10, 20 U indicated above] of DNase I. As indicated, in addition to the germline 26 kb DNA fragment, two new strong DNase I cleaved fragments, 3.2 and 2.5 kb are observed
Ubiquitously expressed MLL2 lacks intron and exon chromatin structural elements
In addition to BCR, ABL and AF4, we investigated a new member of the trx/MLL family of genes, the MLL2 gene located at chromosome 19q13.1 (Fitzgerald et al. 1999; Huntsman et al. 1999). Hybridizing the MLL2 cDNA probe to BamHI-, HindIII- or EcoRI-restricted DNA from VP16- or Dox-treated BV173 cells, no additional topo II cleavage sites were observed (Fig. 4a). In addition, no SAR DNA fragments were identified (data not shown). Interestingly, following the DNase I digestion of BV173 nuclei and hybridization with the MLL2 cDNA probe, two new HindIII (11 kb) and BamHI (8 kb) DNA fragments were detected. This DNase I HS site located ~600 bp upstream of exon 1 in the MLL2 promoter region (Fig. 4b). These results demonstrate that the structural elements found in MLL, but not in MLL2, depend mainly on the different chromatin structure of the introns between the two trx genes (Table 3). MLL2 is ubiquitously expressed in a wide array of adult human tissues including peripheral blood (Fitzgerald et al. 1999). Since it is likely that chromatin accessibility of genes is closely linked with gene expression, we examined MLL2 expression in five hematopoeitic cell lines (BV173, K562, Jurkat, MM6, SupB13) and lung fibroblasts (Fig. 4c). Results showed that MLL2 is highly expressed in all cell lines similar to normal peripheral blood T-lymphocytes and other human tissues (Fitzgerald et al. 1999).
a MLL2 analysis for topo II cleavage sites. Southern blot hybridized with the MLL2 cDNA probe to BV173 HindIII and BamHI restricted DNA isolated from cells treated with increasing concentrations of VP16 (V) and Doxorubicin (D). As indicated, in addition to germline HindIII (7, 12 kb) and BamHI (1.6, 3.0, 10 kb) fragments, no new DNA fragments were detected throughout the gene. b MLL2 gene analysis for DNase I cleavage sites. Southern blot hybridized with the MLL2 cDNA probe to BV173 HindIII and BamHI restricted DNA isolated from nuclei treated with increasing concentrations [0, 5, 10, 20 U indicated above] of DNase I. As indicated, in addition to germline HindIII (7, 12 kb) and BamHI (1.6, 3.0, 10 kb) DNA fragments, two new strong DNase I cleaved fragments (BamHI 8.0 kb; HindIII 10.7 kb) located in the MLL2 promoter region. c MLL2 restriction map. Approximatley 21 kb of MLL2 was screened for structural elements and only one DNase I HS site was located 5′ of exon 1. Top and bottom lines represent BamHI and HindIII restriction sites and fragment sizes, respectively. The DNA probe is indicated below and encompasses exons 11–23, including the zinc finger region homologous to the MLL BCR. d MLL2 gene expression. The 1.5 kb MLL2 cDNA expression pattern is shown on an agarose gel as inverse image. MLL2 is expressed in peripheral blood T-lymphocytes, lung fibroblasts, BV173, SupB13 and MM6 cell lines (M Marker)
Statistical analysis of cloned patient breakpoints to Topo II/DNase I cleavage sites
Similar to the statistical analysis of breakpoint translocation and deletion regions in terms of nucleotide composition (AT or GC richness, recombination motifs) as demonstrated by Abeysinghe et al. (2003), we analyzed the distribution of patient breakpoints to co-localizing topo II/DNase I sites. For each gene, starting from the co-localizing topo II/DNase I sites, the number of cloned breakpoints were assessed in 5 kb steps totaling 35 kb both 5′ and 3′. Results showed no significant clustering of patient breakpoints surrounding the topo II/DNase I sites. However, a statistically significant distribution of patient breakpoints mapping either all 5′ or 3′ of the topo II DNase I HS sites was found. Patients’ breakpoints significantly localized in the 5′ direction of the topo II/DNase I sites in the MLL gene breakpoint cluster region (P=0.001), intron 6 of AF4 (P=0.001), intron 3 of AF4 (P=0.006), intron 4 of AF9 (P=0.002) and intron 4 of AML1 (P=0.002). For the topo II/DNase I site in intron 1a of ABL no significant breakpoint distribution was established. Patient breakpoints significantly localized in the 3′ direction from the topo II/DNase I sites for BCR (P=0.038) and intron 1b of ETO (P=0.001), whereas the ETO topo II/DNase I site in breakpoint cluster region-II was not significant for a specific direction. A significant distribution of patient breakpoints either 5′ or 3′ of the topo II/DNase I sites supports a DNA repair model involving 5′ or 3′ exonucleases.
Discussion
In this report, we determined that the breakpoint cluster regions of BCR, ABL and AF4 have similar chromatin structures as the breakpoint cluster regions of MLL, AF9, AML-1 and ETO (Aplan et al. 1996; Strissel et al. 1998b, 2000; Strick et al. 2000; Bode et al. 2000; Hensel et al. 2001; Zhang et al. 2002; Iarovala et al. 2004). All seven genes are involved in chromosomal translocations found in both acute and chronic de novo and therapy-related leukemia. If these gene rearrangements result in fusion onco-proteins, like BCR-ABL, MLL-AF4, MLL-AF9 and AML1-ETO, cellular transformation leads to tumor development. We propose that three levels of selection could lead to cellular transformation: (1) SARs, topo II and DNase I sites locate throughout the genome approximately every 60–100 kb and determine regionally specific DNA breaks resulting in NHR (Bode et al. 2000); (2) fusion gene exon reading frame must be maintained and; (3) the fusion onco-protein promotes an advantage for cellular growth in specific cell lineages, e.g. stem or other progenitor cells. So far, no MLL fusion gene or protein including exon 10 or telomeric of exon 10 has been identified, limiting the essential transforming MLL exons from exon 1–6 (7+8), with the AT-hooks (exon 2–3) and the MTase-homology region always present.
In contrast to the 87.5 kb genomic size of MLL, MLL2, mainly due to diverged intron sizes, spans only 23 kb (Fitzgerald et al. 1999; Huntsman et al. 1999). MLL2 has a 41% overall amino acid homology with MLL, supporting similar functions. MLL2 has not been demonstrated in translocations, but was amplified in cell lines derived from solid tumors, thus may contribute in carcinogenic transformation (Fitzgerald et al. 1999; Huntsman et al. 1999). In this investigation it was determined that, although MLL2 is expressed, it does not share similar chromatin structural elements within the homologous region of the MLL breakpoint cluster region or with breakpoint regions of other genes. This finding further implicates that chromatin structural elements mediate DNA breakage and NHR, which can lead to leukemia.
Comparison of structural elements to patient breakpoint junctions
MLL, AF4, AF9, ABL, AML1 and ETO have SARs associated with breakpoint cluster regions (Table 3). Two SARs located in AF4, where SAR2 borders the breakpoint region at the 3′ end and SAR1 which locates in the middle, where many infant and childhood leukemia breakpoints map (Reichel et al. 2001). These findings for AF4 are comparable with specific Distamycin competition experiments identifying SAR regions in Raji cells (Hensel et al. 2001). Previously, we showed that the SARs of MLL and AF9 also either bordered or overlapped with patient breakpoints (Strissel et al. 1996b, 2000). In contrast to MLL, AF4 and AF9, we only found one SAR in the middle of the ABL breakpoint cluster region whereas none located in BCR or MLL2. For all genes the range of SAR sizes was, according to the literature, between 0.8 and 6.0 kb. The intervening DNA regions between the SARs for MLL (5.5 kb), AF4 (7.91 kb) and AF9 (15 kb) were smaller than the expected SAR distance in the genome (50–100 kb) (Bode et al. 2000), whereas the distance between the SARs for ABL and BCR must be >50 kb.
Except for MLL2, all genes analyzed have breakpoint cluster regions associated with specific open and accessible chromatin regions as defined by topo II drug-induced DNA cleavage and DNase I sensitivity (Table 3). Thus, especially for MLL and its gene partners the location of structural elements (SARs, topo II and DNase I HS sites) to patient breakpoints tend to define a breakpoint cluster region into a particular chromatin structure where patient breakpoints either map within or between borders of open chromatin (Strissel 1996b; Strissel 2000; Bode et al. 2000; Hensel 2001). Previously, bioflavonoids have been implicated in the etiology of de novo leukemia, specifically in infants (Strick et al. 2000; Ross 2000; Zhang et al. 2002). The MLL/AF4 translocation is the most common translocation noted in >60% of infant ALL. In this report, the 3′ BCR open chromatin site in AF4 was cleaved by topo II after treatment of cells with three common bioflavonoids found in food and supplements: Quercetin, Fisetin and Genistein (Fig. 3c). These bioflavonoids also induced topo II DNA cleavage in the BCR, MLL, AF9 and AML1 genes like, VP16 and Dox (Fig. 1a, b; Strissel 2000; Strick 2000; Zhang 2002). For both infant and therapy-related leukemia patients a significant bias accumulation for breakpoint distribution has been demonstrated in the telomeric region of the MLL BCR, 5′ of the topo II cleavage site (Domer et al. 1995; Strissel et al. 1998b; Reichel et al. 2001; Langer et al. 2003; Zhang et al. 2004) supporting a similar mechanism of DNA breakage. To explain the enrichment of patient breakpoints in the MLL telomeric region, the limiting factors may be the topo II inhibitor dose, the number of topo II molecules in the region and the finding of a strong topo II site co-localizing within a SAR. Thus, at higher dosage levels more topo II may become trapped within the SAR resulting in repair and gene fusion.
“Chromatin matters” in determining the specificity for topo II DNase I DNA cleavage and fusion genes
From Drosophila to humans, topo II DNA cleavage sites have been characterized and sequenced in vitro on naked DNA, in nuclei and in vivo using topo II inhibitor drugs (Spitzner and Muller 1988; Sperry et al. 1989; Udvardy and Schedl 1991; Borgnetto et al. 1996; Lovett et al. 2001; Betti et al. 2001; Tennyson et al. 2002; Blanco et al. 2004). However, topo II cleavages are much more frequent and less specific on naked DNA, and in vivo DNA cleavage site selectivity is determined by the inhibitor used. According to the in vitro DNA consensus for topo II DNA cleavage (Spitzner et al. 1988) eight topo II sites were found in the MLL breakpoint cluster telomeric region (Strissel et al. 1996b) but only one topo II cleavage site has been observed in cells and in nuclei in vivo (Aplan et al. 1996; Strissel et al. 1996b; Stanulla et al. 1997; Strick 2000). Similar findings have been observed for AF4, AF9, BCR, ABL and as well as for AML1 and ETO (Chissoe et al. 1995; Reichel et al. 1999; Strissel et al. 2000; Zhang et al. 2002). Recently, a topo II cleavage site in the human X chromosome alpha satellite locus could shift in location if placed next to telomeric sequences, demonstrating further that chromatin structure dictates DNA cleavage (Spence et al. 2002).
Topo II and DNase I HS sites do not always co-localize, thus must demonstrate differences in structure. For example, BCR and ABL DNase I HS sites locating adjacent to and within the breakpoint cluster regions, respectively, were not cleaved by topo II (Table 3). The open chromatin DNase I HS site found in the MLL2 promoter region was not recognized by topo II. In contrast, strong topo II, but not weaker topo II sites in the breakpoint regions of AF9, AML1, ETO and AF4, co-localized with DNAse I HS sites (Strissel et al. 1998b, 2000; Zhang et al. 2002). Using the chromatin assembled in vitro, similar to topo II sites, DNase I HS sites are not solely determined by inherent DNA sequences, intrinsic DNA curvature or nucleosomal organization. (Langst et al. 1997). All of the above support that the overall chromatin structure including all bound DNA proteins determines topo II recognition and DNA cleavage in the genome.
Structural elements and NHR
There are many examples in the literature implicating topo II and DNase I HS sites in DNA breaks and recombination. In three regions of the yeast genome DNAse I HS sites co-localized with DSBs during meiosis, implicating open chromatin as a prerequisite in initiating recombination (Wu and Lichten 1994). These same DNase I HS sites were also maintained throughout mitotis during the cell cycle. Cell culture experiments using the topo II inhibitor drug m-AMSA showed that both single strand breaks and DSBs associated with topo II and DNAse I HS sites at the replication origin on SV40 chromatin (Yang et al. 1985). Willmore et al. (1998) could visualize both topo II alpha and beta as stabilized cleavable complexes on DNA in a leukemic cell line exposed to VP16. It has also been demonstrated that topo II-induced DSBs resulted in sister chromatid exchanges and chromosomal aberrations (Charron et al. 1991). Treatment of yeast with VP16 increased illegitimate recombination of chromosomes by tenfold (Asami et al. 2002). Following the induction of DSBs within the human MLL breakpoint cluster region transfected into yeast cells, a translocation increase of 100- to 1,000-fold was observed where breakpoint junctions showed minor or extensive repair (Tennyson et al. 2002). Interestingly, following a single MLL cleavage, two different DNA recombination events were observed in both haploid and diploid yeast cells: (1) breakpoint junctions were scattered either throughout the breakpoint cluster region or (2) all breakpoints located telomeric. Furthermore, experiments with murine cells treated with VP16 also induced chromosome translocations with all breakpoints locating telomeric in the mll breakpoint cluster region in intron 9 or 11 (Blanco et al. 2004).
On the other hand, treatment with topo II inhibitors also induces apoptosis leading to multiple DSBs and, after sometime, cell death (Liu 1989). Since many topo II sites associate with DNAse I HS sites, they are most likely the first targets of apoptotic nucleases. In addition, these sites are sensitive to radiation. For example, the topo II sites both in the MLL and BCR breakpoint cluster regions are preferred sites for apoptotic cleavage and damage by ionizing radiation (Stanulla et al. 1997; Betti et al. 2001; Fig. 1c). Experiments using nuclei and the acidic DNAse II, another apoptotic nuclease, also induced DNA cleavage at the MLL breakpoint cluster region topo II/DNase I HS sites (R. Strick and P. L. Strissel, unpublished). Interestingly, the treatment of cell cultures with anti-CD95 antibody or ionizing radiation initiated translocations in the MLL breakpoint cluster region where all breakpoints clustered at the topo II open chromatin cleavage site near exon 9 (Betti et al. 2001).
If during apoptosis, a translocation occurs and results in an in-frame gene fusion, transcription and translation would have to be completed before apoptotic proteases cleave, for example, the essential translation initiation factor eIFG, which is active 1–2 h after the start of apoptosis (Clemens et al. 1998). In addition, a chromosome translocation would have to be completed before multiple DNA DSBs fragment the chromosomes to <200 kb pieces, which is most likely the beginning of irreversible programmed cell death. Higher-order genomic fragmentation by topo II using VP16 and other enzyme inhibitory drugs have yielded different DNA fragments of 50–200 kb and 600 kb, where only the 600 kb DNA fragmentation was reversible (Filipski et al. 1990). Solovyan et al. (2002) could demonstrate that the 200–600 kb fragments were cleaved by topo II, but not by the caspase-activated DNase (CAD). In contrast, the CAD apoptotic DNA fragmentation protein complex has been shown to be initially associated with topo II (Durrieu et al. 2000), but after <50 kb genomic fragments are detectable topo II itself becomes degraded by apoptotic proteases (Filipski et al. 1990). In the case of MLL breakpoint cluster cleavage, we observed that topo II inhibitory drugs induce cleavage very early, at the stage of >500 kb (R. Strick and P. L. Strissel, unpublished) ,and thus could be repaired before apoptosis commitment when many DSBs occur to <50 kb fragments. One interpretation for the above results is that initial DSBs of topo II, which result in 200–600 kb DNA fragments can be repaired by the cellular repair machinery, whereas further DSBs resulting in <50 kb fragments must be a signal leading to committed apoptosis and subsequently nucleosomal fragmentation through DNAse. It is interesting to note that in addition, the main recombinase Rad51 becomes degraded by caspase 3 after initializing apoptosis (Huang et al. 1999), supporting further that a recombination event must occur during pre-apoptosis.
A revised non-homologous chromosome translocation model
We previously published a multi-step non-homologous chromosome translocation (NHCT) model involving MLL and AF9, and AML1 and ETO (Strissel et al. 2000; Strick et al. 2000; Zhang et al. 2002), which accounts for all similar chromatin elements involved in chromosome translocations. Figure 5 represents a revision of the model to include AF4, BCR and ABL and several additional findings from the literature. We propose that breakpoint cluster regions are evolutionary conserved chromatin regions, which are essential for cellular functions including replication, transcription, chromosome condensation and apoptosis. In addition, breakpoint regions become fragile when these processes occur. Recently it was demonstrated that histone H1 depletion along with H4 deacetylated islands occurs in the 3′ half of the MLL breakpoint cluster region further supporting differences in function and a role for topo II and DNase I HS sites in chromatin (Khobta et al. 2004). Although it is still under discussion for topo II inhibitor drugs how the translocation event evolves, we propose that translocations occur only in pre-apoptosis, at HS sites resulting in DNA fragments of over 200–600 kb, which are still reversible due to an active DNA repair. There are other chromosomal breakage and translocation models described in the literature, especially for MLL and its partners. For example, a DNA damage and misguided repair process, which initiates from random DNA damages in hot spot regions (Reichel et al. 1998) and a “positional poison model” where topo II poisons (apurinic, apyrimidinic sites and deaminated cytosine residues) increase the levels of topo II cleavage resulting in DNA repair and illegitimate recombination (Kingma et al. 1997). These models describe that translocation breakpoint junctions result at the site of DNA damage, in contrast to our model involving initial DNA damage and recombination occurring at open chromatin cleavage sites (Fig. 5). The difference to the above models of Reichel et al. 1998 and Kingma et al. 1997 lies in a more continuous flow of chromosomal breakage (at topo II/DNase I HS sites) and DNA repair involving exonucleases, finally resulting in translocation breakpoints 5′ or 3′ of the initial HS site (Fig. 5). Similar to all models is that non-homologous end-joining is a critical step in the chromosome translocation event. For the further understanding of the mechanism of DSB-induced chromosome translocation, some future questions include: (1) Which level of DNA fragmentation leads to the induction of committed apoptosis (<200 or <50 kb)? (2) Can the induction of the apoptotic DNAse be stopped after forming fusion genes or does this represent a “point of no return” to programmed cell death? (3) Does chromatin structure dictate why some genes are the more common translocation partners than others? and (4) Are open chromatin structures found in the breakpoint cluster regions’ active sites of ongoing cellular evolution promoting chromosome fusions and rearrangements.
Non-homologous chromosome translocation model for MLL, AF9 and AF4 involving chromatin structural elements. Left panel shows an MLL and AF9 t(9;11) gene rearrangement. The right panel represents an MLL and AF4 rearrangement. This model for DNA breakage and alignment is also applicable for BCR and ABL; AML1 and ETO. a The first event results in topo II-mediated DNA cleavage (vertical arrows above map) in open chromatin at the MLL, AF9, and AF4 topo II DNA cleavage sites. These regions become recombinogenic due to DNA DSBs resulting from torsional stress due to replication, transcription, condensation or upon exposure to topo II inhibitors or DNA damaging agents [radiation—for example, in CML and the t(9;22) of atomic bomb survivors (see Rassool 2003 for review)]. SAR/SAR distances are indicated below in kb. b Alignment between MLL and chromosomal partners AF9 and AF4 occurs via SAR/SAR chromatin for example, mediated by A/T-tracts and DNA unwinding elements (Bode et al. 2000), along with SAR/SAR proteins. SAR–SAR cooperativity via proteins has been demonstrated at human centromeric regions (Strissel et al. 1996a) and at the IFN and MLL genes (Strissel et al. 1996a, 1998a, b). Specifically, the t(9;22) chromosomal alignment has been proposed to occur between 72 kb repeats and specific dense regions of Alus on chromosomes 9 and 22, associating with the major breakpoint cluster region and the ABL breakpoint cluster region, respectively, as well as Alus reported at breakpoint junctions (Jeffs et al. 1998, 2001; Saglio 2002). There is evidence that Alu repeats can function like SARs (Bode et al. 2000); thus, this also supports that alignment could occur between the ABL SAR and BCR major breakpoint cluster region Alus. Following specific DNA cleavage and chromosome alignment, a complex repair mechanism occurs involving either a 5′ or 3′ exonucleolytic activity extending away from the initial DNA cleavage sites (horizontal arrows on map). At the same time, the reciprocal MLL, AF9 and AF4 alleles may be used to repair gene sequences by gene conversion up to the recombination junctions, which mostly occurs 5′ to the topo II cleavage site. In the case of MLL, AF4, AF9 and AML1 repair of gene sequences up to the recombination junctions, mostly occurs 5′ to the topo II cleavage site. For BCR and ETO repair of gene sequences up to the recombination junctions, mostly occurs 3′ to the topo II cleavage site. In relation to the topo II/DNase I HS sites a range from <0.1 to 20 kb was observed for cloned breakpoint junctions of acute, chronic and therapy-related leukemia patients (Table 3). c The last step involves DNA exchange, ligation and fusion at the junction site. It is possible that NHR could occur through weak sequence homologies or protein–protein interactions. The subunit exchange model mediated by topo II dimers (Bae et al. 1998) could also include the DNA MRE11-RAD50-NBS1 complex which bridges DNA ends resulting in protein/DNA exchanges. Another candidate DNA exchange/repair protein is the heterodimeric Ku protein (Pierce et al. 2001). The recombination junction may be reciprocal in nature, or result in DNA end joining with deletions, duplications or inversions, demonstrating repair (Rassool 2003). Interestingly, the majority of all patient breakpoint translocation junctions found in MLL, AML1, BCR and their partners have hallmarks of non-homologous end joining (Rassool 2003). In addition, up to 25% of MLL breakpoints result in more than one million base pairs of MLL deleted on the derivative other chromosome (van der Burg et al. 1999). Similar findings have also been noted for BCR and ABL rearrangements (Sinclair et al. 2000). The often large distances between topo II HS sites and patient breakpoints further support extensive exonuclease activities of the DNA repair mechanism (Elliott et al. 2002). The proposed NHCT-model can also be used to explain intra-chromosomal deletions noted in solid tumors, where chromosome band 9p21 involving the IFN gene family is deleted (Strissel et al. 1998a). These DNA breaks, alignment and recombination events, which lead to large interstitial deletions could also involve SARs, LINEs or Alu elements (Bode et al. 2000). In contrast to translocation breakpoint junctions, AT-richness was found more frequently at chromosomal deletion breakpoints, which is also present in SARs, LINEs or Alu elements supporting a homologous recombination event (Abeysinghe et al. 2003)
References
Abeysinghe SS, Chuzhanova N, Krawczak M, Ball EV, Cooper DN (2003) Translocation and gross deletion breakpoints in human inherited disease and cancer I: Nucleotide composition and recombination-associated motifs. Hum Mut 22:229–244
Advani AS, Pendergast AM (2002). Bcr-Abl variants: biological and clinical aspects. Leuk Res 26:713–720
Aplan PD, Chervinsky DS, Stanulla M, Burhans WC (1996) Site-specific DNA cleavage within the MLL breakpoint cluster region induced by topoisomerase II inhibitors. Blood 87:2649–2658
Asami Y, Jia DW, Tatebayashi K, Yamagata K, Tanokura M, Ikeda H (2002) Effect of the DNA topoisomerase II inhibitor VP-16 on illegitimate recombination in yeast chromosomes. Gene 291:251–257
Bae YS Kawasaki I, Ikeda H, Liu LF (1988) Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro. Proc Natl Acad Sci USA 85:2076–2080
Baxter EJ, Hochhaus A, Bolufer P, Reiter A, Fernandez JM, Senent L, Cervera J, Moscardo F, Sanz MA, Cross NC (2002) The t(4;22)(q12;q11) in atypical chronic myeloid leukaemia fuses BCR to PDGFRA. Hum Mol Genet 11:1391–1397
Betti CJ, Villalobos MJ, Diaz MO, Vaughan AT (2001) Apoptotic triggers initiate translocations within the MLL gene involving the nonhomologous end joining repair system. Cancer Res 61:4550–4555
Blanco JG, Edick MM, Relling MV (2004) Etoposide induces chimeric Mll gene fusions. FASEB J 18: 173–175
Bloomfield CD, Archer KJ, Mrozek K, Linnington DM, Kanko Y, Head DR, Dai Cin P, Ramondi SC (2002) 11q23 balanced chromosome aberrations in treatment-related myelodysplastic syndromes and acute leukemia: Report from an international workshop. Geneshromosomes Cancer 33:362–378
Bode J, Benham C, Ernst E, Knopp A, Marschalek R, Strick R, Strissel P (2000) Fatal connections: when DNA ends meet on the nuclear matrix. J Cell Biochem 35:3–22
Borgnetto ME, Zunino F, Tinelli S, Kas E, Capranico G (1996) Drug-specific sites of topoisomerase II DNA cleavage in Drosophila chromatin: heterogeneous localization and reversibility. Cancer Res 56:1855–1862
Charron M, Hancock R (1991) Chromosome recombination and defective genome segregation induced in Chinese hamster cells by the topoisomerase II inhibitor VM-26. Chromosoma 100:97–102
Chissoe SL, Bodenteich A, Wang YF, Wang YP, Burian D, Clifton SW, Crabtree J, Freeman A, Iyer K, Jian L, Yichen M, et al (1995) Sequence and analysis of the human ABL gene, the BCR gene, and regions involved in the Philadelphia chromosomal translocation. Genomics 27:67–82
Clemens MJ, Bushell M, Morley SJ (1998) Degradation of eukaryotic polypeptide chain initiation factor (eIF) 4G in response to induction of apoptosis in human lymphoma cell lines. Oncogene 17:2921–2931
Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, Koduru P, Brody JP, Hawson G, Rodwell R and Doody ML, et al (2001) The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: transforming activity and specific inhibition of FGFR1 fusion proteins. Blood 98:3778–3783
Domer PH, Head DR, Renganathan N, Raimondi SC, Yang E, Atlas M (1995) Molecular analysis of 13 cases of MLL/11q23 secondary acute leukemia and identification of topoisomerase II consensus-binding sequences near the chromosomal breakpoint of a secondary leukemia with the t(4;11). Leukemia 9:1305–1312
Durrieu F, Samejima K, Fortune JM, Kandels-Lewis S, Osheroff N, Earnshaw WC (2000) DNA topoisomerase II alpha interacts with CAD nuclease and is involved in chromatin condensation during apoptotic execution. Curr Biol 10:923–926
Echlin-Bell DR, Smith LL, Li L, Strissel PL, Strick R, Gupta V, Banerjee J, Larson R, Relling MV, Raimondi SC, Hayashi Y, Taki T, Zeleznik-Le N, Rowley JD (2003) Polymorphisms in the MLL BCR. Hum Genet 113:80–91
Elliott B, Jasin M (2002) Double-strand breaks and translocations in cancer. Cell Mol Life Sci 59:373–385
Filipski J, Leblanc J, Youdale T, Sikorska M, Walker PR (1990) Periodicity of DNA folding in higher order chromatin structures. EMBO J 9:1319–1327
Fitzgerald KT, Diaz MO (1999) MLL2, A new mammalian member of the trx/MLL family of genes. Genomics 59:187–192
Greaves MF (1996) Infant leukaemia biology, aetiology and treatment. Leukemia 10:372–377
Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Canaani E (1992) The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax to the AF-4 gene. Proc Natl Acad Sci USA 71:701–708
Hensel JP, Gellert E, Fey GH, Marschalek R (2001) Breakpoints of t(4;11) translocations in the human MLL and AF4 genes in ALL patients are preferentially clustered outside of high-affinity matrix attachment regions. J Cell Biochem 82:299–309
Huang Y, Nakada S, Ishiko T, Utsugisawa T, Datta R, Kharbanda S, Yoshida K, Talanian RV, Weichselbaum R, Kufe D, Yuan ZM (1999) Role for caspase-mediated cleavage of Rad51 in induction of apoptosis by DNA damage. Mol Cell Biol 4:2986–2997
Huntsman DG, Chin SF, Muleris M, Batley SJ, Collins VP, Wiedemann L M, Aparicio S, Caldas C (1999) MLL2 the second human homolog of the Drosophila trithorax gene, maps to 19q13.1 and is amplified in solid tumor cell lines. Oncogene 18:7975–7984
Iarovala OV, Shkumatov P, Razin SV (2004) Breakpoint cluster regions of the AML-1 and ETO genes contain MAR elements and are preferentially associated with the nuclear matrix in proliferating HEL cells. J Cell Sci 117:4583–4590
Jeffs AR, Benjes SM, Smith TL, Sowerby SJ, Morris CM (1998) The BCR gene recombines preferentially with Alu elements in complex BCR-ABL translocations of chronic myeloid leukaemia. Hum Mol Gen 7:767–776
Jeffs AR, Wells E, Morris CM (2001) Nonrandom distribution of interspersed repeat elements in the BCR and ABL1 genes and its relations to breakpoint cluster regions. Genes Chromosomes Cancer 92:144–154
Jiang X Trujillo JM, Liang JC (1990) Chromosomal breakpoints within the first intron of the ABL gene are nonrandom in patients with chronic myelogenous leukemia. Blood 76:597–601
Khobta A, Carlo-Stella C, Capranico G (2004) Specific histone patterns and acetylase/deacetylase activity at the breakpoint-cluster region of the human MLL gene. Cancer Res 64:2656–2662
Kingma PS, Greider CA, Osheroff N (1997) Spontaneous DNA lesions poison human topoisomerase II alpha and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry 36:5934–5939
Langer T, Metzler M, Reinhardt D, Viehmann S, Borkhardt A, Reichel M, Stanulla M, Schrappe M, Creutzig U, Ritter J, Leis, et al (2003) Analysis of t(9;11) chromosomal breakpoint sequences in childhood acute leukemia: almost identical MLL breakpoints in therapy-related AML after treatment without etoposides. Genes Chromosomes Cancer 36:393–401
Langst G, Schatz T, Langowski J, Grummt I (1997) Structural analysis of mouse rDNA: coincidence between nuclease hypersensitive sites, DNA curvature and regulatory elements in the intergenic spacer. Nucleic Acids Res 25:511–517
Liu LF (1989) DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem 58:351–375
Lovett BD, Nigro L, Rappaport EF, Blair IA, Osheroff N, Zheng N, Megonigal MD, Williams WR, Nowell PC, Felix CA (2001) Near-precise interchromosomal recombination and functional DNA topoisomerase II cleavage sites at MLL and AF-4 genomic breakpoints in treatment-related acute lymphoblastic leukemia with t(4;11) translocation. Proc Natl Acad Sci USA 98:9802–9807
Mitelman F (1994) Catalog of chromosome aberrations in cancer 5th edn. Wiley–Liss, New York
Mondal N, Parvin JD (2001) DNA topoisomerase II alpha is required for RNA polymerase II transcription on chromatin templates. Nature 413:435–438
Nakamura T, Alder H, Gu Y, Prasad R, Canaani O, Kamada N, Gale RP, Lange B, Crist WM, Nowell PC, Croce CM, Canaani E (1993) Genes on chromosomes 4, 9, and 19 involved in 11q23 abnormalities in acute leukemia share sequence homology and/or common motifs. Proc Natl Acad Sci USA 90:4631–4635
Negrini M, Felix CA, Martin C, Lange BJ, Nakamura T, Canaani E, Croce CM (1993) Potential topoisomerase II DNA-binding sites at the breakpoints of a t(9;11) chromosome translocation in acute myeloid leukemia. Cancer Res 53:4489–4492
Papadopoulos P, Ridge SA, Boucher CA, Stocking C, Wiedemann LM (1995) The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res 55:34–38
Pedersen-Bjergaard J, Brondum-Nielsen K, Karle H, Johansson B (1997) Chemotherapy-related-late occurring-Philadelphia chromosome in AML, ALL and CML. Similar events related to treatment with DNA topoisomerase II inhibitors? Leukemia 11:1571–1574
Pierce AJ, Hu P, Han M, Ellis N, Jasin M (2001) Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev 15: 3237–3242
Rassool FV (2003) DNA double strand breaks (DSB) and non-homologous end joining (NHEJ) pathways in human leukemia. Cancer Lett 193:1–9
Reichel M, Gillert E, Angermuller S, Hensel JP, Heidel F, Lode M, Leis T, Biondi A, Haas OA, Strehl S, Panzer-Grumayer, et al (2001) Biased distribution of chromosomal breakpoints involving the MLL gene in infants versus children and adults with t(4;11) ALL. Oncogene 20:2900–2907
Reichel M, Gillert E, Nilson I, Siegler G, Gre il J, Fey GH, Marschalek R (1998) Fine structure of translocation breakpoints in leukemic blasts with chromosomal translocation t(4;11): the DNA damage-repair model of translocation. Oncogene 17:3035–3044
Reichel M, Gillert E, Breitenlohner I, Repp R, Greil J, Beck JD, Fey GH, Marschalek R (1999) Rapid isolation of chromosomal breakpoints from patients with t(4;11) acute lymphoblastic leukemia. Cancer Res 59:3357–3362
Reilling MV, Yanishevski Y, Nemec I, Evans WE, Boyett JM, Behm FG, Pui C-H (1998) Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia 12:346–352
Ross JA (2000) Dietary flavonoids and the MLL gene: a pathway to infant leukemia? Proc Natl Acad Sci USA 97:4411–4413
Rowley JD (1999) The role of chromosome translocations in leukemogenesis. Semin Hematol 36:59–72
Rowley J, Olney HJ (2002) International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: Overview report. Genes Chromosomes Cancer 33:331–345
Saglio G, Storlazzi CT, Giugliano E, Surace C Anelli L, Rege-Cambrin G, Zagaria A, Jimenez Velasco A, Heiniger A, Scaravaglio P, Torres Gomez A, et al (2002) 76-kb duplicon maps close to the BCR gene on chromosome 22 and the ABL gene on chromosome 9: possible involvement in the genesis of the Philadelphia chromosome translocation. Proc Natl Acad Sci USA 99:9882–9887
Salovoyan VT, Bezvenyuk ZA, Salminen A, Austin CA, Courtney MJ (2002) The role of topoisomeras II in the excision of DNA loop domains during apoptosis. J Biol Chem 277:21458–21467
Schaefer-Rego KE, Leibowitz D, Mears JG (1990) Chromatin alterations surrounding the BCR/ABL fusion gene in K562 cells. Oncogene 5:1669–1673
Schichman S, Caligiuri M, Gu Y, Strout M, Canaani E, Bloomfield CD, Croce CM (1994) ALL-1 partial duplication in acute leukemia. Proc Natl Acad Sci USA 91:6236–6239
Sinclair PB, Nacheva EP, Leversha M, Telford N, Chang J, Reid A, Bench A, Champion K, Huntly B, Green AR (2000) Large deletions at the t(9;22) breakpoint are common and may identify a poor-prognosis subgroup of patients with chronic myeloid leukemia. Blood 95:738–743
Spence JM, Critcher R, Ebersole TA, Valdivia M M, Earnshaw W C, Fukagawa T, Farr CJ (2002) Co-localization of centromere activity, proteins and topoisomerase II within a subdomain of the major human X alpha satellite array. EMBO J 21:5269–5280
Sperry AO, Blasquez VC, Garrard WT (1989) Dysfunction of chromosomal loop attachment sites: Illegitimate recombination linked to matrix association regions and topoisomerase II. Proc Natl Acad Sci USA 86:5497–5501
Spitzner JR, Muller MT (1988) A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids Res 16:5533–5556
Stanulla M, Wang J, Chervinsky DS, Thandla S, Aplan PD (1997) DNA cleavage within the MLL breakpoint cluster region is a specific event which occurs as part of higher-order chromatin fragmentation during the initial stages of apoptosis. Mol Cell Biol 17:4070–4079
Strick R, Laemmli UK (1995) SARs are cis elements of chromosome dynamics: synthesis of a SAR repressor protein. Cell 83:1137–1148
Strick R, Strissel PL, Borgers S, Smith SL, Rowley J D (2000) Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci USA 97:4790–4795
Strick R, Strissel PL, Gavrilov K, Levi-Setti R (2001) Cation-chromatin binding as shown by ion microscopy is essential for the structural integrity of chromosomes. J Cell Biol 155:899–910
Strissel PL, Espinosa RIII, Rowley JD, Swift H (1996a) Scaffold attachment regions in centromere-associated DNA. Chromosoma 105:122–133
Strissel PL, Gill-Super H, Thirman MJ, Pomykala H, Yonebayashi Y, Tanabe S, Zeleznik-Le N, Rowley JD (1996b) Distribution of 11q23 breakpoints within the MLL breakpoint cluster region in de novo acute leukemia and in treatment related acute myeloid leukemia: Correlation with scaffold attachment regions and topoisomerase II consensus binding sites. Blood 87:1912–1922
Strissel PL, Hadan HA, Pomykala HM, Diaz MO, Rowley JD, Olopade OI (1998a) Scaffold associated regions in the human type I interferon gene cluster on the short arm of chromosome 9. Genomics 47:217–229
Strissel PL, Strick R, Rowley JD, Zeleznik-Le NJ (1998b) An in vivo topoisomerase II cleavage site and a DNase I hypersensitive site colocalize near exon 9 in the MLL breakpoint cluster region. Blood 92:3793–3803
Strissel PL, Strick R, Tomek R J, Roe B, Zeleznik-Le N (2000) AF9, a common partner gene in MLL translocations of leukemia patients, demonstrates similar DNA structural properties to MLL that could act as recombination “hot spots”. Hum Mol Genet 9:1671–1679
Taki T, Kano H, Taniwaki M, Sako M, Yanagisawa M, Hayashi Y (1999) AF5q31, a newly identified AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia with ins(5;11)(q31;q13q23). Proc Natl Acad Sci USA 96:14535–14540
Tennyson RB, Ebran N, Herrera AE, Lindsley JE (2002) A novel selection system for chromosome translocations in Saccharomyces cerevisiae. Genetics 160:1363–1373
Timmons MS, Witte ON (1989) Structural characterization of the BCR gene product. Oncogene 4:559–567
Udvardy A, Schedl P (1991) Chromatin structure, not DNA sequence specificity, is the primary determinant of topoisomerase II sites of action in vivo. Mol Cell Biol 11:4973–4984
van der Burg M, Beverloo HB, Langerak AW, Wijsman J, van Drunen E, Slater R, van Dongen JJ (1999) Rapid and sensitive detection of all types of MLL gene translocations with a single FISH probe set. Leukemia 13:2107–2113
von Bergh AR, Beverloo HB, Rombout P, van Wering ER, van Weel MH, Beverstock GC, Kluin PM, Slater RM, Schuuring E (2002) LAF4, an AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia. Genes Chromosomes Cancer 35:92–96
Watt PM, Hickson ID (1994) Structure and function of type II DNA topoisomerases. J Biochem 303:681–695
Wiedeman LM, MacGregor A, Caldas C (1999) Analysis of the region of the 5′ end of the MLL gene involved in genomic duplication events. Brit J Haematol 105:256–264
Willmore E, Frank AJ, Padget K, Tilby MJ, Austin CA (1998) Etoposide targets topoisomerase II alpha and II beta in leukemic cells: isoform specific cleavage complexes visualized and quantified in situ by a novel immunofluorescence technique. Mol Pharmacol 54:78–85
Wu TC, Lichten M (1994) Meiosis-induced double strand break sites determined by yeast chromatin structure. Science 263:515–518
Yang L, Rowe TC, Nelson EM, Liu LF (1985) In vivo mapping of DNA topoisomerase II specific cleavage sites on SV40 chromatin. Cell 41:127–132
Zhang Y, Strissel P, Strick R, Chen J, Nucifora G, Le Beau M, Larson RA, Rowley JD (2002) Genomic DNA breakpoints cluster in AML1/RUNX1 and ETO with topoisomerase II DNA cleavage and DNase I hypersensitive sites in t(8;21) leukemia. Proc Natl Acad Sci USA 99:3070–3075
Zhang Y, Zeleznik-Le N, Emmanuel N, Jayathilaka N, Chen J, Strissel P, Strick R, Li L, Neilly MB, Taki T, Hayashi et al (2004) Characterization of genomic breakpoints in MLL and CBP in leukemia patients with t(11;16). Genes Chromosomes Cancer 41:257–265
Acknowledgements
The authors would like to dedicate this study to Dr. Hewson Swift, from the University of Chicago, deceased in 2004, who supported the idea that “chromatin matters” in chromosomal rearrangements. The authors would also like to thank Dr. Janet Rowley for her continued support with this study. This project was supported by ACS Grant # 01-06 to PLS and RS
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strick, R., Zhang, Y., Emmanuel, N. et al. Common chromatin structures at breakpoint cluster regions may lead to chromosomal translocations found in chronic and acute leukemias. Hum Genet 119, 479–495 (2006). https://doi.org/10.1007/s00439-006-0146-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-006-0146-9