Abstract
The current system for the classification of hereditary defects of tooth dentin is based upon clinical and radiographic findings and consists of two types of dentin dysplasia (DD) and three types of dentinogenesis imperfecta (DGI). However, whether DGI type III should be considered a distinct phenotype or a variation of DGI type II is debatable. In the 30 years since the classification system was first proposed, significant advances have been made regarding the genetic etiologies of inherited dentin defects. DGI type II is recognized as an autosomal dominant disorder with almost complete penetrance and a low frequency of de novo mutations. We have identified a mutation (c.52G→T, p.V18F) at the first nucleotide of exon 3 of the DSPP (dentin sialophosphoprotein) gene in a Korean family (de novo) and a Caucasian family. This mutation has previously been reported as causing DGI type II in a Chinese family. These findings suggest that this mutation site represents a mutational “hot spot” in the DSPP gene. The clinical and radiographic features of these two families include the classic phenotypes associated with both DGI type II and type III. Finding that a single mutation causes both phenotypic patterns strongly supports the conclusion that DGI type II and DGI type III are not separate diseases but rather the phenotypic variation of a single disease. We propose a modification of the current classification system such that the designation “hereditary opalescent dentin” or “DGI type II” should be used to describe both the DGI type II and type III phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary dentin defects have historically been classified into two main groups: dentin dysplasia (DD) and dentinogenesis imperfecta (DGI). According to this classification system, which is based upon clinical and radiographic features (Shields et al. 1973), inherited dentin defects associated with osteogenesis imperfecta (OI) are classified as DGI type I, whereas isolated inherited dentin defects are categorized as DGI types II and III, and DD types I and II. DGI type II is one of the more common genetic tooth disorders, with an estimated incidence of 1:8,000 (Witkop 1957). DGI type II has an autosomal dominant pattern of inheritance and generally shows complete penetrance and a low frequency of de novo mutations (Shields et al. 1973; Witkop 1971).
Recent genetic findings have focused attention on the role of the DSPP (dentin sialophosphoprotein) gene in the etiology of inherited defects of tooth dentin. Mutations in the DSPP gene have been shown to cause DGI type II (Kim et al. 2004; Malmgren et al. 2004; Xiao et al. 2001; Zhang et al. 2001) and DD type II (Rajpar et al. 2002). In addition, an important possible connection between the DSPP gene and the DGI type III phenotype has been established by the characterization of DSPP knock-out mice, which develop tooth defects similar to those seen in human DGI type III (Sreenath et al. 2003). Although there are other candidate genes for isolated inherited malformations of dentin, no disease-causing mutations outside of the DSPP gene have yet been identified. In the eight kindreds with disease-producing DSPP gene mutations characterized to date, the pattern of inheritance is autosomal dominant.
Here, we report the results of mutational analyses of the DSPP gene in two unrelated kindreds with the same disease-causing mutation (c.52G→T, p.V18F), which is located at the first nucleotide of exon 3 in the DSPP gene. Kindred 1 is a Korean family with a de novo mutation in the proband displaying a DGI type III phenotype. Kindred 2 is a Caucasian family displaying a phenotype most consistent with a DGI type II classification. Previously, this DSPP mutation was identified in a Chinese family diagnosed with DGI type II (Xiao et al. 2001). None of the other DSPP mutations have been identified in more than one kindred.
The implications of these findings are twofold. First, the observation of the same DSPP mutation in three unrelated kindreds suggests that the first nucleotide of exon 3 is a mutational “hotspot”. Second, the finding that a single DSPP gene mutation underlies both the DGI type II and DGI type III phenotypes indicates that these DGI types should be combined and recognized as phenotypic variations related more to differences in severity or expressivity than to variations in kind. Therefore, we propose a modification of the current classification system.
Materials and methods
Enrollment of human subjects
The study protocol and subject consent were reviewed and approved by the Institution Review Boards at the University of Michigan and Seoul National University, and appropriate informed consent was obtained from all subjects.
Polymerase chain reaction and sequencing
Peripheral whole blood (10 ml) was obtained from participating family members. Genomic DNA was isolated by using the G-DEX Genomic DNA Extraction Kit (iNtRON Biotechnology, Korea). Conditions for the polymerase chain reaction (PCR) and the DNA sequences of the oligonucleotide primer pairs used for DSPP exon-specific PCR amplifications and DNA sequencing were as previously described (Kim et al. 2004). PCRs were performed with i-StarTaq DNA polymerase (iNtRON Biotechnology). PCR amplification products were purified by the QIAquick PCR Purification Kit and protocol (Qiagen, Valencia, Calif., USA).
Paternity testing
Genomic DNA from the proband and parents in Kindred 1 were analyzed by a commercial laboratory (Affiliated Genetics, Salt Lake City, Utah, USA) by using ten genetic markers (D3S1358, D21S11, vWA, D16S539, D2S1338, D8S1179, D18S51, D19S433, Tho 1, FGA).
Single-stranded conformational polymorphism analysis
After the DSPP mutation was identified in exon 3, we designed PCR primers for single-stranded conformational polymorphism analysis (SSCP) analysis (218-bp amplification product; sense: 5′-gtgtgcacgctcacacacat and antisense 5′-ggagaagttaatggaatgtagc). The PCR products were mixed with formamide loading buffer (95% formamide with bromophenol blue and xylenecyanol), heated to 95°C for 5 min, quenched on ice, and loaded onto a 10% polyacrylamide gel containing 10% glycerol. Electrophoresis was performed at a constant ampere mode of 15 mA for 7 h.
Results
The DNA sequence analyses from two unrelated kindreds showed that the probands had identical mutations (GTT to TTT, c.52G→T) in the first codon of exon 3 in the DSPP gene (Fig. 1). These mutations changed codon 18 from valine to phenylalanine (p.V18F).
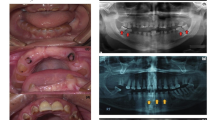
a Pedigree of kindred 1. b, c DNA sequencing chromatograms of the PCR amplification of intron 2/exon 3 from the proband and a wild-type individual, respectivley (red arrowhead position of the mutated nucleotide). d SSCP analysis showing that the mutation (red arrowhead) is only found in the proband. e Pedigree of kindred 2. f Clinical photograph showing amber discoloration of deciduous dentition. g Periapical radiograph showing slightly smaller coronal pulp chambers but normal pulp canals
Kindred 1
The family pedigree for kindred 1 was determined, and eight members spanning three generations were recruited for mutational analyses (Fig. 1a). Only the proband showed the disease phenotype. The mutation in exon 3 was identified in the proband (Fig. 1b, c), and SSCP analysis showed a unique band among the exon 3 amplification products of the proband (Fig. 1d), indicating that only the proband had the mutation. Paternity testing with ten genetic markers gave a probability of paternity of 99.98%, indicating that the father was 6,120 times more likely to be the biological father than a random person. From this, we concluded that the c.52G→T mutation had arisen spontaneously in this family.
The oral and radiographic presentations of the proband from kindred 1 are shown in Fig. 2. The crowns of the primary teeth are uniformly shaded brown. The enamel tends to chip and fracture away causing rapid attrition that exposes the softer malformed dentin to the oral cavity. The sizes of the dental pulp chambers appear to be wide and similar to so-called “shell teeth”. The anterior teeth showed multiple pulp exposures and periapical inflammation. Four primary first molars were restored with stainless steel crowns to prevent further attrition. Physical examination revealed no rickets or other obvious skeletal abnormalities.
Clinical photographs and radiographs from the proband of kindred 1. The clinical photograph taken at age 25 months (a) shows severe attritions and resulting periapical abscess (arrowhead). Panoramic radiograph taken at age 3 (b). Periapical radiograph taken at 15 months old (c) and 25 months old (d–i) show wide pulp chambers
Kindred 2
Kindred 2 is a Caucasian family with a dental history that can be traced back three generations (Fig. 1e). The DSPP mutation is the same as in kindred 1, but some of the phenotypes show different characteristics. The proband is a 22-month-old boy with 16 primary teeth. The chronology of dental development is within normal limits. Because of a digit sucking habit, the proband has an anterior open bite. Radiographically, the coronal pulp chambers are smaller than normal, but the pulp canals look normal. There are no signs of the teeth having pulpal exposures secondary to severe attrition and a lack of dentin structure. The 4-year-old brother of the proband has the same dental condition, but without the open bite, and had all of his primary teeth covered with stainless steel crowns at age 3.5.
There was no apparent hearing loss symptom in either kindred 1 and kindred 2 (data not shown).
Discussion
The effect of the observed mutation (g.1191G→T, c.52G→T, GTT to TTT) on DSPP protein synthesis is not as straightforward as might be expected. Because of the narrow pattern of DSPP gene expression and its association with mineralized structures, there are no tissues that can be easily biopsied to gain DSPP mRNA or protein for analysis. For this reason, the effect of the mutation on DSPP expression cannot be assayed directly. Because this mutation consistently yields a dental phenotype, even though affecting just one of the two DSPP alleles, it can safely be concluded that it causes a significant quantitative or qualitative disturbance in DSPP expression.
The effects on DSPP expression could take any of several forms. Previously, the G to T transversion in the first nucleotide of exon 3 was predicted to result in a substitution of Val by Phe at amino acid position 18, which might interfere with the signal peptide function (to secrete the protein) or with its cleavage (by signal peptidase; Xiao et al. 2001). The signal peptide cleavage site is between Ala15 and Ile16. Alternatively, the p.V18F substitution might interfere with a function of the DSP protein or disrupted its folding.
In addition to altering the DSP protein sequence, the mutation might interfere with normal mRNA splicing. The mutation changes the G in the +1 position of the splicing acceptor sequence to a T (CAG GT to CAG TT). A G is found in the +1 acceptor position in 50% of human GT-AG introns (Clark and Thanaraj 2002), making it plausible that this mutation may also diminish the ability of the splicing machinery to delete intron 2. This would result in the skipping of exon 3, with 28 amino acid being deleted (p.V18-Q45del) from the N-terminal portion of DSP as a consequence.
There are now eight known mutations in the human DSPP gene, seven being associated with DGI type II and one being associated with DD type II (Table 1). In this study, we have found the same mutation in different ethnic populations. Previously, this mutation was reported in a Chinese family (Xiao et al. 2001). We have now identified this same mutation in a Caucasian family and as a rare de novo mutation in a Korean family. We conclude that this site, at the edge of an exon, represents a mutational “hot spot” in the DSPP gene.
The current classification system for dentinogenesis imperfecta was proposed by Shield et al. (1973), and is briefly summarized.
DGI type I
This is the dental phenotype in individuals afflicted with osteogenesis imperfecta. The teeth show marked discoloration and attrition in both the deciduous and permanent dentition. Pulpal obliteration occurs soon after eruption and sometimes even prior to tooth eruption. The degree of expressivity is variable, even within a single individual patient, ranging from total pulpal obliteration to normal-appearing dentin.
DGI type II
This has many clinical and radiographic similarities to DGI type I, but penetrance is almost complete, and expressivity is much more consistent within a family when compared with DGI type I.
DGI type III
This was first found in the Brandywine triracial isolate from southern Maryland and Washington DC. In coloration and shape, the teeth appear variable as in either DGI type I and DGI type II, but unlike those two traits, multiple pulp exposures are observed in the deciduous teeth. Radiographically, the deciduous teeth show considerable variation in appearance, ranging from pulpal obliteration, to normal, even to shell teeth.
According to this classification, our kindred 1 should be designated as DGI type III. Kindred 2 can be classified as DGI type II, although the pulp chambers seem to be normal when compared with the characteristic DGI type II pattern. In the Chinese family with the same mutation, the phenotype was reported as DGI type II. Our study demonstrates that the characteristics that distinguish DGI type II and DGI type III do not correlate with the genetic etiology and that these two types do not exist as separate disease entities.
The “Brandywine isolate” is an inbred population of mixed Caucasian, Black, and Amerindian in USA. Almost 6% of this isolate is known to be affected with inherited dentin defects. A clear example of a founder effect, the ancestry of the diseased gene can be traced to a sea captain from Liverpool who came to Maryland in 1732 (Witkop 1965). The dental phenotype in the Brandywine isolate is the prototype for DGI type III. Before the DGI type III designation was proposed, the very large pulp chambers and root canals in the deciduous dentition were explained as a variation of expression, occurring in about 2% of the Brandywine isolate cases (Witkop 1971), and the permanent teeth of these variants were reported to be the same as in DGI type II (Witkop 1989). Thus, the characteristics that distinguish the DGI type III phenotype are rare, even in the Brandywine isolate.
DGI types II and III were set apart because “multiple pulp exposures were observed in deciduous teeth in DGI type III” and “in all cases reported to have DGI type II, there are no reported instances of shell teeth” (Shields et al. 1973). Unfortunately, the apparent uniqueness of this distinguishing feature of the DGI type III phenotype may have been attributable to a lack of reports published at the time when the classification was proposed. For the same reason, DGI was not believed to exist in pure Mongoloid and Negroid racial groups (Bixler et al. 1969; Reiskin 1981). Furthermore, the classification system was developed without knowledge of the genetic defects underlying these diseases.
The distinguishing DGI type III feature (large pulp chambers resembling shell teeth, especially in young deciduous teeth) was occasionally observed in DGI kindreds outside the Brandywine isolate (Bixler et al. 1969; Esposito and Vergo 1978; Heimler et al. 1985; Sapir and Shapira 2001). Malmgren et al. (1988) have reported a 16-month-old patient with large pulp chambers and periapical radiolucencies in a six-generation DGI type II family and have recently identified (Malmgren et al. 2004) the underlying mutation (p.R68W) in the DSPP gene. In this report, we show that the same DSPP mutation results in a DGI type II or a DGI type III phenotype in different individuals. These results suggest that the phenotypic distinction between DGI types II and III are trivial, and greater emphasis should be placed on the many features common to both the DGI type II and type III phenotypes.
Roberts and Schour (1939) first used the term “dentinogenesis imperfecta” to describe dentin defects related to osteogenesis imperfecta, and Skillen (1937) used “hereditary opalescent dentin” for isolated dentin defects in the absence of bone disease (Witkop 1971). We propose a modification of the current classification system such that the designation “hereditary opalescent dentin” or “DGI type II” should be used to describe both the DGI type II and type III phenotypes.
References
Bixler D, Conneally PM, Christen AG (1969) Dentinogenesis imperfecta: genetic variations in a six-generation family. J Dent Res 48:1196–1199
Clark F, Thanaraj TA (2002) Categorization and characterization of transcript-confirmed constitutively and alternatively spliced introns and exons from human. Hum Mol Genet 11:451–464
Esposito S, Vergo TJ Jr (1978) Removable overdentures in the oral rehabilitation of patients with dentinogenesis imperfecta. J Pedod 2:304–315
Heimler A, Sciubba J, Lieber E, Kamen S (1985) An unusual presentation of opalescent dentin and Brandywine isolate hereditary opalescent dentin in an Ashkenazic Jewish family. Oral Surg Oral Med Oral Pathol 59:608–615
Kim J-W, Nam S-H, Jang K-T, Lee S-H, Kim C-C, Hahn S-H, Hu JC-C, Simmer JP (2004) A novel splice acceptor mutation in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet 115:248–254
Malmgren B, Lundberg M, Lindskog S (1988) Dentinogenesis imperfecta in a six-generation family. A clinical, radiographic and histologic comparison of two branches through three generations. Swed Dent J 12:73–84
Malmgren B, Lindskog S, Elgadi A, Norgren S (2004) Clinical, histopathologic, and genetic investigation in two large families with dentinogenesis imperfecta type II. Hum Genet 114:491–498
Rajpar MH, Koch MJ, Davies RM, Mellody KT, Kielty CM, Dixon MJ (2002) Mutation of the signal peptide region of the bicistronic gene DSPP affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum Mol Genet 11:2559–2565
Reiskin A (1981) Dentinogenesis imperfecta. Quintessence Int 12:617–622
Roberts E, Schour I (1939) Hereditary opalescent dentine (dentinogenesis imperfecta). Am J Orthod Oral Surg 25:267–276
Sapir S, Shapira J (2001) Dentinogenesis imperfecta: an early treatment strategy. Pediatr Dent 23:232–237
Shields ED, Bixler D, el-Kafrawy AM (1973) A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol 18:543–553
Skillen WG (1937) Histologic and clinical study of hereditary opalescent dentin. J Am Dent Assoc 24:1426–1433
Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB (2003) Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem 278:24874–24880
Witkop CJ Jr (1957) Hereditary defects in enamel and dentin. Acta Genet Stat Med 7:236–239
Witkop C Jr (1965) Genetic diseases of the oral cavity. In: Tiecke RW (ed) Oral pathology. McGraw Hill, New York, pp 786–843
Witkop CJ Jr (1971) Manifestations of genetic diseases in the human pulp. Oral Surg Oral Med Oral Pathol 32:278–316
Witkop CJ Jr (1989) Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Path 17:547–553
Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X (2001) Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP (erratum appears in Nat Genet 2001, 27(3):345). Nat Genet 27:201–204
Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y (2001) DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet 27:151–152
Acknowledgements
We thank all the family members for their cooperation. This investigation was supported in part by the Foundation of the American Academy of Pediatric Dentistry, and USPHS Research Grants DE15846 and DE11301 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 29892.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, JW., Hu, J.CC., Lee, JI. et al. Mutational hot spot in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet 116, 186–191 (2005). https://doi.org/10.1007/s00439-004-1223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-004-1223-6