Abstract
MYB transcription factors exist in a large copy number and control various plant phenotypes. We cloned R2R3 MYB-type transcription factors that determine the coloration of basal sheaths in barley and wheat coleoptiles. These genes are highly homologous to maize C1 and rice OsC1, regulators for anthocyanin biosynthesis, but they control seed pigmentation in maize and rice. On the basis of high homology, barley and wheat counterparts are designated HvC1 and TaC1, respectively. HvC1 gene is located on the short arm of chromosome 7H, and TaC1 genes are located on the short arms of chromosomes 7A, 7B, and 7D (TaC1-A1, B1, and D1, respectively). HvC1 is a strong candidate for Ant1 because of (1) complete co-segregation of anthocyanin pigmentation phenotype of the basal sheath with the HvC1 genotype in genetic mapping, and (2) complete deletion of the HvCl gene in two anthocyanin-decreased allelic mutants (ant1.1 and ant1.2) that were induced by irradiation. In contrast, colorless coleoptile wheat lines had lesions in all three genomes consisting of a single-nucleotide substitution or a 1-bp deletion of TaC1-A1, a 1.7-kb insertion of TaC1-B1, and a 2.0-kb insertion of TaC1-D1. At least one normal TaC1 gene appears to be sufficient to produce anthocyanin pigments in wheat coleoptiles. Previous crossing experiments localized Rc (red coleoptile) genes to homoeologous group 7 chromosomes and deduced Rc genotypes of several wheat lines. Their TaC1 gene sequence variation coincided with deduced Rc genotypes; therefore, the present molecular genetic study demonstrates that TaC1 is a strong candidate for Rc in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant pigmentations that are stably expressed in specific growth stages and organs have been used as classical genetic markers because they are easily scored with the naked eye. Particularly, anthocyanin pigmentation has been utilized as a reliable genetic marker in grasses. In wheat (Triticum aestvum L. 2n = 6x = 42, genomes AABBDD), many genes have been reported for anthocyanin pigmentation, such as Pan (Purple anther), Pc (Purple culm), Pg (Purple glume), Plb (Purple leaf blade), Pp (Purple pericarp), R (Red grain), Ra (Red auricle), and Rc (Red coleoptile) (McIntosh et al. 2010). However, among the genes noted above, only the R gene has been molecularly identified to encode an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in grains (Himi et al. 2011; Himi and Noda 2005).
Anthocyanin and proanthocyanidin are synthesized via a common flavonoid biosynthetic pathway. In this pathway, anthocyanin and proanthocyanidin precursors are generated by enzymes encoded by chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′hydroxylase (F3′H), or flavonoid 3′5′ hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR), and then the pathway branches into anthocyanin- or proanthocyanidin-specific synthesis (Winkel-Shirley 2001). These enzymatic genes are grouped into early flavonoid biosynthetic genes (EBG), including CHS, CHI, and F3H, and late flavonoid biosynthetic genes (LBG), including DFR and further downstream catalytic enzymes, for anthocyanin and proanthocyanidin production. These enzymatic genes are controlled by specific regulatory genes. In Arabidopsis (Arabidopsis thaliana), EBGs are transcriptionally activated by R2R3 MYB proteins (MYB11, MYB12, and MYB111); however, several LBGs are activated by the MYB-bHLH-WD40 (MBW) ternary complex (Li 2014). Anthocyanin synthesis of Arabidopsis is controlled by the MBW ternary complex of (1) one R2R3 MYB protein from PRODUCTION OF ANTHOCYANIN PIGMENTS (PAP1), PAP2, MYB113, or MYB114, (2) one bHLH protein from TRANSPARENT TESTA8 (TT8), GLABROUS3 (GL3), or ENHANCER OF GLABRA3 (EGL3), and (3) TRANSPARENT TESTA GLABROUS1 (TTG1) of WD40. In the case of maize (Zea mays), both EBGs and LBGs seemed to be regulated by an MBW complex consisting of C1/Pl of MYB, R/B1/SN1/HOPI1 of bHLH, and PAC1 of WD40 for anthocyanin production (Petroni and Tonelli 2011).
In barley (Hordeum vulgare L. 2n = 2x = 14, HH), a number of anthocyanin/proanthocyanidin-decreased (ant) mutants, with both spontaneous and artificial origins, were isolated. Allelism tests grouped ant mutants into 30 genetically independent loci (Ant1 to Ant30), but the molecular identities of only four loci have been clarified: Ant2 (HvbHLH1) (Cockram et al. 2010), Ant18 (dihydroflavonol 4-reductase; DFR) (Kristiansen and Rohde 1991), Ant28 (Hvmyb10) (Himi et al. 2012), and Ant30 (chalcone isomerase; CHI) (Druka et al. 2003). Ant2 regulates the anthocyanin pigmentation of the auricle, awn, and lemma, but it is not involved in grain proanthocyanidin pigmentation. In contrast, Ant28 specifically regulates proanthocyanidin synthesis in grain.
In rice (Oryza sativa), the allelic series at the C locus controls the degree of anthocyanin pigmentation in the apiculus. The rice C gene encodes an R2R3 MYB-type transcription factor that is an ortholog of maize C1 (Saitoh et al. 2004). Additionally, two R genes were identified in rice, designated Ra and Rb; the active Ra gene showed similarity to the maize R (Lc) gene (Hu et al. 1996).
Wheat Rc genes remain molecularly unidentified, but their recessive (colorless coleoptiles) lines did not express F3H, one of the enzymatic genes. This observation suggests that Rc is a regulatory gene, such as MYB or bHLH (Khlestkina et al. 2008). Wheat Rc genes mapped the short arms of chromosomes 7A (Rc-A1), 7B (Rc-B1), and 7D (Rc-D1) (Khlestkina et al. 2002). Triple recessive genotypes (gene symbol Rc-A1a/Rc-B1a/Rc-D1a) exhibit colorless coleoptiles, while those with at least one dominant genes (Rc-A1b, Rc-B1b, or Rc-D1b) show anthocyanin pigmentation in the coleoptiles (Khlestkina et al. 2010). Interestingly, barley ant1 mutants lack anthocyanin pigmentation in the basal leaf sheath that appears to have a similar phenotype to that of wheat Rc triple recessives with colorless coleoptiles (Jende-Strid and Lundqvist 1978).
In this study, we cloned new members of the R2R3 MYB genes that control the anthocyanin biosynthesis pathway in wheat and barley. These MYB genes were designated TaC1-A1, TaC1-B1, and TaC1-D1 derived from the A, B, and D genomes of hexaploid wheat, respectively, and their barley ortholog was named HvC1. This is because they showed high similarity to the previously reported maize C1 and rice OsC1. TaC1 genes and the HvC1 gene mapped on the short arms of wheat homoeologous group 7 and barley 7H, which are syntenic to the rice chromosome 6 region that harbors the OsC1 gene. Genomic sequencing analysis revealed that a single-nucleotide substitution or a 1-bp deletion was found in the TaC1-A1 gene of recessive Rc-A1a lines, a 1.7-kb insertion was found in the TaC1-B1 gene of recessive Rc-B1a lines, and a 2.0-kb insertion was found in the TaC1-D1 gene of recessive Rc-D1a lines. Substitutions or insertions are predicted to cause amino acid changes or frame-shift, and the resultant protein may be dysfunctional as regulators to activate the flavonoid biosynthetic genes. Additionally, it was found that two independent barley ant1 mutants deleted at least a 7-kb 7HS genomic region, including the entire HvC1 gene. On the basis of multiple mutant analyses, both in wheat and barley, we conclude that wheat Rc genes and barley Ant1 loci may encode new members of the R2R3 MYB transcription factor that show a high homology to the maize C1 gene. The unique aspect of wheat Rc and barley Ant1 genes is their vegetative tissue-specific regulation of anthocyanin pigmentation, in contrast to the grain color regulation of maize C1 and rice OsC1.
Materials and methods
Materials
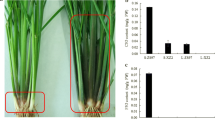
Wild-type barley (Hordeum vulgare L., cv. Bonus) and its anthocyaninless ant1 mutants (ant1.1, ant1.2, ant1.4, and ant1.56) were supplied by Dr. Udda Lundqvist (NordGen) for use in this study (Supplementary Table S1). As compared to the wild type, ant1.1 does not show red pigmentation in leaf sheath (Fig. 1a, b). ant1.2 shows a similar colorless phenotype, but ant1.4 and ant1.56 show a red-colored phenotype that disagrees with the original mutant description (Supplementary Fig. S1a). The latter two dubious mutants were also included in the initial molecular analysis.
Leaf sheath bases of barley (a–c), and coleoptiles of wheat (d, e). Bonus and OUI026, which have dominant Ant1 loci, show red (a and c); whereas ant1.1, which lacks Ant1, does not show the red color (b). NS67genotyped Rc-A1a/Rc-B1b/Rc-D1b accumulates anthocyanin in a coleoptile (d), but CS (Rc-A1a/Rc-B1a/Rc-D1a) does not accumulate red pigment in a coleoptile (e). Ant1, Rc-B1b, and Rc-D1b written in bold represent dominant alleles (color figure online)
Four wheat cultivars with deduced Red coleoptile (Rc) genotypes in a previous study (Khlestkina et al. 2011) were used: Chinese Spring (CS; Rc-A1a/Rc-B1a/Rc-D1a), Hope (Rc-A1b/Rc-B1b/Rc-D1a), Novosibirskaya67 (NS67; Rc-A1a/Rc-B1a/Rc-D1b), and Mironovskaya808 (M808; Rc-A1a/Rc-B1a/Rc-D1b) (Supplementary Table S1). Since CS is a triple recessive Rc genotype, its coleoptile does not have red pigmentation as compared to NS67 (Fig. 1d, e). Moreover, 6 additional lines/cultivars with unknown Rc genotypes were included: 8019R1, Douson1, Cornell595-W, Ardito, AUS1408, and OW104. All these show red pigmentation in the coleoptile.
Wheat cv. CS and barley cv. Betzes chromosome disomic addition lines harboring barley chromosomes 2H, 3H, 4H, 5H, 6H, and 7H as well as ditelosomic addition lines for the short arms of Betzes chromosome 1HS, 7HS, and 7HL (Islam et al. 1981) listed in Supplementary Table S1 were also used. Nulli-tetrasomic lines of Chinese Spring for homoeologous group 7 and CS deletion lines for the short arms of 7A, 7B, and 7D (Endo and Gill 1996) provided by the National BioResource Project were also used.
Analysis of anthocyanin content
Relative anthocyanin levels were determined according to Neff and Chory (1998), with some modification. Auricles of flag leaves and 3 cm of the upper part of the awns of Bonus and ant1.1 were collected at the heading stage. Frozen tissues were ground with liquid N2 and incubated for 16 h in 300 μL of methanol acidified with 1 % HCl at 4 °C. After the addition of 200 μL of distilled water, anthocyanins were separated from chlorophylls with 500 μL of chloroform. Total anthocyanins were determined by measuring the OD535 and OD657 of the aqueous phase using a spectrophotometer (Ubest, Japan Spectroscopic Co.). By subtracting the OD657 from the OD530, the relative amount of anthocyanin was calculated. Anthocyanin extraction was performed in triplicate with different tillers. A t test was used for statistical analyses.
DNA extraction and PCR amplification
DNA was isolated from 0.1 g of leaves with DNeasy Plant Mini Kit (Qiagen) according to instructions from the supplier. Primers used for this study are listed in Supplementary Table S2.
Barley cDNA (accession number AK359777) that showed high similarity to OsC1 (AB111869; Os06g0205100) on rice chromosome 6 was used to design the primers (HvC1-LP1, 2, 3, and HvC1-RP1, 2, 3) (Fig. 2a). The cDNA sequence showed identification with Morex_contig_137164 (IPK Blast Server; http://webblast.ipk-gatersleben.de/barley/), and about 4-kb upper and 1.5-kb lower sequences from the identification area in the contig were used to design the pairs of primers shown in Fig. 2a (137164-LP1 and RP1 and 137164-LP2 and RP2, respectively). Also, pairs of primers (48315-LP1/RP1 and 1562627-LP1/RP1) were designed based on the sequences of Morex_contig_48315 and Morex_contig_1562627, which correspond to Os6t0204800 and Os6t0205600, respectively (Fig. 2a).
a Chromosomal localization of rice C1 (OsC1) and the relation between OsC1 and HvC1. OsC1 (syn.Os06t0205100) is located on the rice chromosome 6 pseudomolecule positioned from 5315163 to 5316640 (+strand). The OsC1 shows high homology to a barley contig, morex_contig_137164, and a cDNA, AK359777. Two neighbor genes of OsC1, Os06t0204800 and Os06t0205600, show high homology to barley contigs, morex_contig_48315 and morex_contig_1562627, respectively. Arrows represent primers based on these contigs (48315-LP1 and 48315-RP1, 1562627-LP1 and 1562627-RP1, 137164-LP1 and 137164-RP1, and 137164-LP2 and 137164-RP2). Black arrowheads represent primers for HvC1. b PCR amplification of HvC1 using wheat cv. CS, barley cv. Betzes, and wheat–barley chromosome addition lines
A partial sequence of TaC1 was obtained from the amplification with a pair of HvC1-LP1 and -RP2. The obtained sequence was compared to Cereals DB (http://www.cerealsdb.uk.net/cerealgenomics/CerealsDB/search_reads.php), and high similarity between contig559744 and contig383159 derived from CS was found. Then, primers for TaC1 were designed based on the obtained sequences.
The PCR mixture (10 μL) included 5 ng of genomic DNA, 1× Ex Taq buffer (TaKaRa), 0.2 mM dNTP, 0.5 μM primers, 10 % glycerol, and 0.25 U of ExTaq (TaKaRa). A total of 35 PCR cycles were performed, with each cycle involving 30 s at 94 °C, 30 s at 60 °C, and 1 min at 72 °C, except for some pairs of primers, for which the annealing temperatures used are mentioned later. PCR products were checked by electrophoresis and then cloned into pGEM-T Easy Vector (Promega) and sequenced with ABI 3100 sequencer. Obtained sequences were analyzed using GENETYX ver. 10.0.3 (GENETYX CORPORATION).
The 5′ regions of TaC1 were amplified by inverse PCR. The genomic DNA (2.5 μg) of NS67 was digested with 25 U of one of the following enzymes: ClaI or PmlI. The digested DNA was self-ligated using T4-ligase (Promega) and used as a template for subsequent PCRs, with TaC1-LP4 and TaC1-RP2 for the first PCR and TaC1-LP5 and TaC1-RP5 for the nested PCR.
TaC1 genotyping
In recessive TaC1-A1 lines such as CS, single-nucleotide substitutions were found in the 3′ terminal region. A dCAPS (Derived Cleaved Amplified Polymorphic Sequences) primer, TaC1-LP15, was designed using the dCAPS Finder ver. 2.0 (http://helix.wustl.edu/dcaps/dcaps.html). TaC1-A1 fragments were first amplified with TaC1-LP11 and TaC1-RP13 with 35 cycles of 30 s at 94 °C, 30 s at 64 °C, and 1 min at 72 °C, and then nested PCR was performed with TaC1-LP15 and TaC1-RP4 with 35 cycles of 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C. Ten μL of the PCR product was incubated with/without 10 U of ApaI and separated in 3 % agarose gel.
Two types of TaC1-B1 were separately amplified with pairs of primers: TaC1-LP10 and TaC1-RP8, and TaC1-LP11 and TaC1-RP8. Also, two types of TaC1-D1 were amplified with combinations of TaC1-LP7 and TaC1-RP11, and TaC1-LP3 and TaC1-RP11.
TaC1-D1 genotyping was performed with 96 F2 plants of NS67 and CS crossing, using the primers for TaC1-D1 mentioned above.
Mapping of HvC1 and TaC1
A total of 96 F2 plants derived from a cross between OUI026 and ant1.1 were used for the molecular mapping of ant1 in relation to HvC1 and other markers. OUI026 shows pigmentation in the leaf sheath base (Fig. 1c). The presence or absence of purple pigmentation at the leaf sheath base was individually scored at the tillering stage. Recombination values were calculated as reported in Taketa et al. (2011).
To map the HvC1 gene, HvC1-LP1 and HvC1-RP1 primers were used. Other molecular markers used for mapping listed in Supplementary Table S2 were from public sources (Li et al. 2003). The map was compared to previously reported maps by Varshney et al. (2007), Rostoks et al. (2005), and BinMap (http://barleygenomics.wsu.edu/).
For chromosomal localization of TaC1-A1, -B1, and -D1 of CS, the amplification specificity from each homoeologous group 7 chromosome was confirmed with nulli-tetrasomic lines. Cytogenetic mapping was also performed with the aid of partial deletion lines of the short arm of homoeologous group 7 chromosomes of CS. The primer pairs for 7A, 7B, and 7D were TaC1-LP3 and TaC1-RP4, TaC1-LP10 and TaC1-RP8, and TaC1-LP7 and TaC1-RP11, respectively. The chromosomal location of TaC1-A1 was compared to maps by Khlestkina et al. (2002) and Somers et al. (2004).
Results
Isolation of HvC1
Rice C1 (OsC1), a homolog of maize C1, was previously isolated and is located on the short arm of chromosome 6 (Saitoh et al. 2004) (Fig. 2a). A predicted amino acid sequence of AK359777 derived from a cDNA library from a germinated shoot of barley cv. Haruna Nijo showed a high homology (59 % identity) to OsC1. It showed a high identity (94 %) to a contig (morex_contig_137164) derived from barley cv. Morex. From the comparison of the genomic and cDNA sequences, this gene consists of two exons and one intron and is predicted to encode a 253-amino acid protein. We designated this gene HvC1 as a homolog of maize C1 and OsC1.
PCR primers specific for HvC1 were designed (Fig. 2a) and used for chromosome localization with wheat–barley chromosome addition lines. As shown in Fig. 2b, an amplified fragment was observed in barley cv. Betzes but not in wheat cv. CS. An amplified fragment of the same size was observed in 7H and the 7HS addition lines. From this result, HvC1 is located on the short arm of chromosome 7H, which was reported to be syntenic to rice chromosome 6.
In barley, a number of anthocyanin-/proanthocyanidin-reduced or -deficient mutants were discovered and classified from Ant1 to Ant30. It was reported that ant1 reduces the anthocyanin content in the stem, and Ant1 is located on chromosome 7H (Jende-Strid 1984). Four alleles of ant1 mutants (ant1.1, ant1.2, ant1.4, and ant1.56) have been isolated. Both ant1.1 and ant1.2 showed anthocyaninless leaf sheaths. However, ant1.4 showed pink pigmentation, and ant1.56 showed dark red pigmentation in the leaf sheath at a level similar to that of the original cv. Bonus (Fig. 1a and Supplementary Fig. S1a). Red pigment is visible in the auricles of Bonus and ant1.1, but the ant1.1 awn appeared anthocyaninless (Supplementary Fig. S1b–e). Quantification of the red pigments in the auricle and awn showed that ant1.1 accumulated reduced amounts of anthocyanin as compared with Bonus in both organs; the ant1.1 awn accumulated a significantly lower amount of red pigment (Supplementary Fig. S1f).
PCR amplification of HvC1 was attempted using three pairs of primers. Bonus, ant1.4, and ant1.56 showed amplification in all primer pairs, whereas no amplification was observed with ant1.1 or ant1.2 (Fig. 3a). Thus, positive or negative PCR amplification corresponded to the presence or absence of anthocyanin pigmentation in the leaf sheath. In ant1.1 and ant1.2, no amplification was also found with primers designed from the regions of 4-kb above the 5′ terminus of HvC1 and 1.3-kb below the 3′ terminus of HvC1 in morex_contig_137164 (Figs. 2a, 3b). Os06t0204800 and Os06t0205600, which show similarities to barley contig (morex_contig_48315 and morex_contig_1562627, respectively), are located 14-kb above and 29-kb below Os06t0205100 (OsC1) (Fig. 2a). Primers designed for each farther distant barley contig amplified the expected size of the fragments in ant1.1 and ant1.2 as their wild type (Fig. 3b). Therefore, at least a 7-kb length of DNA, including the entire HvC1 gene, seemed to be deleted in ant1.1 and ant1.2; both are irradiation-induced mutants (Franckowiak and Lundqvist 2012).
Amplification of the HvC1 gene and the neighbor genes of HvC1. a PCR amplification of HvC1 genes of three sets of primers with Bonus and its mutants, ant1.1, 2, 4, and 56. b PCR amplification of the neighbor genes of HvC1. 137164-LP1 and -RP1 amplified 4-kb above and 137164-LP2 and -RP2 amplified 1.3-kb below the region from HvC1. 48315-LP1 and -RP1, and 1562627-LP1 and -RP1 were designed based on the barley contigs, morex_contig_48315 and morex_contig_1562627, which showed high similarity to Os06t0204800 and Os06t0205600, located on 14-kb above and 29-kb below Os06t0205100 (OsC1). Positions of the primers are depicted in Fig. 2a
Isolation of TaC1
In wheat, a gene for controlling the coleoptile color has been reported and designated the Rc gene. Rc genes have been shown to be located on the short arm of chromosomes 7A, 7B, and 7D (Khlestkina et al. 2002). In this study, CS (colorless coleoptile; Rc-A1a/Rc-B1a/Rc-D1a), NS67 (red coleoptile; Rc-A1a/Rc-B1a/Rc-D1b), and Hope (red coleoptile; Rc-A1b/Rc-B1b/Rc-D1a) were used. A partial sequence was obtained from amplification with a primer pair, HvC1-LP1 and -RP2, using an NS67 DNA template. This sequence showed 83 % identity to HvC1 (deduced amino acid showed 90 % identity), and the position of an intron is identical. Thus, we designated TaC1 as being homologous to HvC1, maize C1, and OsC1. Unique primers for TaC1 were designed, and the primer pair, TaC1-LP3 and -RP4, recognized only wheat chromosome 7A because an amplified fragment was absent in the Nulli7A-tetra7B and Nulli7A-tetra 7D lines but present in other group 7 nulli-tetrasomic lines that retained 7A (Fig. 4a). Therefore, the amplified sequence was named TaC1-A1. PCR using the TaC1-LP3 and -RP4 primer pair was performed with partial chromosome deletion lines for 7AS. TaC1-A1 was amplified in 7AS-1, 9, 12, and 2, but not in 7AS-11, 5, 8, 10, 13, 3, 4, or 6 (Fig. 4b). This result showed that TaC1-A1 is located between fraction lengths 0.66 and 0.73.
The TaC1 sequence was compared to Cereals DB (http://www.cerealsdb.uk.net/cerealgenomics/CerealsDB/search_reads.php) and showed high similarity to contig 559744 and contig 383159 derived from CS. Contig 559744 has a partial sequence of TaC1 that starts from 10-bp above the second exon. An inverse PCR product of NS67 was identical to contig 559744; therefore, primers were designed based on these sequences. A pair of primers (TaC1-LP10 and -RP8) based on the inverse PCR product and contig 559744 recognized only TaC1 on 7B, designated as TaC1-B1 (Fig. 4a). Also, a pair of primers (TaC1-LP7 and -RP11) based on contig 383159 amplified only TaC1 on 7D, designated as TaC1-D1 (Fig. 4a). TaC1-B1 did not amplify in partial chromosome deletion lines 7BS-1, -2, and -3. Similarly, TaC1-D1 did not amplify in any of the five deletion lines tested, 7DS-1, -2, -3, -4, or -5. From these results, TaC1-B1 and D1 appeared to be located on the distal region of chromosomes 7B and 7D, respectively (Supplementary Fig. S2a, b).
In TaC1-A1 of CS (recessive Rc-A1a), one nucleotide deletion was found that causes a frame-shift that disrupts the amino acid sequence from position 239 to the C-terminal (Figs. 5, 6a, and Supplementary Fig. S3).
Alignment of deduced amino acid sequences of C1 genes of wheat (TaC1-A1 of Hope, TaC1-B1 of Hope, and TaC1-D1 of NS67; accession numbers AB983540, AB983541, and AB983542), barley (HvC1; BAJ90986), maize (ZmC1; NP_001147547), Aegilops tauschii (AetC1; EMT07248), Triticum urartu (TuC1; EMS50544). Deduced amino acid sequences of an HvC1-like gene (AK358937) and the seed color regulator of Arabidopsis (AtTT2; CAC40021) are also aligned. Regions shaded light gray and dark gray represent R2 and R3 MYB repeats, respectively. Conserved W (Trp) and I (Ile) in the R2R3 MYB repeats are denoted with black circles. White arrows indicate the positions of single-nucleotide substitution in NS67 and 1-bp deletion in other Rc-A1a lines of the TaC1-A1 gene. White arrowheads represent the insertion of TaC1-B1 and -D1 in Rc-B1a and Rc-D1a, respectively
TaC1 genotyping. a Schematic diagrams of TaC1-A1, B1, and D1 genes. Boxes represent exons, and lines represent introns and untranslated regions. Gray-boxed region encodes R2R3 MYB repeats. Positions and directions of primers are shown by arrows. White arrows on TaC1-A1 indicate the positions of a single-nucleotide substitution in NS67 and a 1-bp deletion in CS and M808. b Partial alignments of TaC1-A1 sequences of Hope (Rc-A1b) and CS (Rc-A1a). Primers for PCR are shown above (TaC1-LP15) and below (TaC1-RP4) with black arrows in alignment. The TaC1-LP15 primer generates a recognition site of ApaI (GGGCCC) with a mismatch base printed in lower case and shown by a gray arrow. c PCR products of TaC1-A1 with TaC1-LP15 and TaC1-RP4 from CS (Rc-A1a), Hope (Rc-A1b), NS67 (Rc-A1a), and M808 (Rc-A1a) with (+) or without (−) ApaI treatment. d PCR product of TaC1-B1 with the pairs of TaC1-LP11 and TaC1-RP8 from Hope (Rc-B1b) (above), and PCR product of TaC1-B1 with the pairs of TaC1-LP10 and TaC1-RP8 from CS (Rc-B1a), NS67 (Rc-B1a), and M808 (Rc-B1a) (below). e PCR products of TaC1-D1 with pairs of TaC1-LP3 and TaC1-RP11 from NS67 (Rc-D1b) and M808 (Rc-D1b), respectively (above), and PCR products of TaC1-D1 with pairs of TaC1-LP7 and TaC1-RP11 from CS (Rc-D1a) and Hope (Rc-D1a), respectively (below)
Interestingly, another recessive Rc-A1a line, Mironovskaya808 (M808), had the same 1-bp deletion at the identical position. On the other hand, NS67 (recessive Rc-A1a) has a normal C-terminal region, but a single-nucleotide substitution that changes an amino acid residue from N (Asn) to D (Asp) in R3 MYB repeats (Figs. 5, 6a, and Supplementary Fig. S3). As compared to the TaC1-B1 of Hope (dominant Rc-B1b), that of CS (recessive Rc-B1a) is longer because of a 1.7-kb insertion sequence in the first exon (Fig. 6a and Supplementary Fig. S3). Transcription of the sequence that is 1.7 kb longer may not have a functional MYB factor. As does TaC1-B1, TaC1-D1 of CS (recessive Rc-D1a) has a 2.0-kb insertion in the second exon (Fig. 6a and Supplementary Fig. S3).
The TaC1 genotype coincides with the Rc genotype
A dCAPS primer, TaC1-LP15, was designed to detect the 1-bp deletion and the TaC1-A1 genotyping (Fig. 6b). PCR products of CS and M808 were not digested after ApaI treatment because their TaC1-A1 lacked 1-bp, and the recognition sites of ApaI were not produced (Fig. 6c). On the other hand, PCR products of Hope and NS67 were digested (Fig. 6c). Undigested upper fragments might be derived from the homoeologous TaC1. This dCAPS marker was also used with other red coleoptile lines which were not genotyped. 8019R1 and Douson1 showed same digested pattern to Hope and NS67 whereas Cornell595-W, Ardito, AUS1408 and OW104 showed same undigested pattern to CS and M808 (Supplementary Fig. S4a). This result indicated that the Rc-A1 genotype of Cornell595-W, Ardito, AUS1408 and OW104 is recessive Rc-A1a. Although NS67 is also an Rc-A1a line, the amplified fragment was digested, since the mutation site of NS67 was different (Fig. 6a, Supplementary Fig. S3). Then, other dCAPS marker, TaC1-RP15, was designed to detect 1-bp substitution of NS67 (Supplementary Fig. S4b). After treatment with EcoRV, PCR fragments of NS67 and Douson1 were digested (Supplementary Fig. S4c). Therefore, Douson1 has same allele of TaC1-A1 of NS67, and is diagnosed as Rc-A1a. However, this NS67-type allele of TaC1-A1 seems to be rare because other colorless coleoptile lines belong to the CS type with a 1-bp deletion in the second exon (data not shown). 8019R1 showed same pattern to Hope with the two dCAPS markers; 8019R1 has dominant Rc-A1b allele. Then, a primer, TaC1-LP10, was designed based on the 1.7-kb insertion sequence of TaC1-B1. Amplified fragments with this primer and TaC1-RP8 were observed only in recessive Rc-B1a lines. In contrast, a primer for the normal sequence of TaC1-B1 (TaC1-LP11) recognized a dominant Rc-B1b line (Hope) (Fig. 6d and Supplementary Fig. S4d). Similarly, TaC1-LP7, designed based on the 2.0-kb insertion sequence of TaC1-D1, amplified fragments in recessive Rc-D1a lines, whereas a primer of the normal sequence of TaC1-D1 (TaC1-LP3) was amplified in dominant Rc-D1b lines (NS67 and M808) (Fig. 6e and Supplementary Fig. S4e). From these results, normal and mutated sequences of TaC1-A1, B1, and D1 were clarified, and the pattern of the TaC1 genotype coincides with the Rc genotype. Additionally, Rc genotypes of other red coleoptile lines were estimated as follows: 8019R1 (Rc-A1b/Rc-B1a/Rc-D1a), Douson1 (Rc-A1a/Rc-B1a/Rc-D1b), Cornell595-W (Rc-A1a/Rc-B1a/Rc-D1b), Ardito (Rc-A1a/Rc-B1a/Rc-D1b), AUS1408 (Rc-A1a/Rc-B1a/Rc-D1b), and OW104 (Rc-A1a/Rc-B1a/Rc-D1b).
Mapping of the HvC1 gene
To map the HvC1 gene, an HvC1-LP1 and -RP1 primer pair was used. No amplification was observed in ant1.1, whereas the expected fragment was amplified from the wild-type mapping parent, OUI026. This is probably due to the irradiation-induced origin of ant1.1, deleting the entire Ant1 gene. Thus, molecular mapping for HvC1 was conducted as a dominant marker.
Mapping of the HvC1 gene was carried out along with the basal leaf sheath color segregating in the F2 population consisting of 96 individuals derived from an OUI026 and ant1.1 cross. The ant1 basal leaf sheath color and the HvC1 gene cosegregated completely and mapped to the same position (Fig. 7a). Ant1 and the HvC1 genes were mapped on chromosome 7HS by comparison with other barley maps (Rostoks et al. 2005; Varshney et al. 2007) (BinMap, http://barleygenomics.wsu.edu/).
a Location of HvC1 as compared with other genetic maps of barley chromosome 7H. Left to right partial linkage map of barley chromosome 7H in the F2 population of OUI026 and ant1.1 (the locations of the ant1 trait and HvC1 are shown in bold); the microsatellite consensus map (Varshney et al. 2007); BinMap from Washington State University (http://barleygenomics.wsu.edu/); and agronomic QTL map (Rostoks et al. 2005). A black triangle indicates the centromere. Numbers at the left side of the left map show the distance in centiMorgans, and numbers on the left side of the three maps on the right show the distance in centiMorgans from the top of each chromosome. b Comparative mapping of wheat TaC1-A1 and rice OsC1. Left to right part of rice chromosome 6 pseudomolecule from the Rice Annotation Project Database (RAP-DB, http://rapdb.dna.affrc.go.jp/); BIN map of wheat chromosome 7AS from the wheat genetics resource center, Kansas University (http://www.k-state.edu/wgrc/Germplasm/Deletions/delindex.html); and the microsatellite consensus maps by Somers et al. (2004) and Khlestkina et al. (2002). OsC1 is located from 5315163 to 5316640 on the short arm of chromosome 6, indicated by a star. White circles show genes that correspond to ESTs on chromosome 7A of wheat, as reported by Sorrells et al. (2003). Chromosomal breaking positions of partial deletion lines are shown with arrows, and numbers in parentheses indicate fraction lengths. c centromere
Chromosomal location of the TaC1 gene
By cytogenetic mapping using CS deletion lines for 7AS, TaC1-A1 was localized in a rather distal region between breaking points 0.66 and 0.73 (Fig. 4b). Previous bin mapping studies localized many wheat EST clones to physical regions delimited by series of partial chromosome deletion lines (http://wheat.pw.usda.gov/wEST/binmaps/). Between 7AS-5 and -1 (fraction length 0.59–0.89), 13 ESTs may correspond to rice chromosome 6 (Fig. 7b). This region contains BE493812, which corresponds to Os06t0138200, located at 2.0 Mb, and BE590476, which corresponds to Os06t0211200, located at 5.7 Mb on a rice chromosome 6 pseudomolecule. OsC1 (syn. Os06t0205100) is located between these two rice genes separated by 3.7 Mb, and TaC1-A1 is located within the region. Rough genetic mapping using SSR markers localized Rc-A1 between Xgwm913 and Xgwm60 on the short arm of chromosome 7A (Khlestkina et al. 2002). Markers on chromosome 7A reported by Somers et al. (2004) were used for PCR with partial chromosome deletion lines and showed that Xgwm471 is located on a region between 0.83 and 0.89; Xgwm60 is located on a region between 0.73 and 0.83, and Xgwm260 is located on a region between 0.32 and 0.45 (Fig. 7b). These results suggest that TaC1-A1 and Rc occupy a similar chromosome map location. Taken together, it is suggested that barley Ant1 and wheat Rc are strong candidates for R2R3-type MYB HvC1 and TaC1 genes, respectively, which are orthologous to maize C1 and rice OsC1.
Discussion
In this paper, four R2R3 MYB-type transcription factors of barley and wheat were isolated, i.e., Hordeum vulgare HvC1, Triticum aestivum TaC1-A1, B1, and D1. These were highly homologous to maize C1 and rice OsC1 genes and are located on barley chromosome 7HS and wheat chromosomes 7AS, 7BS, and 7DS, which are syntenic to rice chromosome 6S. In maize, C1 and Pl determine the tissue-specific synthesis of anthocyanin, i.e., C1 in the aleurone layer of the kernel and Pl in the vegetative and floral tissues (Cone et al. 1993). The amino acid sequences of C1 and Pl are 96 % identical and are located on chromosomes 9S and 6L, respectively. Since it has been considered that these chromosome arms have duplicated segments, Pl is considered to be a duplicate of C1 (Cone et al. 1993). The OsC1 gene in rice is also responsible for anthocyanin pigmentation in the tip of husk that covers the caryopsis. A duplicated OsC1 gene was, however, not discovered in the Rice Annotation Project Database (RAP-DB, http://rapdb.dna.affrc.go.jp/). The expression of OsC1 was detected in the shoot, seedling of the four-leaf stage, anther, and pistil, but not in seed tissues such as immature seeds 5 and 10 days after pollination or embryo and endosperm of 25 days after pollination in RNA-Seq FPKM Expression Values (Ouyang et al. 2007). These results showed that OsC1 controls only plant color synthesis like maize Pl, and OsC1 has no function to regulate seed color. In barley and wheat, HvC1 and TaC1s also seem to act as regulators for plant pigmentation, but they are likely to be active in vegetative tissue and inactive in grain. Between barley and wheat, pigmented tissues differ; thus, the leaf sheath and culm are pigmented in barley, but the coleoptile is pigmented in wheat. Therefore, HvC1 does not control the pigmentation of the coleoptiles of barley, but TaC1 controls the coleoptile color. Every C1 controls anthocyanin pigmentation, but the tissue specificity of the pigmentation varies among plant species. Comparative analyses of C1-orthologs may clarify their functional diversity.
Two full-length cDNA clones of barley, AK359777 and AK358937, are highly similar to maize C1, while AK359777 is a synonym of HvC1, which is located on chromosome 7HS. The other AK358937 of morex_contig_317820 is located on chromosome 4HL. We previously isolated a C1-like gene of wheat, Tamyb9 (AB252148), which showed 72 % identity to AK358937 (data not shown). Although the draft sequences of the wheat D-genome progenitor Aegilops tauschii and the wheat A-genome progenitor Triticum urartu (Ling et al. 2013) include genes annotated as C1, they are more similar to AK358937 than HvC1 (AK359777) (Fig. 5). Furthermore, the deduced amino acid sequence of the C1-like gene of Triticum urartu is ~98 % identical to wheat Tamyb9 (data not shown). Interestingly, Li et al. (1999) found 6 loci which were hybridized to maize C1 probe on chromosome 4B, 4D, 5A, 7A, 7B, and 7D. Moreover, Devos et al. (1995) reported that the translocation of chromosomes 4AL and 5AL was occurred at the diploid level and it is present in hexaploid wheat. These results consist with the results of barley, i.e., HvC1 is located on chromosome 7H and HvC1-like gene is located on chromosome 4HL. And it is presumed that C1-like gene might be located on chromosome 5AL of Triticum urartu and 4DL of Aegilops tauschii. As mentioned, maize C1 and Pl are considered duplicated genes, but rice OsC1 seems to be present as a single gene. These results suggest that the duplication of the C1 gene occurred in barley and wheat, but not in rice. Analysis of duplicated C1 genes in cultivated and wild barley and wheat may reveal their different roles in controlling pigmentation and other biological processes.
In Arabidopsis, anthocyanin synthesis is regulated by the MYB-bHLH-WD40 (MBW) complex composed of TRANSPARENT TESTA GLABROUS1 (TTG1; also termed WD40), one R2R3 MYB protein from PRODUCTION OF ANTHOCYANIN PIGMENTS (PAP1), PAP2, MYB113, or MYB114, as well as one bHLH protein from TRANSPARENT TESTA8 (TT8), GLABROUS3 (GL3), or ENHANCER OF GLABRA3 (EGL3) (Li 2014). In barley, the Ant2 locus was identified as a regulator of anthocyanin biosynthesis in plant tissues such as the awn, the auricle, and the lemma (Cockram et al. 2010). Ant2 encodes HvbHLH1, which shows 35 % identity to TT8. This study also showed that the deletion of Ant1 reduced the anthocyanin content in the awn and the auricle (Supplementary Fig. S1b–f). This result suggests that MYB (Ant1) and bHLH (Ant2) may produce a complex to control anthocyanin production in barley. Although barley WD40 has not been isolated, findings from other model plants suggest that barley also is likely to form an MBW complex to control anthocyanin synthesis. Barley anthocyaninless (Ant) loci have been classified as Ant1 to Ant30, but many loci are not yet determined. It is possible that the WD40 gene is the one of the loci which acts as a regulator of anthocyanin synthesis with Ant1 and Ant2 in barley. Similarly in wheat, the Rc gene of MYB may also act with unknown bHLH and WD40 as a regulator of anthocyanin synthesis.
References
Cockram J, White J, Zuluaga DL, Smith D, Comadran J, Macaulay M, Luo Z, Kearsey MJ, Werner P, Harrap D, Tapsell C, Liu H, Hedley PE, Stein N, Schulte D, Steuernagel B, Marshall DF, Thomas WT, Ramsay L, Mackay I, Balding DJ, Waugh R, O’Sullivan DM (2010) Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome. Proc Natl Acad Sci USA 107:21611–21616
Cone KC, Cocciolone SM, Burr FA, Burr B (1993) Maize anthocyanin regulatory gene Pl is a duplicate of C1 that functions in the plant. Plant Cell 5:1795–1805
Devos KM, Dubcovsky J, Dvorak J, Chinoy CN, Gale MD (1995) Structural evolustion of wheat chromosomes 4A, 5A, and 7B and its impact on recombination. Theor Appl Genet 91:282–288
Druka A, Kudrna D, Rostoks N, Brueggeman R, von Wettstein D, Kleinhofs A (2003) Chalcone isomerase gene from rice (Oryza sativa) and barley (Hordeum vulgare): physical, genetic and mutation mapping. Gene 302:171–178
Endo TR, Gill BS (1996) The deletion stocks of common wheat. J Heredity 87:295–307
Franckowiak J, Lundqvist U (2012) Descriptions of barley genetic stocks for 2012. Barley Genet Newsl 42:36–792
Himi E, Noda K (2005) Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 143:239–242
Himi E, Maekawa M, Miura H, Noda K (2011) Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor Appl Genet 122:1561–1576
Himi E, Yamashita Y, Haruyama N, Yanagisawa T, Maekawa M, Taketa S (2012) Ant28 gene for proanthocyanidin synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica 188:141–151
Hu J, Anderson B, Wessler SR (1996) Isolation and characterization of rice R genes: evidence for distinct evolutionary paths in rice and maize. Genetics 142:1021–1031
Islam AKMR, Shepherd KW, Sparrow DHB (1981) Isolation and characterization of euplasmic wheat–barley chromosome addition lines. Heredity 46:161–174
Jende-Strid B (1984) Coordinator’s report: anthocyanin genes. Barley Genet Newsl 14:76–79
Jende-Strid B, Lundqvist U (1978) Diallelic tests of anthocyanin-deficient mutants. Barley Genet Newsl 8:57–59
Khlestkina EK, Pestsova EG, Roder MS, Borner A (2002) Molecular mapping, phenotypic expression and geographical distribution of genes determining anthocyanin pigmentation of coleoptiles in wheat (Triticum aestivum L.). Theor Appl Genet 104:632–637
Khlestkina EK, Roder MS, Salina EA (2008) Relationship between homoeologous regulatory and structural genes in allopolyploid genome—a case study in bread wheat. BMC Plant Biol 8:88
Khlestkina EK, Roder MS, Pshenichnikova TA, Borner A (2010) Functional diversity at the Rc (rec coleoptile) gene in bread wheat. Mol Breed 25:125–132
Khlestkina EK, Antonova EV, Pershina LA, Soloviev AA, Badaeva ED, Borner A, Salina EA (2011) Variability of Rc (red coleoptile) alleles in wheat and wheat-alien genetic stock collections. Cereal Res Commun 39:465–474
Kristiansen K, Rohde W (1991) Structure of the Hordeum vulgare gene encoding dihydroflavonol-4-reductase and molecular analysis of ant18 mutants blocked in flavonoid synthesis. Mol Gen Genet 230:49–59
Li S (2014) Transcriptional control of flavonoid biosynthesis: fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal Behav 9(1):e27522
Li WL, Faris JD, Chittoor JM, Leach JE, Hulbert SH, Liu DJ, Chen PD, Gill BS (1999) Genomic mapping of defense response genes in wheat. Thero Appl Genet 98:226–233
Li JZ, Sjakste TG, Roder MS, Ganal MW (2003) Development and genetic mapping of 127 new microsatellite markers in barley. Theor Appl Genet 107:1021–1027
Ling HQ, Zhao S, Liu D, Wang J, Sun H, Zhang C, Fan H, Li D, Dong L, Tao Y, Gao C, Wu H, Li Y, Cui Y, Guo X, Zheng S, Wang B, Yu K, Liang Q, Yang W, Lou X, Chen J, Feng M, Jian J, Zhang X, Luo G, Jiang Y, Liu J, Wang Z, Sha Y, Zhang B, Wu H, Tang D, Shen Q, Xue P, Zou S, Wang X, Liu X, Wang F, Yang Y, An X, Dong Z, Zhang K, Zhang X, Luo MC, Dvorak J, Tong Y, Wang J, Yang H, Li Z, Wang D, Zhang A, Wang J (2013) Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 496:87–90
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J, Morris C, Somers DJ, Appels R, Devos KM (2010) Catalogue of gene symbols for wheat National BioResource Project (NBRP)/KOMUGI
Neff MM, Chory J (1998) Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol 118:27–35
Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, Orvis J, Haas B, Wortman J, Buell CR (2007) The TIGR rice genome annotation resource: improvements and new features. Nucleic Acids Res 35:D883–D887
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181:219–229
Rostoks N, Mudie S, Cardle L, Russell J, Ramsay L, Booth A, Svensson JT, Wanamaker SI, Walia H, Rodriguez EM, Hedley PE, Liu H, Morris J, Close TJ, Marshall DF, Waugh R (2005) Genome-wide SNP discovery and linkage analysis in barley based on genes responsive to abiotic stress. Mol Genet Genomics 274:515–527
Saitoh K, Onishi K, Mikami I, Thidar K, Sano Y (2004) Allelic diversification at the C (OsC1) locus of wild and cultivated rice: nucleotide changes associated with phenotypes. Genetics 168:997–1007
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sorrells ME, La Rota M, Bermudez-Kandianis CE, Greene RA, Kantety R, Munkvold JD, Miftahudin MA, Ma X, Gustafson PJ, Qi LL, Echalier B, Gill BS, Matthews DE, Lazo GR, Chao S, Anderson OD, Edwards H, Linkiewicz AM, Dubcovsky J, Akhunov ED, Dvorak J, Zhang D, Nguyen HT, Peng J, Lapitan NL, Gonzalez-Hernandez JL, Anderson JA, Hossain K, Kalavacharla V, Kianian SF, Choi DW, Close TJ, Dilbirligi M, Gill KS, Steber C, Walker-Simmons MK, McGuire PE, Qualset CO (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13:1818–1827
Taketa S, Yuo T, Sakurai Y, Miyake S, Ichii M (2011) Molecular mapping of the short awn 2 (lks2) and dense spike 1 (dsp1) genes on barley chromosome 7H. Breeding Science 61:80–85
Varshney RK, Marcel TC, Ramsay L, Russell J, Roder MS, Stein N, Waugh R, Langridge P, Niks RE, Graner A (2007) A high density barley microsatellite consensus map with 775 SSR loci. Theor Appl Genet 114:1091–1103
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Acknowledgments
This work was supported by the Wesco Scientific Promotion Foundation and the Sapporo Bioscience Foundation. The authors thank Ms. Fumiko Katayama, Mr. Yuichi Kikuchi, and Ms. Noriko Hirose for their technical assistance and Dr. M. Röder (IPK) for barley SSR primer information. CS deletion lines for the short arms of 7A, 7B, and 7D were provided by the National BioResource Project.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
438_2015_991_MOESM1_ESM.pptx
Supplementary material 1 (PPTX 1553 kb) Supplementary Fig. S1 a Base-color comparison of the leaf sheath of Bonus, ant1.1, ant1.2, ant1.4, and ant1.56. Awns of the heading stages of Bonus (b) and ant1.1 (c), and auricles of the flag leaves of Bonus (d) and ant1.1 (e). f Relative anthocyanin content of the auricles (black) of the flag leaves and awns (white) at the heading stage. Error bars represent standard error. *P < 0.05Supplementary Fig. S2 a Schematic diagrams of chromosomes 7BS and 7DS. Chromosomal breaking positions of partial deletion lines are shown with arrows, and numbers in parentheses indicate fraction lengths. b Amplification of TaC1-B1 in CS and its partial deletion lines of 7BS (upper) and TaC1-D1 in CS and its partial deletion lines of 7DS (lower).Supplementary Fig. S3 Alignment of genomic nucleotide sequences of three wheat C1 genes (TaC1-A1 of Hope, TaC1-B1 of Hope, and TaC1-D1 of NS67). Gray-boxed regions represent exons. Putative initiation codons (ATG) and stop codons (TAA) are underlined. White arrowheads indicate the insertion in TaC1-B1 and -D1. White arrows show mutation sites in TaC1-A1. Recognition sites of restriction enzyme (PmlI and ClaI) are boxed.Supplementary Fig. S4 TaC1 genotyping of one colorless coleoptile line (CS) and nine red coleoptile lines (8019R1, Hope, NS67, Douson1, M808, Cornell595-W, Ardito, AUS1408, and OW104). a PCR products of TaC1-A1 using TaC1-LP15 and TaC1-RP4 with (+) or without (-) ApaI treatment. Undigested lines (CS, M808, Cornell595-W, Ardito, AUS1408, and OW104) are genotyped to Rc-A1a. b Partial alignments of TaC1-A1 sequences of Hope (Rc-A1b) and NS67 (Rc-A1a). A primer for PCR is shown with a black arrow in alignment. The TaC1-RP15 primer generates a recognition site of EcoRV (GATATC) with a mismatched base printed in lower case and indicated by a gray arrow. c PCR products of TaC1-A1 using TaC1-LP6 and TaC1-RP15 with (+) or without (-) EcoRV treatment. Digested lines (NS67 and Douson1) are genotyped to Rc-A1a. d PCR product of TaC1-B1 with pairs of TaC1-LP11 and TaC1-RP8 (upper), and the PCR product of TaC1-B1 with pairs of TaC1-LP10 and TaC1-RP8 (lower). e PCR product of TaC1-D1 with pairs of TaC1-LP3 and TaC1-RP11 (upper), and the PCR product of TaC1-D1 with pairs of TaC1-LP7 and TaC1-RP11 (lower)
Rights and permissions
About this article
Cite this article
Himi, E., Taketa, S. Isolation of candidate genes for the barley Ant1 and wheat Rc genes controlling anthocyanin pigmentation in different vegetative tissues. Mol Genet Genomics 290, 1287–1298 (2015). https://doi.org/10.1007/s00438-015-0991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-0991-0