Abstract
The MADS-box gene family is an ancient and well-studied transcription factor family that functions in almost every developmental process in plants. There are a number of reports about the MADS-box family in different plant species, but systematic analysis of the MADS-box transcription factor family in Brassica rapa (Chinese cabbage) is still lacking. In this study, 160 MADS-box transcription factors were identified from the entire Chinese cabbage genome and compared with the MADS-box factors from 21 other representative plant species. A detailed list of MADS proteins from these 22 species was sorted. Phylogenetic analysis of the BrMADS genes, together with their Arabidopsis and rice counterparts, showed that the BrMADS genes were categorised into type I (Mα, Mβ, Mγ) and type II (MIKCC, MIKC*) groups, and the MIKCC proteins were further divided into 13 subfamilies. The Chinese cabbage type II group has 95 members, which is twice as much as the Arabidopsis type II group, indicating that the Chinese cabbage type II genes have been retained more frequently than the type I genes. Finally, RNA-seq transcriptome data and quantitative real-time PCR analysis revealed that BrMADS genes are expressed in a tissue-specific manner similar to Arabidopsis. Interestingly, a number of BrMIKC genes showed responses to different abiotic stress treatments, suggesting a function for some of the genes in these processes as well. Taken together, the characterization of the B. rapa MADS-box family presented here, will certainly help in the selection of appropriate candidate genes and further facilitate functional studies in Chinese cabbage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MADS-box genes encode transcription factors that are involved in developmental control and signal transduction in eukaryotes (Riechmann and Meyerowitz 1997). These genes are found in fungi (Passmore et al. 1988), animals (Norman et al. 1988) and plants (Sommer et al. 1990; Yanofsky et al. 1990). They constitute a large gene family, which is named after a few of its earliest members, MCM1 (from yeast) (Passmore et al. 1988), AGAMOUS (from A. thaliana) (Yanofsky et al. 1990), DEFICIENS (from Antirrhinum majus) (Sommer et al. 1990) and SRF (from Homo sapiens) (Norman et al. 1988). Previous studies of the MADS-box genes have included a thorough comparison analysis of their roles in plant growth and development. However, there are relatively few analyses of the response of these genes to stress conditions. Brassica rapa ssp. pekinensis (Chinese cabbage) is one of the subspecies of Brassica rapa. This subspecies, which originated in China, is one of the most economically significant vegetable crops in Asia. Moreover, Chinese cabbage has become a vegetable that is grown worldwide due to its high yield and good quality. Thus, the growth, development and flowering time of this plant are significant for its yield. Recently, the Chinese cabbage (Chiifu-401-42) genome has been sequenced, and this sequence can help us with the analysis of MADS-box genes from the entire genome (Wang et al. 2011). This genome has undergone triplication events since its divergence from Arabidopsis (13–17 mya) (Wang et al. 2011); however, a high degree of sequence similarity and conserved genome structure remain between these two species, these traits make B. rapa a good species to use to study the retention and ortholog groups of MADS-box genes during genome duplication events. Furthermore, plant growth and development are influenced greatly by numerous plant growth regulators and environmental factors.
MADS proteins are characterised by the presence of a conserved 58–60 amino acids long DNA-binding domain in the N-terminal region, which is known as the MADS domain, and which binds to CArG boxes (Yanofsky et al. 1990). Based on the phylogenetic analysis, the plant MADS gene family is divided into two large lineages, type I and type II, which were generated by an ancestral gene duplication event (Alvarez-Buylla et al. 2000; Becker and Theißen 2003). The type I genes encode SRF-like domain proteins, whereas type II genes encode MEF2-like proteins (De Bodt et al. 2003). The plant type II proteins are named MIKC due to their four domains. In addition to the MADS (M) domain, MIKC type proteins contain the I (intervening), K (keratin-like) and C (C-terminal) domains (Cho et al. 1999). The I domain contributes to dimer formation (Henschel et al. 2002). The K domain is characterised by a coiled-coil structure, which primarily regulates to the dimerisation of MADS proteins (Díaz-Riquelme et al. 2009). The C domain functions in transcriptional activation and in the formation of higher order protein complexes (Honma and Goto 2001). MIKC-type genes have been further divided into two subgroups, MIKCC and MIKC*, based on sequence divergence at the I domain (Henschel et al. 2002). The MIKC* genes encode proteins that tend to have longer I domains and have a duplicated K domain. The type I lineage groups genes with a relatively simple gene structure (only with one or two exons) that lack the K domain and that have common ancestors. The type I genes are subdivided into three groups, Mα, Mβ, Mγ, based on the sequence of the MADS domain and on the presence of additional motifs. The function of the type I genes appears to be restricted to female gametophyte (AGL80 and AGL61) and seed development (PHE1, PHE2, AGL23, AGL28, AGL40, AGL62) (Köhler et al. 2003; Bemer et al. 2010; Colombo et al. 2008; Masiero et al. 2011).
Plant MIKC genes were first identified as floral organ identity genes in Antirrhinum majus and in Arabidopsis (Sommer et al. 1990; Yanofsky et al. 1990). Biologists have made great progress in elucidating the roles of these genes in plant development. Further genetic and molecular analyses regarding their biological functions have focused on flower organogenesis, which acts as the major component in the well-known ABCDE model: sepals (A + E), petals (A + B + E), stamens (B + C + E), carpels (C + E), and ovules (D + E) (Zahn et al. 2006). Briefly, a previous study of Arabidopsis MIKC genes classified these genes into five functional classes as follows: Class A includes APETALA1 (AP1); class B includes PISTILATA (PI) and AP3; class C includes AGAMOUS (AG); class D includes SEEDSTICK/AGAMOUS-LIKE11 (STK/AGL11); and class E includes SEPALLATA (SEP1, SEP2, SEP3, and SEP4) (Pinyopich et al. 2003). Other MIKC genes were later identified as being involved in different regulatory steps, such as: (1) Determination of flowering time genes, which include Suppressor of Overexpression Of Constans1 (SOC1) (Samach et al. 2000; Moon et al. 2003a, b), AGAMOUS-LIKE GENE 24 (AGL24) (Liu et al. 2008), Short Vegetative Phase (SVP) (Lee et al. 2007), MADS Affecting Flowering (MAF1/FLM), Flowering Locus c(FLC) (Michaels and Amasino 1999; Ratcliffe et al. 2003) and AGL15, AGL18 (Adamczyk et al. 2007); (2) Fruit ripening genes, which include SHATTERPROOF 1–2 (SHP1, SHP2) and FUL (Liljegren et al. 2000); (3) Seed pigmentation and embryo development genes, which include TRANSPARENT TESTA16 (TT16) (Nesi et al. 2002). Apart from reproductive development, MIKC genes also function in vegetative development and root development, such as AGL12 and AGL17 genes (Tapia-López et al. 2008).
Some MIKCC genes have already been shown to play key roles to control flowing time in Brassica, such as BrFLC1, 2, 3, BcFLC, BrAGL20 and BnAP3 (Pylatuik et al. 2003; Hong et al. 2012; Liu et al. 2013). For example, the overexpression of BrAGL20 can significantly affect the flowering time of B. napus, and BrFLC genes act similar to AtFLC, with lower expression in early-flowering Chinese cabbage (Hong et al. 2012). Furthermore, plant growth and development are influenced greatly by numerous plant growth regulators and environmental factors. Gibberellin (GA) promotes flower formation and flowering time in biennial plants. Its involvement in flower initiation in plants is well-established, and there is growing insight into the mechanisms by which floral induction is achieved (Mutasa-Göttgens and Hedden 2009). Salicylic acid (SA) also regulates flowering time because SA-deficient plants are late flowering (Martínez et al. 2004). Abscisic acid (ABA) regulates many aspects of plant growth and development (Bezerra et al. 2004; Wilmowicz et al. 2008). As important environmental stress factors, cold and heat also regulate plant growth and development. To learn more about the response of B. rapa MADS-box genes to abiotic stresses, we selected these five treatments to explore in this study.
Flower development is controlled by a complex network of interactions between transcription factors, most of them belonging to the MADS-box family (Airoldi and Davies 2012). To get a better picture about the size and phylogeny of the MADS-box family in plants, we sorted and compared the MADS-box genes from 22 different plant species. To better understand these transcription factors in Chinese cabbage, we determined 160 MADS-box genes and analysed the phylogenetic relationships, conserved motifs, retention and ortholog groups between these Chinese cabbage MADS-box genes and Arabidopsis MADS-box genes. We further studied the chromosomal locations, gene duplication and tissue-specific expression of BrMADS genes. The expression of all of the BrMIKC C genes was also investigated under different treatments, which included GA, SA, ABA, heat and cold.
Materials and methods
Identification of MADS-box gene family in Chinese cabbage
All the files that are related to Brassica genome sequence data that were used for the identification and annotation of MADS proteins were downloaded from the Brassica database (BRAD; http://brassicadb.org/brad/) (Wang et al. 2011). Proteins with SRF-TF domains (PF00319) were retrieved from the Pfam 27.0 database (http://Pfam.sanger.ac.uk/) (Punta et al. 2012). The hidden Markov model (HMM) was used to identify the putative MADS proteins in Chinese cabbage (Finn et al. 2011). To obtain the proteins, first we used the tool hmmsearch, with an expected value (e-value) cut-off 1.0. Then, we verified these sequences using the tool SMART (http://smart.embl-heidelberg.de/) (Letunic et al. 2012), the Pfam database (http://Pfam.sanger.ac.uk/) and the NCBI database (http://www.ncbi.nlm.nih.gov/).
Sequence retrieval
The Arabidopsis thaliana MADS proteins were retrieved from the TAIR database (http://www.arabidopsis.org/) according to a previous report by Parenicova et al. (2003). The dataset of predicted Oryza sativa MADS proteins was retrieved from previous analyses by Arora et al. (Arora et al. 2007). A MADS-box domain was not found in LOC_Os02g01360 (OsMADS60), LOC_Os12g31010 (OsMADS67), and LOC_Os08g20460 (OsMADS69). The MADS proteins of Populus trichocarpa, Medicago truncatula, Glycine max, Cucumls sativus, Citrus sinensis, Citrus clementine, Vitis vinifra, Sorghum bicolor, Zea mays, Selaginella moellendorffi and Physcomitrella paters were retrieved from a previous report.
The Pfam database (http://pfam.sanger.ac.uk/) was used to screen the genome assemblies of Prunus persica, Arabidopsis lyrata, Capsella rubella, Thellungiella halophila, Solanum tuberosum, Solanum lycopersicum, Aquilegia coerulea and Volvox carteri. The genome data were downloaded from the genome browser phytozome (http://www.phytozome.net/), and the evolutionary relationships of these species were determined using the genome browser phytozome and the public database PGDD (http://chibba.agtec.uga.edu/duplication/) (Lee et al. 2013).
Phylogenetic analysis
In the phylogenetic tree, the Arabidopsis MADS proteins were used to classify the Chinese cabbage MADS proteins into different groups. Full-length sequences of MADS proteins of Chinese cabbage and Arabidopsis were aligned using the ClustalW2 program with default parameters (Thompson et al. 1997). Then, a phylogenetic tree was then constructed by the neighbour-joining method, and bootstrap values were calculated with 1,000 replications using MEGA5.2 (Tamura et al. 2011). Additionally, an Arabidopsis MADS proteins phylogenetic tree was used to detect the reliability of this method, and to test and verify the classification, a phylogenetic tree of Chinese cabbage, Arabidopsis, rice and grapevine was built.
To estimate the nucleotide divergence between sequences, all nucleotide sequences of Chinese cabbage MADS-box genes were also analysed by MEGA5.2 using the Jukes-Cantor model. Bootstrap (1,000 replicates) analyses were also performed for this estimation.
Identification of conserved motifs and gene structure
To identify the conserved motifs in full-length Chinese cabbage and Arabidopsis MADS proteins, the Multiple Expectation-maximisations for Motif Elicitation (MEME) program version 4.9.0 (Bailey et al. 2009) was used with default parameters, except for the following parameters: (1) optimum motif width was set to ≥10 and ≤100; and (2) the maximum number of motifs was set to identify 15 motifs. The MEME motifs were annotated using the SMART program (http://smart.embl-heidelberg.de) and the Pfam database.
The coding domain sequences (CDS) and DNA sequences of Chinese cabbage MADS-box genes were used to reveal the gene structure using the tool GSDS (http://gsds.cbi.pku.edu.cn/).
Ortholog groups of MADS-box genes in Brassica and Arabidopsis genome
The program OrthoMCL (http://www.orthomcl.org/cgi-bin/OrthoMclWeb.cgi) (Li et al. 2003) was used to identify the homologous genes of MADS-box between Chinese cabbage and Arabidopsis. Briefly, the tools BLASTP, with an e-value ≤1e−10, and orthomclPairs were applied to find orthologs, inparalogs and coorthologs in these two species. To link these genes to chromosomes, a tool called Circos (Krzywinski et al. 2009) was used. In addition, the Cytoscape software was applied to build the network of these relationships (Shannon et al. 2003).
Chromosome localisation and gene duplications
To determine the physical locations of MADS-box genes, the starting and ending positions of all MADS-box genes on each chromosome were obtained from the BRAD database. The Perl in-house program was used to draw the location images of the Chinese cabbage MADS-box genes. The positions of each Chinese cabbage MADS-box gene on the blocks were verified by searching for homologous genes between Arabidopsis and three B. rapa subgenomes, including least fractionated (LF), medium fractionated (MF1) and most fractionated (MF2) genomes (http://brassicadb.org/brad/searchSynteny.php) (Wang et al. 2011; Cheng et al. 2013).
To determine the gene duplications, first, the CDS sequences of Chinese cabbage MADS-box genes were blasted against each other (evalue <1e−10, identity >85 %), and then Ks values were calculated for all pair-wise alignments of these genes, which previously obtained by blast, using the method of Nei and Gojobori as implemented in KaKs_calculator (Zhang et al. 2006). Lastly, based on phylogenies, the nucleotide divergence (Dist <0.1) was used as the final standard (Lynch and Conery 2000). The purple lines were used to link the duplicate genes on different chromosomes.
Chinese cabbage RNA-seq data analysis
For the expression profiling of Chinese MADS-box genes, we utilised the Illumina RNA-seq data that were previously generated and analysed by Tong et al. (2013). Six tissues of B. rapa accession Chiifu-401-42, including callus, root, stem, leaf, flower, and silique, were analysed. Two samples of root and leaf tissues were generated from different batches of plants. The transcript abundance is expressed as fragments per kilobase of exon model per million mapped reads (FPKM) values. Heat maps for Chinese cabbage MADS-box genes were generated, which have positive FPKM values in at least one or more of the samples.
Plant material and treatments
The Chinese cabbage cultivar Chiifu-401-42 was used for the experiments. Whole genome sequencing has been completed for this cultivar; thus, this cultivar is a typical cultivar for Chinese cabbage research. Seeds were grown in pots containing a soil: vermiculite mixture (3:1) in the greenhouse of Nanjing Agricultural University, and the controlled-environment growth chamber programmed is light 16 h/25 °C, dark 8 h/20 °C (Song et al. 2013). One month later, seedlings at the five-leaf stage were transferred to growth chambers that were set at 4 or 38 °C under identical light intensity and day length as the cold and heat treatments. Simultaneously, for acclimation, some plants were cultured in 1/2 Hoagland’s solution in plastic containers, with the pH at 6.5 (Jensen and Bassham 1966). After 5 days of acclimatisation, plants were cultured in the following four treatments: (1) Control; (2) 100 μM ABA; (3) 100 μM GA; (4) 100 μM SA. At 4 and 12 h after treatment, the young leaf samples were collected, frozen in liquid nitrogen and stored at −70 °C for further analysis.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from 100 mg of frozen tissue using an RNA kit (RNAsimply Total RNA Kit, Tiangen, Beijing, China) according to the manufacturer’s instructions. Five micrograms of each sample were reverse transcribed into cDNA using the PrimeScript RT reagent Kit (TaKaRa). The specific primers of Chinese cabbage MADS-box genes and the housekeeping actin gene (Bra028615) were designed using the Primer Premier 5.0 software (Supplementary Table 11). To verify the primer specificity, we used the program BLAST against the Chinese cabbage genome. The qRT-PCR assays were performed with three biological and three technical replicates. Each reaction was performed in a 20 μL reaction mixture containing a diluted cDNA sample as the template, 2× Power SYBR Green PCR Master Mix (Applied Biosystems), and 400 nM each of forward and reverse gene-specific primers. The reactions were performed using a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with the following cycling profile: 94 °C for 30 s, followed by 40 cycles at 94 °C for 10 s, and 58 °C for 30 s. A melting curve (61 cycles at 65 °C for 10 s) was generated to verify the specificity of the amplification (Song et al. 2013). The relative expression ratio of each gene was calculated using the comparative Ct value method (Heid et al. 1996). The MADS-box gene expression cluster from each stress treatment was analysed using the Cluster program (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm) (Eisen et al. 1998), and the results were shown using the TreeView software (http://jtreeview.sourceforge.net/).
Results
Identification and classification of MADS-box genes in Chinese cabbage and comparative analyses
To identify the putative MADS proteins in the Chinese cabbage genome, a HMM search resulted in the identification of 164 proteins. Subsequently, all 164 protein sequences were subjected to Pfam and SMART analyses, which resulted in the identification of 162 MADS proteins, called BrMADS001 to BrMADS162 according to the hmmsearch e-value (Supplementary Table 1). Simultaneously, by performing a homology search against Arabidopsis and by analyzing the gene structure, two genes were removed. BrMADS047 and BrMADS124 contained other functional domains, while their homologs were non-MADS genes (Supplementary Fig. 1).
To pre-classify the Chinese MADS-box genes, a phylogenetic relationship with Arabidopsis MADS-box genes was built (Supplementary Fig. 2). In total, 95 genes were determined to be type II MADS-box genes (including MIKCc and MIKC*), with twofold more members than that in Arabidopsis. However, 65 of these genes were confirmed to be type I MADS-box genes (including the Mα, Mβ and Mγ groups), which is comparable to that in Arabidopsis.
To perform comparative genomic analyses, we searched for MADS protein-coding sequences in the genomes of 22 other plant species. Some of these genes have been published previously, while others are described in this work for the first time (Supplementary Tables 2 and 3). The evolutionary relationships of the species and the number of MADS-box genes in their genomes are shown in Fig. 1. The data that are coloured green were for the first time analysed in this work. The pre-classified groups of these species were based on their phylogenetic relationships with Arabidopsis MADS-box genes. The data show that the number of MADS-box genes in Alga, Bryophyta and Pteridophyta is less than that in Angiospermae. Since several whole genome duplication (WGD) events happened during angiosperm evolution, it is likely that this higher number is caused by an elevated duplication frequency, in combination with an increased retention of MADS-box genes that were subjected to neofunctionalization and gained important functions in angiosperm flower development (Doebley and Lukens 1998; Theissen et al. 2000; Nam et al. 2003).
The evolutionary relationships of the species and the number detail of the MADS-box family of each species. The left of this figure shows the evolutionary relationships of the species; the right of this figure shows the number detail of the MADS-box family of each species. The data that are coloured blue were described in this work, and the data that are coloured green were published in previous works (colour figure online)
Copy number variation and differential retention of MADS-box genes in Chinese cabbage
A comparison of the homologous MADS-box genes in Arabidopsis and the three B. rapa subgenomes (LF, MF1 and MF2) using the BRAD database revealed that most BrMADSs on the conserved collinear blocks have been well-conserved throughout the divergent evolution of Arabidopsis and B. rapa (Cheng et al. 2012) (Supplementary Table 4). The gene dosage hypothesis predicts that genes whose products are dose-sensitive, interacting either with other proteins or in networks, should be overretained (Thomas et al. 2006; Birchler and Veitia 2007). The type II proteins have been shown to function in large complexes during flower development, while it is still unclear how the type I proteins perform their functions. Interestingly, type II genes have been retained after triplication and fractionation in B. rapa at a significantly higher rate than the type I genes (Supplementary Fig. 3a). Most (74 %) type II genes were retained in two or three copies, which is significantly greater than the retention of type I genes (15 %) (Supplementary Fig. 3a), while more (65 %) of the type I genes were completely lost. The proportion of homoeologs retained varied among the three sub-genomes (Supplementary Fig. 3b). In the LF sub-genome, more MADS-box gene homoeologs were retained than other two sub-genomes. The retention of type II genes homoeologs among the sub-genomes was more than that of the type I genes (Supplementary Fig. 3b).
Phylogenetic and classification analysis of BrMADS genes
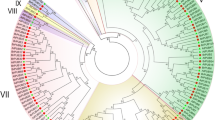
To examine the phylogenetic relationships between BrMADS genes in detail, independent phylogenetic trees were constructed with Arabidopsis and rice type I and type II proteins (Supplementary Fig. 4 and Fig. 2). The type I proteins were divided into three subfamilies Mα (27), Mβ (16), Mγ (22), whereas the type II proteins were divided into 13 subgroups (Supplementary Table 5). Subgroup TM3-like (SOC1) consisted of the highest (16) number of BrMADS type II proteins, whereas subgroup AGL12, AGL6 and Bs (TT16) had the lowest members, with only three. Other subgroups contained from four to ten members (Supplementary Fig. 3c). In addition, in the type II group, there are eleven genes that were identified as MIKC*-type.
Phylogenetic tree of Chinese cabbage, Arabidopsis and rice type II MADS-box proteins. Phylogenetic analysis of 182 type II MADS proteins from Chinese cabbage (95), Arabidopsis (46) and rice (41) showing similar groups in all of the plant species. In total, 13 clades with different colours that were formed by type II MADS proteins are also marked (colour figure online)
Finally, we visualized the phylogenetic relationship of the BrMADS proteins with the Arabidopsis, rice, soybean and grapevine MADS proteins by building an unrooted tree of the full-length MADS protein sequences. The phylogenetic tree divided these proteins into 5 distinct subfamilies (MIKCC, MIKC*, Mα, Mβ, Mγ) (Supplementary Fig. 2c).
Identification of conserved motifs and gene structure
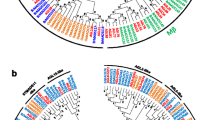
To compare the differences in the protein structure, MEME was used to identify the conserved motifs among the Chinese cabbage and Arabidopsis MADS proteins. The type I and type II MADS proteins of these two species were compared in separate analyses, and for each comparison, fifteen conserved motifs, named motif 1 to motif 15, were identified (Supplementary Fig. 5 and Fig. 3). In general, the MADS proteins were clustered in the same subgroups and shared similar motif composition, which indicates functional similarities among members of the same subgroup (Parenicová et al. 2003). The Arabidopsis and Chinese cabbage MADS proteins were found to have similar structure for every subgroup in type II except BrMADS031, 060, 112 and 103 which with incomplete domains. However, in type I, the protein structure was divergent (Supplementary Fig. 5). This finding indicates that the C-terminal part of the MADS domain in the Mα, Mβ and Mγ groups is more divergent than that in the MIKC group. In type I, except the MADS domain, each of the groups shows a different motif profile, and none of these motifs can be annotated using the tool SMART. The protein motifs shared by the Arabidopsis and Brassica type I proteins within a clade, show that there is also conservation beyond the MADS domain, although proteins of one clade sometimes show some variation in the motif profile, like for example: BrMADS118, 128, 144, 136, 157 in Mγ and BrMADS106, 108, 113 in Mβ (Supplementary Fig. 5).
Simultaneously, the protein structure of BrMADS was analysed using the program MEME. As expected, the commonly shared motifs tend to be in the same group. The motifs were detected by the tool SMART (Supplementary Fig. 6). It will be interesting to characterise the functions of the common motifs within the newly designated groups in relation to the functions of these genes.
In addition to the protein structure, the gene structure was also analysed. We found that all type II BrMADS genes have at least three exons, while the number of exons in the type I genes is at maximum two consistent with AtMADS genes (Parenicová et al. 2003). Furthermore the first exon (approximately 180 bp) of type II genes conservatively codes the MADS domain. Supplementary Fig. 6 gives an overview of the structures of the Chinese cabbage MADS genes and proteins.
Ortholog groups, chromosomal localization and gene duplication of MADS-box genes
Most angiosperm plant lineages have experienced one or more rounds of ancient polyploidy (Lee et al. 2013). Chinese cabbage has undergone genome triplication since its divergence from Arabidopsis (Wang et al. 2011). Generally, the gene number in the Chinese cabbage genome was notably less than three times the Arabidopsis gene number because some genes were lost during polyploidy speciation. Additionally, both segmental and tandem gene duplications have significant impacts on the expansion and evolution of gene families in plant genomes. In this study, we analysed the ortholog groups between Chinese cabbage and Arabidopsis MADS-box genes using the OrthoMCL program. Then, we identified 67 orthologous gene pairs and 120 co-orthologous gene pairs in the MADS proteins of these two species (Supplementary Table 6). Their visualisation was performed using the Circos software (Fig. 4). Among the orthologous gene pairs of Chinese cabbage and Arabidopsis, we found more Chinese cabbage MADS-box homologous genes in Arabidopsis chromosome 5 and chromosome 1 than in other chromosomes. To further obtain insight into the correlation of the MADS-box genes in Chinese cabbage and Arabidopsis, the networks of MADS-box genes were constructed using these two species orthologous (Supplementary Fig. 7). Among the orthologous gene pairs of Chinese cabbage and Arabidopsis, 16 Arabidopsis MADS-box genes were found no ortholog with Chinese cabbage MADS-box genes, these genes have been duplicated in Arabidopsis after the split. Fifty Arabidopsis MADS-box genes have only one ortholog in Chinese cabbage, these genes were present before the split, but two of the three copies have been lost after the B. rapa genome triplication (Supplementary Fig. 7a), and 42 Arabidopsis genes have co-orthologs in Chinese cabbage, these genes were preferentially retained after the triplication (Supplementary Fig. 7b, c and d). Meanwhile, we found 71 and 60 in paralogous gene pairs in Arabidopsis and Chinese cabbage, respectively (Supplementary Table 5 and Fig. 4). From this analysis, we found gene duplication events after the divergence of Chinese cabbage and Arabidopsis resulted in a high number of paralogous and co-orthologous genes in both species. While in Arabidopsis the type I subfamily has predominantly expanded, it is in Chinese cabbage the type II family which has expanded.
Ortholog groups of MADS-box genes in B. rapa and Arabidopsis Genome. Ten Chinese cabbage chromosomes and five Arabidopsis chromosomes are coloured different random colours with their names on the periphery. The lines in the figure represent four pairs. The lines regarding orthologous gene pairs are coloured blue; co-orthologous gene pairs are coloured black; Chinese cabbage paralogous gene pairs are coloured yellow and Arabidopsis paralogous gene pairs are coloured red. The figure was created using the software Circos (colour figure online)
The physical map positions of the MADS-box genes on Chinese cabbage chromosomes were identified (Fig. 5). Among the 160 BrMADS genes, two genes (BrMADS150, BrMADS134) could not be anchored on any of the Chinese cabbage chromosomes. BrMADS150 and BrMADS134 are on Scaffold 000343 and 000385, respectively. The other 158 members of the BrMADS genes were distributed non-randomly on 10 Chinese cabbage chromosomes (Fig. 5a). Chromosomes 2 and 9 contain the most MADS-box genes (15/16 %), whereas chromosome 8 contains the fewest (6 %) (Fig. 5b). We also found that some MADS-box genes cluster together in a region of the chromosome. For example, 16 genes clustered in the end of chromosome 2, and almost of the genes belong to BrMIKC C. Type I and type II also show a differential distribution on Chinese cabbage chromosomes. The type I genes are distributed evenly across all ten chromosomes, whereas genes from type II are located more members that from type I on chromosomes 03, 07 and 08 (Fig 5c). Interestingly, this is different from Arabidopsis, where the MIKC genes are distributed evenly across all five chromosomes, whereas the type I genes are located mainly on chromosomes 1 and 5 (Parenicová et al. 2003).
Distribution of the BrMADS genes on ten Chinese cabbage chromosomes. a The 158 BrMADS genes non-randomly distributed on each conserved collinear blocks of the chromosome. Type I and type II genes are coloured blue and red, respectively Chromosome numbers are indicated above each chromosome followed by type I and type II numbers. The MADS-box genes present on duplicated chromosomal segments are connected by blue lines between the two relevant chromosomes. The tandem duplicated genes are in the box. The conserved collinear blocks on each chromosome are labeled A–X and are colour-coded according to inferred ancestral chromosomes following an established convention. b The percentages of BrMADS genes on each chromosome are demonstrated by the pie. c The percentages of BrMADS type I and type II genes on each chromosome are demonstrated by the doughnut chart (colour figure online)
Duplicated genes from eukaryotic transcription factor families have originated predominantly from inter-chromosomal duplications (Friedman and Hughes 2001). The large size of the gene family MADS-box in B. rapa may suggest that this gene family underwent frequent duplication events during evolution. To learn more regarding the duplication of these genes, we defined the duplicated genes based on their Ks values and phylogenetic criteria (Supplementary Table 7, 8 and 9). Furthermore, these genes, which share similar gene structure and protein structure, were shown in chromosomes and in the phylogenetic tree (Fig. 5 and Supplementary Fig. 8). The duplicated genes were clustered closely together at the extremities of the phylogenetic tree. Most MADS-box genes have undergone segment duplication (39 duplications), whereas others have undergone tandem duplication (6 duplications) (Fig. 5). Tandem duplications have produced MADS-box gene clusters or hotspots, whereas segment duplications have produced many homologs of MADS-box genes on different chromosomes, as indicated with purple lines. The results indicated that the divergence time of duplicated BrMADS gene pairs ranged from 0.18 to 11.29 million years ago (MYA) and averaged 6.43 MYA, which indicates that the duplicated divergence of the MADS family members in B. rapa mostly accompanied the triplication events (5–9 MYA) (Supplementary Table 9) (Wang et al. 2011).
Differential expression of BrMADS genes in various tissues
To identify tissue-specific expression profiles of BrMADS genes, we utilised transcriptome data that were derived from Illumina RNA-Seq reads that were generated and analysed by Tong et al. (2013). The transcript abundance of 160 BrMADSs in 6 different tissues, including callus, root, stem, leaf, flower, and silique, was obtained; however, almost all of the type I BrMADSs either transcribed at too low a level to be detected or have spatial and temporal expression patterns that had no expression in the RNA-seq libraries (Supplementary Table 10). Of the BrMIKC genes, some exhibit tissue-specific expression (Fig. 6). The differences expression levels between typeI and typeII genes in the Chinese cabbage tissues were consistent with that in Arabidopsis (Kofuji et al. 2003). In Chinese cabbage the well-known ABCDE model genes have high expression levels in flowers, except BrMADS046, 052, 057, which only have high expression levels in roots. The CDE genes that are not only in flowers but also in siliques have higher expression levels. Some genes, which include BrMADS077 and 078 (FLC), only had low expression in flowers, suggesting that these genes negatively regulate the flowering time similar to results of a previous report (Michaels and Amasino 1999). BrMADS045, 053, 063, 064, 068 (AGL17) have high expression levels in roots, which may be associated with root development (Zhang and Forde 1998). There are some genes that are expressed in all of the six tissues; these genes are BrMADS023, 039, 032 (TM3-like); BrMADS065, (SVP); BrMADS080, 096, 104 (MAF); and BrMADS055 (AGL18). SOC and SVP control the flowering time, although SOC is a positive regulator, in contrast with SVP, FLC and MAF. AGL18 is a floral transition gene. The expression patterns of these genes may be related to their roles in plant growth and development. The MIKC* group genes had low expression levels in these six tissues, except BrMADS102. Perhaps, this gene is also involved in the mediation of plant growth and development.
Differential expression of BrMADS genes under stresses
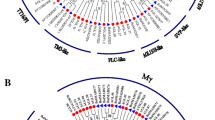
In the process of plant growth and development, plants are subject to various abiotic stresses. Thus, to identify the stress-responsive BrMADS genes under cold, heat, GA, SA, and ABA treatments in the seedling stage, we performed comprehensive expression profiling of BrMIKC genes using qRT-PCR (Supplementary Table 12), and the results are shown using the program TreeView. The expression levels of several genes were upregulated, whereas most genes were downregulated or showed no change under these treatments (Fig. 7). Under ABA treatment, some genes were upregulated at 4 h but were downregulated at 12 h. Because of the many BrMIKC genes and their complicated expression changes, only some were selected for further analysis (Fig. 8).
Expression analysis of Chinese cabbage MIKC genes under five abiotic stresses. Heat map representation and hierarchical clustering of BrMIKC genes during a GA and SA stresses, b ABA, cold and heat stresses. Every stress contains two times, 4 and 12 h. The relative expression levels of BrMIKCs in leaves under these stresses were quantified against the control transcript levels. The bar at the bottom of each heat map represents relative expression values
Three TM3-like ortholog BrMADS genes (BrMADS043, BrMADS036 and BrMADS041) were upregulated at 4 and 12 h under GA treatment. The remaining six genes (BrMADS050, SVP; 079, FLC; 096, MAF; 077, FLC; 078, FLC and 104, MAF) had no response to GA (Fig. 8a). Under SA treatment, BrMADS023, 043, and 041, which are TM3-like ortholog genes, were upregulated at 4 and 12 h. BrMADS079, FLC; 096, MAF; 104, MAF; 078, FLC; 077, FLC and 100, MAF were found with no response to SA treatment (Fig. 8b).
Under the other three abiotic stress treatments, most BrMADS genes have relatively low expression levels (Fig. 7b), whereas several genes were significantly upregulated in these different treatment conditions (Fig. 8c–e). Under cold stress, the MAF ortholog, BrMADS gene BrMADS100, was found with a high expression at 4 h but was downregulated at 12 h. The expression of two BrMADS genes (BrMADS001, AG and 023, SOC) was over 20 times that of the control at 4 and 12 h. In contrast, two genes (BrMADS043, SOC and 036, SOC) were downregulated at 4 and 12 h. Under ABA stress, the relative expression levels of six BrMADS genes (BrMADS063, AGL17; BrMADS043, SOC; BrMADS083, AGL18; BrMADS050, SVP; BrMADS036, SOC and BrMADS041, SOC) were upregulated at 4 h and then decreased rapidly at 12 h. Under heat stress, the expression levels of five BrMADS genes were upregulated; among these genes, BrMADS063, AGL17 and BrMADS050, SVP had a higher expression levels at 4 h than at 12 h. Interestingly, BrMADS043, SOC; BrMADS019, CAL and BrMADS036, SOC had opposite results.
From the results above, 45 duplicated genes were found in the Chinese cabbage MADS-box gene family (Fig. 5 and Supplementary Fig. 8). Based on the expression levels of these genes (34 BrMIKC genes) under different stresses in Chinese cabbage, a line chart was used to show the different trends among them (Fig. 9 and Supplementary Table 13). Interestingly, we found four gene pairs with similar expression, including BrMADS (008:009; 034:035; 017:015; 077:079). Finally, the comparison of expression profiles of the duplicated gene pairs revealed that most duplicated genes had diverged significantly in expression level except for the above-mentioned four gene pairs.
Discussion
Chinese cabbage is one of the most important vegetables that are cultivated worldwide. For successful breeding of biennial plants like Chinese cabbage, flowering time and floral organ development are important traits. MADS-box genes are important regulators of both flowering and floral organ development, and are thus key factors for the breeding of Chinese cabbage. Based on sequence similarities and the complete genome sequence, attempts have been made to predict the function of MADS-box genes in diverse species (Wang et al. 2011). The completion of the B. rapa genome sequence also enabled us to predict a function for the BrMADS genes, based on results of phylogenetic analyses and their high sequence similarity with their Arabidopsis orthologs. In this study, we have presented a number of Chinese cabbage MADS-box gene phylogenetic relationships with Arabidopsis genes, a detailed analysis regarding the MADS-box genes that were collected among 22 species and the expression of BrMADS genes.
First, we identified 160 genes as Chinese cabbage MADS-box family genes. These genes were classified into five subfamilies, including MIKCC, MIKC*, Mα, Mβ and Mγ. Furthermore, the subfamily MIKC can also be divided into 13 subgroups, similar to that of Arabidopsis and rice MIKC genes. However, the number of each subgroup in Chinese cabbage is different compared with Arabidopsis and rice. This result implies that the retention of duplicates in the different clades has been differently in the species, such as Arabidopsis, rice, grapevine and Chinese cabbage. Thus, different groups of MADS-box genes with different gene numbers are under different evolutionary constraints (Nam et al. 2003; Airoldi and Davies 2012).
Most higher organisms pass through different ploidy levels at different development stages (Tang et al. 2008). Genome duplication has permanently shaped the architecture and function of many higher eukaryotic genomes. In the course of evolution, some duplicated genes have been retained, whereas others lost their function (Nakano et al. 2006). Generally, transcriptional regulators within the same group have recent common evolutionary origins and share conserved motifs with similar molecular functions (Doebley and Lukens 1998; Theissen et al. 2000). To understand the evolution of the MADS-box transcription factors family, 1,848 genes were identified and analysed among 22 species in our research. Through the evolution of species, we found that angiosperms contain a relatively large number of MADS-box genes. Thus, some important events during the phylogeny of species, particularly spermatophytes, can be shown through the evolution of the MADS gene family. During the phylogeny of species, after the Gamma whole genome triplication (WGT) event, when the monocots and eudicots diverged, the Brassicaceae genome experienced two whole genome duplication (WGD) events, Arabidopsis beta and Arabidopsis alpha (Bowers et al. 2003). Later, the Chinese cabbage genome experienced one WGT event against Arabidopsis (Wang et al. 2011). Thus, the MADS-box genes experienced WGD events and WGT events, which may be the reason for the different numbers of MADS genes among different species. These genes are the products of two different types of duplications: type II genes have been mainly duplicated through whole genome duplications; type I genes have been mainly duplicated by smaller scale and more recent duplications (Airoldi and Davies 2012). In this study only the number of type II MADS-box genes in Chinese cabbage is more than that of Arabidopsis, and the number of type I genes is similar. For BrMADS genes, we find that type II MADS-box genes are preferentially retained relative to type I genes, whereas most of type I genes were not located in the syntenic regions (Supplementary Table 4). Therefore, type II MADS-box genes are conserved and have accumulated in the Chinese cabbage genome probably by natural and artificial selection. The evolution of the MADS gene family is probably related to its important role in plant growth and development, such as flowering and floral organ development.
Finally, the expression patterns of BrMIKC genes under stresses were identified based on the qRT-PCR analysis. The MADS-box gene family functions in almost every developmental process in plants, and because plants are in the external environment exposed to various abiotic stresses, the MADS-box genes may be affected by some of these stresses. In previous reports, MADS-box genes have been shown to be affected by low temperature stress in tomato (Lozano et al. 1998) and by application of hormones like GA (Moon et al. 2003a, b), SA (Martínez et al. 2004) and ABA (Puig et al. 2013) in other plants. GA functions not only to promote the growth of plant organs but also to induce phase transitions during development. GA promotes flowering in Arabidopsis through the activation of genes that encode the floral integrator Suppressor of Overexpression of Constans 1 (SOC1) in the inflorescence, in floral meristems, and in leaves (Mutasa-Göttgens and Hedden 2009). SA also regulates flowering in non-stressed plants because SA-deficient plants are late flowering. The regulation of the flowering time by SA seems to involve the photoperiod and autonomous pathways; however, SA regulation of flowering time does not require the function of the flowering time gene Flowering Locus C (FLC) (Martínez et al. 2004). In this study, we tested the stress-responsiveness of the BrMADS genes. We found that some SOC1 ortholog genes were highly expressed during GA and SA treatments, while the MAF and FLC ortholog genes were not affected. AGL17 may control plant vegetative development (Zhang and Forde 1998) and was induced by ABA treatment (Puig et al. 2013). We found that the AGL17 ortholog gene BrMADS063 was not only highly expressed under ABA (4 h) treatments but also highly expressed under heat (4 h) treatments. The results suggested that BrMADS063 might be affected by heat regulation. The MAF ortholog gene BrMADS100 was found to have high expression under cold (4 h) but was downregulated at 12 h. Therefore, we speculated that this gene might respond to cold stress. These results show that BrMADS genes may also function in the response to abiotic stresses, an interesting observation that has to be functionally explored further in the future.
In summary, 160 MADS-box transcription factors, including 65 type I and 95 type II genes, were identified in the entire Chinese cabbage genome. Type II MADS genes have been preferentially retained over type I genes, consistent with the gene dosage hypothesis. The isolation and identification of these transcription factors are likely to assist in clarifying the molecular genetic basis for Chinese cabbage genetic improvement and to provide functional gene resources for transgenic research. A comparison of phylogenetic relationships between Chinese cabbage and Arabidopsis MADS-box genes suggested that although most of the basic subfamilies have been retained in Chinese cabbage, the number of each subfamily is different. The distributions and divergence of the duplicated gene pairs in BrMADSs supported the recent triplicated copies of the Chinese cabbage genome In addition, these analyses may also provide new opportunities to discover the tolerance mechanisms of the Chinese cabbage MIKC gene under stress conditions. The bioinformatics analysis results provides basic resources to examine the molecular regulation of Chinese cabbage development and stress resistance. The new information that has been generated is expected to help in the selection of appropriate candidate genes for further functional characterisation.
References
Adamczyk BJ, Lehti-Shiu MD, Fernandez DE (2007) The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J 50:1007–1019
Airoldi CA, Davies B (2012) Gene duplication and the evolution of plant MADS-box transcription factors. J Genet Genomics 39:157–165
Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara-Silva F, Yanofsky MF (2000) MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. Plant J 24:457–466
Arora R, Agarwal P, Ray S, Singh A, Singh V, Tyagi A, Kapoor S (2007) MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genom 8:242
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208
Becker A, Theißen G (2003) The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 29:464–489
Bemer M, Heijmans K, Airoldi C, Davies B, Angenent GC (2010) An atlas of type I MADS box gene expression during female gametophyte and seed development in Arabidopsis. Plant Physiol 154:287–300
Bezerra IC, Michaels SD, Schomburg FM, Amasino RM (2004) Lesions in the mRNA cap-binding gene ABA HYPERSENSITIVE 1 suppress FRIGIDA-mediated delayed flowering in Arabidopsis. Plant J 40:112–119
Birchler JA, Veitia RA (2007) The gene balance hypothesis: from classical genetics to modern genomics. Plant Cell 19:395–402
Bowers JE, Chapman BA, Rong J, Paterson AH (2003) Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422:433–438
Cheng F, Wu J, Fang L, Wang X (2012) Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Front Plant Sci. 3:198
Cheng F, Mandáková T, Wu J, Xie Q, Lysak MA, Wang XW (2013) Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa. Plant Cell 25:1541–1554
Cho S, Jang S, Chae S, Chung KM, Moon YH, An G, Jang SK (1999) Analysis of the C-terminal region of Arabidopsis thaliana APETALA1 as a transcription activation domain. Plant Mol Biol 40:419–429
Colombo M, Masiero S, Vanzulli S, Lardelli P, Kater MM, Colombo L (2008) AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J 54:1037–1048
De Bodt S, Raes J, Van de Peer Y, Theißen G (2003) And then there were many: MADS goes genomic. Trends Plant Sci 8:475–483
Díaz-Riquelme J, Lijavetzky D, Martínez-Zapater JM, Carmona MJ (2009) Genome-wide analysis of MIKCC-type MADS box genes in grapevine. Plant Physiol 149:354–369
Doebley J, Lukens L (1998) Transcriptional regulators and the evolution of plant form. Plant Cell 10:1075–1082
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39:W29–W37
Friedman R, Hughes AL (2001) Gene duplication and the structure of eukaryotic genomes. Genome Res 11:373–381
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6:986–994
Henschel K, Kofuji R, Hasebe M, Saedler H, Münster T, Theißen G (2002) Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol Biol Evol 19:801–814
Hong JK, Kim SY, Kim KS, Kwon SJ, Kim JS, Kim JA, Lee SI, Lee YH (2012) Overexpression of a Brassica rapa MADS-box gene, BrAGL20, induces early flowering time phenotypes in Brassica napus. Plant Biotechnol Rep 7:231–237
Honma T, Goto K (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409:525–529
Jensen R, Bassham J (1966) Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci USA 56:1095
Kofuji R, Sumikawa N, Yamasaki M, Kondo K, Ueda K, Ito M, Hasebe M (2003) Evolution and divergence of the MADS-box gene family based on genome-wide expression analyses. Mol Biol Evol 20:1963–1977
Köhler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U (2003) The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Gene Dev 17:1540–1553
Krzywinski M, Schein J, Birol İ, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645
Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH (2007) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Gene Dev 21:397–402
Lee TH, Tang H, Wang X, Paterson AH (2013) PGDD: a database of gene and genome duplication in plants. Nucleic Acids Res 41:D1152–D1158
Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40:D302–D305
Li L, Stoeckert CJ, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13:2178–2189
Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404:766–770
Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han JH, Liou YC, Yu H (2008) Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135:1481–1491
Liu T, Li Y, Ren J, Zhang C, Kong M, Song X, Zhou J, Hou X (2013) Over-expression of BcFLC1 from non-heading Chinese cabbage enhances cold tolerance in Arabidopsis. Biol Plantarum 57:262–266
Lozano R, Angosto T, Gomez P, Payan C, Capel J, Huijser P, Salinas J, Martinez-Zapater JM (1998) Tomato flower abnormalities induced by low temperatures are associated with changes of expression of MADS-Box genes. Plant Physiol 117:91–100
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155
Martínez C, Pons E, Prats G, León J (2004) Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 37:209–217
Masiero S, Colombo L, Grini PE, Schnittger A, Kater MM (2011) The emerging importance of type I MADS box transcription factors for plant reproduction. Plant Cell 23:865–872
Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11:949–956
Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim SG, Lee I (2003a) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35:613–623
Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim SG, Lee I (2003b) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35:613–623
Mutasa-Göttgens E, Hedden P (2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60:1979–1989
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Nam J, Ma H, Nei M (2003) Antiquity and evolution of the MADS-box gene family controlling flower development in plants. Mol Biol Evol 20:1435–1447
Nesi N, Debeaujon I, Jond C, Stewart AJ, Jenkins GI, Caboche M, Lepiniec L (2002) The TRANSPARENT TESTA16 locus encodes the ARABIDOPSIS BSISTER MADS domain protein and is required for proper development and pigmentation of the seed coat. Plant Cell 14:2463–2479
Norman C, Runswick M, Pollock R, Treisman R (1988) Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell 55:989–1003
Parenicová L, de Folter S, Kieffer M, Horner DS, Favalli C, Busscher J, Cook HE, Ingram RM, Kater MM, Davies B (2003) Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis new openings to the MADS world. Plant Cell 15:1538–1551
Passmore S, Maine GT, Elble R, Christ C, Tye BK (1988) Saccharomyces cerevisiae protein involved in plasmid maintenance is necessary for mating of MATα cells. J Mol Biol 204:593–606
Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF (2003) Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424:85–88
Puig J, Meynard D, Khong GN, Pauluzzi G, Guiderdoni E, Gantet P (2013) Analysis of the expression of the AGL17-like clade of MADS-box transcription factors in rice. Gene Expr Patterns 13:160–170
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J (2012) The Pfam protein families database. Nucleic Acids Res 40:D290–D301
Pylatuik JD, Lindsay DL, Davis AR, Bonham-Smith PC (2003) Isolation and characterization of a Brassica napus cDNA corresponding to a B-class floral development gene. J Exp Bot 54:2385–2387
Ratcliffe OJ, Kumimoto RW, Wong BJ, Riechmann JL (2003) Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15:1159–1169
Riechmann JL, Meyerowitz EM (1997) MADS domain proteins in plant development. Biol Chem 378:1079–1102
Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288:1613–1616
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Sommer H, Beltran JP, Huijser P, Pape H, Lönnig W, Saedler H, Schwarz-Sommer Z (1990) Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J 9:605
Song XM, Huang ZN, Duan WK, Ren J, Liu TK, Li Y, Hou XL (2013) Genome-wide analysis of the bHLH transcription factor family in Chinese cabbage (Brassica rapa ssp. pekinensis). Mol Genet Genomics 289:77–91
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH (2008) Synteny and collinearity in plant genomes. Science 320:486–488
Tapia-López R, García-Ponce B, Dubrovsky JG, Garay-Arroyo A, Pérez-Ruíz RV, Kim SH, Acevedo F, Pelaz S, Alvarez-Buylla ER (2008) An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol 146:1182–1192
Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, Winter KU, Saedler H (2000) A short history of MADS-box genes in plants. Plant Mol Biol 42:115–149
Thomas BC, Pedersen B, Freeling M (2006) Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Res 16:934–946
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tong C, Wang X, Yu J, Wu J, Li W, Huang J, Dong C, Hua W, Liu S (2013) Comprehensive analysis of RNA-seq data reveals the complexity of the transcriptome in Brassica rapa. BMC Genom 14:689
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Wilmowicz E, Kęsy J, Kopcewicz J (2008) Ethylene and ABA interactions in the regulation of flower induction in Pharbitis nil. J Plant Physiol 165:1917–1928
Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM (1990) The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346:35–39
Zahn LM, Feng B, Ma H (2006) Beyond the ABC-model: regulation of Floral Homeotic Gene. Adv Bot Res 44:163–207
Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279:407–409
Zhang Z, Li J, Zhao XQ, Wang J, Wong GK, Yu J (2006) KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinform 4:259–263
Acknowledgments
This work is supported by the National Basic Research Program of China (973 Program, 2012CB113903), the Fundamental Research Funds for the Central Universities of China (KYZ201111), the National Natural Science Foundation of China (31272173, 1301782) and the Jiangsu Province Natural Science Foundation (BK20130673).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, W., Song, X., Liu, T. et al. Genome-wide analysis of the MADS-box gene family in Brassica rapa (Chinese cabbage). Mol Genet Genomics 290, 239–255 (2015). https://doi.org/10.1007/s00438-014-0912-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-014-0912-7