Abstract
To date, epidemiological studies have assessed the association between CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility. However, the results of these studies remained controversial. We aimed to examine the associations by conducting a meta-analysis of case–control studies. A total of 11 studies including 5,093 cases and 5,941 controls evaluated the association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility. No significantly associations were found in all genetic models (CC vs. AA: OR = 1.14, 95 % CI = 0.93–1.40; AC vs. AA: OR = 1.05, 95 % CI = 0.91–1.20; dominant model: OR = 1.08, 95 % CI = 0.95–1.24; recessive model: OR = 1.10, 95 % CI = 0.95–1.28). In the subgroup analysis by ethnicity or source of controls, there were still no significant associations detected in all genetic models. This meta-analysis suggested the CYP1A2-164 A/C polymorphism was not a risk factor for increasing colorectal cancer, further large and well-designed studies are needed to confirm these conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most commonly diagnosed human cancer in the world, with over 143,820 new cancer cases and 50,830 deaths estimated to be occurred in the US in 2013 (Siegel et al. 2013). Modifiable risk factors for colorectal cancer include smoking, physical inactivity, overweight and obesity, red and processed meat consumption, and excessive alcohol consumption (Ferrari et al. 2007). Genetic susceptibility to this disease may result from inherited mutations in genes involved in carcinogen metabolism and DNA repair (Shields and Harris 2000; Goode et al. 2002). It is now commonly accepted that the pathogenesis of colorectal cancer involves the multi-factorial interactions of environmental triggers and genetic susceptibility. A recent study has revealed that approximately 35 % of colorectal cancer cases can be attributed to inherited genetic susceptibility (Markowitz and Bertagnolli 2009).
In recent years, several common low-penetrant genes have been identified as potential colorectal cancer susceptibility genes. Cytochrome P450 enzymes catalyze Phase I metabolism reactions, such as C-, N- and S-oxidation and dealkylation (Sergentanis et al. 2011). Cytochrome P450 1A2 (CYP1A2) is a member of the CYP1 family and it is an important gene in catalyzing 2- and 4-hydroxylations of estrogens (Yamazaki et al. 1998; Nebert and Dalton 2006) and metabolism of carcinogens (Nebert et al. 2004). It is reasonable that CYP1A2 may play an important role in the etiology of colorectal cancer. A single nucleotide polymorphism (CYP1A2-164 A/C or CYP1A2*1F, rs762551) in intron 1 of the CYP1A2 gene at position 734 downstream of the first transcribed nucleotide was identified. The A to C base substitution might influence the inducibility and activity of CYP1A2 (Sachse et al. 1999).
To date, molecular epidemiological studies (Sachse et al. 2002; Landi et al. 2005; Saebø et al. 2008; Rudolph et al. 2011; Eichholzer et al. 2012) have investigated the relationship between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility. However, results of these studies were controversial. Therefore, we performed this meta-analysis of all eligible studies to demonstrate the effect of the CYP1A2-164 A/C polymorphism on colorectal cancer susceptibility.
Materials and methods
Publication search
Prospective cohort and case–control studies on CYP1A2-164 A/C polymorphism and the susceptibility of colorectal cancer published before Oct 13, 2013 were identified through computer-based searches of PubMed, Embase, and Web of Science electronic databases using the terms “cytochrome P-450 1A2”, “CYP1A2”, “CYP1A2*1F”, “polymorphism” and “colon”, “rectum”, “colorectal”, “cancer”, “carcinoma”. All searched studies were retrieved, and their bibliographies were checked for other relevant publications. Review articles and bibliographies of other relevant studies identified were hand searched to find additional eligible studies. Only published studies with full-text articles were included. When more than one of the same patient population was included in several publications, only the most recent or complete study was used in this meta-analysis.
Inclusion criteria
Inclusion criteria were defined as follows: (1) the articles evaluated the association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility, (2) the studies designed as prospective cohorts or case–controls, (3) sufficient data available to estimate an odds ratio (OR) with its 95 % CI, and (4) studies demonstrated that the distribution of genotypes among controls were in Hardy–Weinberg equilibrium.
Data extraction
Information was extracted carefully from all eligible publications independently by two authors according to the inclusion criteria listed above, discrepancies were adjudicated by the other authors until consensus was achieved on every item. For each study, the following characteristics were collected: the first author’s name, country or region, year of publication, study design, method of genotyping, total numbers of cases and controls, and numbers of cases and controls who harbored the CYP1A2-164 A/C polymorphism. The quality of included studies was assessed using the Newcastle-Ottawa Scale (NOS) (Stang 2010) for quality of case control and cohort studies in this meta-analyses, a study awarded seven or more stars was considered as a high-quality study.
Statistical analysis
The strength of association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility were assessed by OR with the corresponding 95 % CI. Although fixed-effect model and random-effects model yielded similar conclusions, we chose to use the random-effects model with Mantel–Haenszel statistics (DerSimonian and Laird 1986; Ades et al. 2005), which assumed that the true underlying effect varied among included individuals. The pooled ORs were performed for co-dominant model (CC vs. AA, AC vs. AA), dominant model (CC + AC vs. AA), and recessive model (CC vs. AA + AC) respectively. Heterogeneity assumptions among studies were checked by the Chi-square test based on Q-statistic (p < 0.05 indicated heterogeneity) (Cochran 1954). Furthermore, we measured the effect of heterogeneity by another measure, I 2 = 100 % ×(Q − df)/Q (Higgins and Thompson 2002). Venice criteria (Ioannidis et al. 2008) for the I 2 test included: ‘I 2 < 25 % represents no heterogeneity, I 2 = 25–50 % represents moderate heterogeneity, I 2 = 50–75 % represents large heterogeneity, and I 2 > 75 % represents extreme heterogeneity’. Funnel plots were used to access publication bias by the method of Begg’s test (Begg and Mazumdar 1994) and Egger’s test (Egger et al. 1997) (p ≥ 0.05 suggests no bias). Statistical analyses were performed using STATA statistical software (version 10.0). A p value <0.05 was considered statistically significant, and all the p values were two sided.
Results
Characteristics of studies
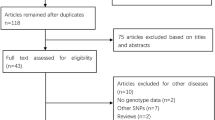
The study selection process is shown in Fig. 1. A total of 11 eligible studies including 5,093 cases and 5,941 controls met the inclusion criteria (Sachse et al. 2002; Landi et al. 2005; Bae et al. 2006; Kiss et al. 2007; Küry et al. 2007; Yoshida et al. 2007; Saebø et al. 2008; Kobayashi et al. 2009; Cleary et al. 2010; Rudolph et al. 2011; Eichholzer et al. 2012). We established a database according to the extracted information from each article. The characteristics of selected studies were summarized in Table 1. There were three studies of Asians, eight studies of Caucasians. Among these studies, seven were hospital-based and four were population based. Controls were mainly healthy populations and matched for age. Genotypes distribution in the controls of each study was in agreement with Hardy–Weinberg equilibrium.
Quantitative analysis
Table 2 lists the main results of this meta-analysis. Overall, no significantly elevated colorectal cancer risk was found in all genetic models when all studies were pooled into the meta-analysis (CC vs. AA: OR = 1.14, 95 % CI = 0.93–1.40, p = 0.06 for heterogeneity, Fig. 2a; AC vs. AA: OR = 1.05, 95 % CI = 0.91–1.20, p = 0.01 for heterogeneity, Fig. 2b; dominant model: OR = 1.08, 95 % CI = 0.95–1.24, p = 0.00 for heterogeneity, Fig. 2c; recessive model: OR = 1.10, 95 % CI = 0.95–1.28, p = 0.30 for heterogeneity, Fig. 2d). In the subgroup analysis by ethnicity or source of controls, there was still no significant association detected in all genetic models.
Sensitivity analysis
Sensitivity analysis was performed according to heterogeneity. We found heterogeneity for CA vs. AA (p = 0.01) and the dominant model (p = 0.00) of CYP1A2-164 A/C polymorphism in overall population, in the stratified analysis by ethnicity and source of control, no heterogeneity was found in Caucasian and population-based groups (Table 2).
Publication bias
Publication bias was examined using Begg’s funnel, the shape of the funnel plot seemed to be approximately symmetrical in the dominant model (Fig. 3a) and the recessive model (Fig. 3b), but there was some uncertainty because the symmetrical degrees were not content. Therefore, the Egger’s test based on linear regression of the standard normal deviate against its precision was used to test the funnel plot symmetry. The Egger’s test suggested that publication biases may not have a significant influence on the results of the CYP1A2-164 A/C polymorphism in the dominant model (p = 0.12), the recessive model (p = 0.79) and other models (data was not shown).
Discussion
The association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility had been studied extensively, but the results were inconsistent. A potential rationale behind these gene–cancer risk associations was that these genetic variants might result in alterations in phenotypes. This meta-analysis suggested that CYP1A2-164 A/C polymorphism was not associated with colorectal cancer susceptibility when all studies were pooled together. In the subgroup analysis by ethnicity or source of controls, there was still no significant association detected in all genetic models.
It was reported that C allele causing decreased activity of the encoded enzyme may lead to decreased metabolism of estradiol. Therefore, C allele carriers might potentially increase the colorectal cancer risk (Sachse et al. 2003). Actually, it might be not uncommon that the epidemiology results were not coincident with the results of functional study. Cancer development was a complicated process involving many genes, different genetic backgrounds might contribute to the discrepancy. The influence of the C allele might be decreased by the presence of other unidentified causal genes involved in colorectal cancer susceptibility.
There was a moderate heterogeneity of studies for CA vs. AA and the dominant model of the CYP1A2-164 A/C polymorphism in the overall population, but when we analysed by ethnicity and source of control, the heterogeneity disappeared in Caucasian and population-based groups. These results suggested that the heterogeneity might be partly due to ethnicity and lacking of sufficient data, large studies should be needed and subgroup should be performed such as according to smoking and other factors.
Some limitations of this meta-analysis should be acknowledged. First, a common limitation of meta-analysis was heterogeneity, heterogeneity was often caused by variation in the environmental and genetic background of study participants, which was unavoidable when combing many studies, and we found evidence of study heterogeneity in our study, presumably due to ethnicity and the small number of included studies. Second, in the subgroup analysis, the number of each subgroup was relatively small, not having enough statistical power to explore the real association. Third, only published articles were included in the meta-analysis, we cannot exclude the possibility of publication bias influencing the results of this meta-analysis, even though statistical analysis indicated no publication bias. Further, the results were based on unadjusted estimates, there would be a more precise estimation on the associations of CYP1A2-164 A/C polymorphism with colorectal cancer susceptibility if the ORs were adjusted for age, diet, tobacco, alcoholism, and other environmental factors, more studies with adjusted ORs are needed to further provide a more precise estimation.
In conclusion, this meta-analysis suggested that the CYP1A2-164 A/C polymorphism was not a risk factor for colorectal cancer susceptibility. Besides, large and adjusted estimates studies are warranted to validate the conclusion from this meta-analysis, furthermore, gene–gene and gene–environment interactions should also be considered, which may eventually lead to comprehensive understanding of the association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility.
References
Ades AE, Lu G, Higgins JP (2005) The interpretation of random-effects meta-analysis in decision models. Med Decis Making 25:646–654
Bae SY, Choi SK, Kim KR, Park CS, Lee SK, Roh HK, Shin DW, Pie JE, Woo ZH, Kang JH (2006) Effects of genetic polymorphisms of MDR1, FMO3 and CYP1A2 on susceptibility to colorectal cancer in Koreans. Cancer Sci 97:774–779
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Cleary SP, Cotterchio M, Shi E, Gallinger S, Harper P (2010) Cigarette smoking, genetic variants in carcinogen-metabolizing enzymes, and colorectal cancer risk. Am J Epidemiol 172:1000–1014
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Eichholzer M, Rohrmann S, Barbir A, Hermann S, Teucher B, Kaaks R, Linseisen J (2012) Polymorphisms in heterocyclic aromatic amines metabolism-related genes are associated with colorectal adenoma risk. Int J Mol Epidemiol Genet 3:96–106
Ferrari P, Jenab M, Norat T, Moskal A, Slimani N, Olsen A, Tjønneland A, Overvad K, Jensen MK, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Rohrmann S, Linseisen J, Boeing H, Bergmann M, Kontopoulou D, Trichopoulou A, Kassapa C, Masala G, Krogh V, Vineis P, Panico S, Tumino R, van Gils CH, Peeters P, Bueno-de-Mesquita HB, Ocké MC, Skeie G, Lund E, Agudo A, Ardanaz E, López DC, Sanchez MJ, Quirós JR, Amiano P, Berglund G, Manjer J, Palmqvist R, Van Guelpen B, Allen N, Key T, Bingham S, Mazuir M, Boffetta P, Kaaks R, Riboli E (2007) Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 121:2065–2072
Goode EL, Ulrich CM, Potter JD (2002) Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev 11:1513–1530
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Ioannidis JP, Boffetta P, Little J, O’Brien TR, Uitterlinden AG, Vineis P, Balding DJ, Chokkalingam A, Dolan SM, Flanders WD, Higgins JP, McCarthy MI, McDermott DH, Page GP, Rebbeck TR, Seminara D, Khoury MJ (2008) Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol 37:120–132
Kiss I, Orsós Z, Gombos K, Bogner B, Csejtei A, Tibold A, Varga Z, Pázsit E, Magda I, Zölyomi A, Ember I (2007) Association between allelic polymorphisms of metabolizing enzymes (CYP 1A1, CYP 1A2, CYP 2E1, mEH) and occurrence of colorectal cancer in Hungary. Anticancer Res 27:2931–2937
Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Sakamoto H, Yoshida T, Tsugane S (2009) Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of colorectal cancer: a hospital-based case-control study in Japan. Scand J Gastroenterol 44:952–959
Küry S, Buecher B, Robiou-du-Pont S, Scoul C, Sébille V, Colman H, Le Houérou C, Le Neel T, Bourdon J, Faroux R, Ollivry J, Lafraise B, Chupin LD, Bézieau S (2007) Combinations of cytochrome P450 gene polymorphisms enhancing the risk for sporadic colorectal cancer related to red meat consumption. Cancer Epidemiol Biomarkers Prev 16:1460–1467
Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, Guino E, Navarro M, de Oca J, Capellà G, Canzian F, Bellvitge Colorectal Cancer Study Group (2005) A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genom 15:535–546
Markowitz SD, Bertagnolli MM (2009) Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med 361:2449–2460
Nebert DW, Dalton TP (2006) The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6:947–960
Nebert DW, Dalton TP, Okey AB, Gonzalez FJ (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279:23847–23850
Rudolph A, Sainz J, Hein R, Hoffmeister M, Frank B, Försti A, Brenner H, Hemminki K, Chang-Claude J (2011) Modification of menopausal hormone therapy-associated colorectal cancer risk by polymorphisms in sex steroid signaling, metabolism and transport related genes. Endocr Relat Cancer 18:371–384
Sachse C, Smith G, Wilkie MJ, Barrett JH, Waxman R, Sullivan F, Forman D, Bishop DT, Wolf CR, Colorectal Cancer Study Group (2002) A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis 23:1839–1849
Sachse C, Brockmöller J, Bauer S, Roots I (1999) Functional significance of a C–>A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 47:445–449
Sachse C, Bhambra U, Smith G, Lightfoot TJ, Barrett JH, Scollay J, Garner RC, Boobis AR, Wolf CR, Gooderham NJ, Colorectal Cancer Study Group (2003) Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br J Clin Pharmacol 55:68–76
Saebø M, Skjelbred CF, Brekke Li K, Bowitz Lothe IM, Hagen PC, Johnsen E, Tveit KM, Kure EH (2008) CYP1A2 164 A–>C polymorphism, cigarette smoking, consumption of well-done red meat and risk of developing colorectal adenomas and carcinomas. Anticancer Res 28:2289–2295
Sergentanis TN, Economopoulos KP, Choussein S, Vlahos NF (2011) Cytochrome P450 1A1 gene polymorphisms and endometrial cancer risk: a meta-analysis. Int J Gynecol Cancer 21:323–331
Shields PG, Harris CC (2000) Cancer risk and low-penetrance susceptibility genes in gene-environment interactions. J Clin Oncol 18:2309–2315
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Yamazaki H, Shaw PM, Guengerich FP, Shimada T (1998) Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol 11:659–665
Yoshida K, Osawa K, Kasahara M, Miyaishi A, Nakanishi K, Hayamizu S, Osawa Y, Tsutou A, Tabuchi Y, Shimada E, Tanaka K, Yamamoto M, Takahashi J (2007) Association of CYP1A1, CYP1A2, GSTM1 and NAT2 gene polymorphisms with colorectal cancer and smoking. Asian Pac J Cancer Prev 8:438–444
Acknowledgments
This study was supported by Natural Science Foundation of The People’s Republic of China (No.81072175, 81102010, 81202096, 81372854), Shanghai Science and Technology Committee (No.114119a7500, No.06DZ19505 and No.13NM141504) and Chanhai Hospital 1255 discipline construction projects (No.CH125530400). The funders had no roles in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Hohmann.
J. Hu, C. Liu and Q. Yin contributed equally to this work and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Hu, J., Liu, C., Yin, Q. et al. Association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility: a meta-analysis. Mol Genet Genomics 289, 271–277 (2014). https://doi.org/10.1007/s00438-013-0806-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-013-0806-0